Crystal Organisation of Muscle Attachment Sites of Bivalved Marine Organisms: A Juxtaposition Between Brachiopod and Bivalved Mollusc Shells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methods
2.4. Terminology
3. Results
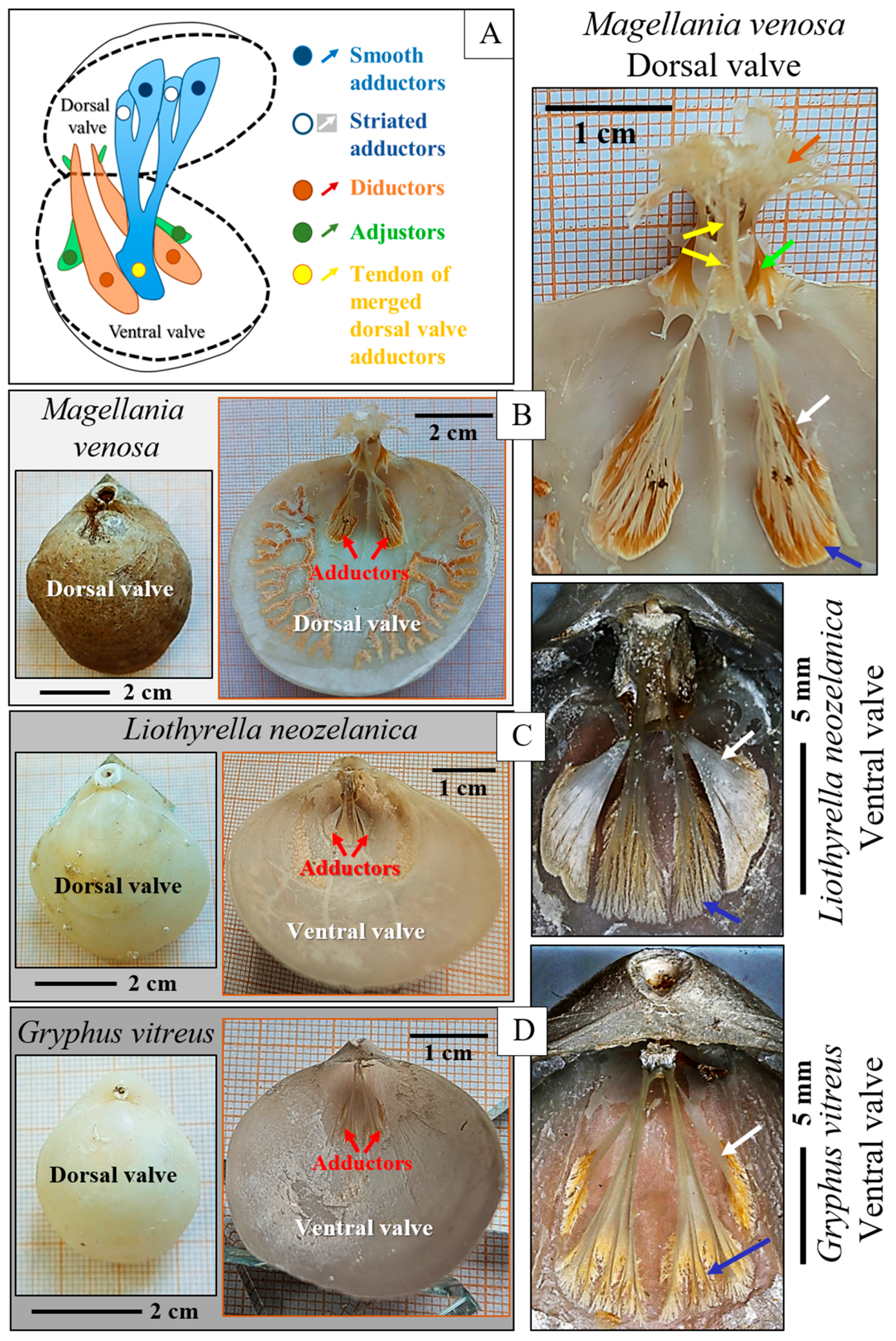
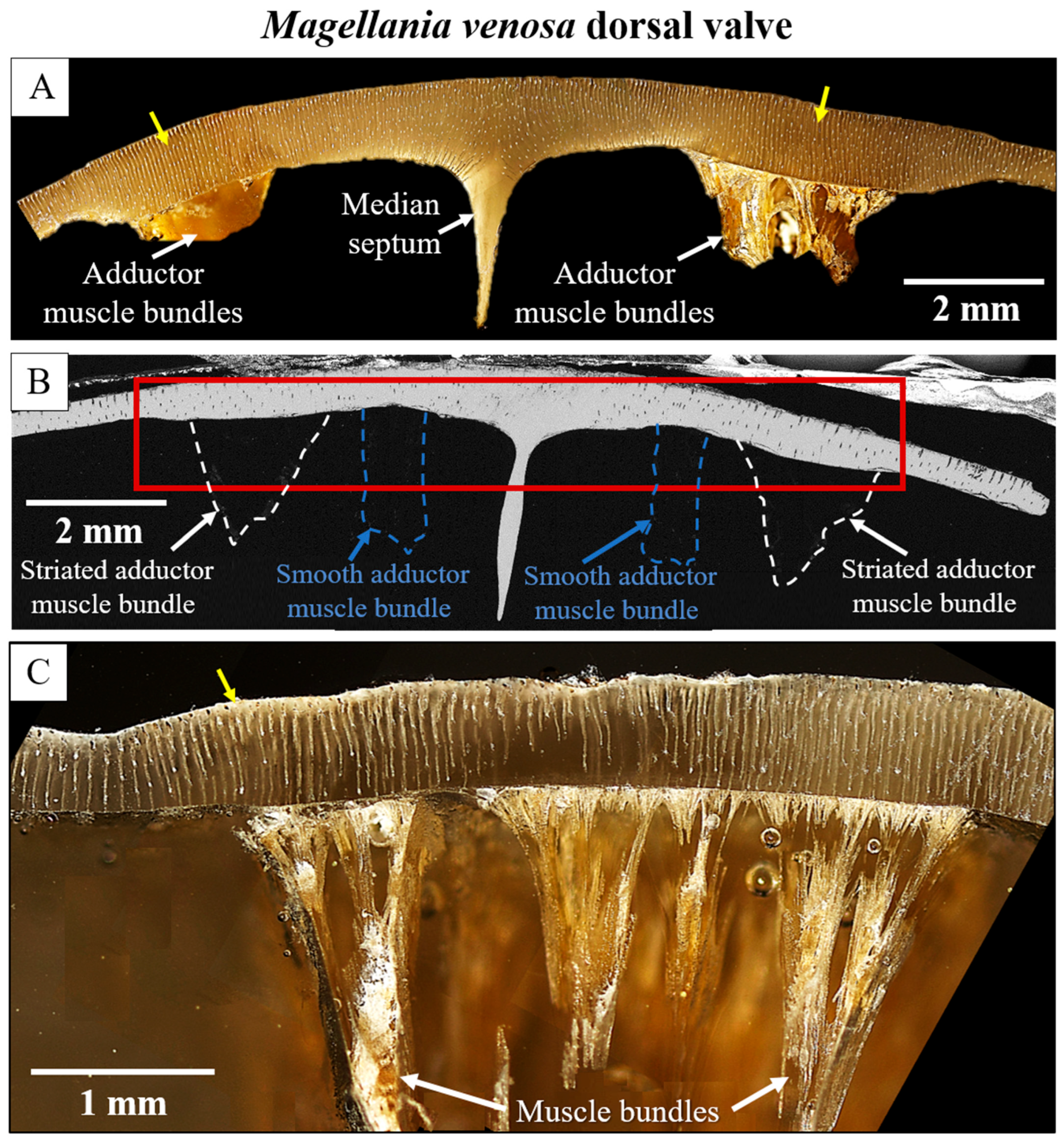
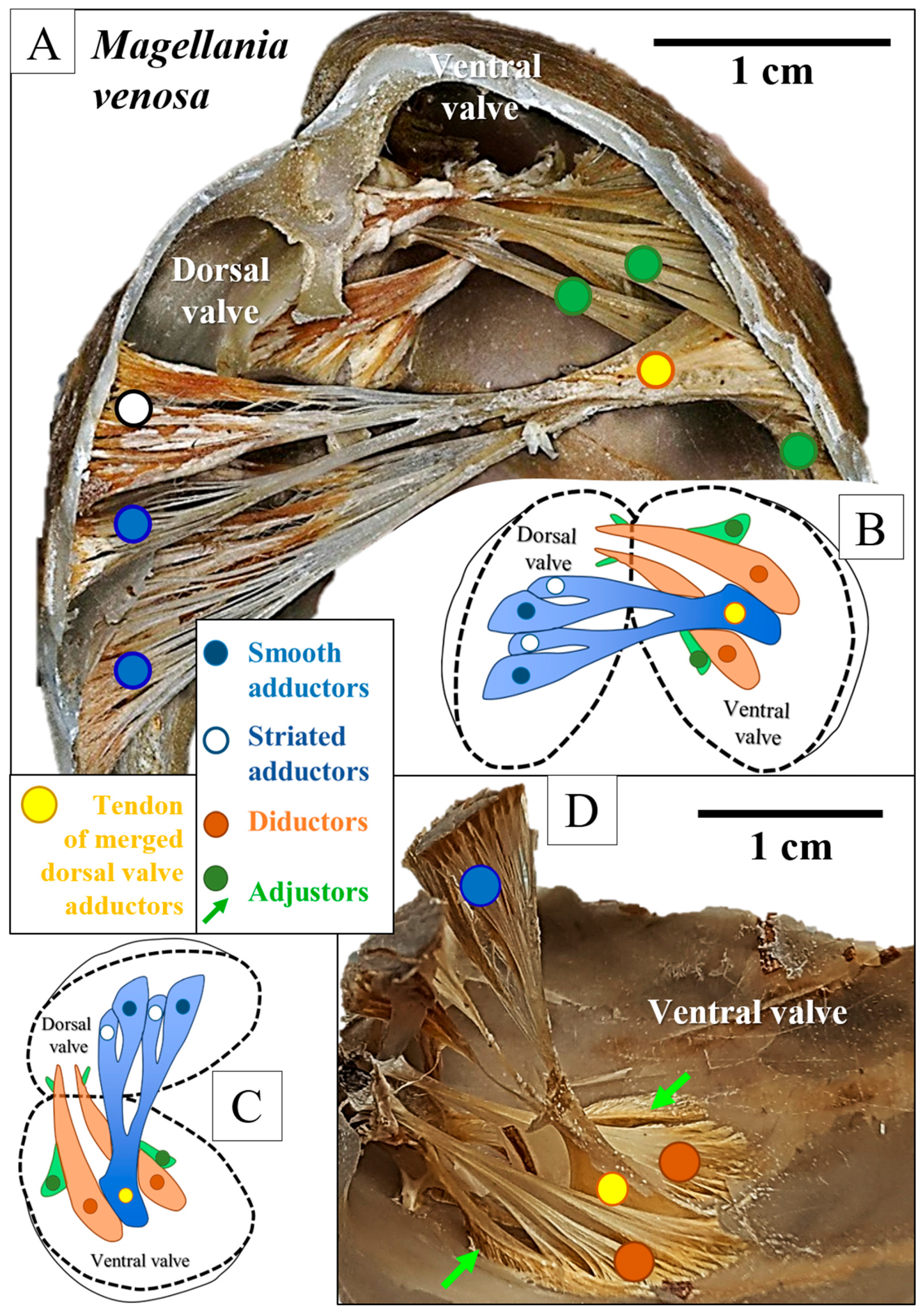
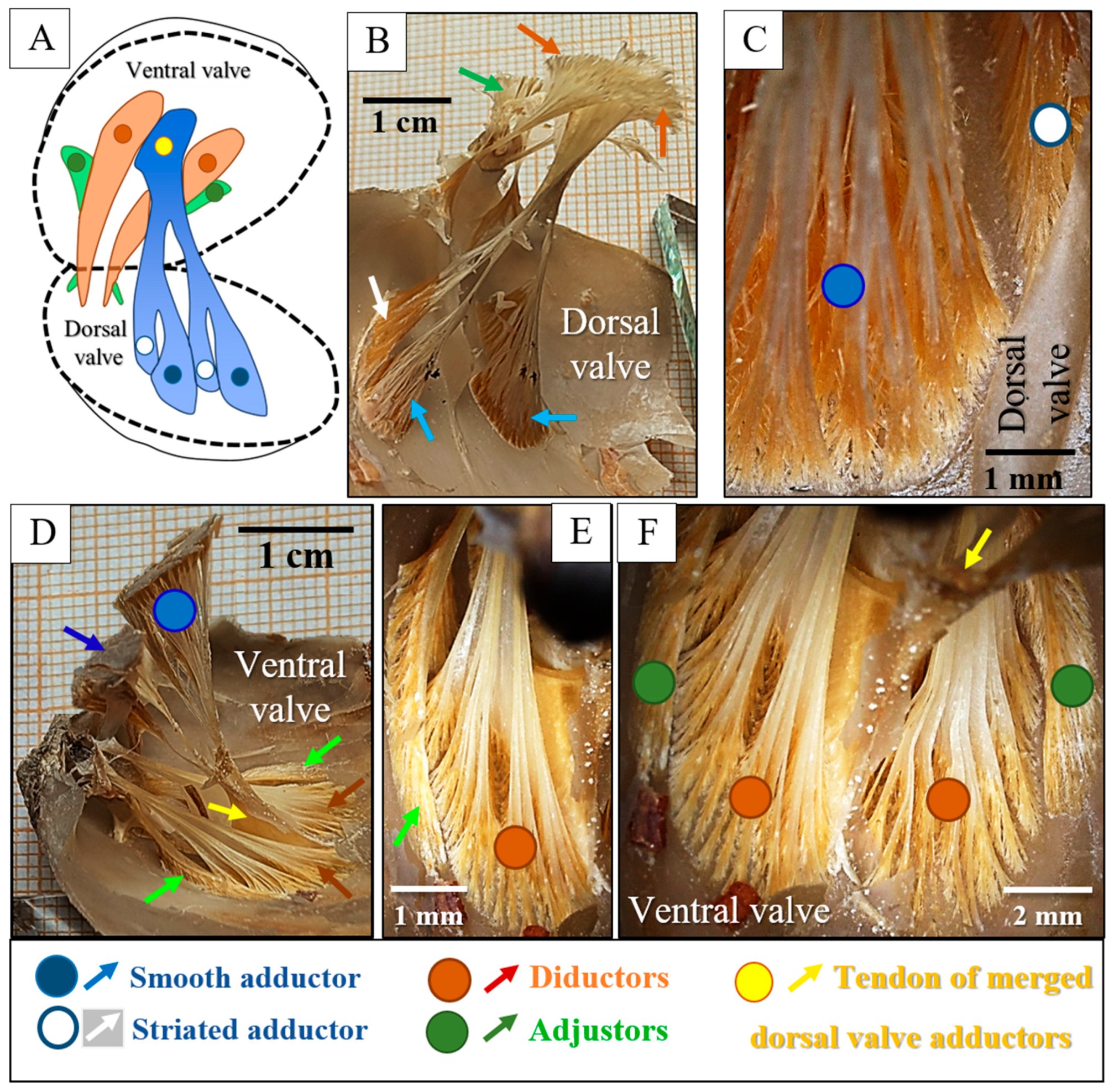
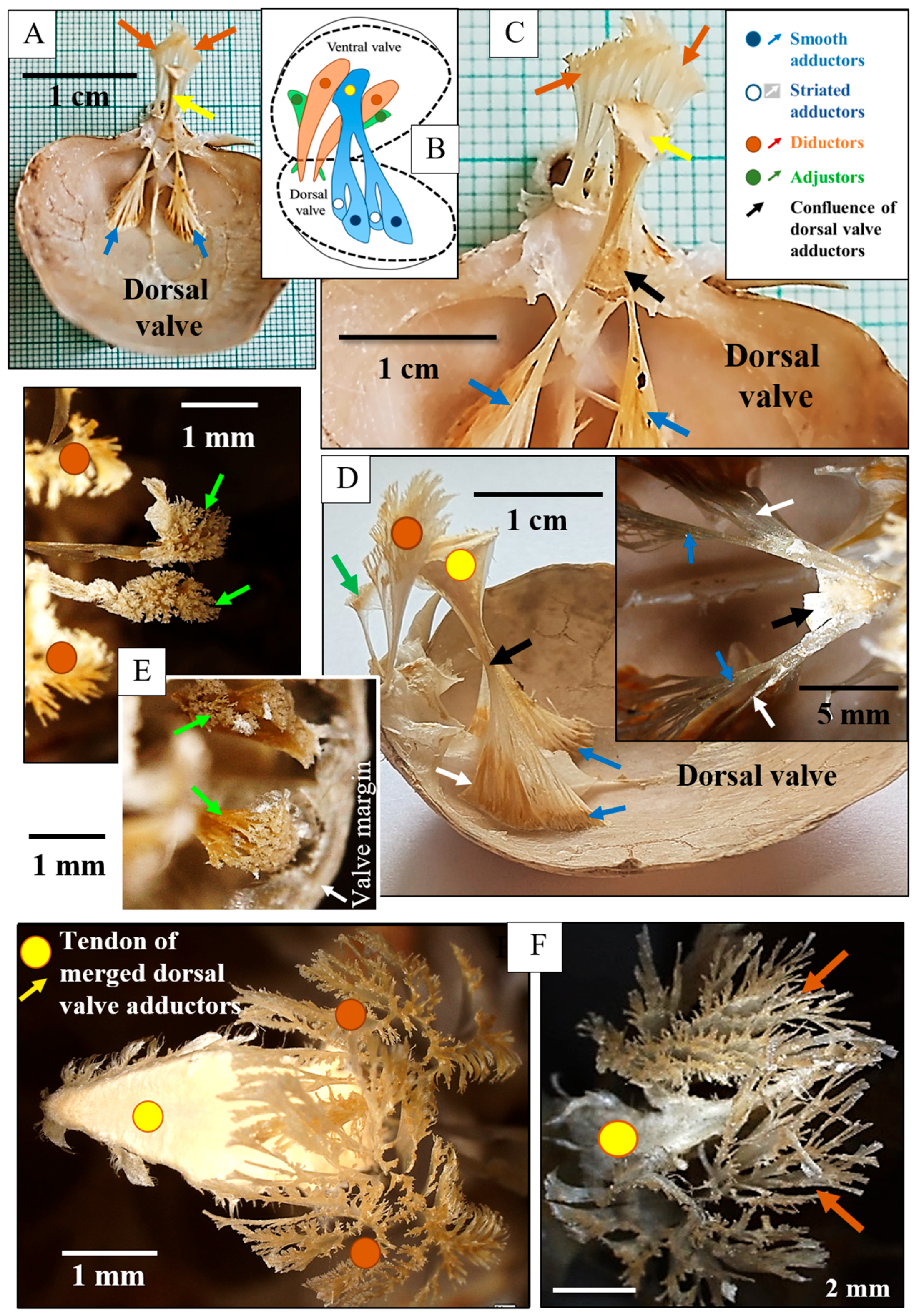
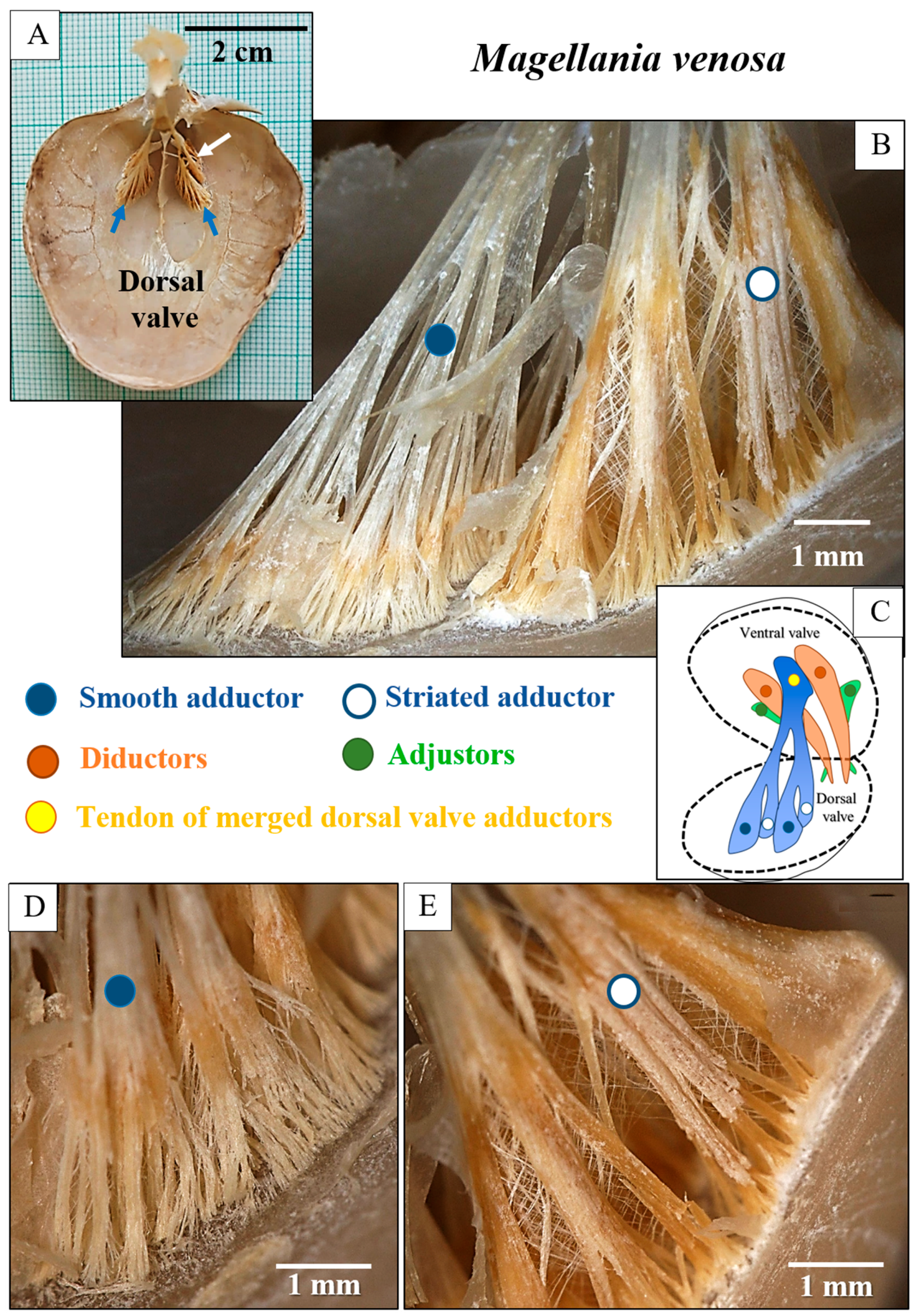

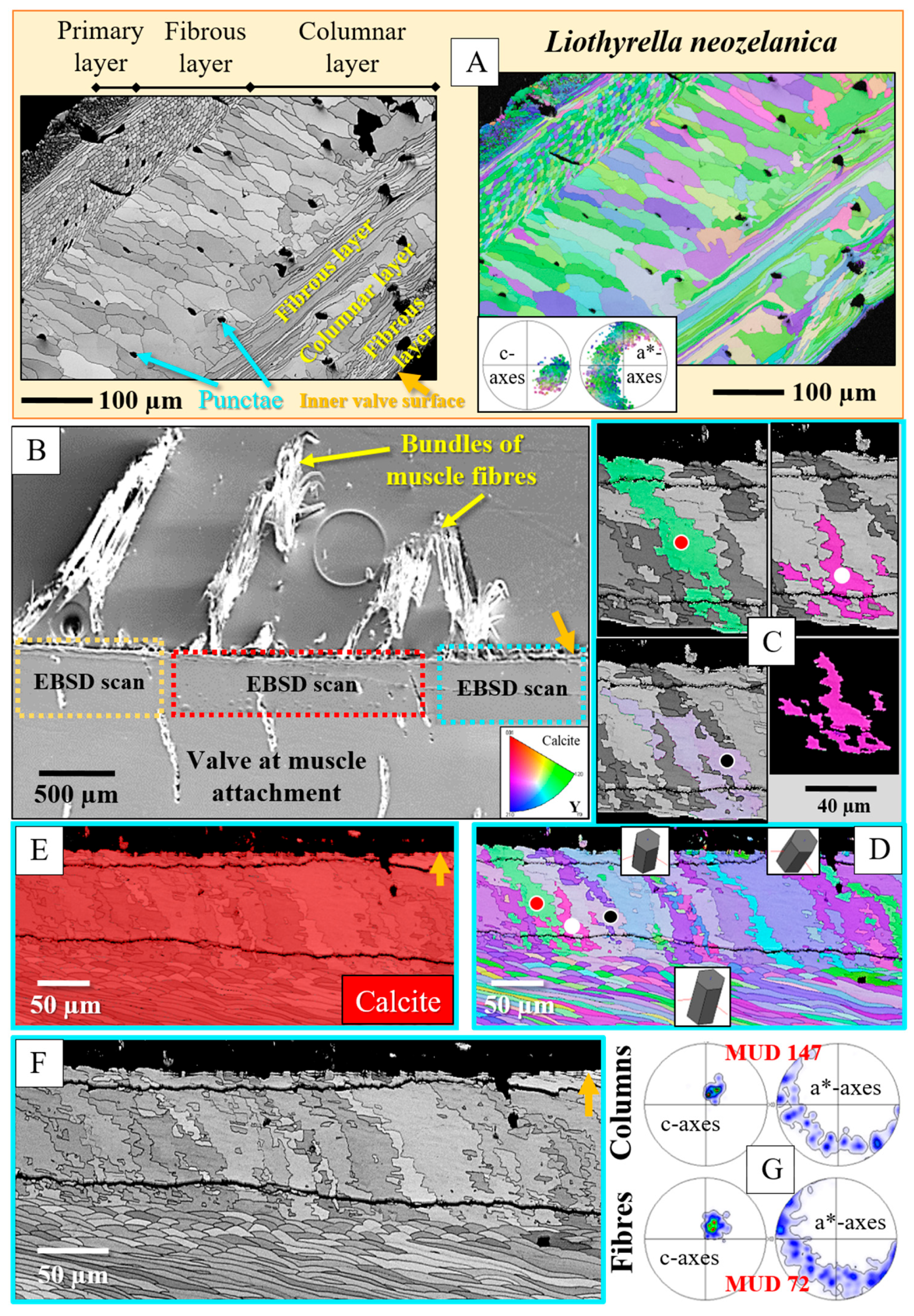
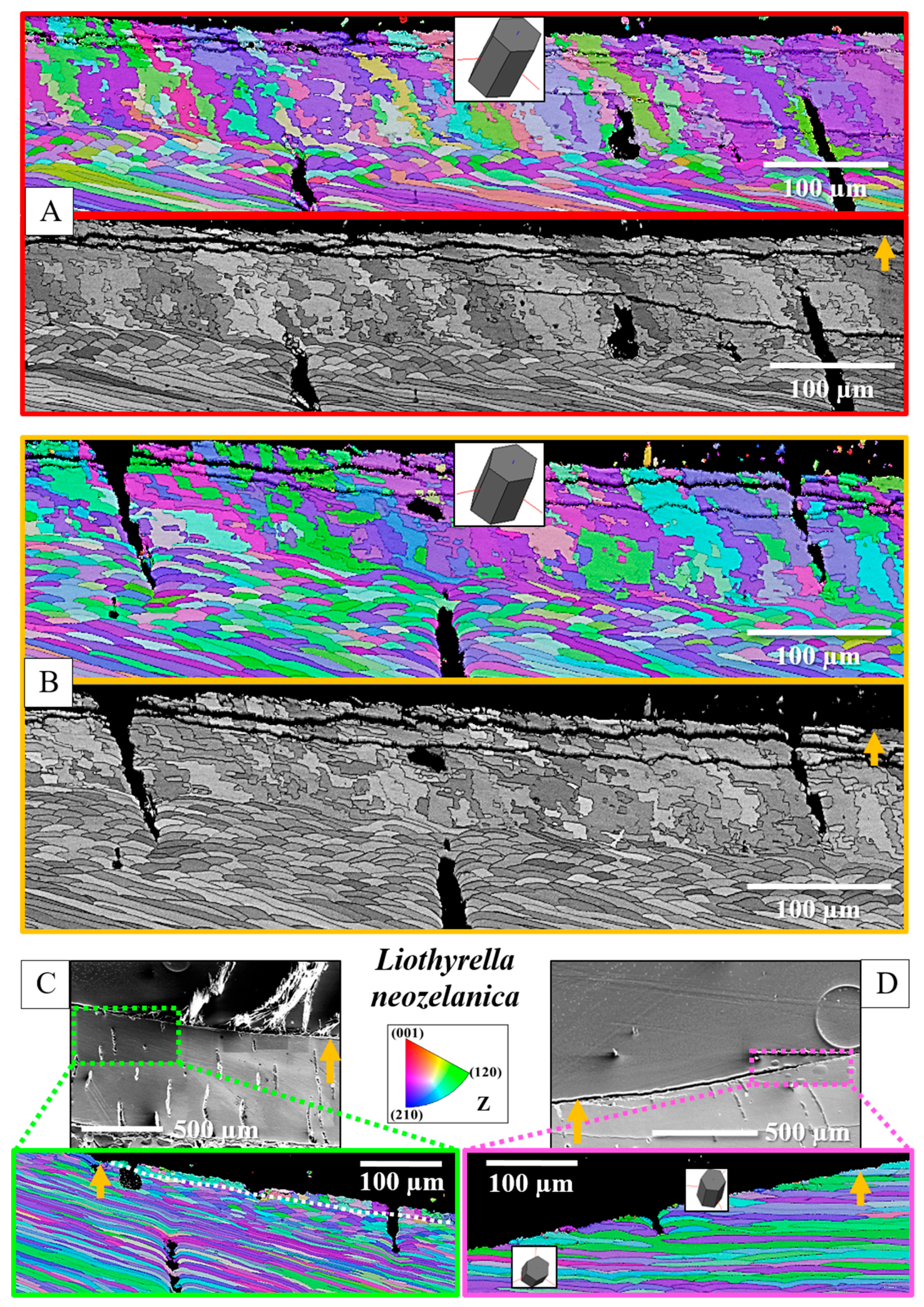
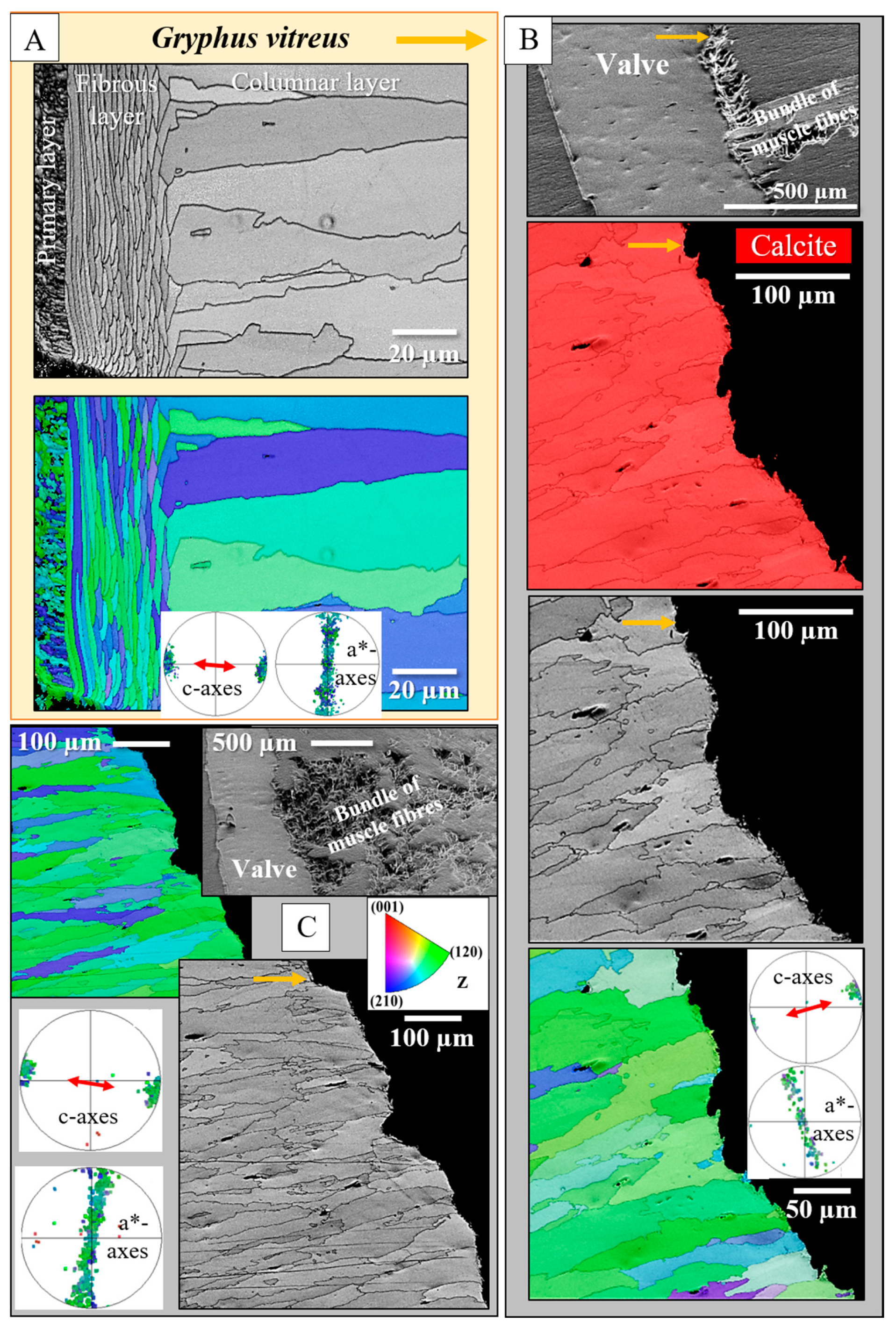
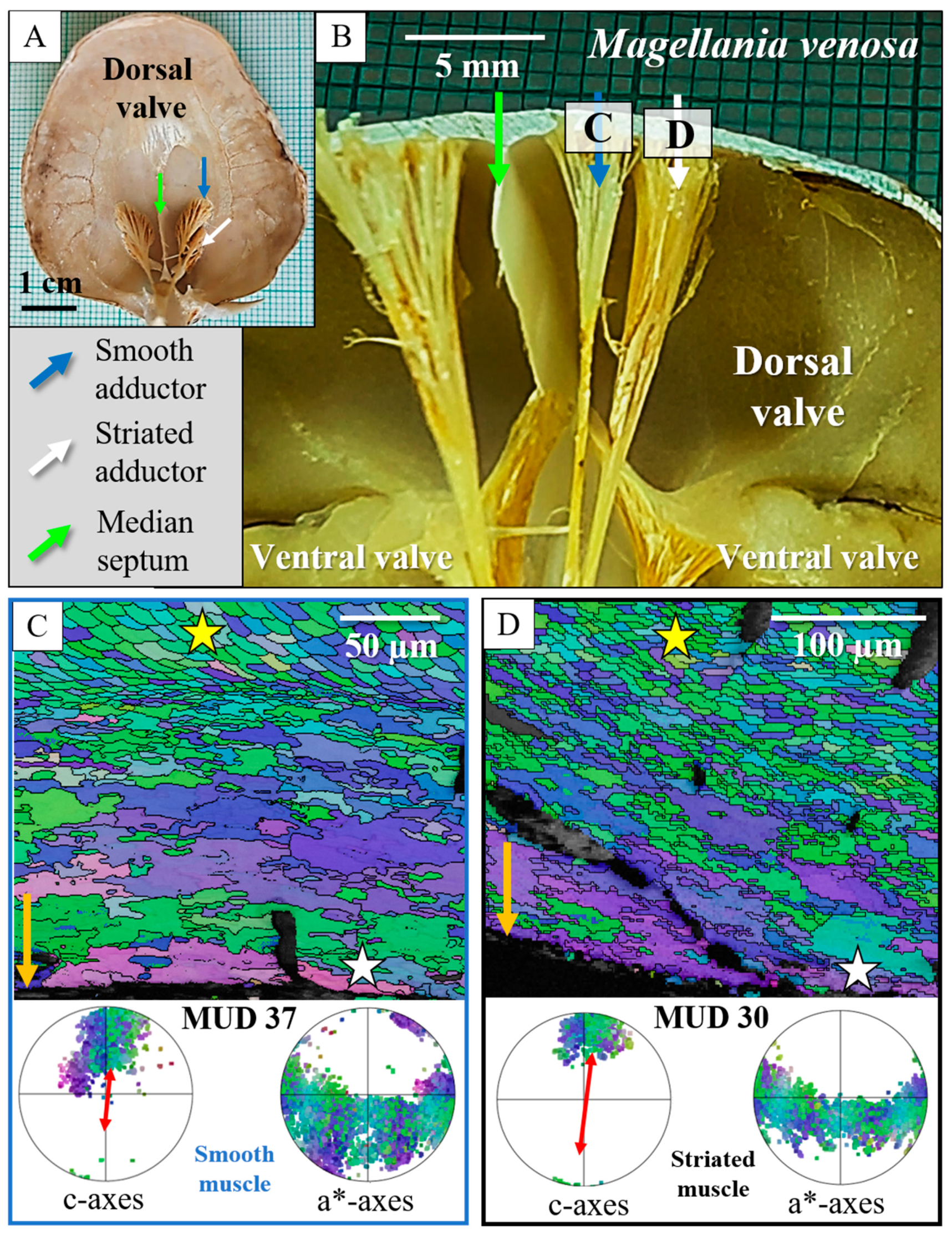
4. Discussion
4.1. The Muscles and Their Attachment to the Attachment Site Crystals
4.2. Are the Muscle Attachment Sites of Rhynchonellata and Bivalvia Convergent in Structure?
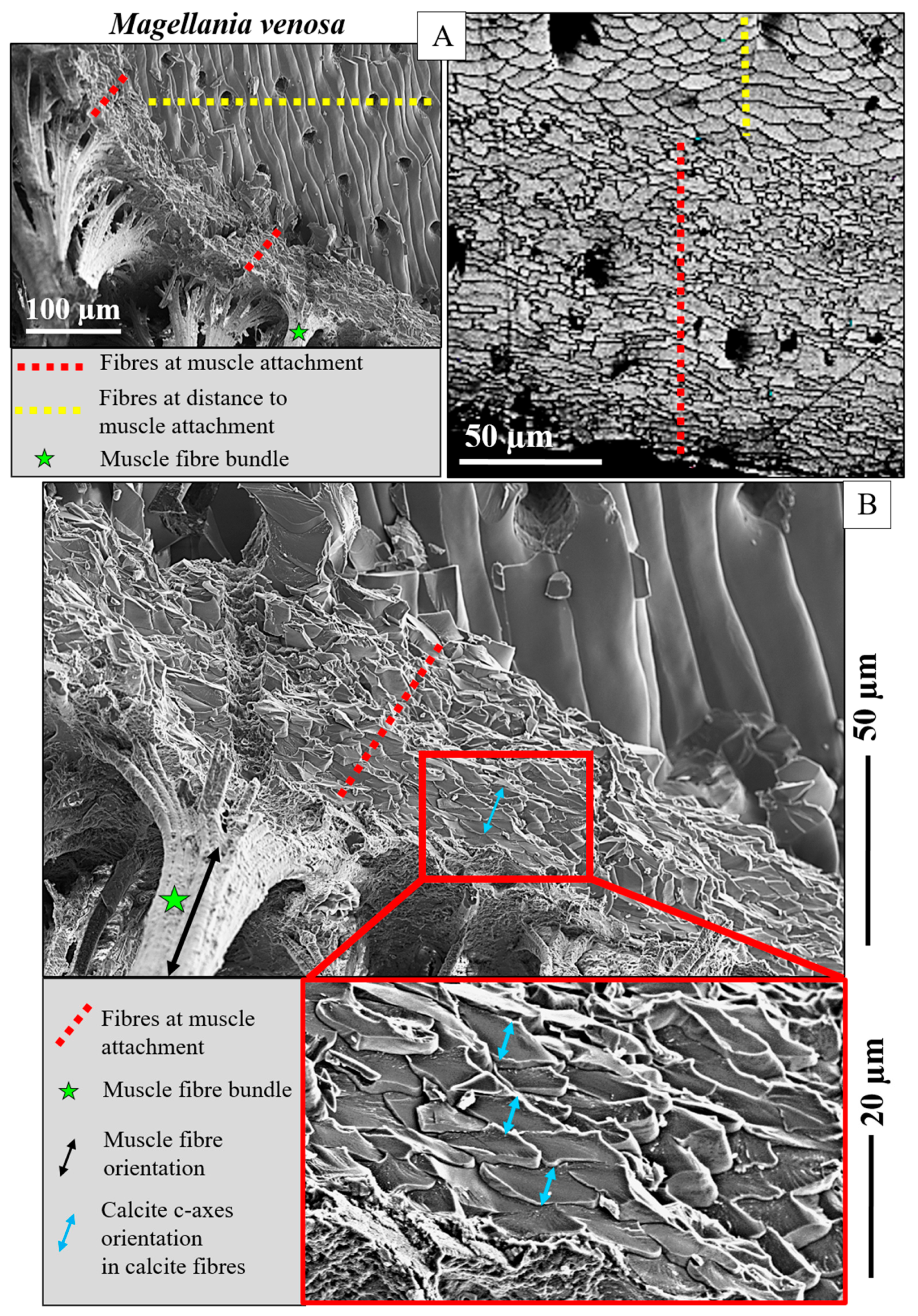
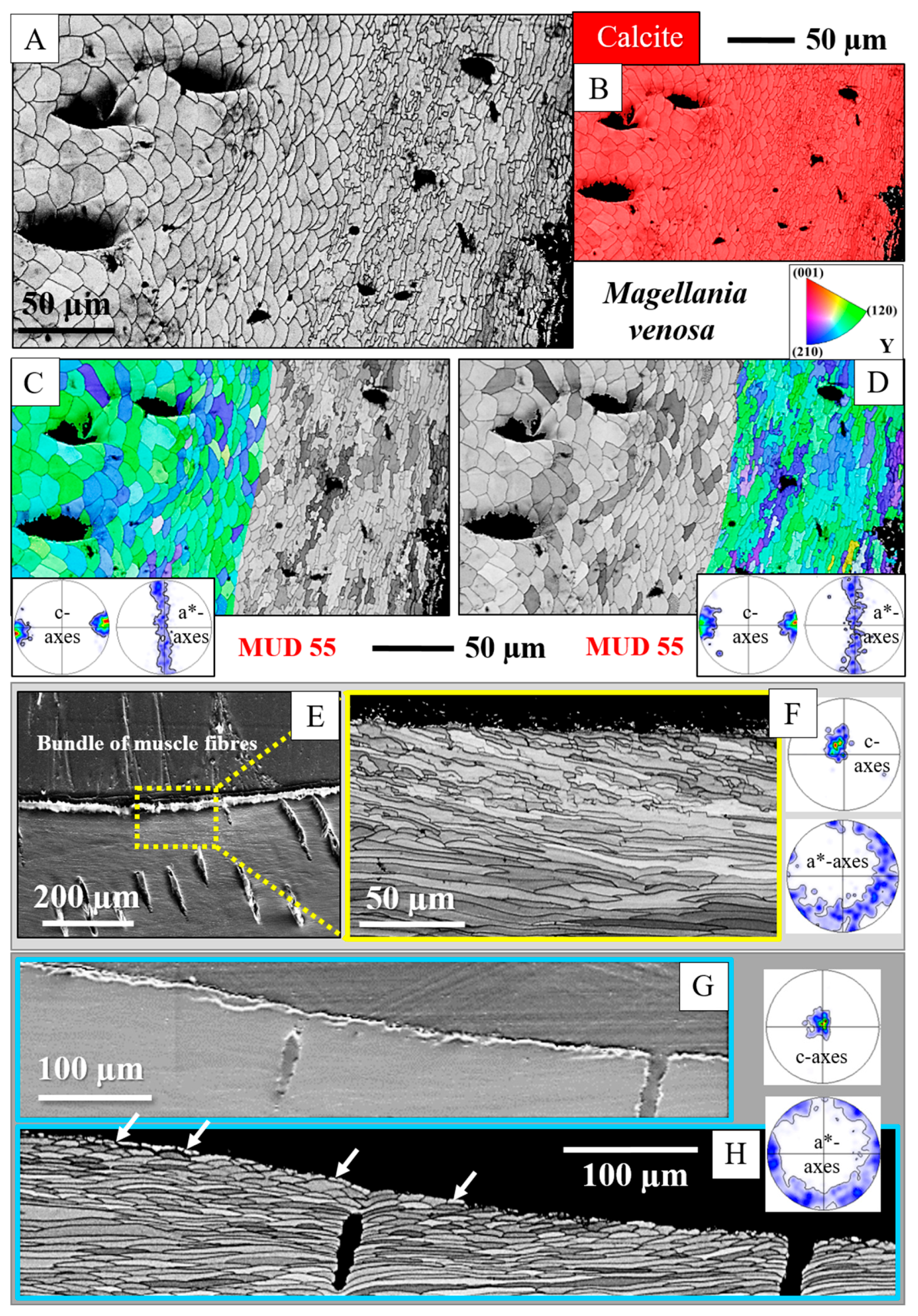
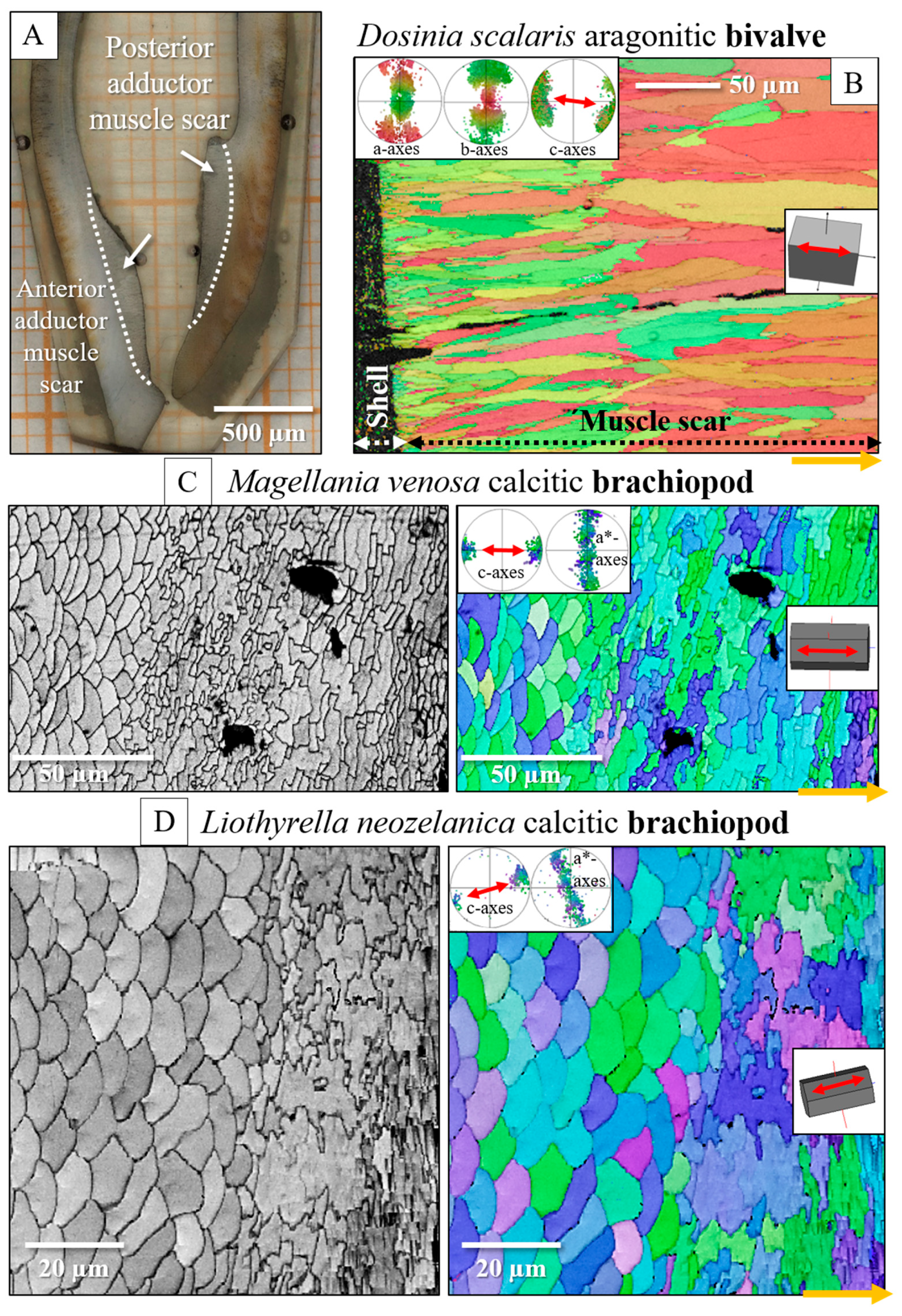
- The muscle scar of bivalves is a prominent, protruding structure (Figure 14A), limited in extent to only those valve sections where the muscle bundles directly attach to the valves. For rhynchonellate brachiopods, we observed extensive thickening of the valve portions at and near muscle attachment sites (see Figure 2 for dorsal valves). However, the latter is not only present at the sites of muscle attachments but also extends to regions that surround those valve portions where the brachiopod muscles attach to (Figure 2A, red rectangle in Figure 2B). The innermost layer of the thickened shell portion shows a disturbed microstructure of fractal-like crystals. With distance from the muscle attachment, the layer with the disturbed microstructure thins out and disappears (white arrows in Figure 9H and Figure 11C).
- One of the most distinctive characteristics is that, in contrast to rhynchonellate brachiopods, the muscle scars of bivalves have a competitive growth-derived microstructure (Figure 14B and [21]). These myostraca consist of prisms with irregular morphologies, assembled in a very specific way that depends upon the microstructure of the adjacent shell layer. This was not observed for the investigated rhynchonellate brachiopod species. As detailed in this contribution, the microstructures of muscle attachment crystals of brachiopod shells are also distinct from the rest of the shell. However, muscle scar crystal formation of brachiopod shells is not generated by competitive growth; the muscle scar crystals have highly irregular morphologies and a very disturbed microstructure (Figure 7, Figure 8 and Figure 14). Irrespective of whether muscle attachment crystals originate from fibres or columns (Figure 11A,B, Figure 12B,C and Figure 13C,D), their morphologies are fractal-like (Figure 10C, Figure 12C and Figure 14C,D) and the crystals interdigitate in 3D,
- The carbonate phase of brachiopod muscle attachment sites is always calcite, while bivalve muscle scars always consist of aragonite, even when the adjacent shell layer is formed of calcite [21]. While the reasons for this are not yet fully understood, it might be due to historical restrictions. The earliest bivalve shells were presumably purely aragonitic, and the ability to secrete calcite microstructures may have developed later in bivalves [87].
- The texture pattern of the brachiopod muscle attachment site crystals is similar to that of the adjacent shell layer, and it is not changed with progressive attachment site crystal growth. At first, bivalves also adopt the texture pattern of the adjacent section of the valves if it contains the same calcium carbonate phase [21]. However, with progressive attachment site crystal growth, the texture of bivalve muscle scar crystals changes slightly [21,23,26].
5. Conclusions
- In rhynchonellate brachiopods, the adductor and diductor muscle bases attach to calcite fibres and to calcite columns.
- The attachment site crystals have very irregular, fractal morphologies. Adjacent attachment site crystals interdigitate markedly in 3D. The attachment site portion of the valves is intimately connected to the non-attachment site sections of the shell.
- There is a marked difference in microstructure between the inner shell surface of the attachment site and the other valve portions. The texture of the attachment site and non-attachment site calcite is similar. We found an axial texture for both.
- Attachment site calcite c-axis orientations are perpendicular to the inner shell surface and parallel to the morphological axis of the muscle bundles. This is a finding we observed for species of Rhynchonellata and Bivalvia and is, most probably, necessary for a strong attachment of muscle base-tendon cell polymer fibril to the crystals.
- The difference in microstructure between attachment site and the other valve portions results from the difference in the ultrastructure and secretory behaviour of the secreting cells. A layer of cuboidal, tendon cells secretes the muscle attachment site crystals, while the crystals of the rest of the shell are mainly secreted by columnar cells.
- Regarding the structural convergence for muscle attachment sites of rhynchonellate brachiopods and bivalves, we could find some structural characteristics of muscle attachment sites that are similar for species of the investigated invertebrate classes. For both invertebrate classes, the texture of the adjacent shell layer continues in the microstructure of the muscle attachment layer. This may derive from the determinants of the similar secreting epithelial cells underlying the muscle attachment sites. However, it should be kept in mind that valve actions are realised differently by rhynchonellate brachiopods and bivalves. Bivalve valve movement is not only the result of muscle action, but also the involvement of the hinge ligament. In contrast, rhynchonellate brachiopods do not involve a ligament in valve motion, but solely utilise their muscles. Thus, different constraints operate on muscle involvement when opening and closing the valves for rhynchonellate brachiopods and bivalves and are a determining factor in the generation of the structural differences that were observed between rhynchonellate and bivalve muscle attachment sites.
- The action of opening and closing the valves is realised differently by rhynchonellate brachiopods and bivalves. Bivalve valve movement not only results from muscle action, but also involves hinge ligament action. In contrast, rhynchonellate brachiopods do not involve a ligament in valve motion; they solely employ muscles. Thus, different constraints operate on muscle involvement at opening and closing the valves for rhynchonellate brachiopods and bivalves. These might be determining factors in the formation of the differences that were observed between rhynchonellate and bivalve muscle attachment sites.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirose, M.; Endo, K. Phylum Brachiopoda. In Invertebrate Zoology: A Tree of Life Approach; CRC Press: Boca Raton, FL, USA, 2021; pp. 329–340. [Google Scholar]
- Angiolini, L.; Crippa, G.; Azmy, K.; Capitani, G.; Confalonieri, G.; Della Porta, G.; Griesshaber, E.; Harper, D.A.T.; Leng, M.J.; Nolan, L.; et al. The giants of the phylum Brachiopoda: A matter of diet? Palaeontology 2019, 62, 889–917. [Google Scholar] [CrossRef]
- Vinn, O.; Holmer, L.E.; Wilson, M.A. Evolution of brachiopod symbiosis in the early Paleozoic. Hist. Biol. 2024, 36, 1274–1294. [Google Scholar] [CrossRef]
- Bengtson, S. Mineralized skeletons and early animal evolution. In Evolving Form and Function: Fossils and Development; Seilacher, A., Ed.; Peabody Museum of Natural History, Yale University: New Haven, CT, USA, 2005; pp. 101–124. ISBN 978-0-912532-72-1. [Google Scholar]
- Peel, J.S. Failed predation, commensalism and parasitism on lower Cambrian linguliformean brachiopods. Alcheringa Australas. J. Palaeontol. 2015, 39, 149–163. [Google Scholar] [CrossRef]
- James, M.A.; Ansell, A.D.; Collins, M.J.; Curry, G.B.; Peck, L.S.; Rhodes, M.C. Biology of living brachiopods. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1992; Volume 28, pp. 175–387. ISBN 978-0-12-026128-4. [Google Scholar]
- Hoel, O.A. Evidence for muscular control of the apical pedicle in a Silurian Leptaenine brachiopod from Gotland, Sweden, and its life position. In Fossils and Strata; Harper, D.A.T., Long, S.L., Nielsen, C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; Volume 54, pp. 53–58. ISBN 978-1-4051-8664-3. [Google Scholar]
- LaBarbera, M. Brachiopod orientation to water movement: Functional morphology. Lethaia 1978, 11, 67–79. [Google Scholar] [CrossRef]
- Alexander, R.R. Functional morphology and biomechanics of articulate brachiopod shells. Paleontol. Soc. Pap. 2001, 7, 145–170. [Google Scholar] [CrossRef]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Biology and Evolution of the Mollusca, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-1-351-11566-7. [Google Scholar]
- Tremblay, I.; Samson-Dô, M.; Guderley, H.E. When behavior and mechanics meet: Scallop swimming capacities and their hinge ligament. J. Shellfish Res. 2015, 34, 203–212. [Google Scholar] [CrossRef]
- Yonge, C.M. Functional morphology with particular reference to hinge and ligament in Spondylus and Plicatula and a discussion on relations within the superfamily Pertinacea (Mollusca: Bivalvia). Phil. Trans. R. Soc. Lond. B 1973, 267, 173–208. [Google Scholar] [CrossRef]
- Kahler, G.A.; Fisher, F.M., Jr.; Sass, R.L. The chemical composition and mechanical properties of the hinge ligament in bivalve molluscs. Biol. Bull. 1976, 151, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.F.; Coates, C.J.; Whitten, M.W. (Eds.) Invertebrate Pathology; Oxford University Press: Oxford, UK, 2022; ISBN 978-0-19-259543-0. [Google Scholar]
- Simkiss, K.; Wilbur, K.M. Biomineralization: Cell Biology and Mineral Deposition; Academic Press: New York, NY, USA, 1989; ISBN 978-0-12-643830-7. [Google Scholar]
- Simonet Roda, M.; Ziegler, A.; Griesshaber, E.; Yin, X.; Rupp, U.; Greiner, M.; Henkel, D.; Häussermann, V.; Eisenhauer, A.; Laudien, J.; et al. Terebratulide brachiopod shell biomineralization by mantle epithelial cells. J. Struct. Biol. 2019, 207, 136–157. [Google Scholar] [CrossRef] [PubMed]
- Simkiss, K. Molluscan skin (excluding cephalopods). In Form and Function; Elsevier: Amsterdam, The Netherlands, 1988; pp. 11–35. [Google Scholar]
- Checa, A.G.; Griesshaber, E.; Salas, C.; Angiolini, L.; Schmahl, W.W. Mollusc and brachiopod carbonate biomineralization. In Biomineralization and Bioinspired Composites; Fratzl, P., Liz-Marzán, L.M., Kotov, N.A., Eds.; 2025; submitted. [Google Scholar]
- Castro-Claros, J.D.; Checa, A.; Lucena, C.; Pearson, J.R.; Salas, C. Shell-adductor muscle attachment and Ca2+ transport in the bivalves Ostrea stentina and Anomia ephippium. Acta Biomater. 2021, 120, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Huang, J.; Liu, C.; Wang, H.; Zhang, G.; Xie, L.; Zhang, R. Characterization of the myostracum layers in molluscs reveals a conservative shell structure. Front. Mar. Sci. 2022, 9, 862929. [Google Scholar] [CrossRef]
- Hoerl, S.; Micheletti, C.; Amini, S.; Griesshaber, E.; Hess, K.-U.; Checa, A.G.; Peharda, M.; Schmahl, W.W. Correlation between nanomechanical properties and microstructural design concepts of bivalve muscle attachment sites. Mater. Des. 2025, 253, 113845. [Google Scholar] [CrossRef]
- Lee, S.-W.; Jang, Y.-N.; Kim, J.-C. Characteristics of the aragonitic layer in adult oyster shells, Crassostrea gigas: Structural study of myostracum including the adductor muscle scar. Evid.-Based Complement. Altern. Med. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hoerl, S.; Le Moine, T.; Peter, N.J.; Amini, S.; Griesshaber, E.; Wang, J.; Harper, E.M.; Salas, C.; Checa, A.G.; Schwaiger, R.; et al. Crystal organisation and material properties of Chama and Glycymeris myostraca and shells. Materialia 2024, 36, 102149. [Google Scholar] [CrossRef]
- Hoerl, S. Calcium Carbonate Biomaterials–Architecture, Design and Nanomechanical Properties of Selected Mollusc, Brachiopod and Echinoderm Skeletal Elements. Ph.D. Thesis, Ludwig-Maximilians-Universität, München, Germany, 2025. [Google Scholar]
- Stevens, K.; Griesshaber, E.; Schmahl, W.; Casella, L.A.; Iba, Y.; Mutterlose, J. Belemnite biomineralization, development, and geochemistry: The complex rostrum of Neohibolites minimus. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 468, 388–402. [Google Scholar] [CrossRef]
- Crippa, G.; Griesshaber, E.; Checa, A.G.; Harper, E.M.; Roda, M.S.; Schmahl, W.W. Orientation patterns of aragonitic crossed-lamellar, fibrous prismatic and myostracal microstructures of modern Glycymeris shells. J. Struct. Biol. 2020, 212, 107653. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Navas, A.; Martin-Algarra, A.; Sanchez-, M.; Jimenez-Lopez, C.; Nieto, F.; Ruiz-Bustos, A. Crystal growth of inorganic and biomediated carbonates and phosphates. In Advanced Topics on Crystal Growth; Ferreira, S., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1010-1. [Google Scholar]
- Nakahara, H.; Bevelander, G. The formation and growth of the prismatic layer of Pinctada radiata. Calcif. Tissue Res. 1971, 7, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, H.; Bevelander, G. An electron microscope study of the muscle attachment in the mollusc Pinctada radiata. Tex. Rep. Biol. Med. 1970, 28, 279–286. [Google Scholar] [PubMed]
- Le Moine, T. Characterization of the Microstructure and the Texture of Calcium Carbonate Crystals in Bivalve Shells. Master’s Thesis, Ludwig-Maximilians-Universität, München, Germany, 2022. [Google Scholar]
- Rathi, P.A. Modes of Carbonate Crystal Organization at Muscle Attachment Sites of Bivalve Shells. Master’s Thesis, Ludwig-Maximilians-Universität, München, Germany, 2023. [Google Scholar]
- Taylor, J.; Kennedy, W.; Hall, A. Nuculacea-Trigonacea. Bull. Br. Mus. (Nat. Hist.) Zool. Suppl. 1969, 3, 1–125. [Google Scholar]
- Taylor, J.; Kennedy, W.; Hall, A. The shell structure and mineralogy of the Bivalvia. II. Lucinacea-Clavagellacea, conclusions. Bull. Br. Mus. (Nat. Hist.) Zool. 1973, 22, 255–294. [Google Scholar] [CrossRef]
- Garbelli, C.; Angiolini, L.; Shen, S. Biomineralization and global change: A new perspective for understanding the end-Permian extinction. Geology 2017, 45, 19–22. [Google Scholar] [CrossRef]
- Williams, A.; Ager, D.V.; Grant, R.E.; Rowell, A.J.; Stehli, F.G.; Muir-Wood, H.M.; Elliott, G.F. Part H, Brachiopoda. In Treatise on Invertebrate Paleontology; Moore, R.C., Ed.; The Geological Society of America: Boulder, CO, USA; University of Kansas: Lawrence, KS, USA, 1965; Volume 1. [Google Scholar]
- Cusack, M.; Williams, A. Chemico-structural differentiation of the organocalcitic shells of rhynchonellate brachiopods. In Brachiopods; CRC Press: Boca Raton, FL, USA, 2001; pp. 31–41. [Google Scholar]
- Harper, D.A.T.; Popov, L.E.; Holmer, L.E. Brachiopods: Origin and early history. Palaeontology 2017, 60, 609–631. [Google Scholar] [CrossRef]
- Harper, D.A.T.; Cocks, L.R.M.; Popov, L.E.; Sheehan, P.M.; Bassett, M.G.; Copper, P.; Holmer, L.E.; Jin, J.; Jiayu, R. Brachiopods. In The Great Ordovician Biodiversification Event; Webby, B.D., Paris, F., Droser, M.L., Percival, I.G., Eds.; Columbia University Press: New York, NY, USA, 2004; pp. 157–178. ISBN 978-0-231-12678-6. [Google Scholar]
- Ye, F.; Jurikova, H.; Angiolini, L.; Brand, U.; Crippa, G.; Henkel, D.; Laudien, J.; Hiebenthal, C.; Šmajgl, D. Variation in brachiopod microstructure and isotope geochemistry under low-pH–ocean acidification conditions. Biogeosciences 2019, 16, 617–642. [Google Scholar] [CrossRef]
- Cross, E.L.; Harper, E.M.; Peck, L.S. A 120-year record of resilience to environmental change in brachiopods. Glob. Change Biol. 2018, 24, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Bitner, M.A. Lower Eocene (Middle Ilerdian) brachiopods from the Campo region, Central Pyrenees, north-eastern Spain. Span. J. Palaeontol. 2021, 15, 117–128. [Google Scholar] [CrossRef]
- Peck, L.S.; Brockington, S.; Brey, T. Growth and metabolism in the Antarctic brachiopod Liothyrella uva. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1997, 352, 851–858. [Google Scholar] [CrossRef]
- Peck, L.S.; Harper, E. Variation in size of living articulated brachiopods with latitude and depth. Mar. Biol. 2010, 157, 2205–2213. [Google Scholar] [CrossRef]
- Williams, A.; Cohen, B.L.; Cusack, M.; Long, S.L. Provenance of Atlantic lingulid brachiopods. Palaeontology 2000, 43, 999–1018. [Google Scholar] [CrossRef]
- Richardson, J.R. Brachiopods. Sci. Am. 1986, 255, 100–107. [Google Scholar] [CrossRef]
- Robinson, J. The muscles, body wall and valve-opening mechanism of extant craniid (inarticulated) brachiopods. J. Nat. Hist. 2014, 48, 1231–1252. [Google Scholar] [CrossRef]
- MacKinnon, D.I. The formation of muscle scars in articulate brachiopods. Phil. Trans. R. Soc. Lond. B 1977, 280, 1–27. [Google Scholar] [CrossRef]
- Paniagua, R.; Royuela, M.; Garcia-Anchuelo, R.; Fraile, B. Ultrastructure of invertebrate muscle cell types. Histol. Histopathol. 1996, 11, 181–201. [Google Scholar] [PubMed]
- Helmkampf, M.; Bruchhaus, I.; Hausdorf, B. Phylogenomic analyses of lophophorates (brachiopods, phoronids and bryozoans) confirm the Lophotrochozoa concept. Proc. R. Soc. B 2008, 275, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Tompa, A.S.; Watabe, N. Ultrastructural investigation of the mechanism of muscle attachment to the gastropod shell. J. Morphol. 1976, 149, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Simonet Roda, M.; Griesshaber, E.; Angiolini, L.; Rollion-Bard, C.; Harper, E.M.; Bitner, M.A.; Milner Garcia, S.; Ye, F.; Henkel, D.; Häussermann, V.; et al. The architecture of Recent brachiopod shells: Diversity of biocrystal and biopolymer assemblages in rhynchonellide, terebratulide, thecideide and craniide shells. Mar. Biol. 2022, 169, 4. [Google Scholar] [CrossRef]
- Crippa, G.; Jurikova, H.; Leng, M.J.; Zanchi, M.; Harper, E.M.; Rae, J.W.B.; Savickaite, K.; Viaretti, M.; Angiolini, L. Brachiopods as archives of intrannual, annual, and interannual environmental variations. Limnol. Ocean. Lett. 2025, 10, 390–402. [Google Scholar] [CrossRef]
- Goetz, A.J.; Griesshaber, E.; Neuser, R.D.L.; Harper, E.; Schmahl, W.W. Calcite morphology, texture and hardness in the distinct layers of rhynchonelliform brachiopod shells. EJM 2009, 21, 303–315. [Google Scholar] [CrossRef]
- Bubel, A. Epidermal cells. In Biology of the Integument; Bereiter-Hahn, J., Matoltsy, A.G., Richards, K.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 400–447. ISBN 978-3-642-51595-8. [Google Scholar]
- Dove, P.M. Biomineralization, 1st ed.; Reviews in Mineralogy and Geochemistry Series; Walter de Gruyter GmbH: Boston, MA, USA, 2018; ISBN 978-1-5015-0934-6. [Google Scholar]
- Weiner, S.; Dove, P.M. An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Gilbert, P.U.P.A.; Bergmann, K.D.; Boekelheide, N.; Tambutté, S.; Mass, T.; Marin, F.; Adkins, J.F.; Erez, J.; Gilbert, B.; Knutson, V.; et al. Biomineralization: Integrating mechanism and evolutionary history. Sci. Adv. 2022, 8, eabl9653. [Google Scholar] [CrossRef] [PubMed]
- Wernström, J.V.; Gąsiorowski, L.; Hejnol, A. Brachiopod and mollusc biomineralisation is a conserved process that was lost in the phoronid–bryozoan stem lineage. EvoDevo 2022, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Chantler, P. Biochemical and structural aspects of molluscan muscle. In The Mollusca; Elsevier: Amsterdam, The Netherlands, 1983; pp. 77–154. [Google Scholar]
- Schwartz, A.J.; Kumar, M.; Adams, B.L.; Field, D.P. (Eds.) Electron Backscatter Diffraction in Materials Science; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-88135-5. [Google Scholar]
- Wilkens, J.L. Adductor muscles of brachiopods: Activation and contraction. Can. J. Zool. 1978, 56, 315–323. [Google Scholar] [CrossRef]
- Ackerly, S.C. Hydrodynamics of rapid shell closure in articulate brachiopods. J. Exp. Biol. 1991, 156, 287–314. [Google Scholar] [CrossRef]
- Ackerly, S.C. Mechanical couplings in the shell closing mechanism of articulate brachiopods: Implications for the evolution of skeleto-muscular architecture. Paleobiology 1993, 19, 420–432. [Google Scholar] [CrossRef]
- McCammon, H.M. Behavior in the brachiopod Terebratulina septentrionalis (Couthouy). J. Exp. Mar. Biol. Ecol. 1971, 6, 35–45. [Google Scholar] [CrossRef]
- Watabe, N. Shell Repair. In The Mollusca; Elsevier: Amsterdam, The Netherlands, 1983; pp. 289–316. ISBN 978-0-12-751404-8. [Google Scholar]
- Lemaire-Gony, S.; Boudou, A. Mantle and gill fine structure in the freshwater Asiatic clam, Corbicula fluminea (Müller). Ann. Limnol.-Int. J. Lim. 1997, 33, 163–178. [Google Scholar] [CrossRef]
- Fraiser, M.L.; Bottjer, D.J. When bivalves took over the world. Paleobiology 2007, 33, 397–413. [Google Scholar] [CrossRef]
- Checa, A.G.; Salas, C.; Harper, E.M.; Bueno-Pérez, J.d.D. Early Stage Biomineralization in the Periostracum of the ‘Living Fossil’ Bivalve Neotrigonia. PLoS ONE 2014, 9, e90033. [Google Scholar] [CrossRef] [PubMed]
- Audino, J.A.; Marian, J.E.A.R.; Wanninger, A.; Lopes, S.G.B.C. Mantle margin morphogenesis in Nodipecten nodosus (Mollusca: Bivalvia): New insights into the development and the roles of bivalve pallial folds. BMC Dev. Biol. 2015, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Rudwick, M.J.S. ‘Quick’ and ‘catch’ adductor muscles in brachiopods. Nature 1961, 191, 1021. [Google Scholar] [CrossRef]
- Thayer, C.W. Diductor muscles of brachiopods: Active or passive? Paleobiology 1975, 1, 44–47. [Google Scholar] [CrossRef]
- Hamaya, A.; Fujisaki, K.; Sasagawa, K.; Miura, K. A novel method for Mytilus galloprovincialis adductor muscle activity measurement during and after physical stimulation. J. Biomech. 2025, 187, 112754. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, W.P.; Wilkens, J.L.; Cavey, M.J. Electrophoretic and electron microscopic examination of the adductor and diductor muscles of an articulate brachiopod, Terebratalia transversa. Can. J. Zool. 1982, 60, 550–559. [Google Scholar] [CrossRef]
- Niemiec, M.; Kim, K. Lifetime engineering of bioelectronic implants with mechanically reliable thin film encapsulations. Prog. Biomed. Eng. 2024, 6, 012001. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Y.; Zhang, Q.; Fan, L.; Han, D.-D.; Li, L.; Yan, L.; Hou, P.-Y. Engineering the interface of organic/inorganic composite solid-state electrolyte by amino effect for all-solid-state lithium batteries. J. Colloid Interface Sci. 2022, 628, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Gong, X.; Zhang, Y.; Sun, Z.; Xia, N.; Zhang, H.; Wang, J.; Zhang, X. Simulation analysis of organic–inorganic interface failure of scallop under ultra-high pressure. Coatings 2022, 12, 963. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, R.U.; Silberschmidt, V.V. Assessing pseudo-ductile behavior of woven thermoplastic composites under tension and bending. Compos. Sci. Technol. 2024, 248, 110465. [Google Scholar] [CrossRef]
- Seilacher, A. Constructional morphology of sand dollars. Paleobiology 1979, 5, 191–221. [Google Scholar] [CrossRef]
- Vyatchin, I.; Dyachuk, V. The unique biology of catch muscles: Insights into structure, function, and robotics innovations. Front. Bioeng. Biotechnol. 2025, 13, 1478626. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, L.; Zhou, L.; Zhang, T.; Liu, Z.; Chen, L.; Wu, B.; Jing, H.; Sun, X. Phalloidin fluorescence and confocal microscopy reveal the musculature development of clam Ruditapes philippinarum. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2022, 258, 110693. [Google Scholar] [CrossRef] [PubMed]
- Cragg, S.M. The adductor and retractor muscles of the veliger of Pecten maximus (L.) (Bivalvia). J. Molluscan Stud. 1985, 51, 276–283. [Google Scholar] [CrossRef]
- Nunzi, M.G.; Franzini-Armstrong, C. The structure of smooth and striated portions of the adductor muscle of the valves in a scallop. J. Ultrastruct. Res. 1981, 76, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Guderley, H.E.; Tremblay, I. Chapter 12-Swimming in Scallops. In Scallops; Shumway, S.E., Parsons, G.J., Eds.; Developments in Aquaculture and Fisheries Science; Elsevier: Amsterdam, The Netherlands, 2016; Volume 40, pp. 535–566. [Google Scholar]
- Funabara, D.; Kanoh, S.; Siegman, M.J.; Butler, T.M.; Hartshorne, D.J.; Watabe, S. Twitchin as a regulator of catch contraction in molluscan smooth muscle. J. Muscle Res. Cell Motil. 2006, 26, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.; Lowy, J. Structure and function of the contractile apparatus in the muscles of invertebrate animals. In Structure and Function of Muscle; Academic Press: New York, NY, USA, 1960; Volume 1, pp. 265–335. [Google Scholar]
- Kennedy, W.J.; Taylor, J.D.; Hall, A. Environmental and biological controls on bivalve shell mineralogy. Biol. Rev. 1969, 44, 499–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Zhu, Y.; Song, X.; Fang, X.; Huang, R.; Que, H.; Zhang, G. Aragonite shells are more ancient than calcite ones in bivalves: New evidence based on omics. Mol. Biol. Rep. 2014, 41, 7067–7071. [Google Scholar] [CrossRef] [PubMed]
- Yonge, M. Form, Habit and Evolution in the Chamidae (Bivalvia) with Reference to Conditions in the Rudists (Hippuritacea). Philos. Trans. R. Soc. Lond. B Biol. Sci. 1967, 252, 49–105. [Google Scholar]
- Carlson, S.J. The articulate brachiopod hinge mechanism: Morphological and functional variation. Paleobiology 1989, 15, 364–386. [Google Scholar] [CrossRef]
- Carriker, M. The shell and ligament. In The Eastern Oyster Crassostrea virginica; Kennedy, V.S., Newell, R.I.E., Eds.; Maryland Sea Grant College: College Park, MD, USA, 1996; pp. 76–168. [Google Scholar]
- Wainwright, S.A. Mechanical Design in Organisms; Princeton University Press: Princeton, NJ, USA, 1982; ISBN 0-691-08308-8. [Google Scholar]
- Shadwick, R.E.; Gosline, J.M. Molecular biomechanics of protein rubbers in molluscs. In Metabolic Biochemistry and Molecular Biomechanics; Elsevier: Amsterdam, The Netherlands, 1983; pp. 399–430. ISBN 978-0-12-751401-7. [Google Scholar]
- Schmahl, W.W.; Griesshaber, E.; Kelm, K.; Goetz, A.; Jordan, G.; Ball, A.; Xu, D.; Merkel, C.; Brand, U. Hierarchical structure of marine shell biomaterials: Biomechanical functionalization of calcite by brachiopods. Z. Für Krist.-Cryst. Mater. 2012, 227, 793–804. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoerl, S.; Griesshaber, E.; Weller, D.; Amini, S.; Häussermann, V.; Bitner, M.A.; Achterhold, K.; Pfeiffer, F.; Schmahl, W.W. Crystal Organisation of Muscle Attachment Sites of Bivalved Marine Organisms: A Juxtaposition Between Brachiopod and Bivalved Mollusc Shells. Crystals 2025, 15, 649. https://doi.org/10.3390/cryst15070649
Hoerl S, Griesshaber E, Weller D, Amini S, Häussermann V, Bitner MA, Achterhold K, Pfeiffer F, Schmahl WW. Crystal Organisation of Muscle Attachment Sites of Bivalved Marine Organisms: A Juxtaposition Between Brachiopod and Bivalved Mollusc Shells. Crystals. 2025; 15(7):649. https://doi.org/10.3390/cryst15070649
Chicago/Turabian StyleHoerl, Sebastian, Erika Griesshaber, Daniel Weller, Shahrouz Amini, Verena Häussermann, Maria A. Bitner, Klaus Achterhold, Franz Pfeiffer, and Wolfgang W. Schmahl. 2025. "Crystal Organisation of Muscle Attachment Sites of Bivalved Marine Organisms: A Juxtaposition Between Brachiopod and Bivalved Mollusc Shells" Crystals 15, no. 7: 649. https://doi.org/10.3390/cryst15070649
APA StyleHoerl, S., Griesshaber, E., Weller, D., Amini, S., Häussermann, V., Bitner, M. A., Achterhold, K., Pfeiffer, F., & Schmahl, W. W. (2025). Crystal Organisation of Muscle Attachment Sites of Bivalved Marine Organisms: A Juxtaposition Between Brachiopod and Bivalved Mollusc Shells. Crystals, 15(7), 649. https://doi.org/10.3390/cryst15070649








