Enhancing Electrochemical Kinetics and Stability of Biodegradable Mg-Y-Zn Alloys with LPSO Phases via Strategic Micro-Alloying with Ca, Sr, Mn, and Zr
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Microstructure Characterization
2.3. Corrosion Test
3. Results and Discussion
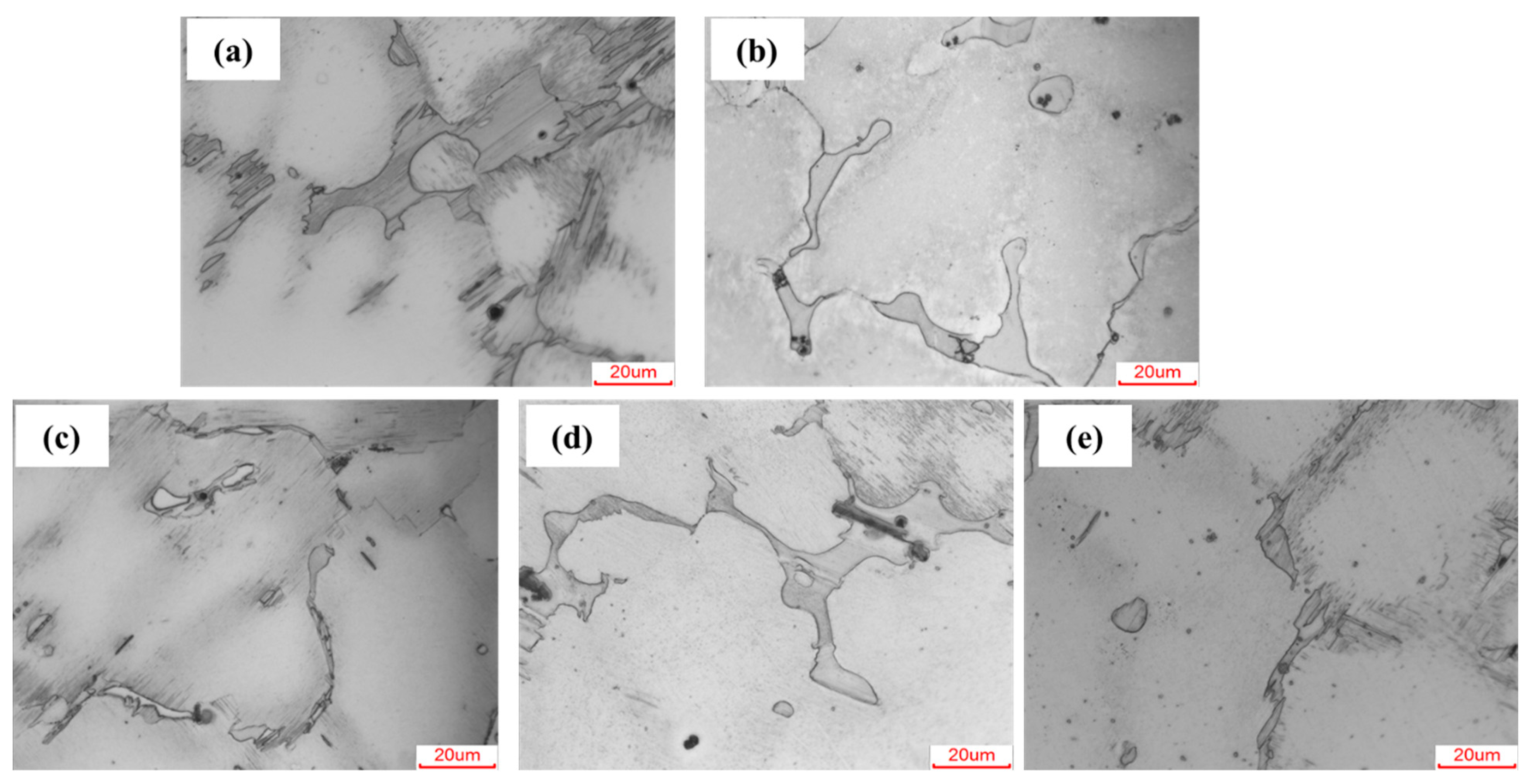
3.1. Microstructure Evolution
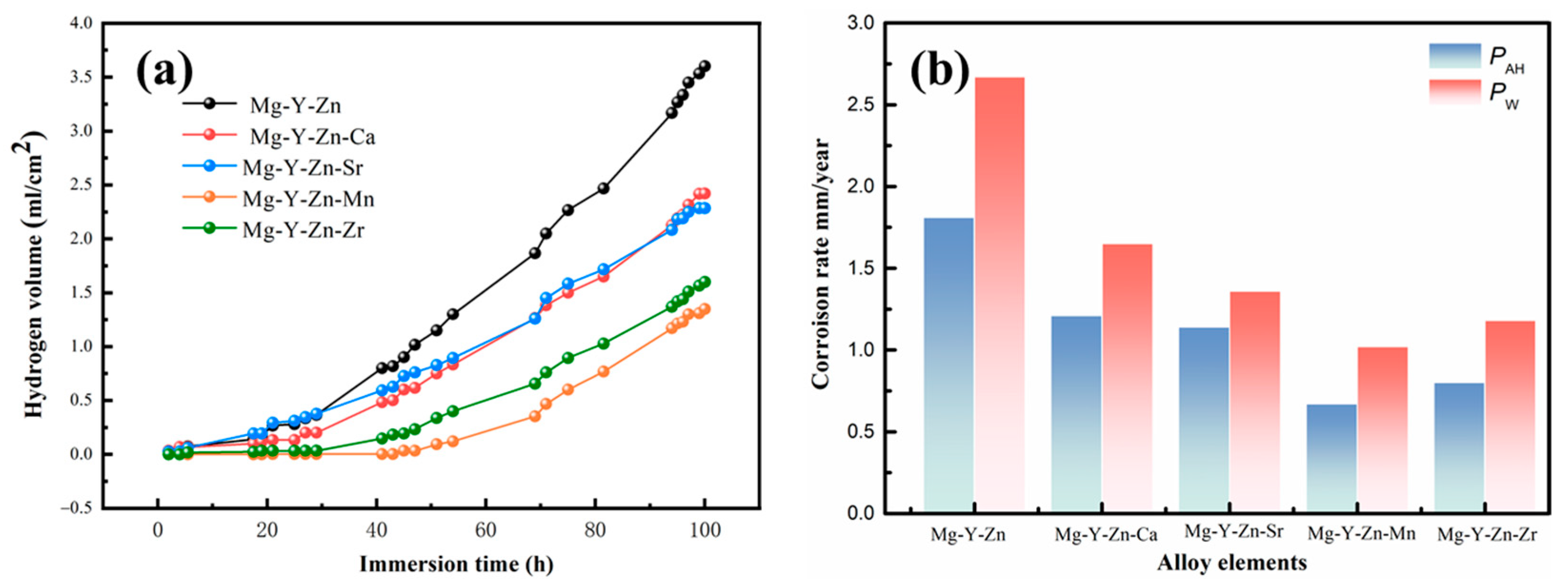
3.2. Corrosion Rates
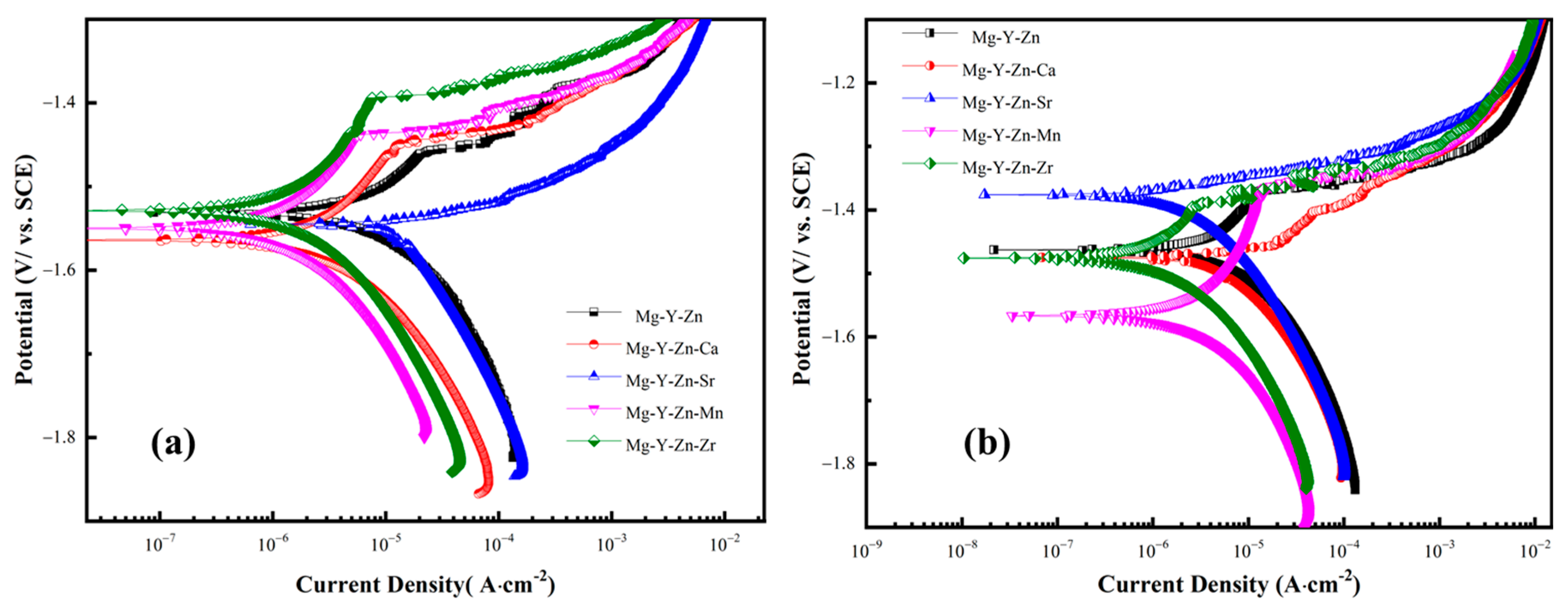
3.3. Electrochemical Responses as a Function of Processing
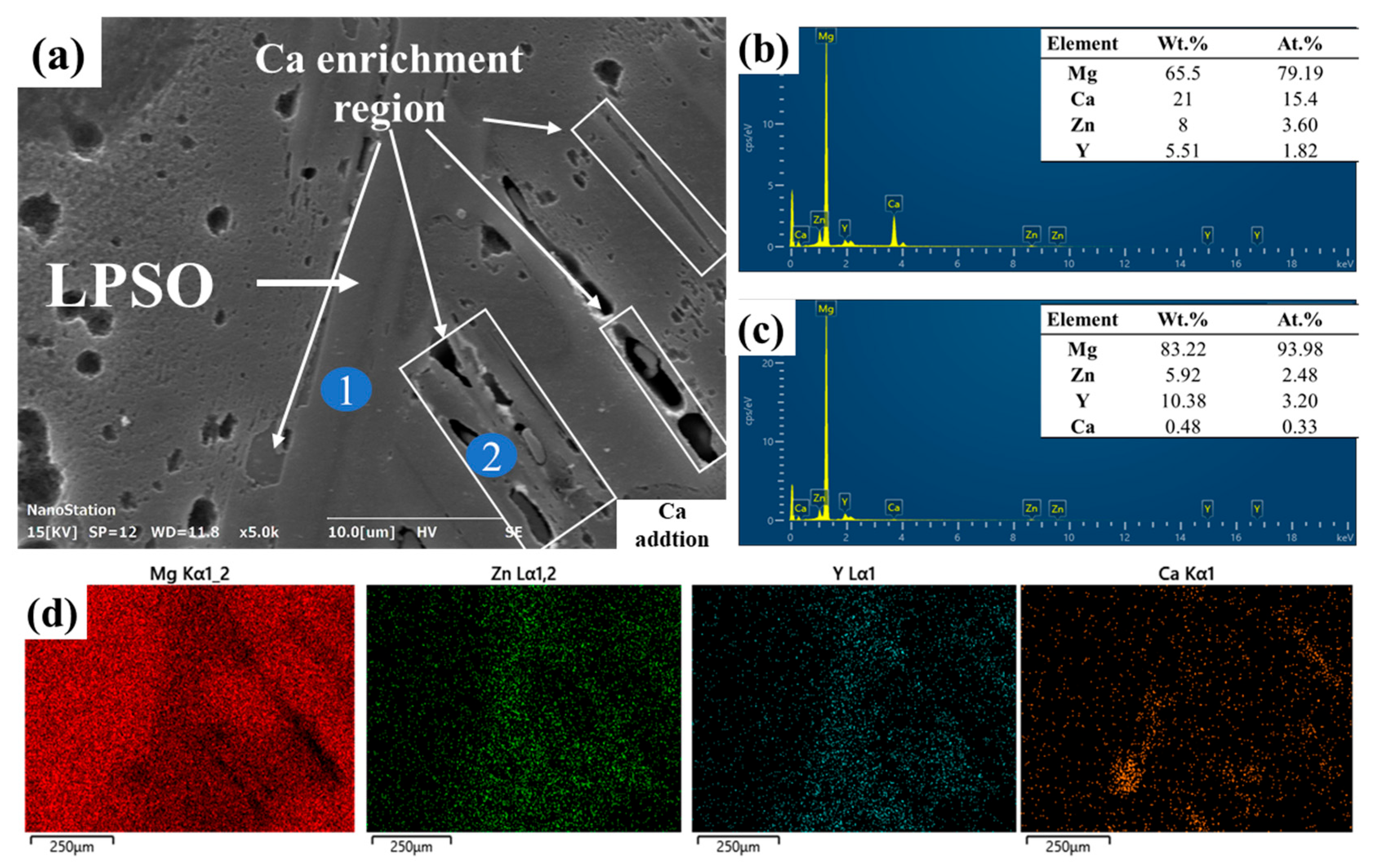
3.4. Corrosion Morphology
4. The Influence of Microalloying on the Corrosion Process
5. Conclusions
- (1)
- Microalloying modified the precipitation behavior of the second phase. Ca and Sr induced the formation of Mg2Ca and Mg17Sr2, while Mn and Zr refined the LPSO structure without generating new phases. All four elements promoted LPSO formation and distribution refinement.
- (2)
- Corrosion resistance was significantly enhanced by microalloying, with corrosion rates decreasing in the following order: Mg-Y-Zn > Mg-Y-Zn-Ca > Mg-Y-Zn-Sr > Mg-Y-Zn-Zr > Mg-Y-Zn-Mn.
- (3)
- In the Ca-containing alloy, Mg2Ca initially acts as a sacrificial anode, delaying corrosion. However, galvanic coupling between the α-Mg matrix and LPSO structure eventually dominates, accelerating localized attacks.
- (4)
- Sr addition leads to uniform Mg17Sr2 distribution and refined LPSO networks, facilitating uniform corrosion and suppressing deep penetration over time by inhibiting cathodic kinetics.
- (5)
- Mn microalloying yields the best corrosion resistance by suppressing both anodic and cathodic reactions, forming a stable protective film. Zr also improves general resistance but induces localized corrosion due to dislocation-related activity and LPSO phase disruption.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akbarzadeh, F.Z.; Sarraf, M.; Ghomi, E.R.; Kumar, V.V.; Salehi, M.; Ramakrishna, S.; Bae, S. A state-of-the-art review on recent advances in the fabrication and characteristics of magnesium-based alloys in biomedical applications. J. Magnes. Alloys 2024, 12, 2569–2594. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2022. J. Magnes. Alloys 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- Singh, N.; Batra, U.; Kumar, K.; Ahuja, N.; Mahapatro, A. Progress in bioactive surface coatings on biodegradable Mg alloys: A critical review towards clinical translation. Bioact. Mater. 2023, 19, 717–757. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, L.; Yin, M.; Xu, S.; Liang, Z. Review on magnesium and magnesium-based alloys as biomaterials for bone immobilization. J. Mater. Res. Technol. 2023, 23, 4396–4419. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Zhang, M.; Zhang, Y.; Fang, D.; Wei, Y.; Liu, B. Microstructure and mechanical behavior of Mg–Y–Zn alloys with respect to varying content of LPSO phase. Int. J. Mater. Res. 2018, 109, 944–950. [Google Scholar] [CrossRef]
- Lin, X.; Saijilafu; Wu, X.; Wu, K.; Chen, J.; Tan, L.; Witte, F.; Yang, H.; Mantovani, D.; Zhou, H.; et al. Biodegradable Mg-based alloys: Biological implications and restorative opportunities. Int. Mater. Rev. 2023, 68, 365–403. [Google Scholar] [CrossRef]
- Jiang, J.; Geng, X.; Zhang, X. Stress corrosion cracking of magnesium alloys: A review. J. Magnes. Alloys 2023, 11, 1906–1930. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, Y.; Wu, Q.; Xue, Y.; Zhang, Y.; Peng, L.; Ding, W. Microstructure evolution and mechanical properties of a high-strength Mg-10Gd-3Y–1Zn-0.4Zr alloy fabricated by laser powder bed fusion. Addit. Manuf. 2022, 49, 102517. [Google Scholar] [CrossRef]
- Peng, P.; Zhang, K.; She, J.; Tang, A.; Zhang, J.; Song, K.; Yang, Q.; Pan, F. Role of second phases and grain boundaries on dynamic recrystallization behavior in ZK60 magnesium alloy. J. Alloys Compd. 2021, 861, 157958. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, X.; Li, M.; Lu, X.; Chen, X.; Pang, X.; Li, J.; Zhang, J. Effect of zn content and homogenization treatment on mechanical properties and corrosion behavior of mg-9y-xzn alloys. Met. Mater. Int. 2025, 31, 770–786. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Y.; Li, J.; Yue, H.; Li, C.; Guo, S.; Chen, Q. Improving strength-ductility of mg-8.5gd-4.5y-0.8zn-0.4zr magnesium alloy due to bimodal lpso and <c + a> dislocations. J. Rare Earths 2025, 43, 832–842. [Google Scholar]
- Wang, Z.; Shen, Z.; Liu, Y.; Zhao, Y.; Zhu, Q.; Chen, Y.; Wang, J.; Li, Y.; Lozano-Perez, S.; Zeng, X. The effect of lpso phase on the high-temperature oxidation of a stainless mg-y-al alloy. J. Magnes. Alloys 2024, 12, 4045–4052. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhari, A.; Gupta, A.K.; Kumar, A. Rare-earth based magnesium alloys as a potential biomaterial for the future. J. Magnes. Alloys 2024, 12, 3841–3897. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Yao, W.; Wu, Y.; Gao, Y.; Yang, Y.; Wu, L.; Serdechnova, M.; Blawert, C.; Pan, F. Recent developments in coatings on biodegradable mg alloys: A review. J. Magnes. Alloys 2025, 13, 1405–1427. [Google Scholar] [CrossRef]
- Avey, T.; Cho, D.; Zhang, J.; Miao, J.; Dean, D.; Luo, A.A. Determining critical zn/ca atomic ratio and its role in mechanical and corrosion properties of biodegradable mg-ca-zn-mn alloys. Materialia 2024, 37, 102203. [Google Scholar] [CrossRef]
- Felten, M.; Chaineux, V.; Zhang, S.; Tehranchi, A.; Hickel, T.; Scheu, C.; Spille, J.; Lipińska-Chwałek, M.; Mayer, J.; Berkels, B.; et al. The effect of laves phases and nano-precipitates on the electrochemical corrosion resistance of mg-al-ca alloys under alkaline conditions. J. Magnes. Alloys 2024, 12, 2447–2461. [Google Scholar] [CrossRef]
- Gong, C.; He, X.; Yan, X. Corrosion behavior of mg–ca–zn alloys with high zn content. J. Phys. Chem. Solids 2021, 152, 109952. [Google Scholar] [CrossRef]
- Zander, D.; Zumdick, N.A. Influence of ca and zn on the microstructure and corrosion of biodegradable Mg-Ca-Zn alloys. Corros. Sci. 2015, 93, 222–233. [Google Scholar] [CrossRef]
- Akhmetshina, T.; Berger, L.; Basu, I.; Montibeller, S.; Rubin, W.; Rich, A.M.; Schäublin, R.E.; Löffler, J.F. High-performance ultra-lean biodegradable mg–ca alloys and guidelines for their processing. Acta Mater. 2024, 278, 120247. [Google Scholar] [CrossRef]
- Seong, J.W.; Kim, W.J. Mg-Ca binary alloy sheets with ca contents of ≤1wt.% with high corrosion resistance and high toughness. Corros. Sci. 2015, 98, 372–381. [Google Scholar] [CrossRef]
- Deng, M.; Wang, L.; Höche, D.; Lamaka, S.V.; Wang, C.; Snihirova, D.; Jin, Y.; Zhang, Y.; Zheludkevich, M.L. Approaching “stainless magnesium” by ca micro-alloying. Mater. Horiz. 2021, 8, 589–596. [Google Scholar] [CrossRef]
- Gu, X.N.; Xie, X.H.; Li, N.; Zheng, Y.F.; Qin, L. In vitro and in vivo studies on a mg–sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Wang, F.; Venezuela, J.; Wang, Y.; Shi, Z.; Chen, W.; Dargusch, M. Contrasting the mechanisms of high corrosion resistance in ultra-high purity mg-sr alloys in hanks’ and nacl solutions. Electrochim. Acta 2025, 518, 145716. [Google Scholar] [CrossRef]
- Munir, K.; Lin, J.; Wen, C.; Wright, P.F.A.; Li, Y. Mechanical, corrosion, and biocompatibility properties of Mg-Zr-Sr-Sc alloys for biodegradable implant applications. Acta Biomater. 2020, 102, 493–507. [Google Scholar] [CrossRef]
- Li, Y.; Wen, C.; Mushahary, D.; Sravanthi, R.; Harishankar, N.; Pande, G.; Hodgson, P. Hodgson. Mg-Zr-Sr alloys as biodegradable implant materials. Acta Biomater. 2012, 8, 3177–3188. [Google Scholar] [CrossRef]
- Benzarti, Z.; Itani, S.; Castro, J.D.; Carvalho, S.; Ramos, A.S. Design multifunctional Mg–Zr coatings regulating mg alloy bioabsorption. J. Magnes. Alloys 2024, 12, 1461–1478. [Google Scholar] [CrossRef]
- Niu, R.; Yan, F.; Wang, Y.; Duan, D.; Yang, X. Effect of Zr content on damping property of Mg-Zr binary alloys. Mater. Sci. Eng. A 2018, 718, 418–426. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, J.; Zhao, C.; Wen, M.; Wang, R.; Wu, D.; Wu, Z.; Yang, F.; Sheng, L. The recent developments of thermomechanical processing for biomedical mg alloys and their clinical applications. Materials 2025, 18, 1718. [Google Scholar] [CrossRef]
- Rogachev, S.O.; Bazhenov, V.E.; Komissarov, A.A.; Li, A.V.; Ten, D.V.; Yushchuk, V.V.; Drobyshev, A.Y.; Shin, K.S. Effect of hot rolling on structure and mechanical properties of Mg-Y-Zn-Mn alloys. Metals 2023, 13, 223. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Li, A.V.; Rogachev, S.O.; Bazlov, A.I.; Statnik, E.S.; Tavolzhanskii, S.A.; Komissarov, A.A.; Redko, N.A.; Korsunsky, A.M.; Shin, K.S. Structure and mechanical properties of hot-extruded Mg-Y-Zn-Mn biodegradable alloys. Mater. Today Commun. 2024, 40, 110166. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Recently deepened insights regarding mg corrosion and advanced engineering applications of mg alloys. J. Magnes. Alloys 2023, 11, 3948–3991. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of recent developments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.; Cao, F.; Shi, Z.; Bowen, P.K. Advances in mg corrosion and research suggestions. J. Magnes. Alloys 2013, 1, 177–200. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Saleh, B.; Xie, Q.; Xu, Q.; Liu, H.; Ma, A. Controlling corrosion resistance of a biodegradable mg–y–zn alloy with lpso phases via multi-pass ecap process. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 1180–1190. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Liu, H.; Saleh, B.; Ma, A. Microstructure characterization and corrosion behavior of mg–y–zn alloys with different long period stacking ordered structures. J. Magnes. Alloys 2020, 8, 1208–1220. [Google Scholar] [CrossRef]
- Song, D.; Li, C.; Liang, N.; Yang, F.; Jiang, J.; Sun, J.; Wu, G.; Ma, A.; Ma, X.S. Simultaneously improving corrosion resistance and mechanical properties of a magnesium alloy via equal-channel angular pressing and post water annealing. Mater. Des. 2019, 166, 107621. [Google Scholar] [CrossRef]
- Abdel-Gawad, S.A.; Shoeib, M.A. Corrosion studies and microstructure of mg−zn−ca alloys for biomedical applications. Surf. Interfaces 2019, 14, 108–116. [Google Scholar] [CrossRef]
- Wang, L.; Bing, L.; Xiuyuan, Z.; Ying, L.; Mei, Z.; Yang, L.; Fudong, W.; Lei, W.; Pengyu, M. Effects of lpso mg12yzn-phase on coefficient of thermal expansion and mechanical properties of mg–y–zn alloys. Phys. Met. Metallogr. 2024, 125, 1785–1790. [Google Scholar] [CrossRef]
- Rastegaev, I.A.; Khrustalev, A.K.; Merson, D.L.; Rastegaeva, I.I.; Eva, O.V.M.; Ev, V.V.M.; Vladykin, A.L. Influence of secondary phase on elastic and acoustic characteristics of magnesium alloys of the mg–zn–y–gd system. Russ. J. Non-Ferrous Metals 2024, 65, 185–198. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, B.; Lin, Y.; Zhou, X. Experimental study of low–cycle fatigue behavior in a mg–y–zn alloy with initial lpso phase. Mater. Sci. Eng. A 2024, 899, 146414. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, T.; Lu, G.J. Atomistic simulations on liquid mg–sr alloys assisted with deep learning potential. Mater. Sci. 2024, 59, 13558–13574. [Google Scholar] [CrossRef]
- Predko, P.; Rajnovic, D.; Grilli, M.L.; Postolnyi, B.O.; Zemcenkovs, V.; Rijkuris, G.; Pole, E.; Lisnanskis, M. Promising methods for corrosion protection of magnesium alloys in the case of mg-al, mg-mn-ce and mg-zn-zr: A recent progress review. Metals 2021, 11, 1133. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Li, A.V.; Bautin, V.A.; Plegunova, S.V.; Vadekhina, V.V.; Ten, D.V.; Komissarov, A.A.; Koltygin, A.V.; Drobyshev, A.Y.; Shin, K.S. Corrosion properties and cytotoxicity of hot-extruded mg-zn-y-mn biodegradable alloys. JOM 2025, 77, 4363–4373. [Google Scholar] [CrossRef]
- Cao, X.; Sun, B.; Zhong, F.; Wang, T.; Pi, L.; Zhang, J.; Cheng, X.; Liang, M.; Li, J. The influence of duplex-phase structure evolution and second phase on corrosion behavior of Mg-Li-Zn-Y alloys. Mater. Today Chem. 2025, 43, 102487. [Google Scholar] [CrossRef]
- Kiani, F.; Lin, J.; Munir, K.; Wen, C.; Li, Y. Improvements in mechanical, corrosion, and biocompatibility properties of mg–zr–sr–dy alloys via extrusion for biodegradable implant applications. J. Magnes. Alloys 2023, 11, 3840–3865. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Wang, J.; Wu, L.; Cao, F.; Zhang, L.; Pan, F. Controllable degradation behavior of mg-sr-y alloys for the bio-applications. npj Mater. Degrad. 2023, 7, 45. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Yin, M.; Xiao, Y.; Wang, X.H.; He, J.X. Comparison of corrosion behavior of al-mn and al-mg alloys in chloride aqueous solution. Mater. Corros. 2016, 67, 51–59. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.R.; Zhang, S.; Shi, Z.L.; Wei, C.; Ma, M.Z.; Liu, R.P. Effect of cu content on the corrosion behavior of ti-based bulk amorphous alloys in hcl solution. Mater. Lett. 2023, 337, 133742. [Google Scholar] [CrossRef]
- Atrens, A.; Shi, Z.; Mehreen, S.U.; Johnston, S.; Song, G.; Chen, X.; Pan, F. Review of mg alloy corrosion rates. J. Magnes. Alloys 2020, 8, 989–998. [Google Scholar] [CrossRef]
- Song, G. and Xu, Z. Effect of microstructure evolution on corrosion of different crystal surfaces of AZ31 Mg alloy in a chloride containing solution. Corros. Sci. 2012, 54, 97–105. [Google Scholar] [CrossRef]
- Südholz, A.D.; Kirkland, N.T.; Buchheit, R.G.; Birbilis, N. Electrochemical properties of intermetallic phases and common impurity elements in magnesium alloys. Electrochem. Solid-State Lett. 2011, 14, C5. [Google Scholar] [CrossRef]
- Roh, H.; Park, J.; Lee, S.; Kim, D.; Lee, G.; Jeon, H.; Chae, M.; Lee, K.; Sun, J.; Lee, D.; et al. Optimization of the clinically approved mg-zn alloy system through the addition of ca. Biomater. Res. 2022, 26, 41. [Google Scholar] [CrossRef]
- Liu, S.; Diao, H.; Chai, L.; Song, B. On the microstructure and mechanical property of as-extruded mg-gd-y-zn alloy with sr addition. Mater. Sci. Eng. A 2017, 679, 183–192. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Saleh, B.; Fathi, R.; Huang, H.; Liu, H.; Ma, A. Optimization of the experimental parameters affecting the corrosion behavior for mg–y–zn–mn alloy via response surface methodology. Met. Mater. Int. 2021, 27, 5095–5107. [Google Scholar] [CrossRef]
- Yuan, Y.; Ma, A.; Gao, Z.; Wu, H.; Gu, Y.; Wang, J.; Chen, J.; Ji, C.; Jiang, J. Improving microstructure and corrosion resistance of lpso-containing mg–y–zn–mn alloy through ecap integrated with prior solution treatment. J. Mater. Res. Technol. 2022, 19, 2275–2286. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Zhao, R.; Zhang, J.; Xu, C. Research of the microstructure, mechanical property and corrosion behaviours of mg–y–zn–mn(–mo) alloy with solution treatment. Corros. Eng. Sci. Technol. 2021, 56, 427–438. [Google Scholar] [CrossRef]
- Cao, F.; Xiao, B.; Wang, Z.; Ying, T.; Zheng, D.; Atrens, A.; Song, G. A Mg alloy with no hydrogen evolution during dissolution. J. Magnes. Alloys 2023, 11, 2084–2095. [Google Scholar] [CrossRef]
- Nie, Y.; Dai, J.; Li, X.; Zhang, X. Recent developments on corrosion behaviors of mg alloys with stacking fault or long period stacking ordered structures. J. Magnes. Alloys 2021, 9, 1123–1146. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Yuan, T.; Xie, Q.; Liu, H.; Ma, A. Recent progress on corrosion behavior and mechanism of mg–re based alloys with long period stacking ordered structure. Met. Mater. Int. 2020, 26, 551–563. [Google Scholar] [CrossRef]



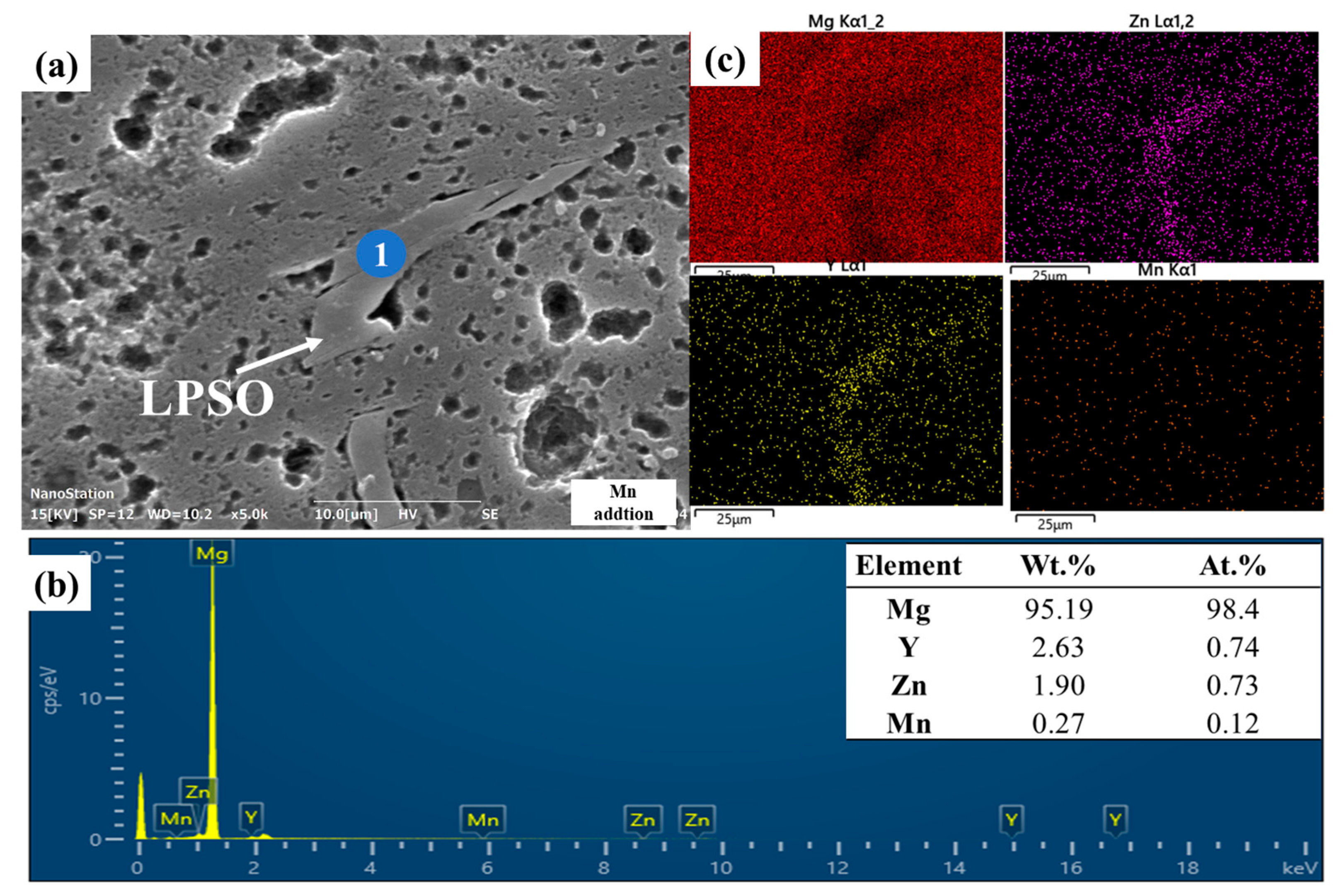

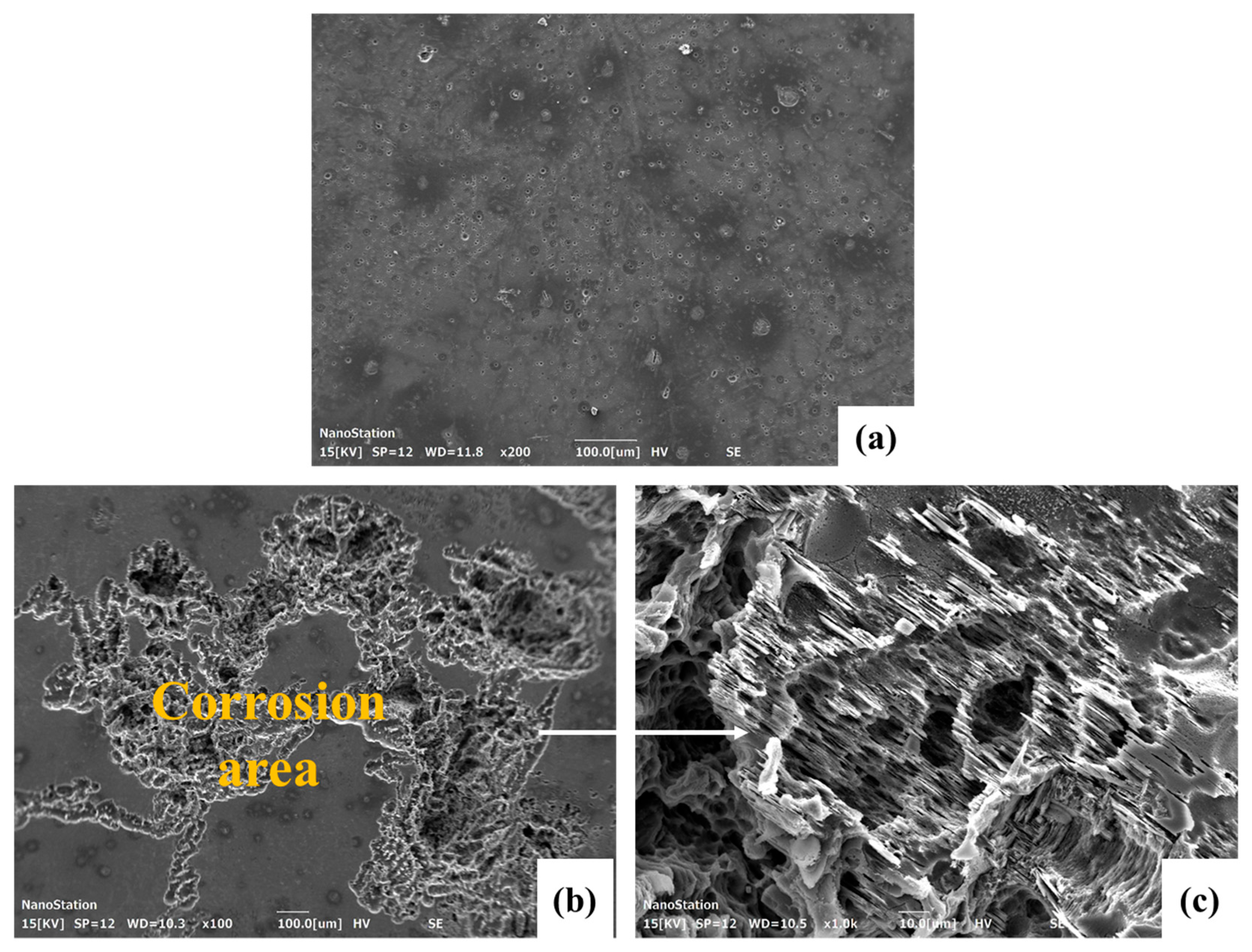

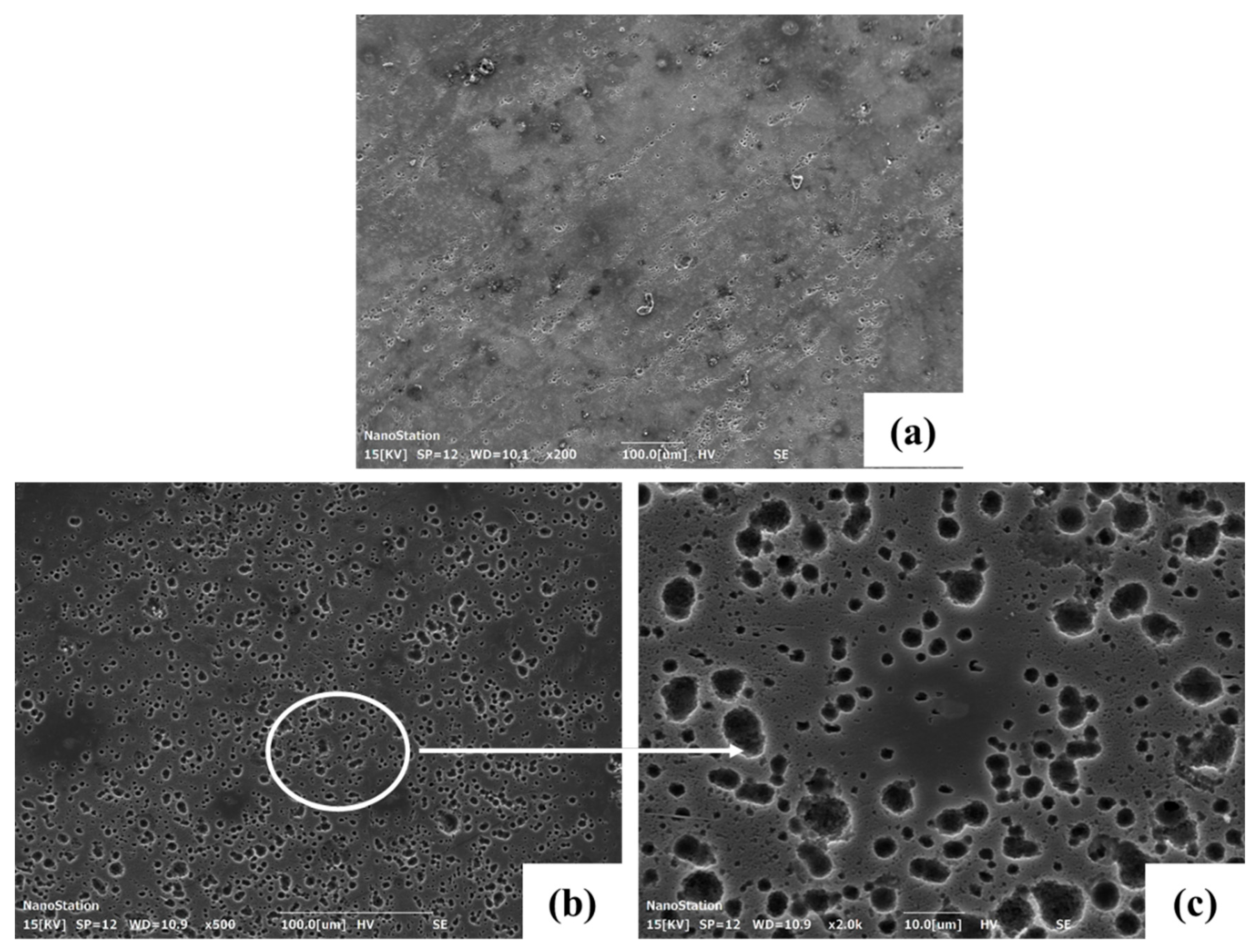
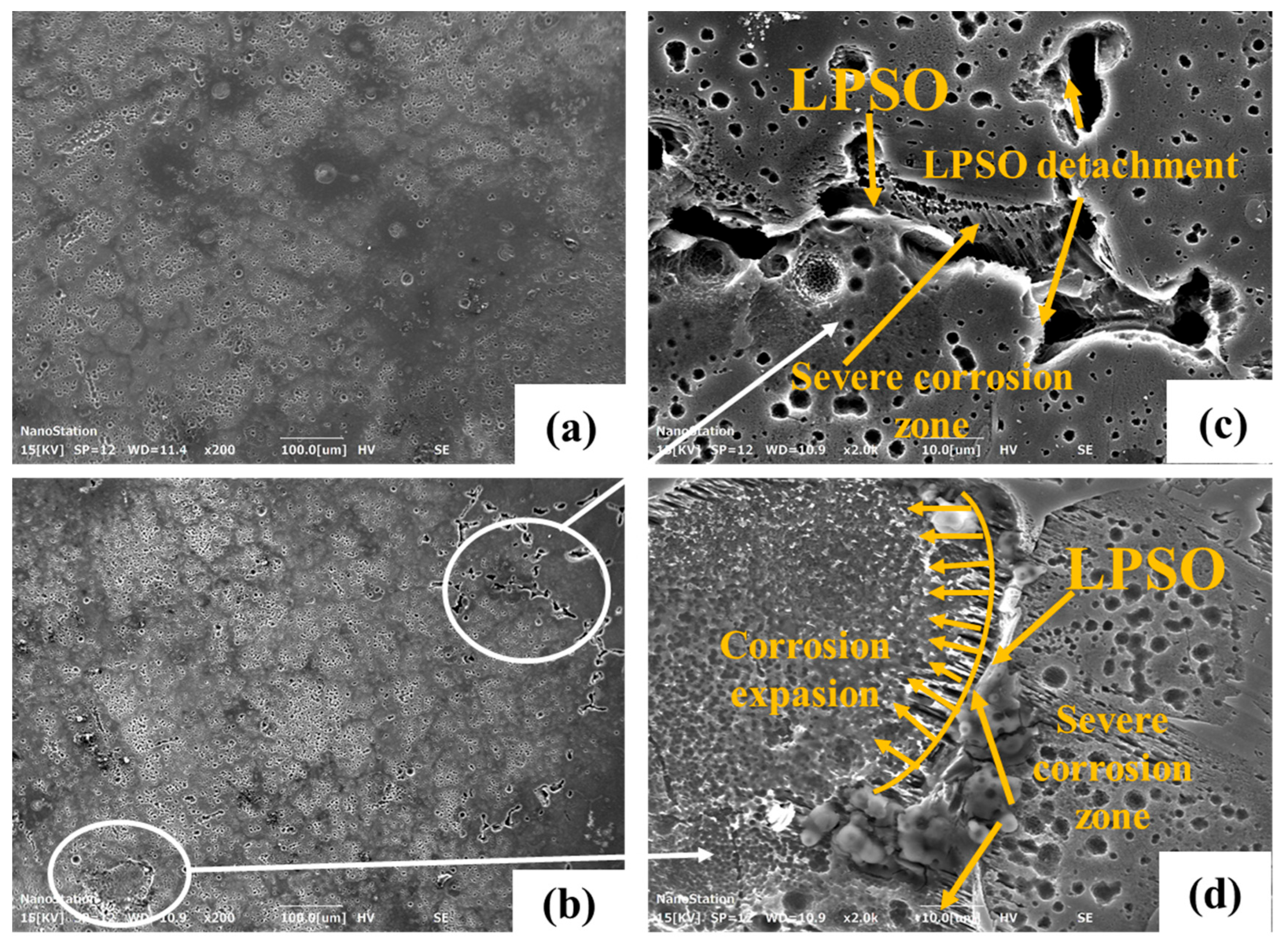
| Nominal Alloys | Mg | Y (wt.%) | Zn (wt.%) | Ca (wt.%) | Sr (wt.%) | Mn (wt.%) | Zr (wt.%) |
|---|---|---|---|---|---|---|---|
| Mg-Y-Zn | Bal. | 2.829 | 1.420 | ||||
| Mg-Y-Zn-Ca | Bal. | 2.407 | 1.323 | 0.5 | |||
| Mg-Y-Zn-Sr | Bal. | 2.219 | 1.463 | 0.5 | |||
| Mg-Y-Zn-Mn | Bal. | 2.227 | 1.435 | 0.45 | |||
| Mg-Y-Zn-Zr | Bal. | 2.196 | 1.359 | 0.3 |
| Alloys | Ecorr (VSCE) | icorr (μA·cm−2) | Epit (VSCE) | Pi (mm/Year) | PAH (mm/Year) | PW (mm/Year) |
|---|---|---|---|---|---|---|
| Mg-Y-Zn | −1.529 | 26.2 | 1.461 | 0.53 | 1.81 | 2.67 |
| Mg-Y-Zn-Ca | −1.564 | 10.06 | −1.45 | 0.31 | 1.21 | 1.65 |
| Mg-Y-Zn-Sr | −1.544 | 18.9 | - | 0.19 | 1.14 | 1.36 |
| Mg-Y-Zn-Mn | −1.55 | 3.39 | −1.43 | 0.12 | 0.67 | 1.02 |
| Mg-Y-Zn-Zr | −1.53 | 3.88 | −1.39 | 0.1 | 0.8 | 1.18 |
| Alloys | Ecorr (VSCE) | icorr (μA·cm−2) | Epit (VSCE) |
|---|---|---|---|
| Mg-Y-Zn | −1.46 | 23.2 | −1.37 |
| Mg-Y-Zn-Ca | −1.47 | 13.6 | - |
| Mg-Y-Zn-Sr | −1.38 | 8.3 | - |
| Mg-Y-Zn-Mn | −1.56 | 5.41 | −1.36 |
| Mg-Y-Zn-Zr | −1.47 | 4.52 | −1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, H.; Zhang, C.; Sun, W.; Wang, Y.; Wang, L.; Kang, X. Enhancing Electrochemical Kinetics and Stability of Biodegradable Mg-Y-Zn Alloys with LPSO Phases via Strategic Micro-Alloying with Ca, Sr, Mn, and Zr. Crystals 2025, 15, 639. https://doi.org/10.3390/cryst15070639
Wang L, Wang H, Zhang C, Sun W, Wang Y, Wang L, Kang X. Enhancing Electrochemical Kinetics and Stability of Biodegradable Mg-Y-Zn Alloys with LPSO Phases via Strategic Micro-Alloying with Ca, Sr, Mn, and Zr. Crystals. 2025; 15(7):639. https://doi.org/10.3390/cryst15070639
Chicago/Turabian StyleWang, Lisha, Huiping Wang, Chenchen Zhang, Wei Sun, Yue Wang, Lijuan Wang, and Xiaoyan Kang. 2025. "Enhancing Electrochemical Kinetics and Stability of Biodegradable Mg-Y-Zn Alloys with LPSO Phases via Strategic Micro-Alloying with Ca, Sr, Mn, and Zr" Crystals 15, no. 7: 639. https://doi.org/10.3390/cryst15070639
APA StyleWang, L., Wang, H., Zhang, C., Sun, W., Wang, Y., Wang, L., & Kang, X. (2025). Enhancing Electrochemical Kinetics and Stability of Biodegradable Mg-Y-Zn Alloys with LPSO Phases via Strategic Micro-Alloying with Ca, Sr, Mn, and Zr. Crystals, 15(7), 639. https://doi.org/10.3390/cryst15070639






