Abstract
Cubic phase SnSe-based materials have great potential in the field of thermoelectricity due to their reduced carrier scattering, increased band degeneracy, and ultra-low lattice thermal conductivity. Nevertheless, systematic studies on the influence of element doping on the thermoelectric properties of cubic SnSe-based materials are still relatively scarce. To enrich the research in this field, this work investigates the effects of Ge doping on the phase composition, electrical and thermal transport properties of cubic Sn0.50Ag0.25Bi0.25Se0.50Te0.50 thermoelectric materials. X-ray diffraction (XRD) analysis confirmed that the Ge-doped samples exhibited a single cubic phase structure, while scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) revealed a uniform distribution of elements within the samples. The results indicate that increasing the Ge doping content substantially enhances their electrical conductivity, albeit at the expense of elevated thermal conductivity. By optimizing the content of Ge-doping, the thermoelectric figure of merit (ZT) reached 0.74 at 750 K. Notably, while moderate Ge doping enhances electrical transport properties, excessive doping leads to a significant rise in thermal conductivity, ultimately constraining further thermoelectric performance gains.
1. Introduction
Thermoelectric materials can directly convert heat into electricity or vice versa, making them highly promising for applications in energy conversion and refrigeration technologies. With the continuous growth in global energy demand and increasing emphasis on environmentally friendly technologies, the development of high-efficiency thermoelectric materials has become a significant focus in materials science [1,2,3,4,5]. The performance of thermoelectric materials is primarily determined by the dimensionless figure of merit (ZT), which is a function of the Seebeck coefficient (S), electrical conductivity (σ), and thermal conductivity (κ) [1,5]. Although traditional Bi2Te3-based materials exhibit high ZT values in low-temperature ranges, their performance declines significantly at mid-to-high temperatures, limiting their potential for broader applications [6,7,8]. Therefore, the development of new materials that can maintain excellent thermoelectric performance in the mid-to-high temperature range (500–800 K) is of paramount importance.
In recent years, SnSe-based thermoelectric materials have garnered considerable attention due to their exceptional ZT values in the mid-to-high temperature range [9,10,11]. Notably, the small formation energy difference between the Pnma phase and the Fm-3m phase of SnSe suggests that the cubic SnSe can be thermally stabilized. This highly symmetric cubic crystal structure offers two advantages for TE materials: reduced carrier scattering for enhanced carrier mobility and increased band degeneracy that improves the Seebeck coefficient. In addition, the anharmonic bonds and ultra-low lattice thermal conductivity of Sn-Se are still retained in the cubic SnSe. These characteristics indicate that cubic SnSe is a promising thermoelectric material. Nevertheless, it is challenging to obtain stable cubic SnSe-based materials under ambient conditions by conventional methods. Researchers have been committed to modifying the crystal structure and increasing the crystal structure symmetry by introducing second-phase alloying [12,13]. Recently, various successful stabilizations of cubic phase SnSe-based materials through alloying with I-V-VI2 compound family (I = Ag, Na, Cu; V = Sb, Bi, VI = S, Se, Te) have been demonstrated [12,14,15]. AgBiTe2, as a leader of the I-V-VI2 compound family, exhibits a rock salt cubic phase crystal structure at high temperatures. In addition, it also possesses relatively low thermal conductivity owing to the presence of lone pairs of electrons. Therefore, it has been extensively applied in stabilizing the cubic structure and improving the thermoelectric properties of SnSe-based materials [15,16]. High-quality cubic-phase SnSe-based materials can now be reliably prepared in large quantities, through the unremitting efforts of the researchers. However, the influence of metal element doping on the thermoelectric performance of cubic SnSe-based materials remains limited, although it has been proven to be an effective method for improving the thermoelectric performance of SnSe-based materials [17,18,19,20].
Germanium (Ge), as a Group IV element, shows good lattice compatibility with the SnSe matrix, and its doping can optimize electrical transport properties by modulating carrier concentration and mobility [20,21]. Furthermore, Ge doping may introduce new scattering mechanisms, potentially reducing thermal conductivity and enhancing the ZT value [20,21,22]. Herein, a series of Ge-doped Sn0.50Ag0.25Bi0.25Se0.50Te0.50 samples were prepared using ball milling and rapid hot-pressing sintering in this work. The phase composition and microstructure of the prepared samples were comprehensively characterized using X-ray diffraction (XRD) and scanning electron microscopy (SEM). The effects of varying Ge doping levels on the electrical and thermal transport behaviors of the samples were systematically investigated. Our objective is to elucidate the mechanisms by which Ge doping affects the crystal structure, carrier concentration, mobility, and overall thermoelectric performance of these materials, thereby providing a theoretical foundation and experimental basis for designing high-efficiency thermoelectric materials.
2. Experimental Procedures
The Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 materials with various stoichiometric ratios were synthesized by cold pressing-microwave melting. The tin (Aladdin, ≥99.99%), selenium (Aladdin, ≥99.99%), silver (Aladdin, ≥99.99%), bismuth (Aladdin, ≥99.99%), germanium (Aladdin, ≥99.99%) and tellurium (Aladdin, ≥99.99%) powders were mixed uniformly by ball milling and then cold pressed into a bulk. The pressed bulk was placed in a carbon crucible enclosed in evacuated ampoules. The sealed silica ampoules were heated in a microwave apparatus for 10 min and cooled slowly in air. The ingots obtained by microwave melting were ground into powder and densified by rapidly hot-pressing sintering (RHP) at 773 K for 30 min under an axial compressive stress of 60 MPa to form the high-density disk-shaped samples.
The electrical conductivity and Seebeck coefficient of the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 bulk samples (with a diameter of ~12.7 mm and a thickness of 1.0~1.2 mm) were measured using a commercially available electrical performance test platform (ZEM-3, ULVAC-RIKO). Archimedes’ drainage method was used to measure the density (ρ) of the sintered Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 bulk samples. The phase composition of Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples was measured by X-ray diffraction (XRD, SmartLab 9KW with Cu Ka radiation, Japan) with a scanning speed of 4°/min at the range of 10–80°. The XRD patterns of the optimized Sn0.50Ag0.25(Bi0.94Ge0.06)0.25Se0.50Te0.50 sample under different temperatures were obtained on an in situ XRD instrument (Empyrean, PANalytical) in an Ar atmosphere. The thermal conductivity (κ) of the prepared samples was measured using a flash laser thermal conductivity meter (LFA-1000, Netzsch, Selb, Germany) under a flowing argon atmosphere according to the following equation:
where λ, ρ and Cp are the thermal diffusivity, density, and specific heat capacity, respectively. Owing to the experimental uncertainties in the measurement of transport properties, the estimated errors for the conductivity, Seebeck coefficient, and thermal conductivity are approximately 5%. Consequently, the combined uncertainty in the calculated ZT is estimated to be about 8.6%. The Hall coefficient (RH) of the sample was determined at room temperature under a 0.8 T reversible magnetic field using the Van der Pauw method. The carrier concentration (n) and carrier mobility (μ) of the prepared samples were calculated using the following formulas:
κ = λρCp
3. Results and Discussion
Figure 1 presents the crystal structure (a) and XRD pattern (b) of the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples. All Bragg peaks of the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples can be well indexed to the cubic SnSe standard card (PDF#89-4781) [15,16], confirming the successful formation of a single-phase cubic structure without detectable secondary phases. Derived from doping with the Ge element, the most obvious change in the XRD patterns was the shift in Bragg diffraction angle to lower angles, which was attributed to the lower ionic radius of the Ge element than the Bi element [20,21]. According to Vegard’s law, the uniform distribution of point defects typically results in lattice contraction to minimize strain energy. The absence of secondary phases confirms that Ge doping did not trigger phase segregation or structural transformation, indicating the successful formation of a homogeneous solid solution within the cubic SnSe lattice. Furthermore, the incorporation of the Ge element can provide additional charge carriers while causing lattice distortion, thereby improving thermoelectric performance [20,21].
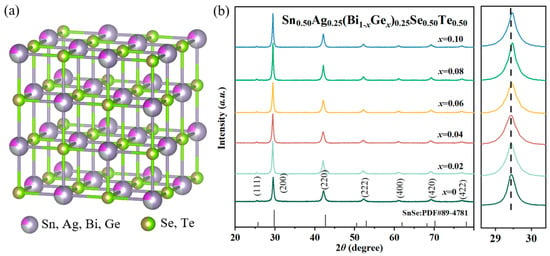
Figure 1.
Crystal structure (a) and XRD pattern (b) of the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples.
Figure 2a presents representative scanning electron microscope (SEM) images of the fresh fracture surface of Sn0.50Ag0.25(Bi0.90Ge0.10)0.25Se0.50Te0.50 samples at varying magnifications. The Ge-doped Sn0.50Ag0.25Bi0.25Se0.50Te0.50 sample exhibited a typical blocky grain, which is consistent with the cubic SnSe-based materials [15]. The grain size (~1 ± 0.5 μm) and morphology were similar to those of the Sn0.50Ag0.25Bi0.25Se0.50Te0.50 samples that we previously reported [15,16], which suggests that Ge doping does not markedly affect the grain growth process. Additionally, a large number of dispersed pores and voids were observed in the images. The presence of these voids can effectively reduce their thermal conductivity [23,24,25]. Figure 2b shows the backscattered electron (BSE) image and energy-dispersive X-ray spectroscopy (EDS) elemental distribution maps for Sn0.50Ag0.25(Bi0.90Ge0.10)0.25Se0.50Te0.50 samples. The EDS elemental distribution maps demonstrate that various elements are uniformly distributed throughout the sample, with no evident element segregation. This indicates that the Ge element is uniformly incorporated into the lattice, without precipitating out or reacting with other elements to form impurity phases.
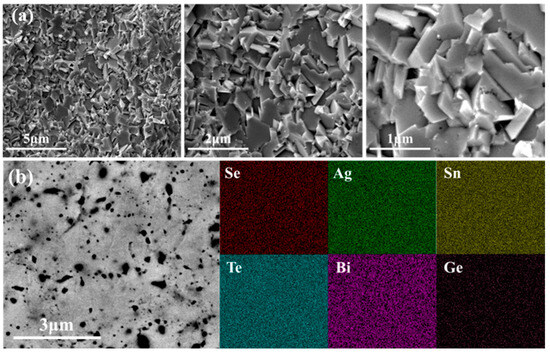
Figure 2.
SEM images of the prepared bulk Sn0.50Ag0.25(Bi0.90Ge0.10)0.25Se0.50Te0.50 sample: (a) SEM images of fresh fracture surface; (b) SEM images of polished surface and various elemental distribution mapping images.
Figure 3a illustrates the electrical conductivity of Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples as a function of temperature. The trend in electrical conductivity with temperature for Ge-doped Sn0.50Ag0.25Bi0.25Se0.50Te0.50 samples is similar to that of the undoped sample. The electrical conductivity of the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples exhibited an increasing trend with the increase of Ge element content at room temperature, which is mainly attributed to the significant increase in carrier concentration (from 1.11 to 5.43 × 1021 cm−3) caused by Ge element doping (as shown in Table 1). The increase in carrier concentration caused by Ge doping also leads to a significant increase in electrical conductivity across the entire temperature range. In the low-temperature range (300 K to 450 K), the electrical conductivity decreased with increasing temperature, which was primarily attributed to the semi-metallic conductive characteristics introduced by alloying [15,16]. Notably, the electrical conductivity of all synthesized Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples exhibited a sharp decrease with increasing temperature in the range of 450~550 K. To elucidate the underlying physical mechanism responsible for this abrupt decline in electrical conductivity, XRD patterns of the optimized Sn0.50Ag0.25(Bi0.94Ge0.06)0.25Se0.50Te0.50 sample under different temperatures were analyzed, as displayed in Figure 4. The absence of secondary phases (including other phase structures of SnSe and oxides) within this temperature range suggests that neither phase transformation nor chemical reactions occurred. Consequently, the observed conductivity reduction is likely attributable to a significant decrease in carrier concentration, arising from diminished valence band participation in electrical transport [26,27]. In contrast, at elevated temperatures (approximately 550 K to 750 K), the electrical conductivity increased with further temperature rise, which is attributed to the bipolar effect [28]. The bipolar effect generated the enhancement of electrical conductivity due to the generation of electron-hole pairs at high temperatures.
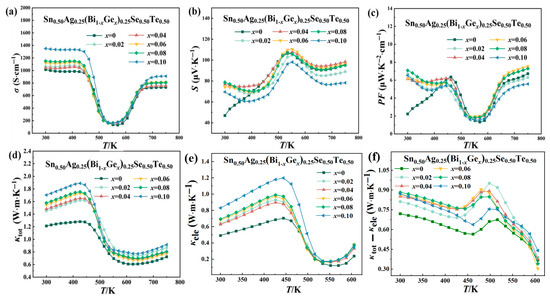
Figure 3.
Temperature-dependent curves of electrical and thermal transport properties of Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 thermoelectric materials: (a) electrical conductivity σ; (b) Seebeck coefficient S; (c) power factor PF; (d) total thermal conductivity κₜₒₜ; (e) electronic thermal conductivity κele; (f) κtot-κele.

Table 1.
Density, relative density, carrier concentration (n), and carrier mobility (μ) of the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 thermoelectric materials.
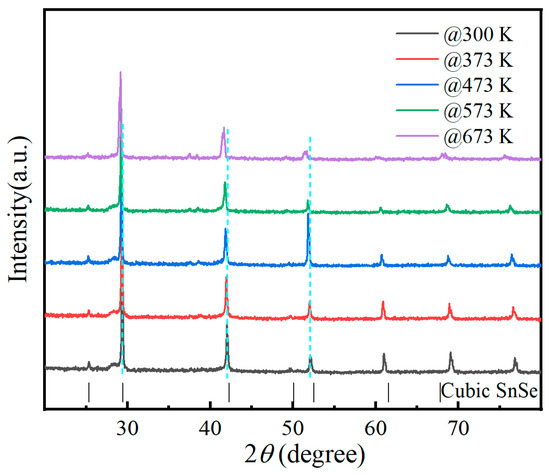
Figure 4.
XRD pattern of the prepared Sn0.50Ag0.25(Bi0.94Ge0.06)0.25Se0.50Te0.50 sample under different temperatures.
Figure 3b shows the Seebeck coefficient as a function of temperature for the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples. All Ge-doped samples exhibit positive Seebeck coefficients, indicating that these samples are p-type semiconductors. With temperature increasing, the Seebeck coefficient initially increases to a peak and then decreases due to the bipolar effect. With increasing Ge doping amount, a slight rise in carrier concentration (n) was observed in the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples (as shown in Table 1). In SnSe-based compounds, alloying with metal elements typically promotes energy band convergence, which enhances the density of states near the Fermi level and consequently increases the carrier effective mass (m*) [29,30]. The Seebeck coefficient (S) depends on both carrier concentration and effective mass according to the following relationship:
where h is Planck’s constant and kB is Boltzmann’s constant. Equation (1) clearly shows that S is inversely proportional to n2/3 but directly proportional to m*. Therefore, the synergistic effect of both carrier concentration and carrier effective mass leads to the Seebeck coefficient of the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples remaining at relatively high values.
Figure 3c presents the temperature dependence curves of power factor for the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples. In samples with low Ge doping levels, the increase in electrical conductivity is not significant, and the slight reduction in the Seebeck coefficient means that the power factor is not notably optimized [28]. However, when the Ge doping level reaches x = 0.06, there is a substantial increase in electrical conductivity, and the decrease in the Seebeck coefficient is relatively small, leading to a significant improvement in the power factor. For instance, at 750 K, the power factor of Sn0.50Ag0.25(Bi0.94Ge0.06)0.25Se0.50Te0.50 sample is increased by 13% compared to the undoped samples. This phenomenon indicates that appropriate Ge doping not only enhances electrical conductivity but also maintains a high Seebeck coefficient, ultimately achieving an optimized power factor. However, when the Ge doping level is further increased, such as x = 0.10, the sharp rise in carrier concentration leads to a significant decrease in the Seebeck coefficient, resulting in a reduction in the power factor.
Figure 3d displays the temperature-dependent total thermal conductivity (κtot) of the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples. Obviously, the total thermal conductivity exhibited a strong dependence on Ge doping content, increasing monotonically from 1.21 Wm−1K−1 (x = 0) to 1.70 Wm−1K−1 (x = 0.10) at room temperature, which seems to be contrary to the phenomenon observed in the doping of conventional metal elements. To elucidate the underlying mechanism, the electronic thermal conductivity (κele) contributions via the Wiedemann-Franz law (as displayed in Figure 3e) [31,32]:
where is the electrical conductivity, T is the absolute temperature, and L is the Lorenz number, calculated from the measured Seebeck coefficient as L(10−8 W Ω K−2) = 1.5 + exp [−|S|/116]. κele of the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples exhibited a significant increase with Ge doping concentration across the entire temperature range. This enhancement arises because Ge doping introduces a high hole concentration (Table 1), leading to a substantial improvement in electrical conductivity. Furthermore, the lattice thermal conductivity (κlat) of the prepared Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 sample was derived by subtracting κele from κtot as displayed in Figure 3f. Notably, κtot-κele remained comparable in magnitude to κele, attributable to the sharp rise in electrical conductivity. These results indicate nearly equivalent contributions from electronic and lattice thermal conductivity.
Figure 5 presents the dimensionless thermoelectric figure of merit (ZT) as a function of temperature for Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 samples. The observed ZT enhancement with Ge doping mainly stems from improved electrical conductivity, though this improvement is partially offset by the concomitant increase in total thermal conductivity. Optimal Ge doping (x = 0.06) achieves an effective balance between electrical conductivity and Seebeck coefficient, yielding a maximum ZT of 0.74 at 750 K. This represents a 35% enhancement compared to pristine SnSe. Notably, excessive doping increases carrier concentration beyond the optimal level, causing substantial thermal conductivity elevation that ultimately degrades thermoelectric performance.
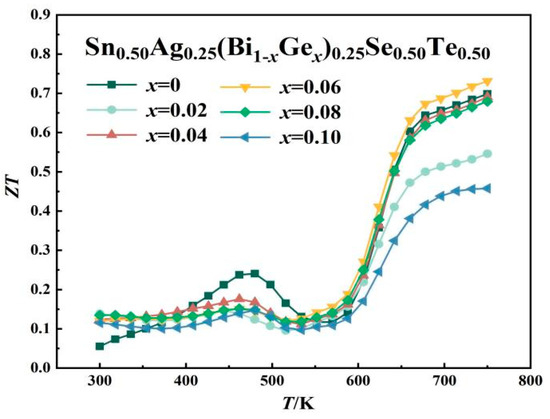
Figure 5.
Thermoelectric figure of merit (ZT) of Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 materials with various Ge doping levels.
4. Conclusions
In summary, this study thoroughly investigated the impact of Ge doping on the performance of Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50 thermoelectric materials. In terms of electrical transport properties, Ge doping significantly increased the electrical conductivity and kept the Seebeck coefficient at relatively high values, which is probably owing to the enhancement in carrier concentration and density of states near the Fermi level caused by the introduction of the Ge element. Appropriate Ge doping effectively enhances the thermoelectric figure of merit (ZT) of Sn0.50Ag0.25(Bi1−xGex)0.25Se0.50Te0.50, yielding a maximum ZT of 0.74 at 750 K. This result indicates that by optimizing the Ge doping content, the conductivity of cubic SnSe-based materials can be significantly enhanced while maintaining a high Seebeck coefficient, thereby achieving a significant improvement in thermoelectric performance.
Author Contributions
H.Z. and J.Z. completed the experiment and wrote the manuscript. Z.Z., L.B., W.W. and X.L. provide advice and support during the thermoelectric performance evaluation and paper writing process. C.L. and D.Z. supervised the project and conceived the idea. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Taishan Scholar Program of Shandong Province (No. tsqn202306225), Shandong Postdoctoral Science Foundation (No. SDBX2023025), Leader of Scientific Research Studio Program of Jinan (No. 2021GXRC082), University of Jinan Disciplinary Cross-Convergence Construction Projects 2023 (Nos. XKJC-202301 and XKJC-202311), the Natural Science Foundation of Shandong Province (Nos. ZR2024QF181 and ZR2024QE044), the Youth Innovation Team Project of Shandong Province (2024KJH108), Jinan City-School Integration Development Strategy Project (Nos. JNSX2023015 and JNSX2023018), Postdoctoral Fellowship Program of CPSF (No. GZC20230956), and China Postdoctoral Science Foundation (No. 2024M751104).
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Shi, X.-L.; Zou, J.; Chen, Z.-G. Advanced thermoelectric design: From materials and structures to devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef]
- Han, H.; Zhao, L.; Wu, X.; Zuo, B.; Bian, S.; Li, T.; Liu, X.; Jiang, Y.; Chen, C.; Bi, J.; et al. Advancements in thermoelectric materials: Optimization strategies for enhancing energy conversion. J. Mater. Chem. A 2024, 12, 24041–24083. [Google Scholar] [CrossRef]
- Wua, Z.; Zhang, S.; Liu, Z.; Mu, E.; Hu, Z. Thermoelectric converter: Strategies from materials to device application. Nano Energy 2022, 91, 106692. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, Y.; Zhang, H.; Qin, Y.; Wang, Z.-Y.; Zhan, S.; Liu, D.; Lin, N.; Tao, Y.; Hong, T.; et al. Large mobility enables higher thermoelectric cooling and power generation performance in n-type agpb18+xsbte20 crystals. J. Am. Chem. Soc. 2023, 145, 24931–24939. [Google Scholar] [CrossRef]
- He, J.; Tritt, T.M. Advances in thermoelectric materials research: Looking back and moving forward. Science 2017, 357, eaak9997. [Google Scholar] [CrossRef]
- Mamur, H.; Bhuiyan, M.R.A.; Korkmaz, F.; Nil, M. A review on bismuth telluride (Bi2Te3) nanostructure for thermoelectric applications. Renew. Sustain. Energy Rev. 2018, 82, 4159–4169. [Google Scholar] [CrossRef]
- Saberi, Y.; Sajjadi, S.A. A comprehensive review on the effects of doping process on the thermoelectric properties of Bi2Te3 based alloys. J. Alloys Compd. 2022, 904, 163918. [Google Scholar] [CrossRef]
- Puthran, S.; Hegde, G.S.; Prabhu, A.N. Review of chalcogenide-based materials for low-, mid-, and high-temperature thermoelectric applications. J. Electron. Mater. 2024, 53, 5739–5768. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, X.; Zhao, L.-D. Rethinking SnSe thermoelectrics from computational materials science. Acc. Chem. Res. 2023, 56, 3065–3075. [Google Scholar] [CrossRef]
- Siddique, S.; Abbas, G.; Yaqoob, M.M.; Zhao, J.; Chen, R.; Larsson, J.A.; Cao, Y.; Chen, Y.; Zheng, Z.; Zhang, D.; et al. Optimization of thermoelectric performance in p-type SnSe crystals through localized lattice distortions and band convergence. Adv. Sci. 2025, 12, 2411594. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Tan, G.; Hao, S.; He, J.; Pei, Y.; Chi, H.; Wang, H.; Gong, S.; Xu, H.; Dravid, V.P.; et al. Ultrahigh power factor and thermoelectric performance in hole-doped single-crystal SnSe. Science 2016, 351, 141–144. [Google Scholar] [CrossRef]
- Lin, N.; Han, S.; Ghosh, T.; Schön, C.F.; Kim, D.; Frank, J.; Hoff, F.; Schmidt, T.; Ying, P.; Zhu, Y.; et al. Metavalent bonding in cubic SnSe alloys improves thermoelectric properties over a broad temperature range. Adv. Funct. Mater. 2024, 1, 2315652. [Google Scholar] [CrossRef]
- Qin, B.; Wang, D.; Hong, T.; Wang, Y.; Liu, D.; Wang, Z.; Gao, X.; Ge, Z.-H.; Zhao, L.-D. High thermoelectric efficiency realized in SnSe crystals via structural modulation. Nat. Commun. 2023, 14, 1366. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, S.; Shi, H.; Cao, Q.; Qin, B.; Zhao, L. Modulating structures to decouple thermoelectric transport leads to high performance in polycrystalline SnSe. J. Mater. Chem. A 2024, 12, 144–152. [Google Scholar] [CrossRef]
- Zhu, J.; Bo, L.; Kong, J.; Hou, Y.; Zhao, L.; Li, C.; Zhao, D. Enhanced thermoelectric and mechanical properties of polycrystalline cubic snse by AgBiTe2 alloying. J. Alloys Compd. 2024, 971, 172754. [Google Scholar] [CrossRef]
- Tan, Z.; Zeng, Z.; Zhu, J.; Wang, W.; Bo, L.; Liu, X.; Li, C.; Zhao, D. Enhanced thermoelectric properties in cubic Sn0.50Ag0.25Bi0.25Se0.50Te0.50 via MWCNTs incorporation. Crystals 2025, 15, 365. [Google Scholar] [CrossRef]
- Chandra, S.; Dutta, P.; Biswas, K. High-performance thermoelectrics based on solution-grown SnSe nanostructures. ACS Nano 2021, 16, 7–14. [Google Scholar] [CrossRef]
- Shi, X.; Wu, A.; Feng, T.; Zheng, K.; Liu, W.; Sun, Q.; Hong, M.; Pantelides, S.T.; Chen, Z.-G.; Zou, J. High thermoelectric performance in p-type polycrystalline Cd-doped SnSe achieved by a combination of cation vacancies and localized lattice engineering. Adv. Energy Mater. 2019, 9, 1803242. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, K.; Hong, M.; Liu, W.; Moshwan, R.; Wang, Y.; Qu, X.; Chen, Z.-G.; Zou, J. Boosting the thermoelectric performance of p-type heavily Cu-doped polycrystalline SnSe via inducing intensive crystal imperfections and defect phonon scattering. Chem. Sci. 2018, 9, 7376–7389. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, S.; Hou, Y.; Li, S.; Wang, C.; Xiong, W.; Zhang, Q.; Miao, X.; Liu, J.; Cao, Y.; et al. Enhanced density of states facilitates high thermoelectric performance in solution-grown Ge- and In-codoped SnSe nanoplates. ACS Nano 2022, 17, 801–810. [Google Scholar] [CrossRef]
- Gong, Y.; Ying, P.; Zhang, Q.; Liu, Y.; Huang, X.; Dou, W.; Zhang, Y.; Li, D.; Zhang, D.; Feng, T.; et al. Realizing the high thermoelectric performance of highly preferentially oriented snse based nanorods via band alignment. Energy Environ. Sci. 2024, 17, 1612–1623. [Google Scholar] [CrossRef]
- Liu, M.; Guo, M.; Lyu, H.; Lai, Y.; Zhu, Y.; Guo, F.; Yang, Y.; Yu, K.; Dong, X.; Liu, Z.; et al. Doping strategy in metavalently bonded materials for advancing thermoelectric performance. Nat. Commun. 2024, 15, 8286. [Google Scholar] [CrossRef]
- Shi, X.; Wu, A.; Liu, W.; Moshwan, R.; Wang, Y.; Chen, Z.-G.; Zou, J. Polycrystalline SnSe with extraordinary thermoelectric property via nanoporous design. ACS Nano 2018, 12, 11417–11425. [Google Scholar] [CrossRef]
- Wu, J.; Chen, K.; Reece, M.J.; Huang, Z. Porous thermoelectric materials for energy conversion by thermoelectrocatalysis. Energy Technol. 2024, 1, 2400973. [Google Scholar] [CrossRef]
- Ijaz, U.; Siyar, M.; Park, C. The power of pores: Review on porous thermoelectric materials. RSC Sustain. 2024, 2, 852–870. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, J.; Bo, L.; Zhou, W.; Liu, X.; Li, C.; Zhang, Z.; Zhao, D. Synergistically optimized electronic and phonon transport properties in cubic SnSe thermoelectric materials via pb doping. Rare Met. 2025, 44, 3339–3350. [Google Scholar] [CrossRef]
- Qin, B.; Wang, D.; Liu, X.; Qin, Y.; Dong, J.-F.; Luo, J.; Li, J.-W.; Liu, W.; Tan, G.; Tang, X.; et al. Power generation and thermoelectric cooling enabled by momentum and energy multiband alignments. Science 2021, 373, 556–561. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Jiang, X.-D.; Duan, Y.-X.; Wang, X.; Ge, Z.-H.; Cai, J.-M.; Cai, X.-M.; Tan, H.-L. P-type Sn0.98Ag0.02Se with low thermal conductivity synthesized by hydrothermal method. J. Eur. Ceram. Soc. 2024, 44, 1636–1646. [Google Scholar] [CrossRef]
- Li, C.; Lan, X.; Liu, P.; Xu, J.; Jiang, Q.; Liu, C.; Liu, C.; Jiang, F. Core/hybrid-shell structures boost thermoelectric performance of flexible inorganic/organic nanowire films. Nano Res. 2023, 16, 5702–5708. [Google Scholar] [CrossRef]
- Sun, P.; Li, C.; Xu, J.; Jiang, Q.; Wang, W.; Liu, J.; Zhao, F.; Ding, Y.; Hou, J.; Jiang, F. Effect of Sn element on optimizing thermoelectric performance of Te nanowires. Sustain. Energy Fuels 2018, 2, 2636–2643. [Google Scholar] [CrossRef]
- Mandal, P.; Maitra, S.; Ghorui, U.K.; Chakraborty, P.; Adhikary, B.; Banerjee, D. Effects of co-doping on tin selenide nanomaterials to enhance the thermoelectric performance above the ambient temperature range. J. Mater. Chem. C 2023, 11, 8577–8589. [Google Scholar] [CrossRef]
- Yu, J.; Li, F.; Zhu, J.; Hao, M.; Li, C.; Zhao, D. Modulating structures and nanocomposites to boost thermoelectric properties of polycrystalline SnSe by Ag/In co-doping. J. Mater. Eng. Perform. 2025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).