Eu-Tb-Doped Y-BTC MOF: A Step Towards Optimization of an Energy Conversion System
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Reagents
2.2. Y-BTC: Eu (10%), Tb (10%) Synthesis
2.3. Characterizations
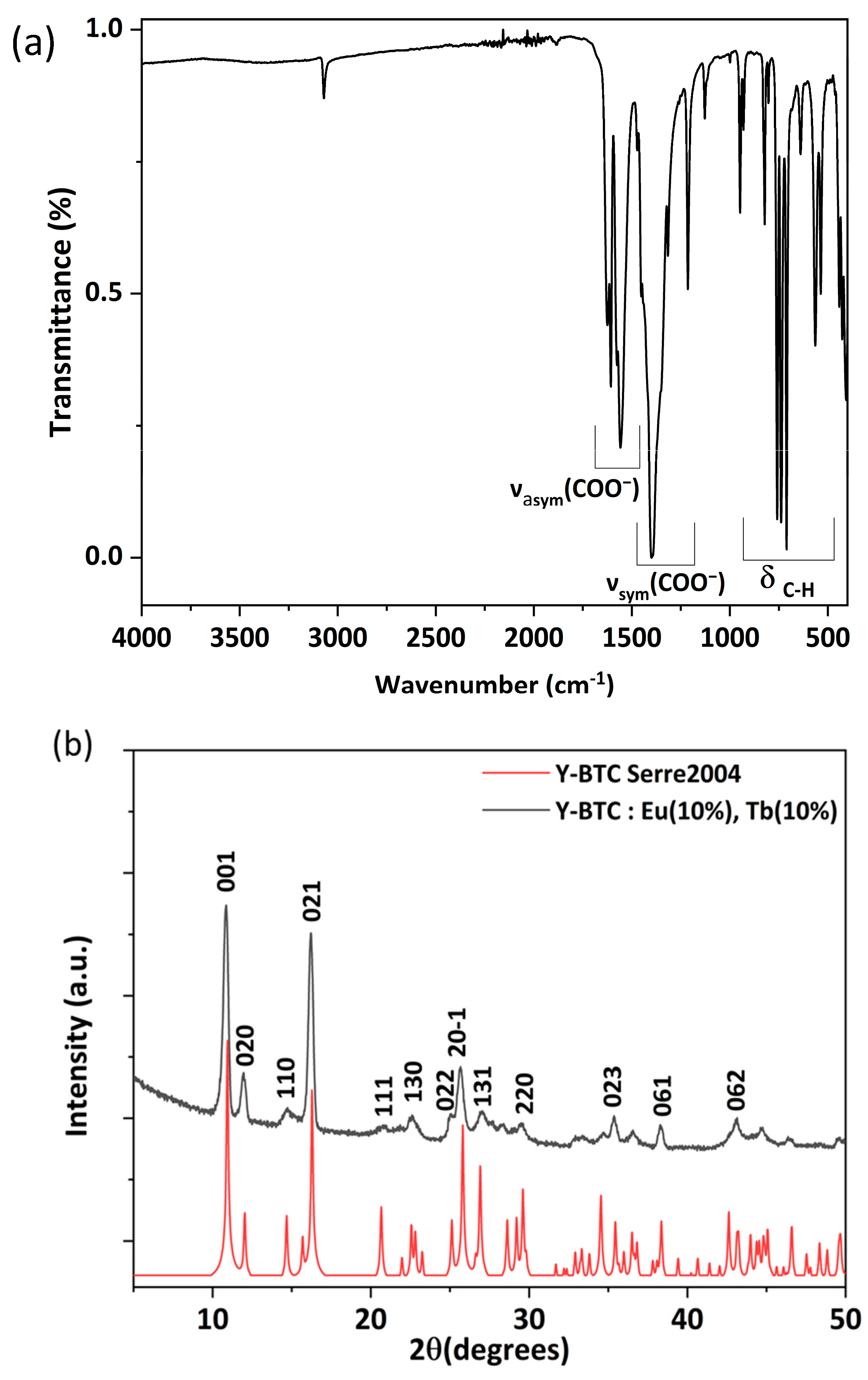
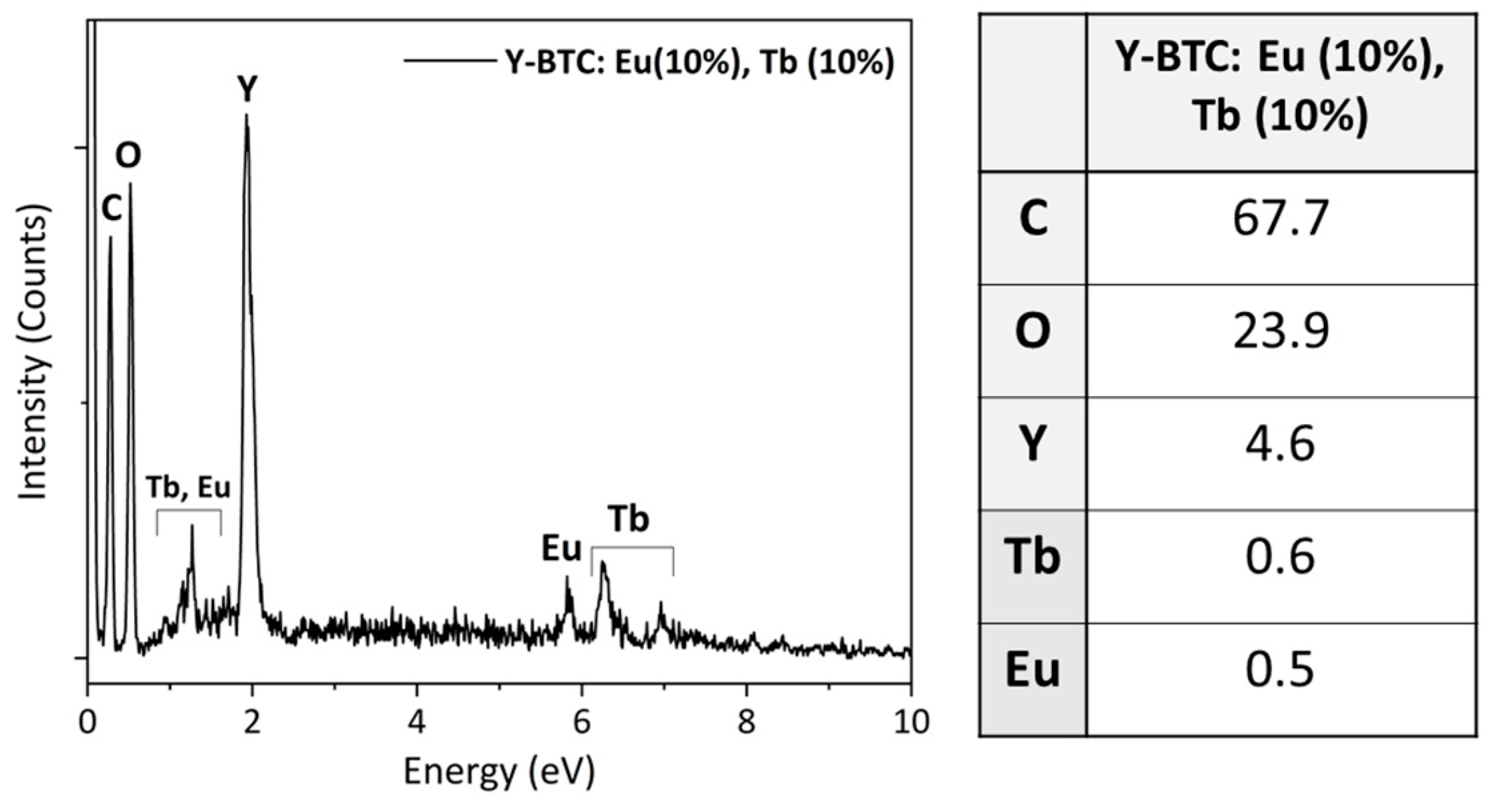
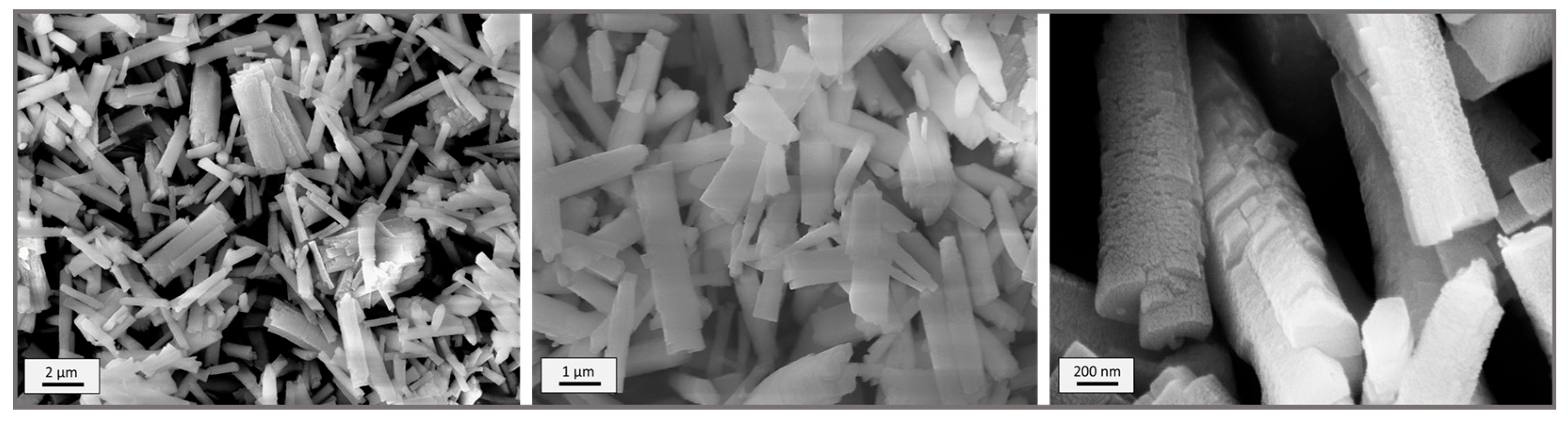
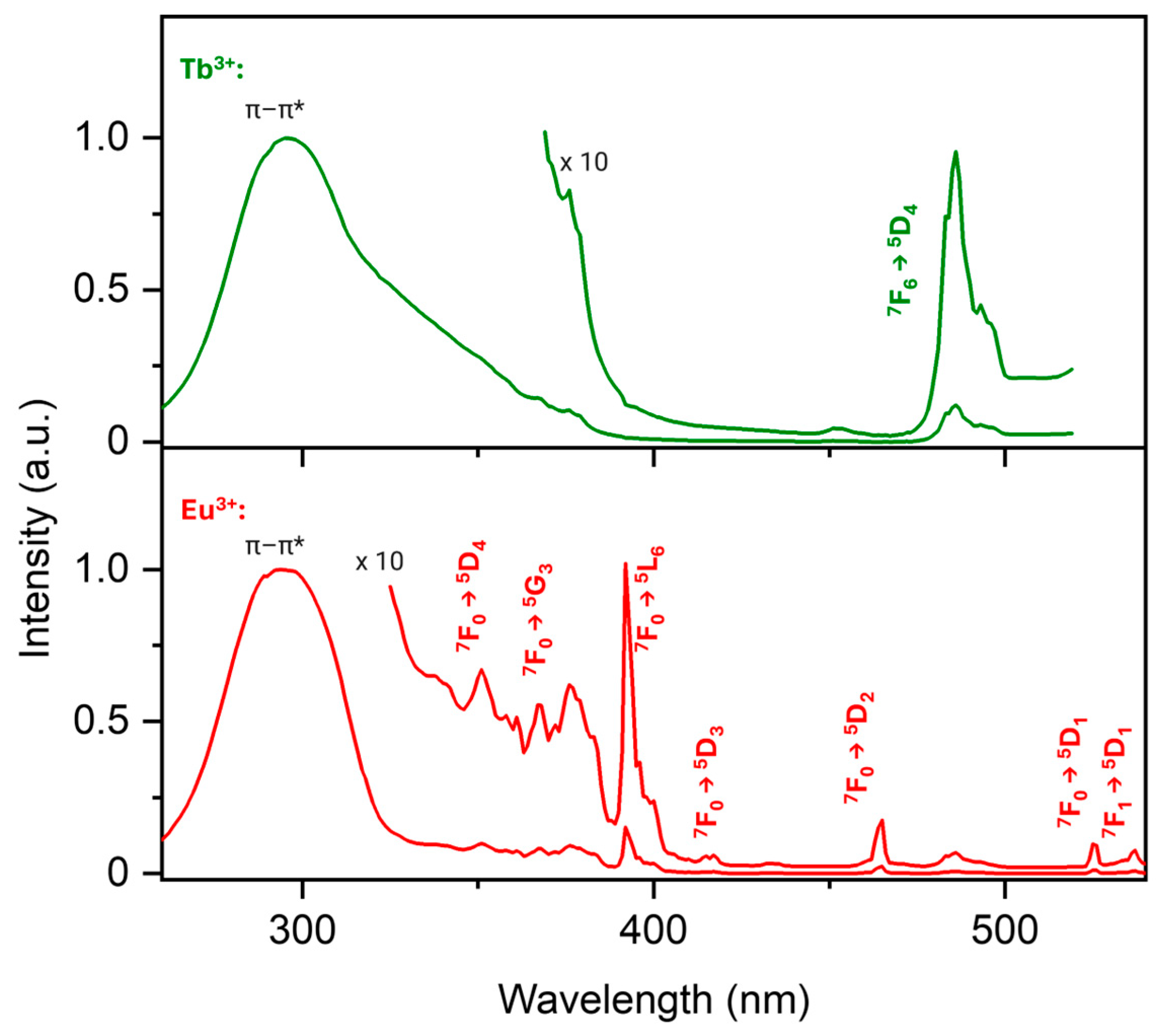
3. Results
3.1. Y-BTC: Eu (10%), Tb (10%)
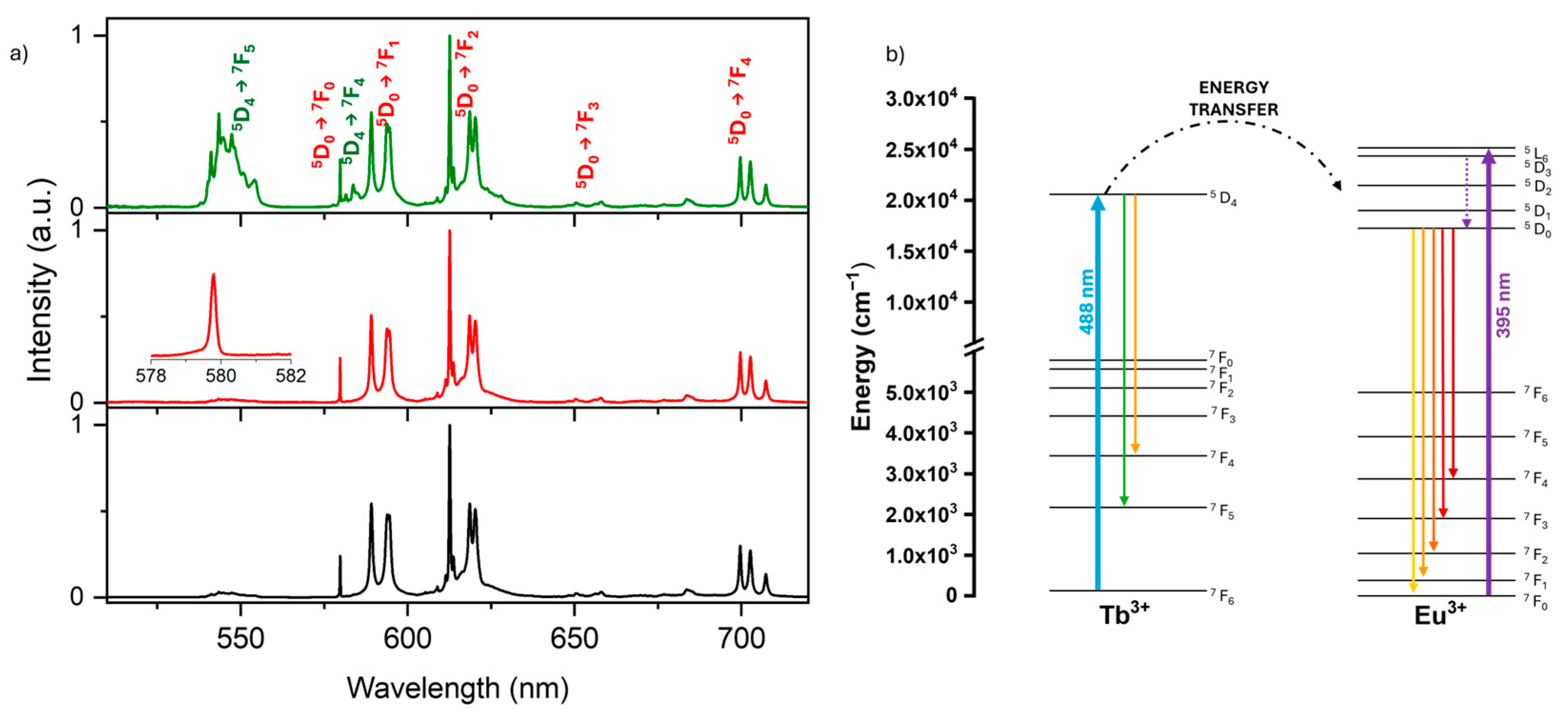
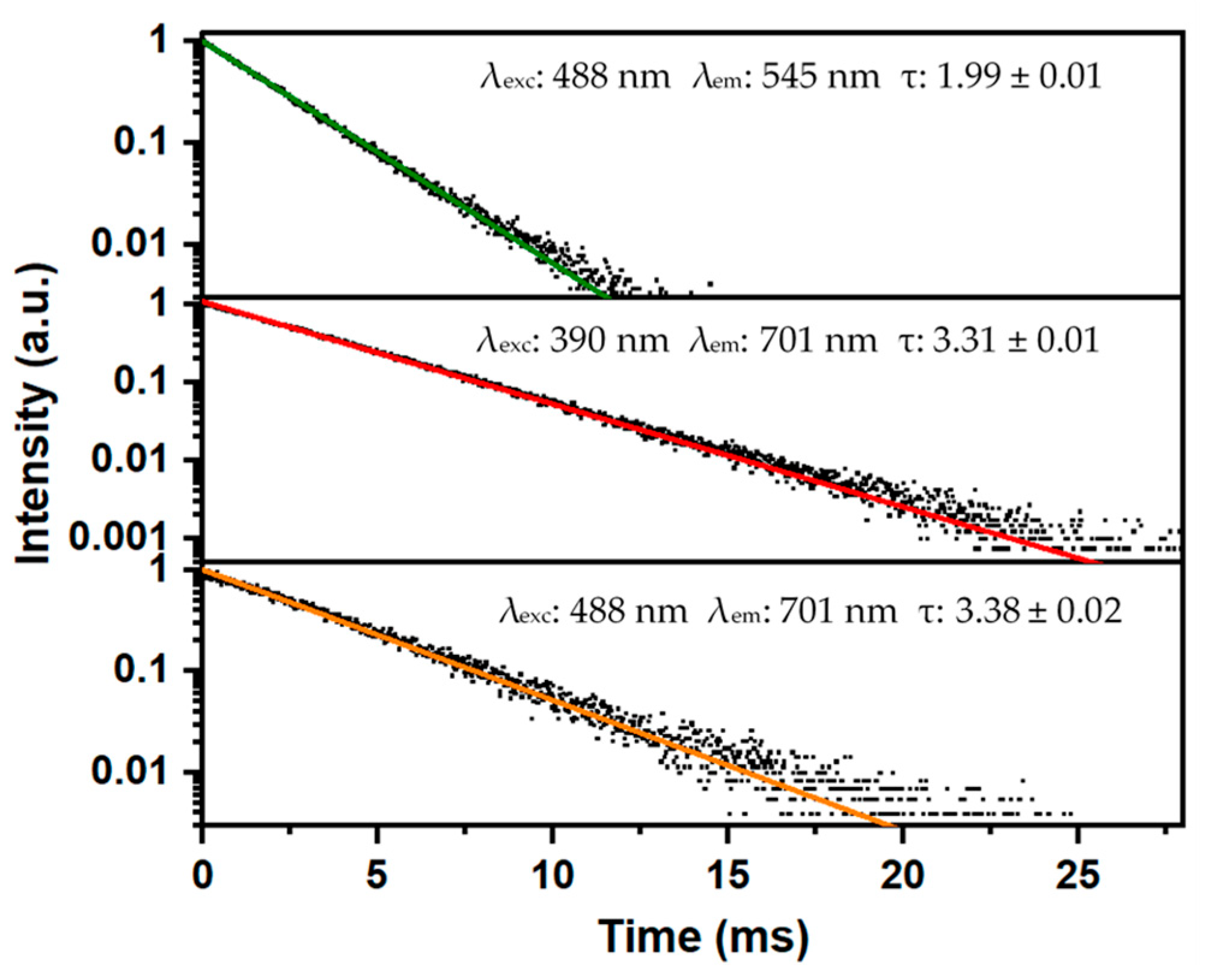
3.2. Luminescence Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younis, S.A.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Rare Earth Metal–Organic Frameworks (RE-MOFs): Synthesis, Properties, and Biomedical Applications. Coord. Chem Rev. 2021, 429, 213620. [Google Scholar] [CrossRef]
- Ay, B.; Yildiz, E.; Enomoto, M.; Okazawa, A.; Kojima, N. Crystal Structures, Gas Storage and Magnetic Properties of Lanthanide-Organic Frameworks Built up from Dicarboxylates, [Ln2(2,5-Pydc)2(2,5-Pipdc)(H2O)2]n (Ln = Ce, Pr, Eu) and (H2pip)n[Ln2(2,6-Pydc)4(H2O)2]n (Ln = Ce, Pr, Eu, Sm). Polyhedron 2022, 226, 116110. [Google Scholar] [CrossRef]
- Patra, K.; Pal, H. Lanthanide-Based Metal–Organic Frameworks (Ln-MOFs): Synthesis, Properties and Applications. RSC Sustain. 2025, 3, 629–660. [Google Scholar] [CrossRef]
- Alzard, R.H.; Alsaedi, S.; Alseiari, S.; Aljasmi, S.; El-Maghraby, H.F.; Poulose, V.; Hassan, A.; Kamel, M.; Ali, A.; Abdel-Hafiez, M.; et al. Heterogeneous Acetalization of Benzaldehyde over Lanthanide Oxalate Metal–Organic Frameworks. ACS Omega 2024, 9, 37386–37395. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Zhao, L.; Liu, H.; Li, Y. Lanthanide-Based Photocatalysts for CO2 Conversion: Are They a Better Choice for Realizing Sustainability? Coord. Chem. Rev. 2025, 522, 216223. [Google Scholar] [CrossRef]
- Barroso, N.; Andreo, J.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S.; Wuttke, S. Magnetic Sustentation as an Adsorption Characterization Technique for Paramagnetic Metal-Organic Frameworks. Commun. Chem. 2023, 6, 4. [Google Scholar] [CrossRef]
- Puglisi, R.; Pellegrino, A.L.; Fiorenza, R.; Scirè, S.; Malandrino, G. A Facile One-Pot Approach to the Synthesis of Gd-Eu Based Metal-Organic Frameworks and Applications to Sensing of Fe3+ and Cr2O72− Ions. Sensors 2021, 21, 1679. [Google Scholar] [CrossRef]
- Wu, N.; Bo, C.; Guo, S. Luminescent Ln-MOFs for Chemical Sensing Application on Biomolecules. ACS Sens. 2024, 9, 4402–4424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, Y.; He, C.; Duan, C. Synthesis of a Lanthanide Metal–Organic Framework and Its Fluorescent Detection for Phosphate Group-Based Molecules Such as Adenosine Triphosphate. Inorg. Chem. 2022, 61, 3132–3140. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y.; Zou, W.; Wu, Q.; Sun, R.; Liu, H.; Zhang, Z.; Zhao, Q. An Acid-Resistant Lanthanide Metal–Organic Framework Based on Tetraphenylethylene as an Electrochemical Nitrite Sensor. Inorg. Chem. 2024, 63, 23354–23362. [Google Scholar] [CrossRef]
- Liu, C.; Eliseeva, S.V.; Luo, T.-Y.; Muldoon, P.F.; Petoud, S.; Rosi, N.L. Near Infrared Excitation and Emission in Rare Earth MOFs via Encapsulation of Organic Dyes. Chem. Sci. 2018, 9, 8099–8102. [Google Scholar] [CrossRef] [PubMed]
- Imran, T.; Aziz, H.S.; Iftikhar, T.; Ahmad, M.; Xie, H.; Su, Z.; Yan, P.; Liu, Z.; Liang, G.; Chen, W.; et al. Interfacial Band Bending and Suppressing Deep Level Defects via Eu-MOF-Mediated Cathode Buffer Layer in an MA-Free Inverted Perovskite Solar Cell with High Fill Factor. Energy Environ. Sci. 2024, 17, 7234–7246. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, C.-I.; Liao, Y.-T.; Wu, K.C.-W.; Chueh, C.-C. Enhancing Efficiency and Stability of Photovoltaic Cells by Using Perovskite/Zr-MOF Heterojunction Including Bilayer and Hybrid Structures. Adv. Sci. 2019, 6, 1801715. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2024; ISBN 978-1-118-87346-5. [Google Scholar]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Machida, K. Luminescence of Lanthanide Complexes: From Fundamental to Prospective Approaches Related to Water- and Molecular-Stimuli. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100484. [Google Scholar] [CrossRef]
- Hu, S.; Liu, J.; Wang, Y.; Liang, Z.; Hu, B.; Xie, J.; Wong, W.-L.; Wong, K.-Y.; Qiu, B.; Peng, W. A New Fluorescent Biosensor Based on Inner Filter Effect and Competitive Coordination with the Europium Ion of Non-Luminescent Eu-MOF Nanosheets for the Determination of Alkaline Phosphatase Activity in Human Serum. Sens. Actuators B Chem. 2023, 380, 133379. [Google Scholar] [CrossRef]
- Mayeuski, A.V.; Poloneeva, D.Y.; Toshcheva, E.A.; Bardakova, A.V.; Shuruhina, A.V.; Emeline, A.V.; Bahnemann, D.W. UV-Induced Alteration of Luminescence Chromaticity of Ln-Based MOF-76. J. Lumin. 2021, 235, 117970. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Liang, M.; Sun, M.; Xia, L.; Qu, F. Fabrication of a Two-Dimensional Bi-Lanthanide Metal-Organic Framework as a Ratiometric Fluorescent Sensor Based on Energy Competition. Talanta 2024, 278, 126456. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Y.; Xia, T.; Cui, Y.; Yang, Y.; Qian, G. A Highly Sensitive Luminescent Metal–Organic Framework Thermometer for Physiological Temperature Sensing. J. Rare Earths 2018, 36, 561–566. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D. Lanthanide-Functionalized Metal–Organic Frameworks as Ratiometric Luminescent Sensors. J. Mater. Chem. C 2020, 8, 12739–12754. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, P.; Yan, K.; Pan, A.; Chen, F.; Hong, J.; Zhao, C.; Chen, X.; Xiong, W. Europium/1,3,5-Benzenetricarboxylic Acid Metal–Organic Framework Nanorods Decorated with CdSe Quantum Dots as Coatings for Noncontact Ratiometric Optical Temperature Sensing. ACS Appl. Nano Mater. 2023, 6, 12087–12094. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, S.-T.; Zhao, S.-S.; Zhang, H.; Tian, P.; Zhao, Z. Facile Preparation of Codoped Lanthanide Metal–Organic Frameworks with Efficient Energy Transfer for Ratiometric Temperature Sensing. J. Mater. Sci. 2023, 58, 7690–7701. [Google Scholar] [CrossRef]
- Rocha, J.; Brites, C.D.S.; Carlos, L.D. Lanthanide Organic Framework Luminescent Thermometers. Chem. A Eur. J. 2016, 22, 14782–14795. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Chen, L.; Lin, Q.; Wang, L.; Zhang, Z.; Ye, Y.; Chen, B. Nanoscale Metal–Organic Frameworks as a Photoluminescent Platform for Bioimaging and Biosensing Applications. Small 2024, 20, 2402641. [Google Scholar] [CrossRef]
- Yu, X.; Ryadun, A.A.; Pavlov, D.I.; Guselnikova, T.Y.; Potapov, A.S.; Fedin, V.P. Ln-MOF-Based Hydrogel Films with Tunable Luminescence and Afterglow Behavior for Visual Detection of Ofloxacin and Anti-Counterfeiting Applications. Adv. Mater. 2024, 36, 2311939. [Google Scholar] [CrossRef]
- Simões, R.; Rodrigues, J.; Granadeiro, C.M.; Rino, L.; Neto, V.; Monteiro, T.; Gonçalves, G. Boosting the Optical Properties of Polylactic Acid/ Lanthanide-Based Metal-Organic Framework Composites. Mater. Today Chem. 2023, 29, 101436. [Google Scholar] [CrossRef]
- Ajoyan, Z.; Bicalho, H.A.; Donnarumma, P.R.; Antanovich, A.; Howarth, A.J. Tuning the Rare-Earth UiO-66 Metal–Organic Framework Platform for White Light Emission. J. Mater. Chem. C 2023, 11, 8929–8934. [Google Scholar] [CrossRef]
- Kang, H.; Peng, J.; Zhang, Z.; Zhou, W. Fluorescent Strengthening Effect of Co-Doped Inert Rare Earth Ions (La3+, Gd3+, Lu3+) on White-Light-Emitting of Eu–Tb(Btc) Coordination Polymers. J. Lumin. 2022, 247, 118904. [Google Scholar] [CrossRef]
- Psalti, A.E.; Andriotou, D.; Diamantis, S.A.; Chatz-Giachia, A.; Pournara, A.; Manos, M.J.; Hatzidimitriou, A.; Lazarides, T. Mixed-Metal and Mixed-Ligand Lanthanide Metal–Organic Frameworks Based on 2,6-Naphthalenedicarboxylate: Thermally Activated Sensitization and White-Light Emission. Inorg. Chem. 2022, 61, 11959–11972. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart | Photovoltaic Research|NREL. Available online: https://www2.nrel.gov/pv/cell-efficiency (accessed on 16 April 2025).
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-Earth Doping in Nanostructured Inorganic Materials. Chem. Rev. 2002, 122, 5519. [Google Scholar] [CrossRef]
- Sun, J.; Yang, X.; Sun, S.; Zhao, L.; Wang, S.; Li, Y. Recent Progress of Rare Earth Conversion Material in Perovskite Solar Cells: A Mini Review. Inorg. Chem. Comm. 2022, 143, 109731. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, T.; He, X.; Pu, Y.; Zhu, D. Ratiometric Fluorescence Temperature Sensing of Dual-Lanthanide MOFs (Eu1-xTbx-BTC) Prepared by Solvothermal Method. Mater. Chem. Phys. 2024, 319, 129379. [Google Scholar] [CrossRef]
- Djanffar, E.; Bicalho, H.A.; Ajoyan, Z.; Howarth, A.J.; Serier-Brault, H. Rare-Earth UiO-66 for Temperature Sensing near Room Temperature. J. Mater. Chem. C 2024, 12, 8024–8029. [Google Scholar] [CrossRef]
- Guan, H.; Qi, M.; Shi, L.; Liu, W.; Yang, L.; Dou, W. Ratiometric Luminescent Thermometer Based on the Lanthanide Metal–Organic Frameworks by Thermal Curing. ACS Appl. Mater. Interfaces 2023, 15, 18114–18124. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, L.; Feng, Z.; Jiang, S.; Lin, J.; Pang, Y.; Li, L.; Xiang, G. Color Tunable Emission and Low-Temperature Luminescent Sensing of Europium and Terbium Carboxylic Acid Complexes. Inorg. Chim. Acta 2018, 469, 576–582. [Google Scholar] [CrossRef]
- Wu, R.-Z.; Yang, X.; Zhang, L.-W.; Zhou, P.-P. Luminescent Lanthanide Metal–Organic Frameworks for Chemical Sensing and Toxic Anion Detection. Dalton Trans. 2017, 46, 9859–9867. [Google Scholar] [CrossRef]
- Brunckova, H.; Mudra, E.; Rocha, L.; Nassar, E.; Nascimento, W.; Kolev, H.; Kovalcikova, A.; Molcanova, Z.; Podobova, M.; Medvecky, L. Preparation and Characterization of Isostructural Lanthanide Eu/Gd/Tb Metal-Organic Framework Thin Films for Luminescent Applications. Appl. Surf. Sci. 2021, 542, 148731. [Google Scholar] [CrossRef]
- Lo Presti, F.; Borzì, A.; Pellegrino, A.L.; Rossi, P.; Paoli, P.; Malandrino, G. Morphology Controlled Synthesis of Yttrium Metal–Organic Frameworks with a Tritopic Ligand. Results Chem. 2022, 4, 100640. [Google Scholar] [CrossRef]
- Kumari, P.; Kareem, A.; Jhariat, P.; Senthilkumar, S.; Panda, T. Phase Purity Regulated by Mechano-Chemical Synthesis of Metal–Organic Frameworks for the Electrocatalytic Oxygen Evolution Reaction | Inorganic Chemistry. Available online: https://pubs.acs.org/doi/10.1021/acs.inorgchem.2c03609 (accessed on 3 March 2025).
- Zhao, L.; Song, X.; Ren, X.; Wang, H.; Fan, D.; Wu, D.; Wei, Q. Ultrasensitive Near-Infrared Electrochemiluminescence Biosensor Derived from Eu-MOF with Antenna Effect and High Efficiency Catalysis of Specific CoS2 Hollow Triple Shelled Nanoboxes for Procalcitonin. Biosens. Bioelectron. 2021, 191, 113409. [Google Scholar] [CrossRef]
- Serre, C.; Millange, F.; Thouvenot, C.; Gardant, N.; Pellé, F.; Férey, G. Synthesis, Characterisation and Luminescent Properties of a New Three-Dimensional Lanthanide Trimesate: M((C6 H3)–(CO2)3) (M = Y, Ln) or MIL-78. J. Mater. Chem. 2004, 14, 1540–1543. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Liu, K.; You, H.; Zheng, Y.; Jia, G.; Zhang, L.; Huang, Y.; Yang, M.; Song, Y.; Zhang, H. Facile Shape-Controlled Synthesis of Luminescent Europium Benzene-1,3,5-Tricarboxylate Architectures at Room Temperature. CrystEngComm 2009, 11, 2622–2628. [Google Scholar] [CrossRef]
- Li, Z.; Núñez, R.; Light, M.E.; Ruiz, E.; Teixidor, F.; Viñas, C.; Ruiz-Molina, D.; Roscini, C.; Planas, J.G. Water-Stable Carborane-Based Eu3+/Tb3+ Metal–Organic Frameworks for Tunable Time-Dependent Emission Color and Their Application in Anticounterfeiting Bar-Coding. Chem. Mater. 2022, 34, 4795–4808. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Roscini, C.; Núñez, R.; Teixidor, F.; Viñas, C.; Ruiz, E.; Planas, J.G. Rationalizing the Carborane versus Phenyl-Driven Luminescence in Related Dicarboxylic Ligands and Their Antenna Effect for Their Eu3+ and Tb3+ Metal–Organic Frameworks: A Combined Experimental and Computational Study. J. Mater. Chem. C 2024, 12, 2101–2109. [Google Scholar] [CrossRef]
- Lucena, M.A.M.; Oliveira, M.F.L.; Arouca, A.M.; Talhavini, M.; Ferreira, E.A.; Alves, S., Jr.; Veiga-Souza, F.H.; Weber, I.T. Application of the Metal–Organic Framework [Eu(BTC)] as a Luminescent Marker for Gunshot Residues: A Synthesis, Characterization, and Toxicity Study. ACS Appl. Mater. Interfaces 2017, 9, 4684–4691. [Google Scholar] [CrossRef]
- Meng, D.; Zhao, T.; Busko, D.; Cosgun Ergene, A.; Richards, B.S.; Howard, I.A. Tb and Eu in MOF-76: Elucidating the Mechanisms Responsible for the Divergent Excellent and Poor Photoluminescence Quantum Yields. Adv. Opt. Mater. 2024, 12, 2300867. [Google Scholar] [CrossRef]
- Werts, M.H.V.; Jukes, R.T.F.; Verhoeven, J.W. The Emission Spectrum and the Radiative Lifetime of Eu3+ in Luminescent Lanthanide Complexes. Phys. Chem. Chem. Phys. 2002, 4, 1542–1548. [Google Scholar] [CrossRef]
- Chong, X.; Kim, K.-J.; Ohodnicki, P.R.; Li, E.; Chang, C.-H.; Wang, A.X. Ultrashort Near-Infrared Fiber-Optic Sensors for Carbon Dioxide Detection. IEEE Sens. J. 2015, 15, 5327–5332. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Ebrahim, F.M.; Stylianou, K.C. Photoluminescent, Upconversion Luminescent and Nonlinear Optical Metal-Organic Frameworks: From Fundamental Photophysics to Potential Applications. Coord. Chem. Rev. 2018, 377, 259–306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Presti, F.; Pellegrino, A.L.; Loschi, F.; Milan, E.; Speghini, A.; Malandrino, G. Eu-Tb-Doped Y-BTC MOF: A Step Towards Optimization of an Energy Conversion System. Crystals 2025, 15, 572. https://doi.org/10.3390/cryst15060572
Lo Presti F, Pellegrino AL, Loschi F, Milan E, Speghini A, Malandrino G. Eu-Tb-Doped Y-BTC MOF: A Step Towards Optimization of an Energy Conversion System. Crystals. 2025; 15(6):572. https://doi.org/10.3390/cryst15060572
Chicago/Turabian StyleLo Presti, Francesca, Anna Lucia Pellegrino, Francesca Loschi, Emil Milan, Adolfo Speghini, and Graziella Malandrino. 2025. "Eu-Tb-Doped Y-BTC MOF: A Step Towards Optimization of an Energy Conversion System" Crystals 15, no. 6: 572. https://doi.org/10.3390/cryst15060572
APA StyleLo Presti, F., Pellegrino, A. L., Loschi, F., Milan, E., Speghini, A., & Malandrino, G. (2025). Eu-Tb-Doped Y-BTC MOF: A Step Towards Optimization of an Energy Conversion System. Crystals, 15(6), 572. https://doi.org/10.3390/cryst15060572









