Thermally Stable Anilate-Based 3D CPs/MOFs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of [Ni(trz2An)]·2.5H2O (1) and [Mn(trz2An)(H2O)]·1.5H2O (2)
2.3. X-Ray Diffraction (Single Crystal and Powder)
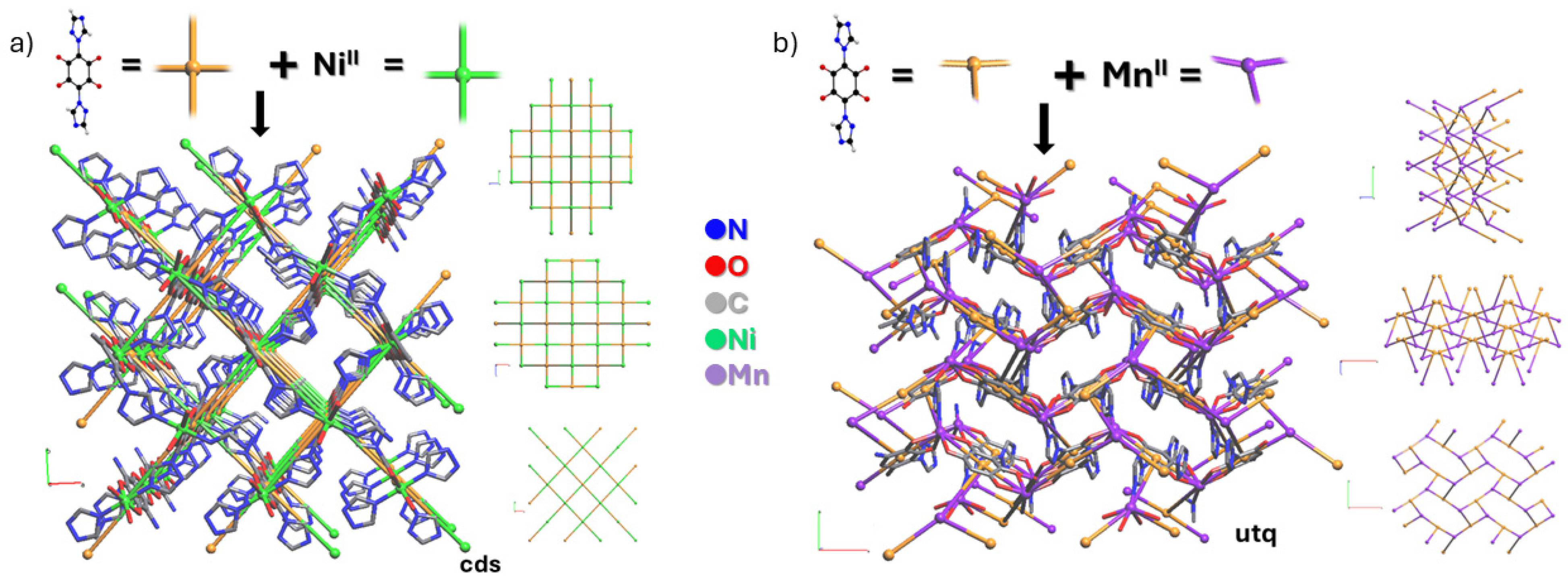
2.4. Topological Analysis
2.5. Magnetic Measurements
2.6. Electrochemical Measurements
2.7. Spectroscopic Measurements
2.8. Thermogravimetric Analysis (TGA)
3. Results
3.1. Synthesis
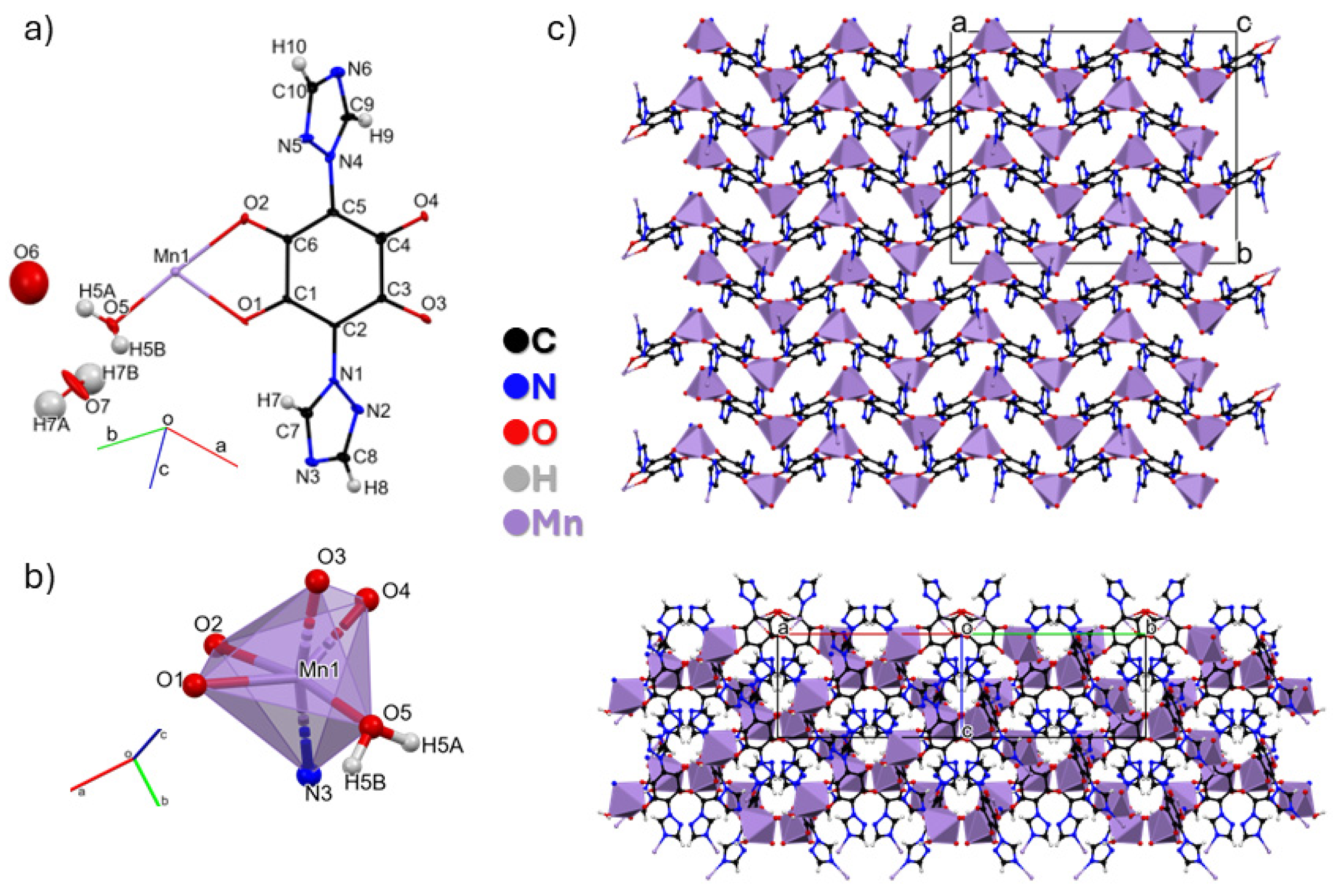
3.2. Crystal Structure
3.3. FT-IR Spectroscopy
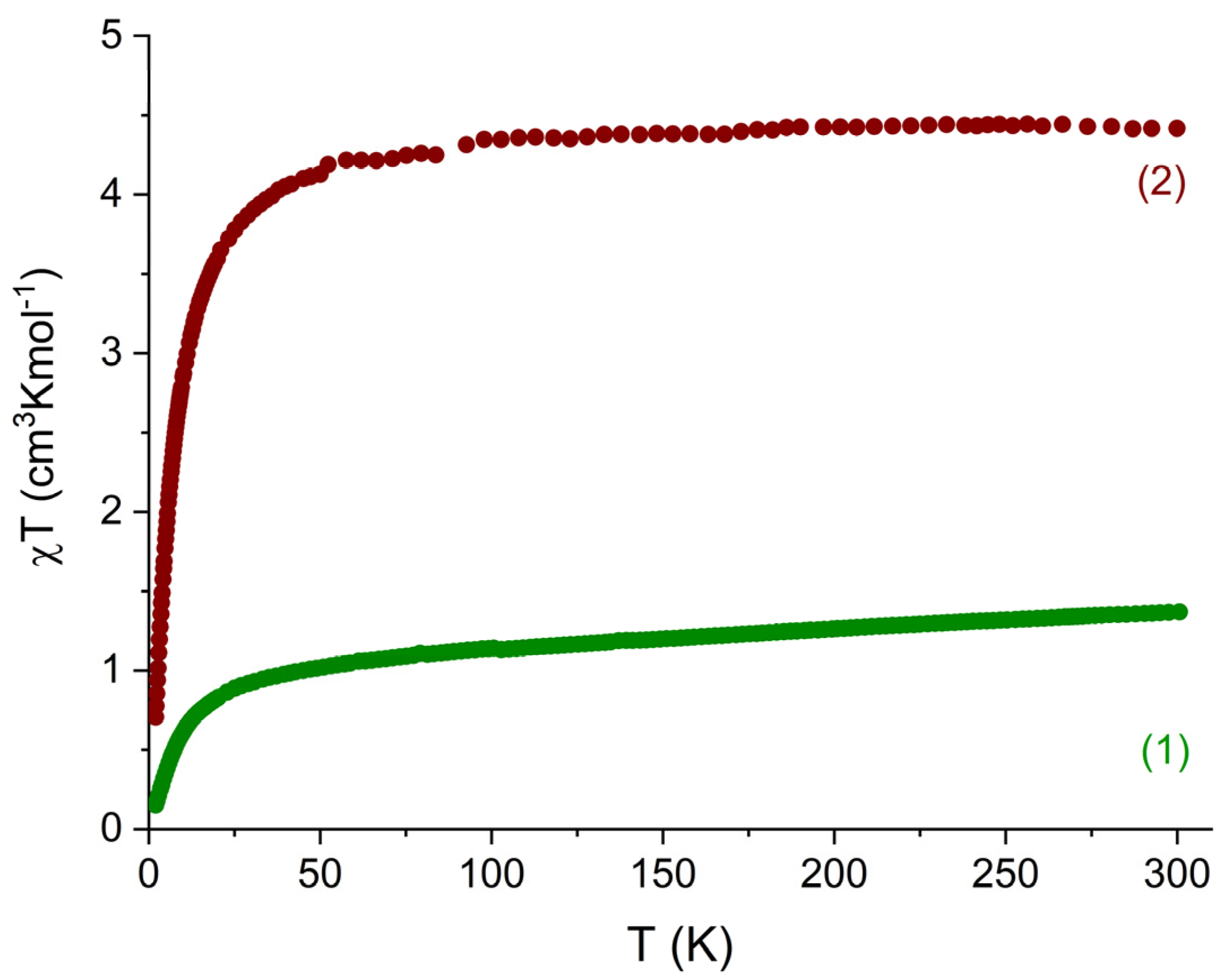
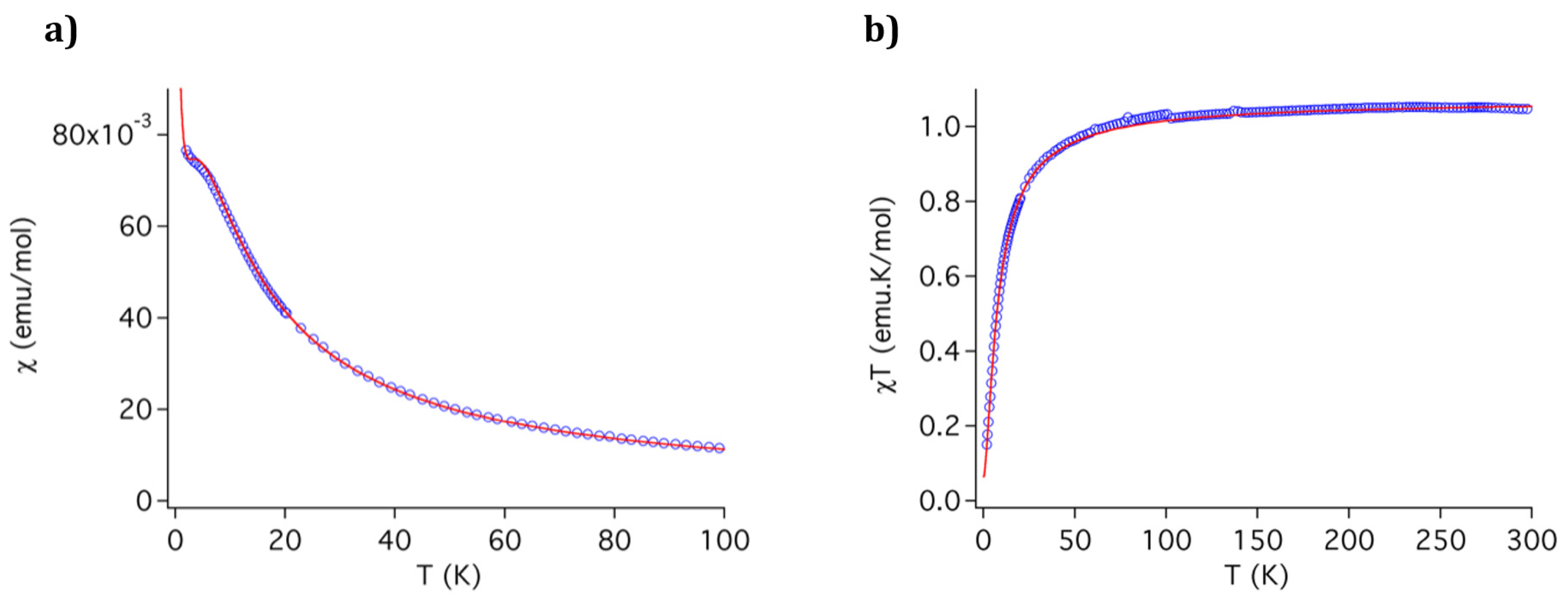
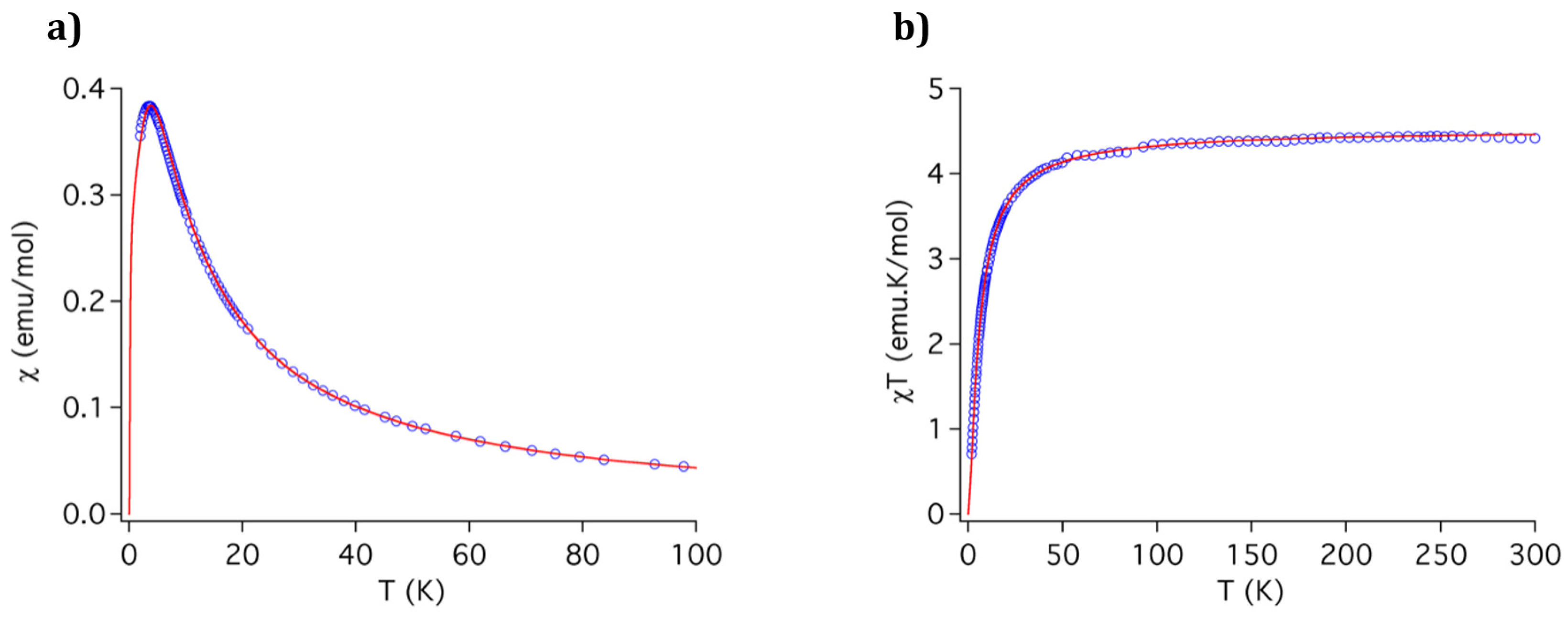
3.4. Magnetic Properties
3.5. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowsell, J.L.C.; Yaghi, O.M. Metal–Organic Frameworks: A New Class of Porous Materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Coordination Polymers, Metal–Organic Frameworks and the Need for Terminology Guidelines. CrystEngComm 2012, 14, 3001. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal Organic Frameworks for Sensing Applications. TrAC Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Li, L.; Jung, H.S.; Lee, J.W.; Kang, Y.T. Review on Applications of Metal–Organic Frameworks for CO2 Capture and the Performance Enhancement Mechanisms. Renew. Sustain. Energy Rev. 2022, 162, 112441. [Google Scholar] [CrossRef]
- Benny, A.; Kalathiparambil Rajendra Pai, S.D.; Pinheiro, D.; Chundattu, S.J. Metal Organic Frameworks in Biomedicine: Innovations in Drug Delivery. Results Chem. 2024, 7, 101414. [Google Scholar] [CrossRef]
- Xu, C.; Fang, R.; Luque, R.; Chen, L.; Li, Y. Functional Metal–Organic Frameworks for Catalytic Applications. Coord. Chem. Rev. 2019, 388, 268–292. [Google Scholar] [CrossRef]
- Manna, F.; Oggianu, M.; Avarvari, N.; Mercuri, M.L. Lanthanide-Based Metal–Organic Frameworks with Single-Molecule Magnet Properties. Magnetochemistry 2023, 9, 190. [Google Scholar] [CrossRef]
- Ashoka Sahadevan, S.; Monni, N.; Oggianu, M.; Abhervé, A.; Marongiu, D.; Saba, M.; Mura, A.; Bongiovanni, G.; Mameli, V.; Cannas, C.; et al. Heteroleptic NIR-Emitting Yb III /Anilate-Based Neutral Coordination Polymer Nanosheets for Solvent Sensing. ACS Appl. Nano Mater. 2020, 3, 94–104. [Google Scholar] [CrossRef]
- Ashoka Sahadevan, S.; Monni, N.; Abhervé, A.; Marongiu, D.; Sarritzu, V.; Sestu, N.; Saba, M.; Mura, A.; Bongiovanni, G.; Cannas, C.; et al. Nanosheets of Two-Dimensional Neutral Coordination Polymers Based on Near-Infrared-Emitting Lanthanides and a Chlorocyananilate Ligand. Chem. Mater. 2018, 30, 6575–6586. [Google Scholar] [CrossRef]
- Oggianu, M.; Manna, F.; Ashoka Sahadevan, S.; Avarvari, N.; Abhervé, A.; Mercuri, M.L. Metal-Organic Framework vs. Coordination Polymer—Influence of the Lanthanide on the Nature of the Heteroleptic Anilate/Terephtalate 3D Network. Crystals 2022, 12, 763. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Chen, W.; Zhou, H.-C.; Morsali, A. Tuning Redox Activity in Metal–Organic Frameworks: From Structure to Application. Coord. Chem. Rev. 2024, 517, 216004. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–Organic Framework Magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef] [PubMed]
- Monni, N.; Angotzi, M.S.; Oggianu, M.; Sahadevan, S.A.; Mercuri, M.L. Redox-Active Benzoquinones as Challenging “Non-Innocent” Linkers to Construct 2D Frameworks and Nanostructures with Tunable Physical Properties. J. Mater. Chem. C 2022, 10, 1548–1572. [Google Scholar] [CrossRef]
- Sahadevan, S.A.; Abhervé, A.; Monni, N.; Sáenz de Pipaón, C.; Galán-Mascarós, J.R.; Waerenborgh, J.C.; Vieira, B.J.C.; Auban-Senzier, P.; Pillet, S.; Bendeif, E.-E.; et al. Conducting Anilate-Based Mixed-Valence Fe(II)Fe(III) Coordination Polymer: Small-Polaron Hopping Model for Oxalate-Type Fe(II)Fe(III) 2D Networks. J. Am. Chem. Soc. 2018, 140, 12611–12621. [Google Scholar] [CrossRef]
- Monni, N.; Dey, S.; García-López, V.; Oggianu, M.; Baldoví, J.J.; Mercuri, M.L.; Clemente-León, M.; Coronado, E. Tunable SIM Properties in a Family of 3D Anilato-Based Lanthanide-MOFs. Inorg. Chem. Front. 2024, 11, 5913–5923. [Google Scholar] [CrossRef]
- Monni, N.; Baldoví, J.J.; García-López, V.; Oggianu, M.; Cadoni, E.; Quochi, F.; Clemente-León, M.; Mercuri, M.L.; Coronado, E. Reversible Tuning of Luminescence and Magnetism in a Structurally Flexible Erbium–Anilato MOF. Chem. Sci. 2022, 13, 7419–7428. [Google Scholar] [CrossRef]
- Monni, N.; Andres-Garcia, E.; Caamaño, K.; García-López, V.; Clemente-Juan, J.M.; Giménez-Marqués, M.; Oggianu, M.; Cadoni, E.; Mínguez Espallargas, G.; Clemente-León, M.; et al. A Thermally/Chemically Robust and Easily Regenerable Anilato-Based Ultramicroporous 3D MOF for CO2 Uptake and Separation. J. Mater. Chem. A 2021, 9, 25189–25195. [Google Scholar] [CrossRef]
- Gaub, W.; Heitzer, H.; Petersen, S. Justus Liebigs. Ann. Chem. 1973, 764, 131–144. [Google Scholar]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A Kit of Tools for Phasing Crystal Structures from Powder Data. J. Appl. Crystallogr. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Altomare, A.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Rizzi, R.; Werner, P.-E. New Techniques for Indexing: N-TREOR in EXPO. J. Appl. Crystallogr. 2000, 33, 1180–1186. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Shevchenko, A.P.; Shabalin, A.A.; Karpukhin, I.Y.; Blatov, V.A. Topological Representations of Crystal Structures: Generation, Analysis and Implementation in the TopCryst System. Sci. Technol. Adv. Mater. Methods 2022, 2, 250–265. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Oggianu, M.; Abhervé, A.; Marongiu, D.; Quochi, F.; Galán-Mascarós, J.R.; Bertolotti, F.; Masciocchi, N.; Avarvari, N.; Mercuri, M.L. Terbium and Europium Chlorocyananilate-Based 2D Coordination Polymers. Molecules 2023, 28, 6453. [Google Scholar] [CrossRef]
- Martí-Rujas, J. Structural Elucidation of Microcrystalline MOFs from Powder X-Ray Diffraction. Dalt. Trans. 2020, 49, 13897–13916. [Google Scholar] [CrossRef]
- Oggianu, M.; Mameli, V.; Hernández-Rodríguez, M.A.; Monni, N.; Souto, M.; Brites, C.D.S.; Cannas, C.; Manna, F.; Quochi, F.; Cadoni, E.; et al. Insights into Nd III to Yb III Energy Transfer and Its Implications in Luminescence Thermometry. Chem. Mater. 2024, 36, 3452–3463. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, C.J.; Abrahams, B.F.; Robson, R. CCDC 1568063: Experimental Crystal Structure Determination; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Kingsbury, C.J.; Abrahams, B.F.; Robson, R. CCDC 1568062-1568063 Experimental Crystal Structure Determination; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Mohsenpour Tehrani, M.; Chehrazi, E. Metal–Organic-Frameworks Based Mixed-Matrix Membranes for CO2 Separation: An Applicable-Conceptual Approach. ACS Appl. Mater. Interfaces 2024, 16, 32906–32929. [Google Scholar] [CrossRef] [PubMed]
- Pawlukojć, A.; Bator, G.; Sobczyk, L.; Grech, E.; Nowicka-Scheibe, J. Inelastic Neutron Scattering, Raman, Infrared and DFT Theoretical Studies on Chloranilic Acid. J. Phys. Org. Chem. 2003, 16, 709–714. [Google Scholar] [CrossRef]
- Billes, F.; Ziegler, I.; Mikosch, H. Vibrational Spectroscopic Study of Sodium-1,2,4-Triazole, an Important Intermediate Compound in the Synthesis of Several Active Substances. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2016, 153, 349–362. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Jayamani, N.; Mathammal, R. Molecular Structure, Vibrational Spectral Studies of Pyrazole and 3,5-Dimethyl Pyrazole Based on Density Functional Calculations. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2011, 79, 1959–1968. [Google Scholar] [CrossRef]
- Yadav, M.; Sharma, S.; Devi, J. Designing, Spectroscopic Characterization, Biological Screening and Antioxidant Activity of Mononuclear Transition Metal Complexes of Bidentate Schiff Base Hydrazones. J. Chem. Sci. 2021, 133, 21. [Google Scholar] [CrossRef]
- Kawata, S.; Kitagawa, S.; Kumagai, H.; Ishiyama, T.; Honda, K.; Tobita, H.; Keiichi Adachi, I.; Katada, M. Novel Intercalation Host System Based on Transition Metal (Fe2+, Co2+, Mn2+)-Chloranilate Coordination Polymers. Single Crystal Structures and Properties. Chem. Mater. 1998, 10, 3902–3912. [Google Scholar] [CrossRef]
- Kawata, S.; Kitagawa, S.; Kumagai, H.; Kudo, C.; Kamesaki, H.; Ishiyama, T.; Suzuki, R.; Kondo, M.; Katada, M. Rational Design of a Novel Intercalation System. Layer-Gap Control of Crystalline Coordination Polymers, {[Cu(CA)(H2O)m](G)}n (m = 2, G = 2,5-Dimethylpyrazine and Phenazine; m = 1, G = 1,2,3,4,6,7,8,9-Octahydrophenazine). Inorg. Chem. 1996, 35, 4449–4461. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Almenar, J.J.; Clemente-Juan, J.M.; Coronado, E.; Tsukerblar, B.S. MAGPACK1 A Package to Calculate the Energy Levels, Bulk Magnetic Properties, and Inelastic Neutron Scattering Spectra of High Nuclearity Spin Clusters. J. Comput. Chem. 2001, 22, 985–991. [Google Scholar] [CrossRef]
- Manna, F.; Oggianu, M.; Galán-Mascarós, J.R.; Pop, F.; Le Guennic, B.; Mercuri, M.L.; Avarvari, N. Tuning the Slow Magnetic Relaxation with the Substituents in Anilate Bridged Bis(Dysprosium) Complexes. Dalt. Trans. 2024, 53, 8369–8381. [Google Scholar] [CrossRef]




| Species | Symmetry | Space Group | a (Å) | b (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|---|
| (1) | Orthorhombic | Pnnm | 9.4650 (17) | 10.0556 (19) | 7.8015 (14) | 742.5 (2) |
| [Co(trz2An)] | Orthorhombic | Pnnm | 9.529 (2) | 10.157 (2) | 7.903 (2) | 764.9 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manna, F.; Monni, N.; Oggianu, M.; Clemente-Juan, J.M.; Clemente-León, M.; Mercuri, M.L. Thermally Stable Anilate-Based 3D CPs/MOFs. Crystals 2025, 15, 570. https://doi.org/10.3390/cryst15060570
Manna F, Monni N, Oggianu M, Clemente-Juan JM, Clemente-León M, Mercuri ML. Thermally Stable Anilate-Based 3D CPs/MOFs. Crystals. 2025; 15(6):570. https://doi.org/10.3390/cryst15060570
Chicago/Turabian StyleManna, Fabio, Noemi Monni, Mariangela Oggianu, Juan Modesto Clemente-Juan, Miguel Clemente-León, and Maria Laura Mercuri. 2025. "Thermally Stable Anilate-Based 3D CPs/MOFs" Crystals 15, no. 6: 570. https://doi.org/10.3390/cryst15060570
APA StyleManna, F., Monni, N., Oggianu, M., Clemente-Juan, J. M., Clemente-León, M., & Mercuri, M. L. (2025). Thermally Stable Anilate-Based 3D CPs/MOFs. Crystals, 15(6), 570. https://doi.org/10.3390/cryst15060570







