1. Introduction
The behavior of powders is typically influenced by three primary factors: (1) intrinsic physical properties, including particle size, density, shape, roughness, and porosity; (2) bulk powder properties, such as size distribution, bulk density, distribution of forces, and cohesive and frictional interactions; and (3) external conditions or processing environments, including temperature and humidity, bed voidage, and state of compaction [
1,
2]. These factors collectively influence powder behavior, which in turn affects the stability of the powder flow. The stability of powder flow plays a crucial role in numerous industrial processes, affecting both the efficiency of operations and the quality of final products. Unstable powder flow can lead to issues such as segregation, caking, and uneven distribution of materials, which can compromise product integrity and process reliability. However, achieving and maintaining stable powder flow presents several challenges. To address these challenges and ensure stable powder flow, it is crucial to understand the underlying factors that influence flow behavior. This understanding is particularly important in industrial settings, where powder flow characteristics directly affect equipment design and processing efficiency. By incorporating this knowledge into process design and equipment selection, industries can mitigate flow-related issues and optimize their operations accordingly. Engineers and process designers must consider flow properties when developing equipment such as hoppers, conveyors, feeders, and mixers to prevent issues such as bridging, ratholing, and irregular discharge. By maintaining stable powder flow, industries can reduce downtime, minimize waste, and improve overall product consistency, ultimately enhancing operational efficiency and product quality across a wide range of applications [
2].
The surface composition significantly influences the cohesion and adhesion properties of the powders. The chemical and physical characteristics of particle surfaces determine the strength and nature of interparticle interactions, which, in turn, affect the flowability, compressibility, and tendency of the powder to agglomerate. Factors such as surface energy, polarity, and the presence of functional groups contribute to the cohesive and adhesive forces between particles and between particles and surrounding surfaces. The impact of surface composition on powder behavior extends to numerous industrial and scientific domains. For instance, in pharmaceutical manufacturing, the surface properties of drug particles can affect their dissolution rates, bioavailability, and drug stability. In materials science, the surface composition influences the sintering behavior of metal and ceramic powders. In food processing, the surface characteristics of powdered ingredients can affect their dispersibility, wettability, and overall product quality. Therefore, understanding and controlling the surface composition is crucial for optimizing powder performance and developing tailored solutions for specific applications.
In industrial contexts, a filler is defined as a material incorporated into a product, mixture, or process to enhance its properties, reduce costs, or achieve specific functionality without substantially altering the primary characteristics of the product. Fillers are typically used in powder form to ensure uniform dispersion within the host material, enhance processability, and achieve consistent performance in the final product. One of the most commonly used fillers is calcium carbonate (CaCO
3), also known as calcite [
3,
4]. Although initially employed primarily for cost reduction, it has evolved into a material tailored to meet the diverse needs of contemporary products [
5]. Its widespread adoption stems from its cost-effectiveness, abundance, ease of production, whiteness, reinforcing qualities, and ability to enhance the mechanical properties of composites, such as strength, modulus, and toughness [
6,
7]. The interface between the inorganic calcite surface and polymer matrix plays a crucial role in determining the properties and performance of composite materials. Enhanced interfacial adhesion and better dispersion of the calcite particles in the polymer matrix can improve the mechanical properties (e.g., tensile strength and impact resistance) of the final composite material. The interfacial adhesion strength depends on the compatibility between the calcite surface and polymer matrix. Fatty acids, particularly stearic acid (SA), are commonly used as surface modifiers for calcite particles [
3,
4,
6,
8,
9,
10,
11,
12]. Industrial surface modification of calcite using fatty acids employs two main approaches: dry and wet methods. The carboxylic acid group of stearic acid interacts with calcium ions on the calcite surface, forming a self-assembled monolayer. This coating creates a hydrophobic surface, improving compatibility with nonpolar polymer matrices [
4,
13]. The optimal SA concentration is critical for forming a monolayer in which each SA molecule binds to a single Ca
2+ ion on the surface (
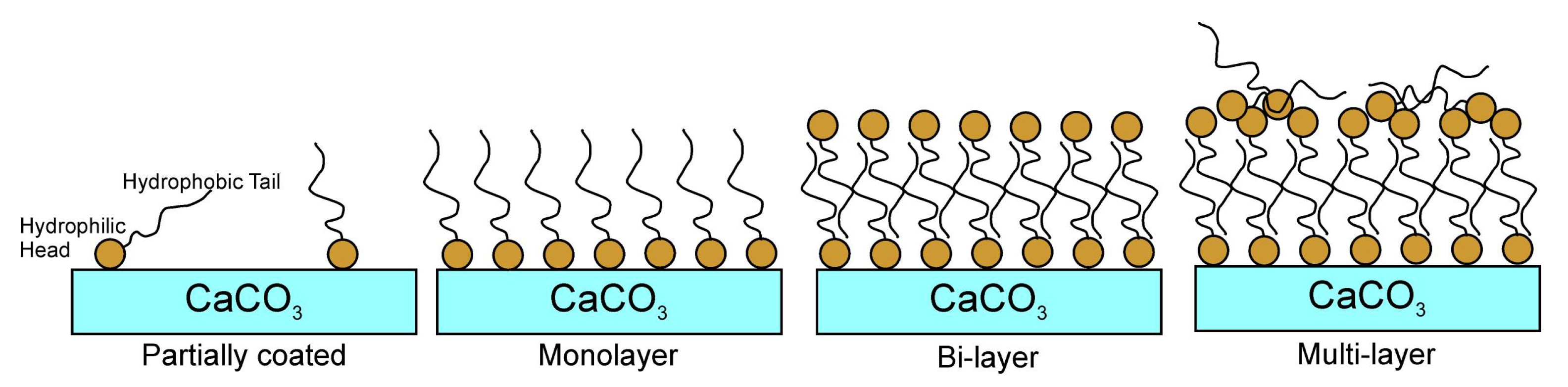
Figure 1). The alkyl chains of these molecules are vertically oriented, restricting their movement and providing a uniform and stable coating on the surface. Excess SA results in unattached molecules, disrupting the coating properties and causing surface inconsistencies. Thus, precise control of the SA concentration is essential for achieving the desired monolayer and optimizing the surface properties of the coated calcite particles [
4,
14].
The measurement of the flow performance of surface-coated calcite powders, which are widely used in industry, is crucial for optimizing industrial processes and product quality. Various testing methods have been developed and refined to address these issues. Reliable prediction of flow performance requires reliable information on the volume, flowability, and processability of the powders [
15]. The absence of a universally effective, consistent, and versatile flowability test can be attributed to the extensive range of granular substances and the effects of handling procedures on measurement accuracy [
16]. Despite this qualitative understanding, quantifying the kinematics of dry powders with different surface compositions is a highly complex problem, given the extensive range of parameters that affect the bulk cohesion [
17,
18]. The enhancement of traditional powder testing techniques, such as shear and bulk property testing, through modern instrumentation and methodologies has maintained their relevance in contemporary analytical toolkits. Shear properties are particularly valuable for hopper design and the characterization of consolidated, cohesive powders. Conversely, bulk properties, such as density, permeability, and compressibility, provide general insights into powder behavior, essential data for process design, and predictions of performance in specific processes. Recently, dynamic powder testing has emerged, offering significant opportunities for a more comprehensive understanding of powder behavior. Dynamic characterization involves measuring the forces acting on a blade in both the axial and rotational directions as it moves through a sample along a predetermined helical path [
19]. Powder rheometers include various dynamic characterization methods that allow the measurement of the powder response to various environments, thus simulating the range of processing conditions more closely [
2,
15].
This study systematically investigates how stearic acid coating concentrations (0.85–1.15 wt.%) and post-coating storage duration (up to 30 days) influence the flow properties of micronized calcite powder. The primary objectives of this study were to (1) quantify the time-dependent rheological changes in surface-modified calcite, (2) establish the relationship between coating concentration and powder flowability, and (3) provide practical guidelines for industries utilizing calcite fillers in polymer composites and other applications where consistent powder flow is critical. By elucidating these processing–structure–property relationships, this study aims to enable manufacturers to optimize both the coating parameters and storage conditions to achieve the target flow characteristics while advancing the fundamental understanding of mineral powder surface modification dynamics.
2. Materials and Methods
Calcite (CaCO3) powder (Niğtaş-65) was supplied by Niğtaş Co. (Niğde, Türkiye). The sample was ground by Niğtaş in a horizontal ball mill in dry form using grinding aid chemicals. Triple-pressed stearic acid (C18-65) was obtained from Suriachem Sdn. Bhd. (Selangor, Malaysia).
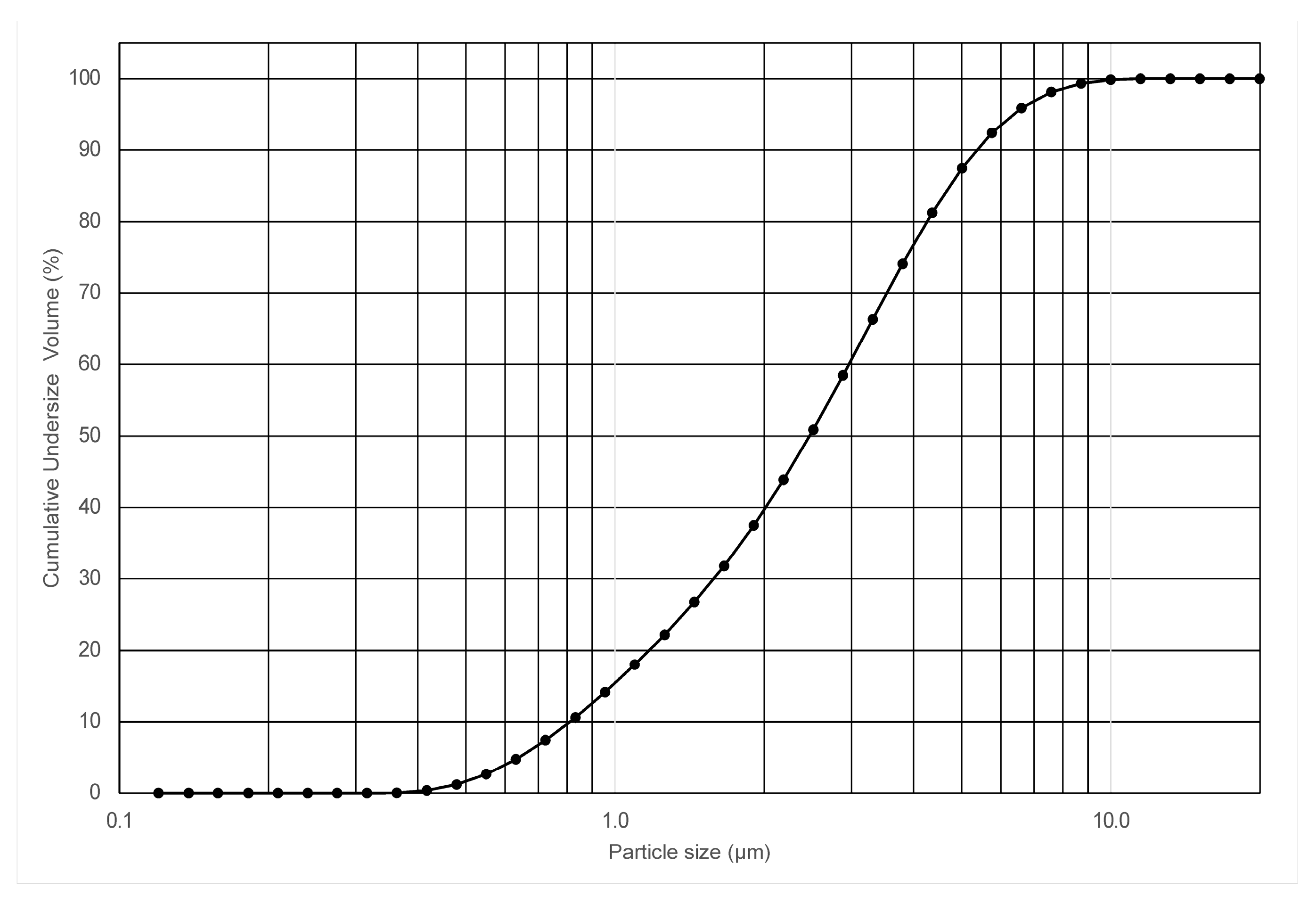
The particle size distribution of the calcite powder samples was analyzed using a laser particle size diffraction analyzer (Mastersizer 2000, Malvern Instruments Ltd., Worcestershirecity, UK).
The color parameters of the powder were determined using a color spectrophotometer (Elrepho, DataColor, Trenton, NJ, USA) as defined by the Commission Internationale de l’Eclairage (CIE). L* indicates whiteness, a* indicates the red/green coordinate, and b* indicates the yellow/blue coordinate [
20]. Ry is the luminous reflectance factor, expressed as a % ratio, and is primarily used for white or neutral colors as an overall indicator of reflectance. Ry is equivalent to Y Brightness [
21].
Brunauer–Emmett–Teller (BET) measurements were used to assess the precise surface area of the CaCO3 powder sample using the gas (nitrogen) adsorption method and a surface area analyzer (Quadrasorb-Evo, Quantachrome, Boynton Beach, FL, USA).
For the dry surface treatment of CaCO3, a high-speed mixer designed for laboratory use (MTI Mischtechnik, Zeppelin Systems GmbH, Detmold, Germany) was employed. CaCO3 was initially agitated at 2000 rpm in the mixer until the friction caused the temperature to increase to 70 °C. Subsequently, stearic acid was added, and the mixture was agitated at 2000 rpm for 5 min. The temperature inside the mixer reached approximately 90 °C. The coated CaCO3 was then left in the mixer and cooled to room temperature before its removal.
The vertical orientation of the alkyl chains of the SA molecules on the CaCO
3 surface ensures that the alkyl chains are positioned close to the CaCO
3 surface, forming a monolayer and a stable coating. This is possible by coating with an optimum amount of SA. Coating with less than the optimum amount of stearic acid leaves uncoated areas on the CaCO
3 surface, whereas coating with more stearic acid causes the alkyl chains to align parallel to the CaCO
3 surface and form a bilayer. In this study, we attempted to estimate the optimum amount of SA that could be adsorbed on the calcite surface. The calculated value is an estimate that approaches the actual optimal value. The following equation was used to determine the optimum amount of stearic acid [
4,
12]:
where Γ is the amount of SA that can be adsorbed as a monolayer on the CaCO
3 surface (μmolg
−1),
σ is the surface area per SA molecule (nm
2),
SA is the specific surface area of CaCO
3 (m
2g/1), and
NA is the Avogadro constant. The specific surface area of the uncoated calcite powder was measured as 5.28 m
2/g. The molecular weight of SA (
Mw) was used to calculate the optimum coating amount of stearic acid by weight % as follows:
The surface area per SA molecule was assumed to be 2.80 nm
2 for the dry coating of the CaCO
3 surface [
22]. As a result of this assumption, the optimum SA coating amount was calculated as 1% by weight. CaCO
3 powders were coated with SA at an optimal concentration of 1% by weight, as well as at concentrations 15% lower (0.85% by weight) and 15% higher (1.15% by weight) than the optimal level. The uncoated calcite powder was designated as “UC”, whereas the coated calcite powder samples were designated as “C-0.85%”, “C-1%”, and “C-1.15%” to denote the respective coating ratios.
A floating test, as described by Sheng et al. [
23], was used to assess the impact of surface modification. This method involves calculating the proportion of the product that floats relative to the total sample weight after vigorous agitation in water. This proportion is referred to as the active ratio (AR).
Each coated powder sample (600 g) was placed in a polyethylene sample bag and stored in a closed container for 30 days at 20 °C and 10% relative humidity. The samples were placed side by side, and no load was applied during the 30-day waiting period to prevent crushing or attrition. The moisture content of the samples was determined using a halogen moisture analyzer (MB45, OHAUS Europe GmbH, Uster, Switzerland).
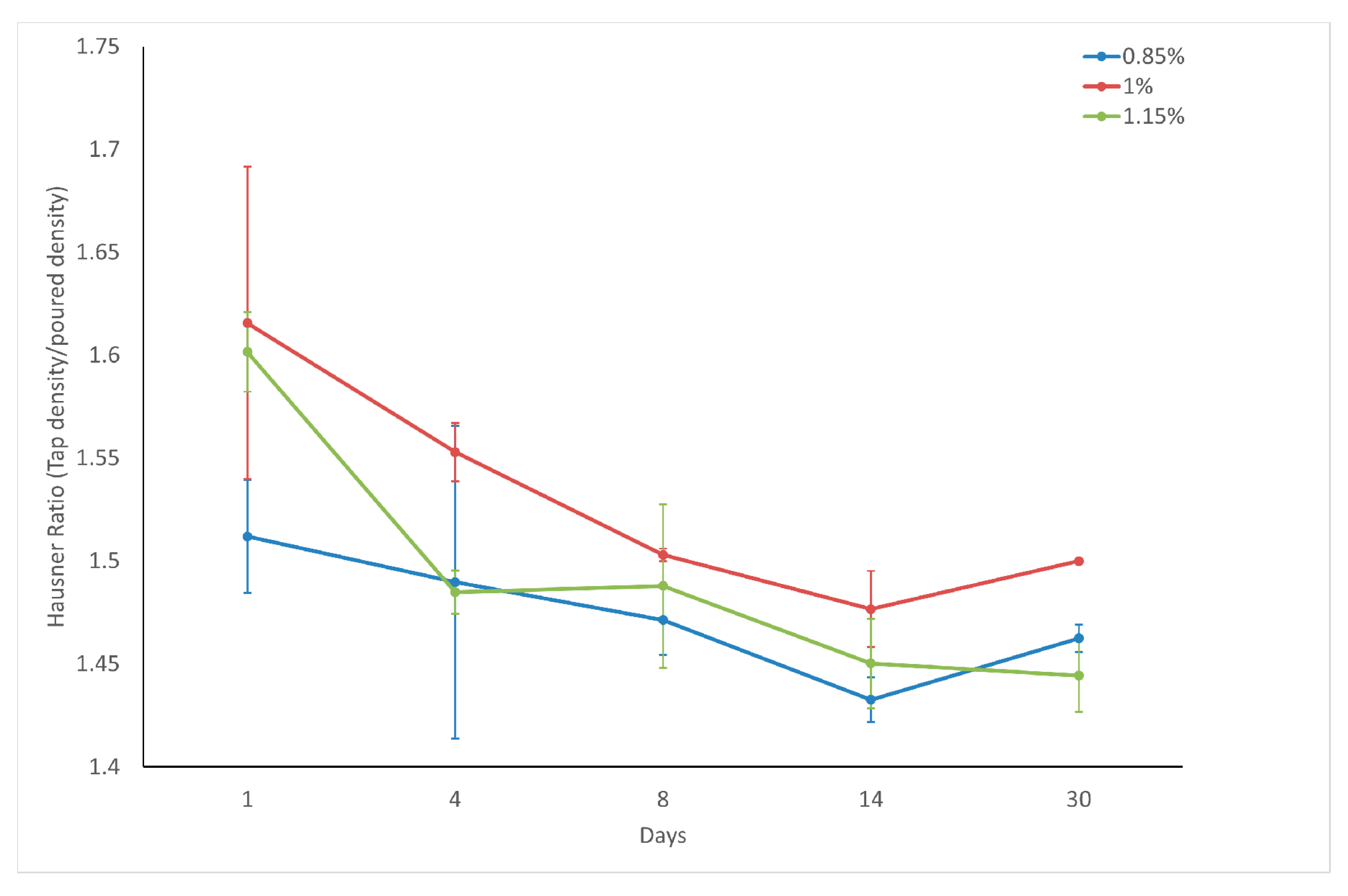
The mass of the powder divided by its volume is known as the bulk density. This characteristic varies depending on the consolidation state of the powder, resulting in significant differences between the aerated and tapped density values [
24]. The bulk volume variation during tapping was measured using a tap density meter (Autotap, Quantachrome, Boynton Beach, FL, USA). The fluidization behavior of fine powders can be effectively assessed using the Hausner Ratio (HR) [
25,
26]. This ratio reflects the particle–particle friction in a moving mass of powder rather than in a static state [
24]. This ratio was calculated by dividing the tapped density by the poured (aerated) density, providing valuable insights into the powder characteristics. The classification of powders based on their HR values is as follows: those with HR values exceeding 1.4 are considered non-flowing or cohesive; powders with HR values ranging from 1.25 to 1.4 are categorized as fairly free-flowing; and those with HR values between 1 and 1.25 are deemed free-flowing [
26].
A powder rheometer (FT4, Freeman Technology Ltd., Tewkesbury, UK) was used to determine the powder rheology. The system employs dynamic measurement technology that simultaneously monitors axial and rotational forces while the blade penetrates the powder bed at controlled velocities (maximum 30 mm/s). The modular design allows interchangeable tooling (blades, shear heads and pistons) for different test protocols, with real-time measurement of normal and shear stresses under user-defined control modes (velocity, force, or torque) [
15]. In total, 25 mL of powdered samples was tested in a 25 mm bore borosilicate glass vessel using 23.5 mm diameter twisted blade. The instrument’s ‘conditioning’ method was used to prepare the samples. This process involves a gentle blade movement that disrupts the powder bed, resulting in a uniformly and lightly compacted test sample that can be consistently replicated. All rheological parameter measurements were performed in triplicate (
n = 3), and mean values with standard deviations were reported. The error bars in the respective figures represent the standard deviation of the repeated measurements.
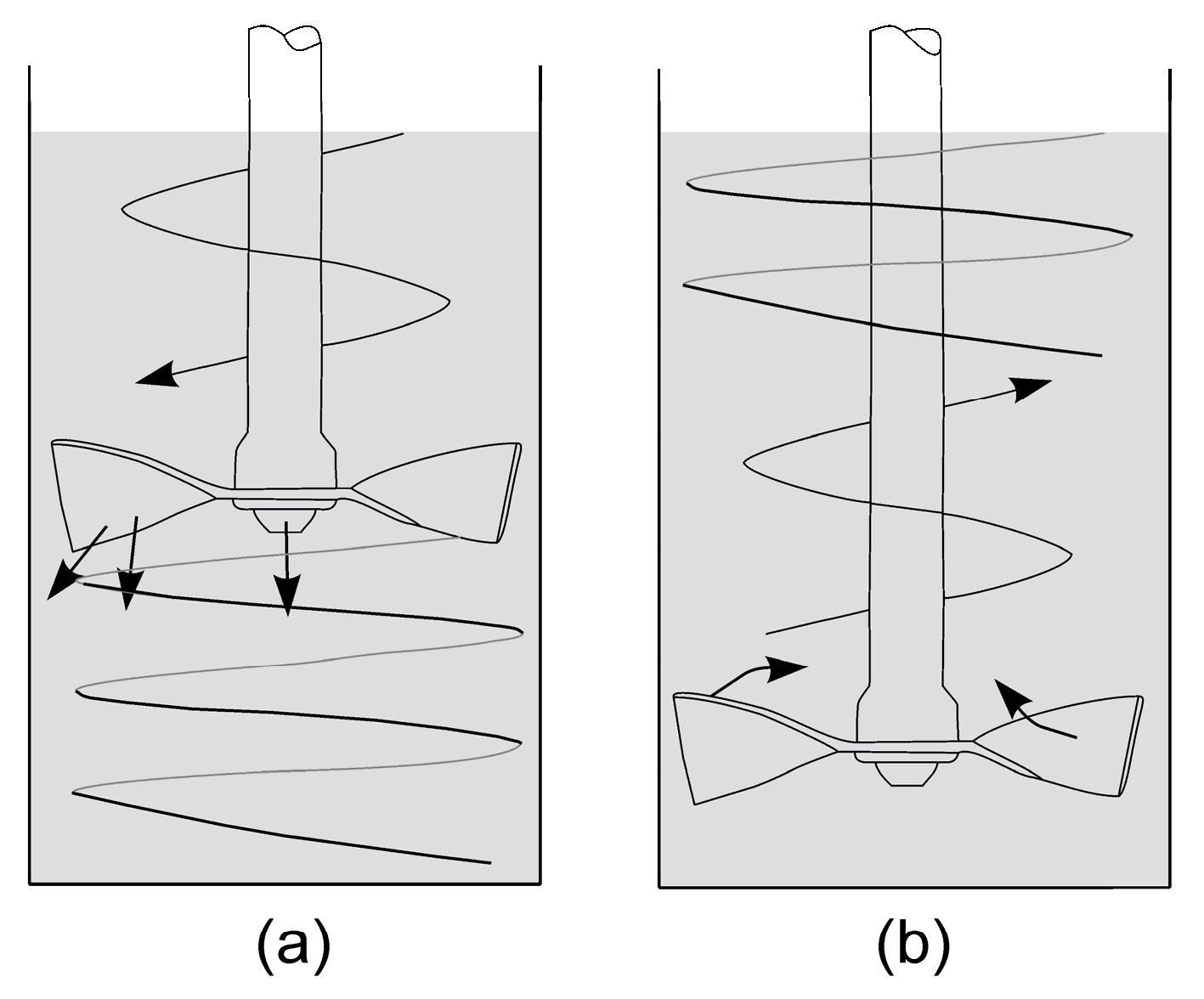
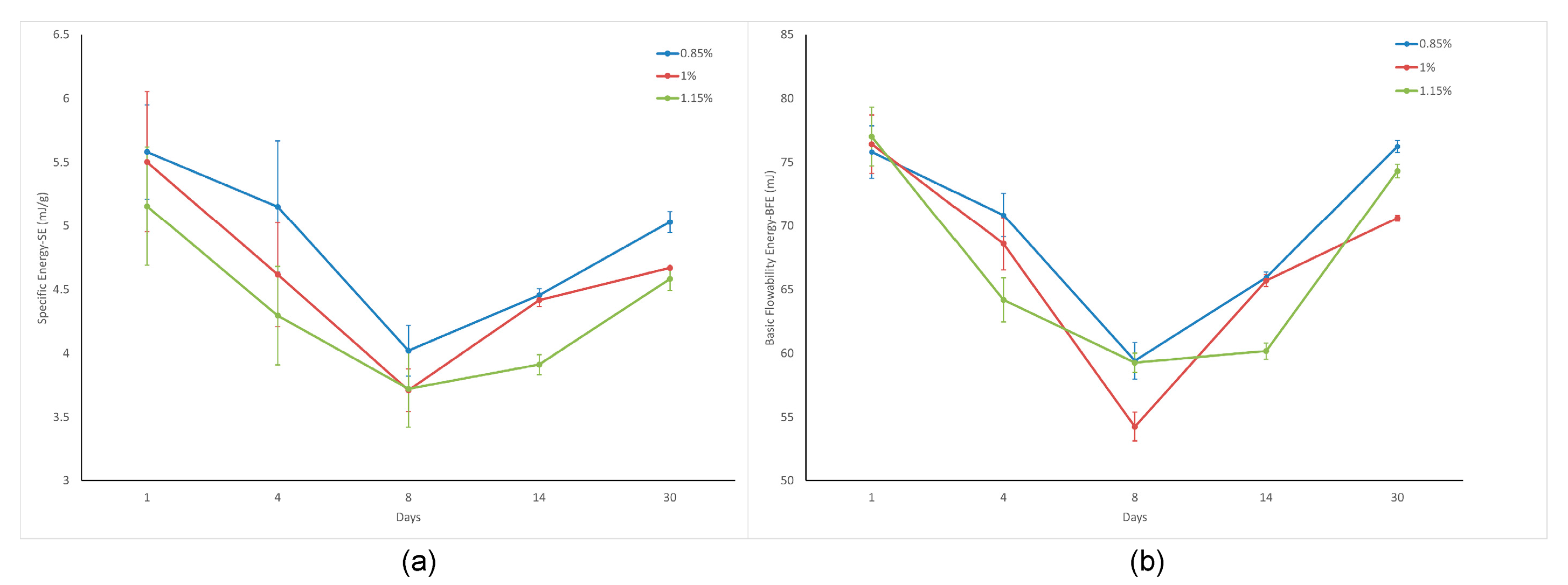
The Basic Flowability Energy (BFE) measures the amount of energy required to create a particular flow pattern in a conditioned, precise volume of powder. This pattern involved the blade moving downward in a counterclockwise direction, resulting in a compressive flow mode that subjected the powder to a relatively high stress (
Figure 2a). The BFE indicates the extent to which a powder resists downward flow. BFE measurements are highly differentiating and are a good indicator of how the powder will flow under ‘forced’ conditions, such as when it is distributed in a feeder or extruded [
19,
27].
The Specific Energy (SE) represents the energy of the flow measured as the blade moves upward through a prepared, unconfined sample (
Figure 2b). Owing to the unconfined nature of the powder, the measured energies were primarily influenced by interparticle friction and mechanical interlocking rather than by factors such as compressibility. The SE effectively indicates how the powder flows without applied stress, such as when it is poured from a container or flows into an empty die [
19].
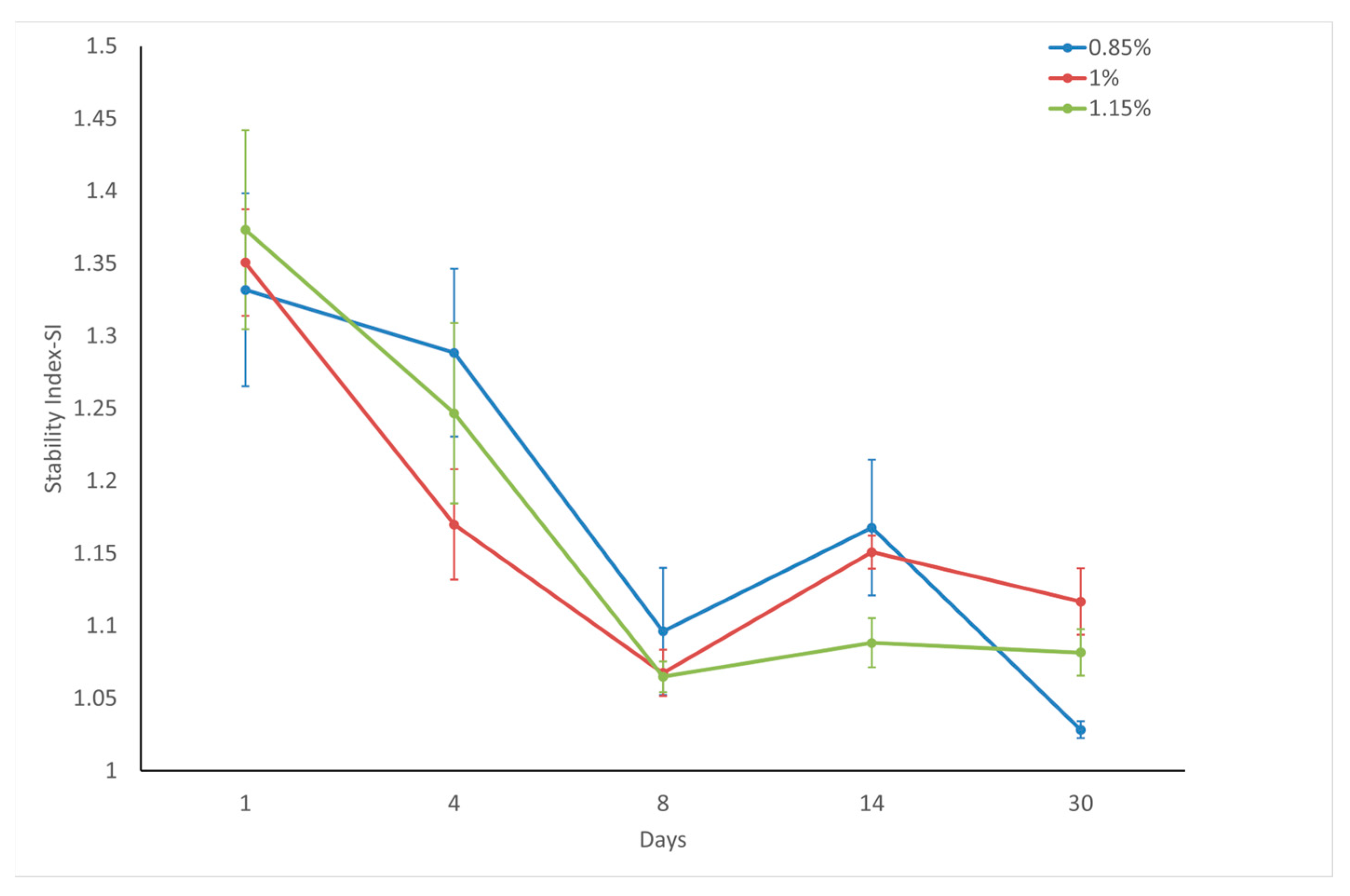
The stability index (SI) measures the consistency of the powder flow after repeated handling, with SI ≈ 1 indicating stability, SI > 1 suggesting increased resistance due to changes such as agglomeration, and SI < 1 indicating decreased resistance due to factors such as attrition or deagglomeration. External variables such as flow rate and air velocity were not considered in this study. Consequently, alterations in flow energy are directly linked to changes in the flow characteristics of the powders [
29]. For the stability measurements, all test cycles were conducted at a blade tip speed of 100 mm/s, with the blades moving transversely down the vessel. The SI was calculated using the following equation:
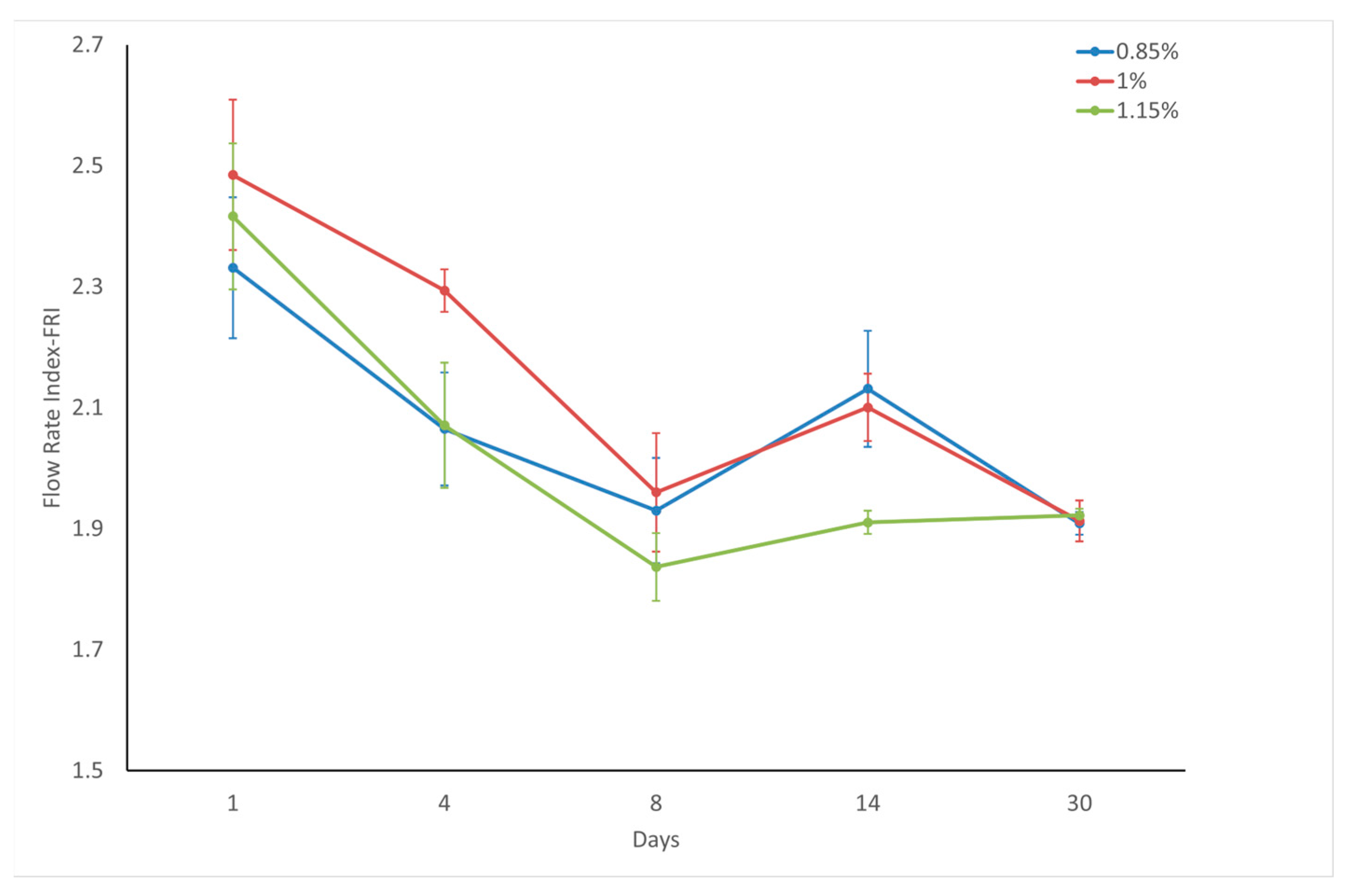
The Flow Rate Index (FRI) quantifies the reaction of powders to alterations in discharge speed. It was calculated by comparing the energy consumed in the final cycle at varying tip velocities to that used in the last cycle at a fixed tip speed of 100 mm/s. Powders with cohesive properties tend to exhibit greater sensitivity to flow rate changes than their non-cohesive counterparts. This heightened responsiveness is primarily attributed to the substantial air content within the cohesive powder beds.
4. Conclusions
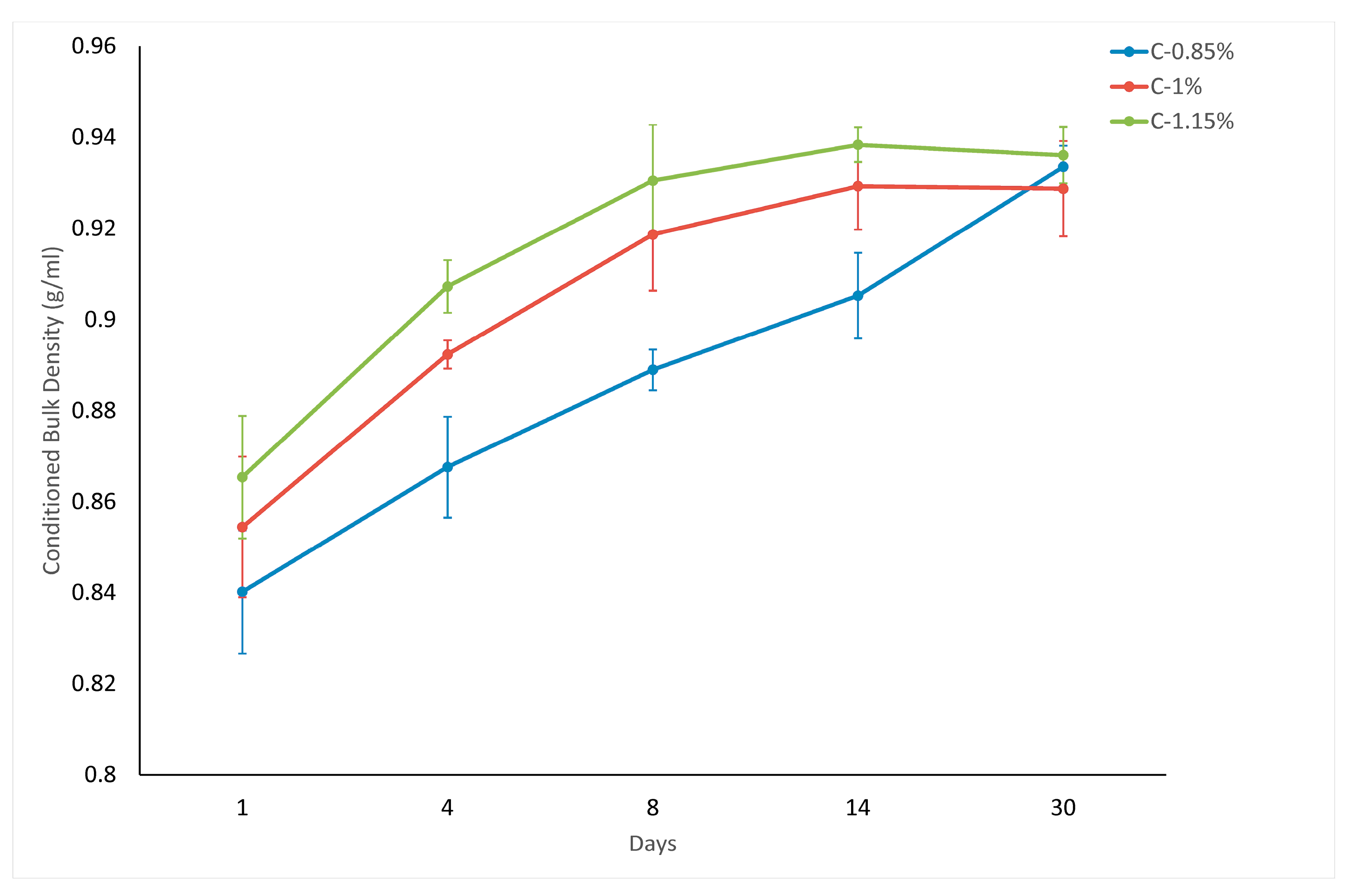
In this study, the effects of stearic acid coating concentration (0.85%, 1%, and 1.15% wt.) and storage duration (up to 30 days) on the flow properties of surface-modified micronized calcite powder were investigated. The results revealed significant changes in the rheological properties over a 30-day period, indicating that the calcite surfaces possessed dynamic characteristics. The Conditioned Bulk Density (CBD) values increased substantially from day 1 to day 30, with the highest coating rate (1.15 wt.% stearic acid) showing a density increase from 0.86 g/cm3 to 0.94 g/cm3, representing an 8.2% rise. This trend confirmed that higher coating rates consistently yielded greater densification over time, leading to more efficient particle packing.
The Specific Energy (SE) and Basic Flowability Energy (BFE) values both decreased progressively until day 8 (e.g., SE: from 5.1 to 3.7 mJ/g; BFE: from 77 to 59.3 mJ for C-1.15%), indicating improved flowability during this initial period. However, from days 8 to 30, both parameters rebounded (SE: 4.6 mJ/g; BFE: 74.3 mJ), suggesting a partial reversal of flow enhancement. This non-monotonic behavior underscores the dynamic nature of the surface-modified calcite powders during prolonged storage.
Although the powders exhibited varying cohesiveness (FRI: 2.3–3.1) immediately after surface modification, all samples converged to ~1.9 FRI values after 30 days of storage, demonstrating both reduced and unified cohesiveness behavior over time. Immediately after coating, all samples exhibited stability index (SI) values > 1.3 (range: 1.33–1.37), indicating significant flow instability across all stearic acid concentrations (0.85–1.15 wt.%). However, after 30-day storage, the SI values converged toward 1.0 (1.03–1.12), demonstrating remarkable stabilization of the powder flow. This evolution from initially heterogeneous/unstable to homogeneous/stable behavior underscores the time-dependent reorganization of the modified calcite surfaces. Considering factors such as storage costs, operational difficulties, and customer satisfaction, it is suggested that eight days may be an appropriate waiting period for coated calcite powders, where many flow parameters offer suitable and acceptable values. Considering the flow stability on the 8th day, it was predicted that coating with 1 wt.% stearic acid would be sufficient for economic reasons, since calcite powders coated with 1 to 1.15 wt.% stearic acid had the same stability index.
This study indicates that allowing stearic acid-treated calcite powders to rest can enhance their flow characteristics and stability, which is crucial for optimizing coating procedures and storage times in industrial processes using calcite fillers. These findings highlight the need to consider time-dependent behavior when working with surface-modified calcite in the future. The interaction between calcite surfaces and SA significantly affects the long-term stability and performance of calcite-based materials. This behavior has implications for industrial applications, including experimental design, product formulation, and quality control.
Further research is required to fully understand the mechanisms underlying these time-dependent changes and their implications for various applications. Manufacturers and researchers should consider these findings when developing and implementing calcite-based formulations to maximize the potential of calcite fillers.