Abstract
A straightforward and economical engraving diode laser with a 455 nm visible wavelength was employed for the first time in a pulsed laser fragmentation in liquid (PLFL) technique coupled simultaneously with a chemical reduction method to synthesize silver nanoparticles (AgNPs) in an Origanum majorana extract liquid, as a natural reduction agent. The chemical reduction correlated with the PLFL method to control the NP size by examining the effect of irradiation times. The AgNPs were characterized by X-Ray diffraction (XRD), UV–vis spectrophotometry, dynamic light scattering (DLS), transmission electron microscopy (TEM), and Fourier transform infrared (FTIR) spectroscopy. The lattice diffraction Bragg’s planes (111), (200), (220), (311), and (222) were found by XRD. The AgNPs had a surface plasmon resonance (SPR) peak at around 432–409 nm. The position of this SPR peak moves toward shorter wavelengths, by around 23 nm, with increased laser irradiation. When exposure times were increased, a drop in Ag NP size was revealed, from 22 nm when only a chemical reduction approach was used to 12 nm when the PLFL technique was associated. The DLS and TEM confirmed the UV–vis results. Such consideration suggests that combining the chemical reduction and PLFL methods could enable the tuning of the Ag NP size to be tailored for specific applications. This work could open the field for synthesizing NPs and controlling their size using an easy and handy engraving laser.
1. Introduction
Silver nanoparticles (AgNPs), have recently received significant consideration in fields of physics, chemistry, medicine, and biology owing to their properties. They are distinguished by surface plasmon resonance (SPR) at wavelengths around 400 nm. This SPR results from the electrons in the conduction band responding to incident light on the particle surface, causing oscillations in certain vibration modes [1]. AgNPs showed their efficient role in terms of their anticancer [2,3], antimicrobial [4], antibacterial [5], and photocatalytic activities [6]. Moreover, AgNPs treated dye wastewater [7] and were used for various applications.
The interest in innovative synthesis techniques is rising along with the expanding need for nanomaterials [8]. AgNPs can be produced by several chemical, physical, and biological methods. In addition, different plant extracts have been used in AgNPs synthesis due to their ability to act as a capping and green reducing agents due to their chemical components, such as fatty acids, flavonoids, polyphenols [9], and others. These plants include Mentha crispa L. [7], green tea (Camellia sinensis) [10,11], Hibiscus rosa Sinensis [12], Hagenia abyssinica [13], ginger [14], and Origanum majorana) [15,16]. The pulsed laser ablation/fragmentation in liquid (PLAL/PLFL) technique is a significant example of a top–down physical method. It simply irradiates a laser beam on a bulk or suspended sample in a liquid medium. Lately, it has attracted much attention from physicist researchers for many reasons. This technique is straightforward, fast, and can be performed at room temperature in a standard atmosphere. Furthermore, it is effective at producing the desired size and shape of nanoparticles as the application requires by controlling the experimental conditions either through laser parameters such as wavelength, power, repetition rate, pulse length, irradiation time, or via the type of liquid, its temperature, concentration, sonication, and many other parameters [17].
In 1997, Prochazka et al. [18] proposed a laser fragmentation method in order to reduce the size of NPs. They started by using the pulse Nd: YAG laser with a wavelength of 1064 nm, a 10 Hz repetition rate, and a 20 ns pulse length to ablate silver foil in water, and as a result, AgNPs of 60 to 140 nm were produced. Applying the laser irradiation to the Ag colloidal solution for a further period of time reduced the size of the NPs to around 5–20 nm. Recently, in 2024, AgNPs with sizes ranging from 33 to 51 nm were fabricated via focused Q-switched Nd: YAG laser on a Ag bulk by applying different pulse energies of 200 and 400 mJ and using several pulse numbers [19]. The authors studied the post-irradiation effect with ultraviolet-C (UVC) irradiations (100–280 nm) on the produced AgNPs. Their results show that, before irradiation with UVC, increasing the pulse numbers led to an increase in the band gap while decreasing the SPR position. However, after subjecting the AgNPs to UVC radiation, the band gap decreased, and the SPR intensity increased. Moreover, Alattar [11] recently prepared AgNPs experimentally using both PLFL and chemical reduction on green tea extract. In his work, he applied 500 pulses from a Nd:YAG laser with a 355 nm wavelength and a 25 J/cm2 fluence. According to his results, Ag NP sizes were reduced from 27 to 8 nm. Additionally, Ganash and Altuwirqi [5] used a Q-switched Nd: YAG laser and chemical reduction simultaneously to reduce the size of AgNPs fabricated in Origanum majorana extract, and the results proved the effectiveness of this method in controlling Ag NP size. The Q-switched Nd:YAG laser used in previous work is incredibly costly, sensitive, and demands an advanced setup. Moreover, it requires extensive knowledge and practice to use. From this point, the novelty of this work arises from the use of simple, portable, and low-cost engraving lasers.
This work seeks to demonstrate the ability to synthesize AgNPs using simultaneously a chemical reduction method and the PLFL technique. For the PLFL technique, an economical laser engraving module employing a diode laser with 455 nm wavelength, 1 ms pulse duration, a 5 W average power, and a 1 kHz frequency was utilized as a laser source, while a green-dried Origanum majorana extract was used as a reduction liquid medium. To our knowledge, no research has employed a commercial low-cost diode laser with a 455 nm visible wavelength with the Origanum majorana extract to fabricate and control AgNPs size.
2. Materials and Methods
2.1. Sample Preparation
Initially, acetone, followed by distilled water, was used to clean all glassware used in this experiment. An identical method was previously mentioned by [15] and was used to prepare the samples for chemical reduction. One stock of silver nitrate (AgNO3) was prepared, as around 0.17 g (10−2 M) of AgNO3 (Aldrich, Burlington, MA, USA) was dissolved in 100 mL of distilled water. On the other hand, the second stock of Origanum majorana extract was prepared as follows: dried Origanum majorana leaves were cleaned and allowed to air dry. Then, 250 mL of distilled water was added to around 20 g of Origanum majorana. The ingredients had been heated to a boil in a water bath for approximately two hours. Later, the mixture was filtered by 11 μm Whatman filter paper after being refrigerated overnight to ensure its stability. A constant volume of 2.5 mL of AgNO3 solution (the first stock) was mixed with 1.2 mL of Origanum majorana extract solution (the second stock) in a 25 mL volumetric flask. Then, the distilled water was added to this flask, making the AgNO3 concentration in the samples be 10−3 M. It should be noted that the concentration was chosen based on previous work that investigated the effect of Origanum majorana concentration on the produced AgNPs [5]. With the same procedures, six samples of a 25 mL volumetric flask were prepared to consider the effect of fragmentation time on the size of the produced nanoparticles. The first sample was not exposed to the laser beam, and the Ag NP solution was only synthesized by chemical reduction. In contrast, the other samples were instantaneously exposed to laser radiation at different irradiation times. Finally, a polyethersulfone (PES) 0.20 μm filter was used to filter each Ag NP sample. A schematic diagram of the experimental setup is shown in Figure 1.
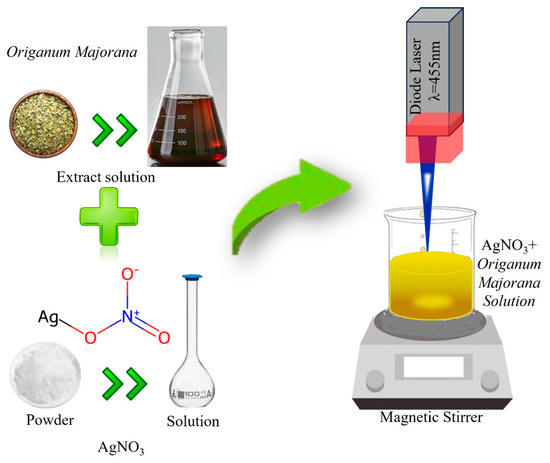
Figure 1.
A schematic diagram of the experimental setup.
2.2. Experimental Setup
The experiment was conducted at room temperature. The laser engraving machine (ATOMSTACK A5 M Pro, laser module, model No M40) with a visible diode laser with a 455 nm wavelength and a maximum power of 5 W was applied to the solution samples. The samples were immediately exposed to laser radiation when they were prepared, as detailed above, to initiate both the chemical reduction process and the laser irradiation simultaneously. The pulse duration of the laser beam is 1 ms (a fixed value), the frequency is 1 kHz, and a lens with a 30 mm focal length was used to focus the laser beam (fixed focus). Magnetic stirring is maintained throughout the irradiation process to ensure the entire sample is evenly exposed to radiation.
2.3. Characterization
The present study applied many characterization techniques to investigate the produced nanostructures. For example, a UV–vis spectrophotometer (Thermo, Waltham, MA USA, Genesys 10 S model) was employed to study the optical properties. Moreover, a transmission electron microscope (TEM) (JEOL-JEM-2100 electron Microscope, Vmax = 200 KV, ultra-high TEM resolution = 0.19 nm, Tokyo, Japan) was applied to acquire the structural properties of the resulting NPs, such as their size and shape. To investigate the structure and crystallization of the created particles, X-ray diffraction (XRD) (Rigaku, Tokyo, Japan Model: Ultima IV, CuKα radiation, λ = 1.54 Å, with continuous scan mode, scanning angles spanning 5° and 90°, 2 degree/min scan speed and 0.02° scan step, working at 40 kV and 30 mA) was used. For the XRD analysis, a thin film of the sample was prepared by depositing drops of the solution onto a glass substrate and gently spreading them with a glass rod. At the same time, the glass substrate was heated to around 75 C until it dried, forming a homogeneous thin film. Furthermore, the size and distribution of the produced nanoparticles was found using a Zeta-sizer (A Nono-zeta sizer, ZS90-Malvern Panalytical, Malvern, UK). Fourier transform infrared (FTIR) spectroscopy (IRSpirit with ATR, SHIMADZU, Kyoto, Japan, Max Resolution = 0.9 cm−1) was employed to identify chemical bonding and the functional groups.
3. Results and Discussion
Illustrated in Figure 2 are the Ag NP colloidal solution samples prepared in this work using 1.2 mL of Origanum majorana extract at several irradiation times: 0, 15, 30, 60, 90, and 120 min. It is clear from this figure that the sample produced only by chemical reduction, without being irradiated by a laser (0 min), exhibited a lighter color. This indicates that only a low concentration of AgNPs was produced. However, when laser irradiation was integrated with the chemical reduction process, the number of fabricated AgNPs increased, hence their concentration increased, giving the samples a much darker color.
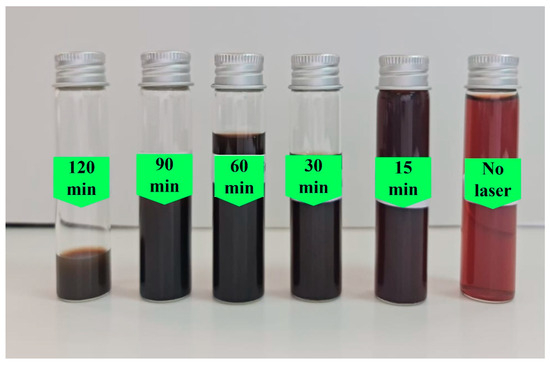
Figure 2.
The produced samples at various irradiation times.
XRD is an effective analytical technique to describe the crystalline nature of the resulting materials and their phase. As shown in Figure 3, the XRD spectrum spans from 20° to 90° and the Miller index (h k l) values of prominent peaks are noted, with the select area electron diffraction (SAED) pattern in the inset. The sharp peaks are due to constructive interference at 2 37.9°, 44.2°, 64.4°,77.5°, and 81.5°. These angles indicate that Ag crystal structures are present and correspond to lattice diffraction Bragg’s planes (111), (200), (220), (311), and (222), respectively. This is also evidence of the purity of the produced nanoparticles and agrees with other studies [5,20,21]. In addition, a peak that appears at 2, with a plane (202), is assigned to AgO NPs, and results from silver oxidation [22,23]. The slight broadening in the peaks is due to polycrystalline structures. The SAED pattern displays a concentric ring shape of discrete bright spots that indicate a polycrystalline structure. Each ring in the SAED pattern represents one peak in the XRD spectrum, and the SAED result confirms the XRD findings.
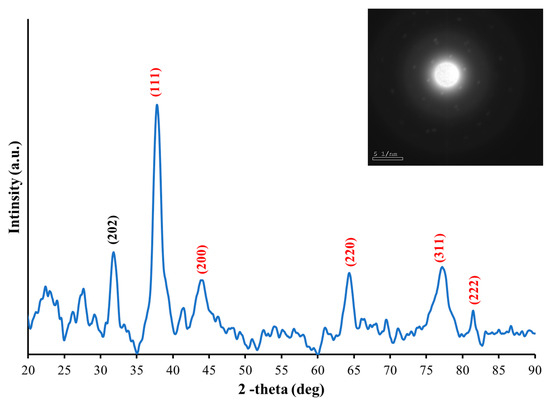
Figure 3.
The XRD spectrum of the fabricated AgNPs, the inset is the SAED pattern, which shows a concentric ring shape of discrete bright spots that imply a polycrystalline structure.
Additionally, an investigation was conducted to study the optical characteristics of the fabricated AgNPs. A UV–vis spectrophotometer was used to measure the absorption spectra of the samples. Figure 4 shows three spectra: one for the Origanum majorana extract, the second for the AgNPs produced solely from a chemical reduction, and the third for the AgNPs produced from the simultaneous effect of both the chemical reduction and the PLFL methods with 15 min of laser beam irradiation (Ag15). It is clear from the figure that there is no absorption peak in the Origanum majorana extract spectrum. However, the other spectra have an absorption peak at around 400 nm. The peak occurrence is attributed to the distinctive SPR peak of the synthesized AgNPs. It originates from an interaction involving the conduction electrons of silver material and a beam of resonant light on the surface of the NPs [24]. The SPR has a significant role in estimating the size and shape of the nanoparticles. The SPR peak position may shift to the short or long wavelength regions indicating small or large nanoparticles, respectively [25,26,27]. The absorption peak is around 432 nm in the spectrum of the produced AgNPs when only a chemical reduction occurs. Nevertheless, the peak underwent a blue shift of around 13 nm toward shorter wavelengths, to about 419 nm, when the PLFL technique was coupled instantaneously with the chemical reduction method and the sample was irradiated for 15 min. This result is evidence of the effectiveness of using the PLFL and chemical reduction techniques together in terms of the shrinking of the synthesized AgNPs compared to those particles created by the chemical reduction method alone. The PLFL process is associated with cavitation bubble formation in the liquid, which can expand and collapse, releasing nanoparticles. The reduced size in the PLFL process depends on photothermal vaporization or Coulomb explosion [28]. The first case occurs when the temperature of the particle is higher than the boiling point of the bulk material. The particles will be removed and confined in a cavitation bubble—this bubble aids in condensing the removed atoms to reduce the particle size. Moreover, according to the Coulomb explosion and due to the internal charge repulsion, the ionized nanoparticles will suffer spontaneous breaking up, producing nanoparticles [28]. In addition, laser irradiation influenced the concentration of the AgNPs. As can be seen from Figure 4, the absorption of the sample that was irradiated by the laser beam for 15 min, was larger than that of the sample that was not irradiated by the laser beam. The increase in absorption indicates an increase in the concentration of the produced NPs.
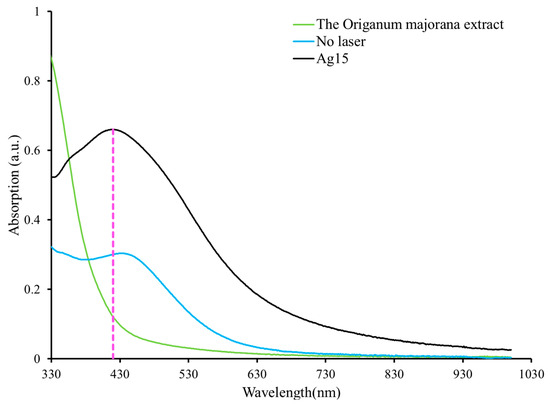
Figure 4.
UV–vis absorption spectra of the Origanum majorana extract and AgNPs with and without laser irradiation.
The influence of the laser irradiation time on the nanoparticles’ size was investigated. Figure 5 shows the spectra of AgNPs produced at different exposure times; namely, 15, 30, 60, 90, and 120 min. As seen from the figure, the position of the SPR peak shifted to a shorter wavelength with increasing irradiation time. To illustrate, the peak’s location at an irradiation time equal to 15 min is 419 nm compared to 409 nm when the irradiation time was increased to 120 min. This shift to the blue side of the spectrum by 10 nm signifies the size reduction of the AgNPs. That is, the size of the procured NPs decreased with increasing irradiation time. This observation is consistent with earlier studies [5,29]. Another feature that can be seen from the SPR peaks of the different samples is that, with increasing radiation time, the FWHM of the SPR peaks became narrower (table inset, Figure 5). For example, the peak’s FWHM at an irradiation time equal to 15 min is 238 nm. However, by increasing radiation time to 120 min, this dropped significantly to 120 nm. This indicates a narrower size distribution of the produced NPs with increasing irradiation time. Clearly, the drop in size and the size distribution are ascribed to the longer interaction duration between the laser beam and the material being studied. This enables the NPs to be fragmented into smaller particles with a more uniform size over time. Consequently, by adjusting the ablation duration, the size of the generated NPs may be modified according to the application requirement. It is essential to point out that after 60 min, no noticeable shift was seen. Hence, it can be concluded that there is a limit in size reduction after an extended irradiation time for a specific laser power. However, a further increase in radiation time reduced the size distribution of the formed AgNPs.
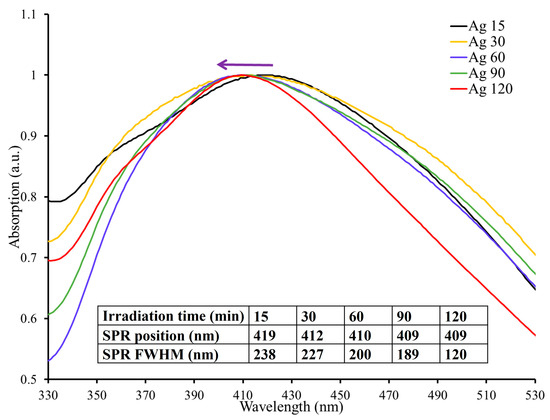
Figure 5.
UV–vis absorption spectra of AgNPs at different irradiation times. The SPR position, FWHM, and irradiation time are listed in the table; the arrow indicates the blue shift.
The dynamic light scattering technique (DLS) is rapid, simple, and non-invasive. It is based on the measurement of the Brownian motion of the distributed particles and is applied to measure the particles’ average size and size distribution [30]. The mean size of the AgNPs produced by the chemical reduction method was around 43.17 nm, and the polydispersity index (PDI), a parameter that measures the particle distribution width, was approximately 0.6, which is considered somewhat polydisperse. The size dropped to about 38.12 and 32.64 nm after 15 and 90 min of irradiation, respectively, while the PDI remained the same. Hence, no significant difference was found regarding the dispersity when laser radiation was employed, as can be seen from the multiple peaks shown in Figure 6. The zeta potential measurements of the produced AgNPs signify the charge on the surface of the particles, which in turn indicates their stability. The zeta potential of the AgNPs produced by the chemical reduction method was −21.4 mV, and after 15 and 90 min of laser irradiation, it was −19.4 mV and −17.7 mV, respectively. This can be considered relatively stable where the repulsive force between the nanoparticles hinders their aggregation [31]. Such results demonstrate that laser radiation reduced the size of the fabricated NPs but did not improve their stability. The electrical conductivity was also higher in the 90 min irradiation case, reaching 0.662 mS/cm2 as compared to 0.375 mS.cm2 when no laser irradiation was applied. Hence, the electric properties can be improved when the chemical reduction method is coupled with laser irradiation.
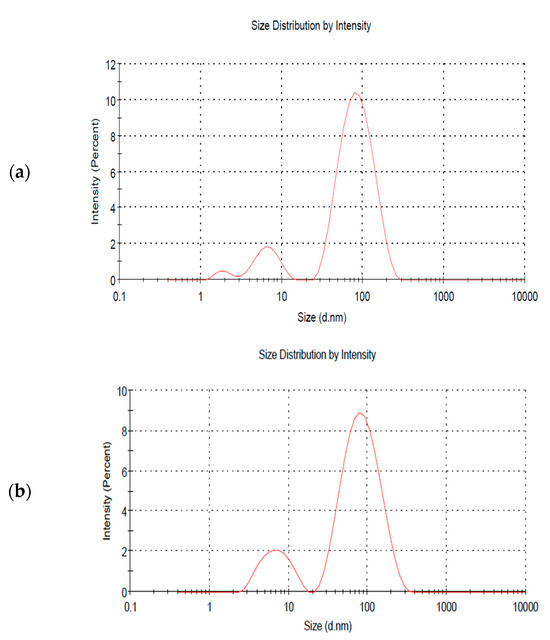
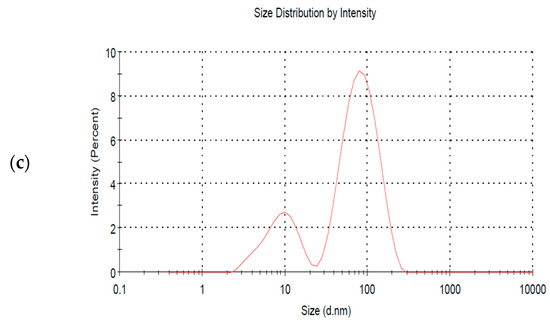
Figure 6.
DLS size distribution spectra of AgNPs at different irradiation times of (a) 0, (b) 15, and (c) 90 min, respectively.
It is clear from Figure 6a that particles with sizes around 100 nm (major peak) dominate the sample, while a minority of particles have a size of around 10 nm (minor peak). However, as shown in Figure 6b,c, the major peak’s intensity decreased while the minor peak’s intensity increased. This implies that the number of larger particles decreased, and the number of smaller particles increased when simultaneously integrating a chemical reduction method with the PLFL technique. However, it should be noted that the high intensity of the major peak might indicate larger particles, as well as a large aggregation of smaller particles that were not fully dispersed in the sample. Hence, a more accurate technique for determining the size of the AgNPs, like transmission electron microscopy (TEM), is required.
TEM is applied to study the size and morphology of the samples under investigation. The size distribution of the spherical AgNPs formed only using the chemical reduction method is shown in Figure 7a, along with their morphological images. As can be seen, the size changes from around 6 to 53 nm, where the average diameter of these particles was calculated to be 22 nm. Similarly, Figure 7b shows the size distribution of AgNPs and their TEM image when the chemical reduction method was combined with laser irradiation for 15 min. It is clear from the figure that the size of the AgNPs varied from around 5 to 34 nm, with a 16 nm average diameter. By increasing the fragmentation time to 90 min, the average diameter of the AgNPs was reduced to 12 nm, and their size distribution was found to be around from 5 to 31 nm, as displayed in Figure 7c. As can be observed from the results, the average diameter of the AgNPs reduced when both the chemical reduction and laser irradiation were coupled, and the size decreased with an increase in exposure time from 15 to 90 min. Moreover, the range of the size distribution also decreased.
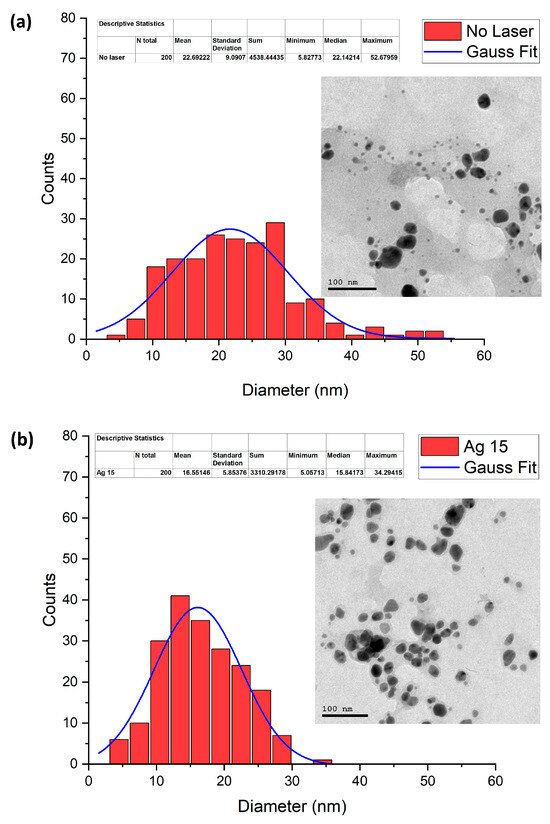
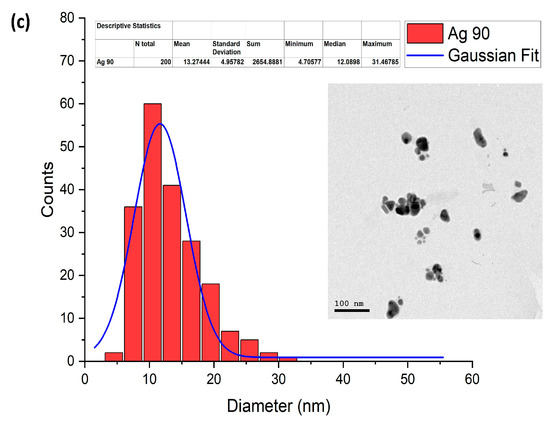
Figure 7.
Size distribution of AgNPs and their TEM images at different irradiation times of (a) 0, (b) 15, and (c) 90 min, respectively.
The difference between the size values found by the TEM and DLS techniques is usual and has been reported on previously, as each has its own basic measurement method for calculating the number of particles; for further details, read [32]. However, as can be noticed in both methods, the resulting pattern of the size distribution change is still the same, which means that, in general, the size of AgNPs decreases in TEM and DLS measurements when the laser exposure time increases.
The effect of PLFL on size reduction, when simultaneously combined with a chemical reduction method, was previously confirmed by the authors [5]. However, comparing the average size of the AgNPs produced in this work, where a long pulse duration (1 ms) was used, with the previous work [5], which used short laser pulses (5 ns), confirmed the effect of pulse duration on the produced NPs. Interestingly, longer laser pulses gave a larger average size of AgNPs than shorter pulses. Hence, the tenability of NPs can be achieved by controlling the pulse duration of the laser beam, as it is a crucial factor when compared to, for example, the laser wavelength.
4. Conclusions
In conclusion, AgNPs were produced via a green route using chemical reduction and laser irradiation simultaneously in an Origanum majorana extract. In the present work, the PLFL process, for the first time to our knowledge, is fabricated by applying a simple and efficient engraving diode laser with a visible wavelength of 455 ± 5 nm. The size of the AgNPs varied between 5 and 31 nm, with an average diameter of 12 nm, when 90 min or laser irradiation was coupled with chemical reduction, compared to 22 nm when only the chemical reduction occurred. The key insight drawn from the current study is the effectiveness of coupling a chemical reduction process with PLFL. The PLFL process has been demonstrated to be an effective means of controlling nanoparticle size. Moreover, this work highlights the potential of using an economical engraving visible diode laser, as well as the exposure laser time parameter and pulse duration in the PLFL process. Such a method can significantly alter the size of AgNPs and allow for tunability.
Author Contributions
Conceptualization, E.A.G. and R.M.A.; Methodology, E.A.G. and R.M.A.; Validation, E.A.G. and R.M.A.; Formal analysis, E.A.G. and R.M.A.; Investigation, E.A.G. and R.M.A.; Resources, E.A.G. and R.M.A.; Data curation, E.A.G. and R.M.A.; Writing—original draft, E.A.G.; Writing—review & editing, E.A.G. and R.M.A.; Visualization, E.A.G.; Supervision, R.M.A.; Project administration, R.M.A.; Funding acquisition, E.A.G. and R.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mukherji, S.; Bharti, S.; Shukla, G.; Mukherji, S. Synthesis and characterization of size- and shape-controlled silver nanoparticles. Phys. Sci. Rev. 2019, 4, 20170082. [Google Scholar] [CrossRef]
- Oves, M.; Aslam, M.; Rauf, M.A.; Qayyum, S.; Qari, H.A.; Khan, M.S.; Alam, M.Z.; Tabrez, S.; Pugazhendhi, A.; Ismail, I.M. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater. Sci. Eng. C 2018, 89, 429–443. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Park, C.; Yoo, H.; Kim, J.-H.; Hong, K. Cytotoxic potential and molecular pathway analysis of silver nanoparticles in human colon cancer cells HCT116. Int. J. Mol. Sci. 2018, 19, 2269. [Google Scholar] [CrossRef] [PubMed]
- Ajaykumar, A.P.; Sabira, O.; Sebastian, M.; Varma, S.R.; Roy, K.B.; Binitha, V.S.; Rasheed, V.A.; Jayaraj, K.N.; Vignesh, A.R. A novel approach for the biosynthesis of silver nanoparticles using the defensive gland extracts of the beetle, Luprops tristis Fabricius. Sci. Rep. 2023, 13, 10186. [Google Scholar] [CrossRef] [PubMed]
- Ganash, E.A.; Altuwirqi, R.M. Size Control of Synthesized Silver Nanoparticles by Simultaneous Chemical Reduction and Laser Fragmentation in Origanum majorana Extract: Antibacterial Application. Materials 2021, 14, 2326. [Google Scholar] [CrossRef]
- Attri, P.; Garg, S.; Ratan, J.K.; Giri, A.S. Silver nanoparticles from Tabernaemontana divaricate leaf extract: Mechanism of action and bio-application for photo degradation of 4-aminopyridine. Environ. Sci. Pollut. Res. 2021, 30, 24856–24875. [Google Scholar] [CrossRef]
- Dinh, T.M.; Tran, K.K.; Le, A.T.; Nguyen, T.M.N.; Do, T.H.; Liau, I. Green synthesis of silver nanoparticles using Mentha crispa L. leaf extract for treatment of dye wastewater. Vietnam J. Sci. Technol. Eng. 2023, 65, 14–20. [Google Scholar] [CrossRef]
- Ye, F.; Musselman, K.P. Synthesis of low dimensional nanomaterials by pulsed laser ablation in liquid. APL Mater. 2024, 12, 050602. [Google Scholar] [CrossRef]
- Mosaviniya, M.; Kikhavani, T.; Tanzifi, M.; Tavakkoli Yaraki, M.; Tajbakhsh, P.; Lajevardi, A. Facile green synthesis of silver nanoparticles using Crocus Haussknechtii Bois bulb extract: Catalytic activity and antibacterial properties. Colloid Interface Sci. Commun. 2019, 33, 100211. [Google Scholar] [CrossRef]
- Widatalla, H.A.; Yassin, L.F.; Alrasheid, A.A.; Ahmed, S.A.R.; Widdatallah, M.O.; Eltilib, S.H.; Mohamed, A.A. Green synthesis of silver nanoparticles using green tea leaf extract, characterization and evaluation of antimicrobial activity. Nanoscale Adv. 2022, 4, 911–915. [Google Scholar] [CrossRef]
- Alattar, A.M. The influence of pulsed laser on the structural and optical properties of green tea extract leaf produced with silver nanoparticles as antimicrobial. J. Mol. Liq. 2024, 398, 124287. [Google Scholar] [CrossRef]
- Lu, L.; Zhuang, Z.; Fan, M.; Liu, B.; Yang, Y.; Huang, J.; Da, X.; Mo, J.; Li, Q.; Lu, H. Green formulation of Ag nanoparticles by Hibiscus rosa-sinensis: Introducing a navel chemotherapeutic drug for the treatment of liver cancer. Arab. J. Chem. 2022, 15, 103602. [Google Scholar] [CrossRef]
- Melkamu, W.W.; Bitew, L.T. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) JF Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon 2021, 7, e08459. [Google Scholar] [CrossRef]
- Mehata, M.S. Green route synthesis of silver nanoparticles using plants/ginger extracts with enhanced surface plasmon resonance and degradation of textile dye. Mater. Sci. Eng. B 2021, 273, 115418. [Google Scholar] [CrossRef]
- Ganash, A.A. Electrochemical Properties and Mechanistic Study of the Green Synthesis of Silver Nanoparticles Using Bardaqush Extract Solution. Mater. Res. Express 2019, 6, 065024. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile green synthesis of silver nanoparticles using aqueous leaf extract of Origanum majorana with potential bioactivity against multidrug resistant bacterial strains. Crystals 2022, 12, 603. [Google Scholar] [CrossRef]
- Ganash, E.A. Synthesis of silver nanoparticles using pulsed laser ablation in liquid: A review. Laser Phys. Lett. 2022, 20, 013001. [Google Scholar] [CrossRef]
- Procházka, M.; Mojzeš, P.; Štěpánek, J.; Vlčková, B.; Turpin, P.-Y. Probing applications of laser-ablated Ag colloids in SERS spectroscopy: Improvement of ablation procedure and SERS spectral testing. Anal. Chem. 1997, 69, 5103–5108. [Google Scholar] [CrossRef]
- Ajaj, K.; Al-Jubbori, M.A.; Ali, A.M. Effect of ultraviolet irradiation on the optical properties and biological activity of silver nanoparticles prepared by pulsed laser ablation. Radiat. Phys. Chem. 2024, 216, 111384. [Google Scholar] [CrossRef]
- Menazea, A.A. Femtosecond Laser Ablation-Assisted Synthesis of Silver Nanoparticles in Organic and Inorganic Liquids Medium and their Antibacterial Efficiency. Radiat. Phys. Chem. 2020, 168, 108616. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Menazea, A.A. Polyvinyl Alcohol/Silver Nanoparticles Film Prepared via Pulsed Laser Ablation: An Eco-friendly Nano-Catalyst for 4-Nitrophenol Degradation. J. Mol. Struct. 2020, 1212, 128125. [Google Scholar] [CrossRef]
- Yang, H.; Ren, Y.-y.; Wang, T.; Wang, C. Preparation and antibacterial activities of Ag/Ag + /Ag 3+ nanoparticle composites made by pomegranate (Punica granatum) rind extract. Results Phys. 2016, 6, 299–304. [Google Scholar] [CrossRef]
- Gauri, B.; Vidya, K.; Sharada, D.; Shobha, W. Synthesis and characterization of Ag/AgO nanoparticles as alcohol sensor. Res. J. Chem. Env. 2016, 20, 1–5. [Google Scholar]
- Alghoraibi, I.; Zein, R. Silver Nanoparticles: Advances in Research and Applications is Approaching. In Silver Nanoparticles: Advances in Research and Applications; Edwards, B., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017. [Google Scholar]
- Rafique, M.; Rafique, M.S.; Kalsoom, U.; Afzal, A.; Butt, S.H.; Usman, A. Laser Ablation Synthesis of Silver Nanoparticles in Water and Dependence on Laser Nature. Opt. Quantum Electron. 2019, 51, 179. [Google Scholar] [CrossRef]
- Hajiesmaeilbaigi, F.; Mohammadalipour, A.; Sabbaghzadeh, J.; Hoseinkhani, S.; Fallah, H.R. Preparation of Silver Nanoparticles by Laser Ablation and Fragmentation in Pure Water. Laser Phys. Lett. 2006, 3, 252–256. [Google Scholar] [CrossRef]
- Moura, C.G.; Pereira, R.S.F.; Andritschky, M.; Lopes, A.L.B.; Grilo, J.P.d.F.; Nascimento, R.M.d.; Silva, F.S. Effects of Laser Fluence and Liquid Media on Preparation of Small Ag Nanoparticles by Laser Ablation in Liquid. Opt. Laser Technol. 2017, 97, 20–28. [Google Scholar] [CrossRef]
- Cortes, F.R.U.; Falomir, E.; Lancis, J.; Mínguez-Vega, G. Pulsed laser fragmentation synthesis of carbon quantum dots (CQDs) as fluorescent probes in non-enzymatic glucose detection. Appl. Surf. Sci. 2024, 665, 160326. [Google Scholar] [CrossRef]
- Altuwirqi, R.M.; Albakri, A.S.; Al-Jawhari, H.; Ganash, E.A. Green Synthesis of Copper Oxide Nanoparticles by Pulsed Laser Ablation in Spinach Leaves Extract. Optik 2020, 219, 165280. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vazquez, M.; Brabazon, D. A Review of Bimetallic and Monometallic Nanoparticle Synthesis via Laser Ablation in Liquid. Crystals 2023, 13, 253. [Google Scholar] [CrossRef]
- Rezaei, A.; Abdollahi, H.; Derikvand, Z.; Hemmati-Sarapardeh, A.; Mosavi, A.; Nabipour, N. Insights into the Effects of Pore Size Distribution on the Flowing Behavior of Carbonate Rocks: Linking a Nano-Based Enhanced Oil Recovery Method to Rock Typing. Nanomaterials 2020, 10, 972. [Google Scholar] [CrossRef]
- Kaasalainen, M.; Aseyev, V.; von Haartman, E.; Karaman, D.S.; Makila, E.; Tenhu, H.; Rosenholm, J.; Salonen, J. Size, Stability, and Porosity of Mesoporous Nanoparticles Characterized with Light Scattering. Nanoscale Res. Lett. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).