The Influence of Annealing Temperature on the Microstructure and Performance of Cold-Rolled High-Conductivity and High-Strength Steel
Abstract
1. Introduction
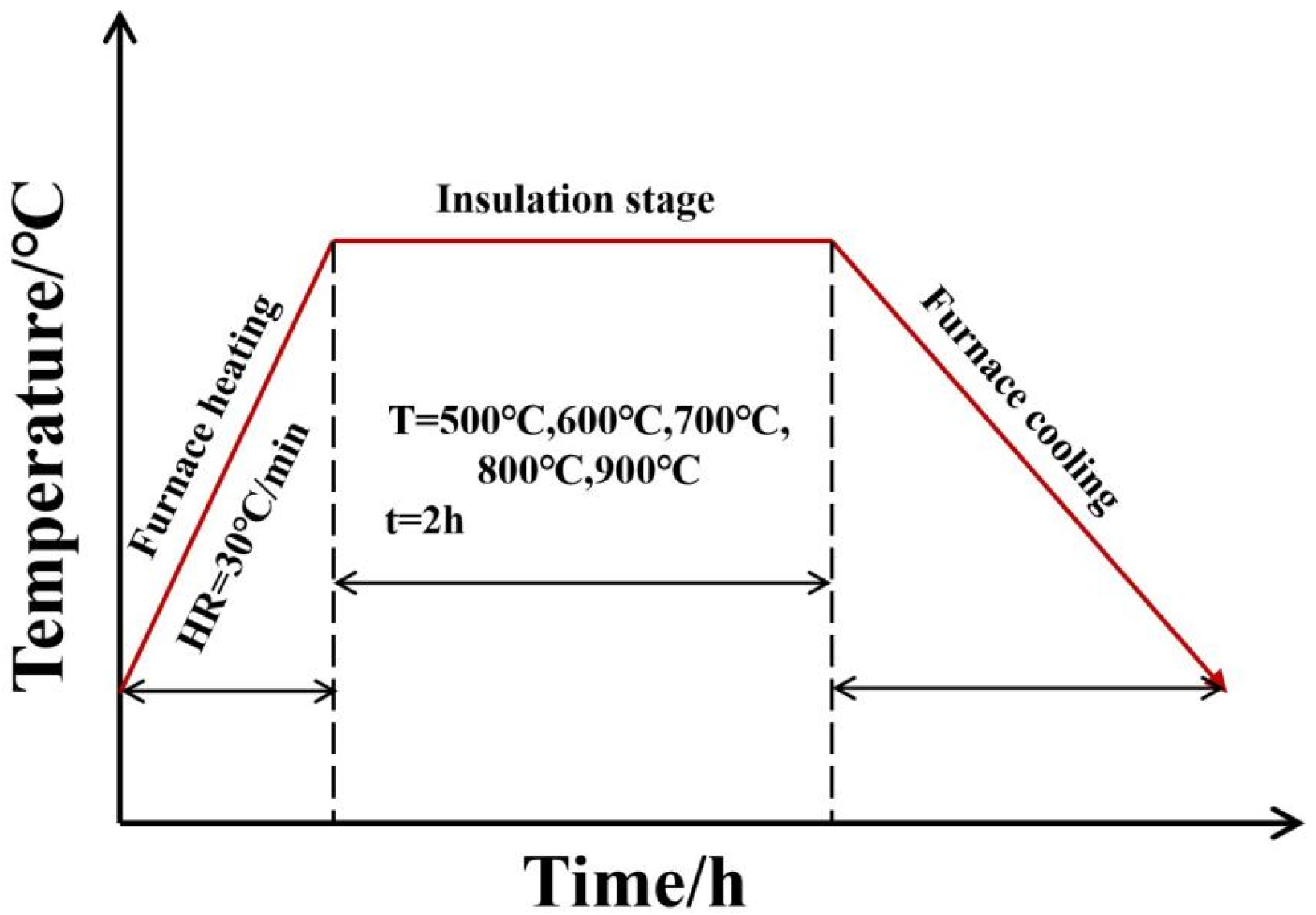
2. Experimental Materials and Methods
2.1. Experimental Material
2.2. Microstructure Characterization
2.3. Transmission Electron Microscopy Analysis
2.4. Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) Analysis
2.5. Comprehensive Performance Test
3. Results
3.1. Microstructure Analysis
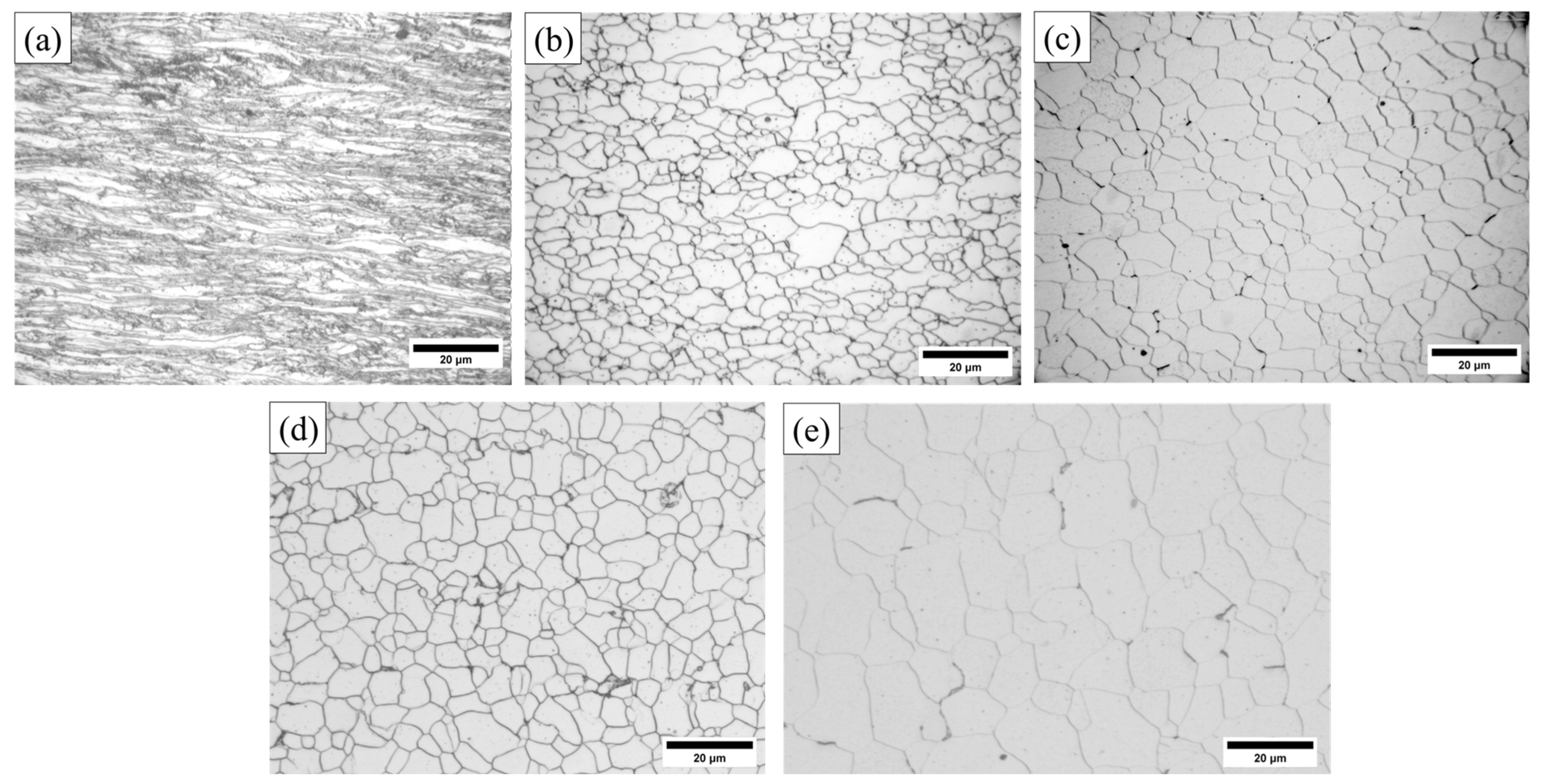
3.1.1. Metallographic Structure
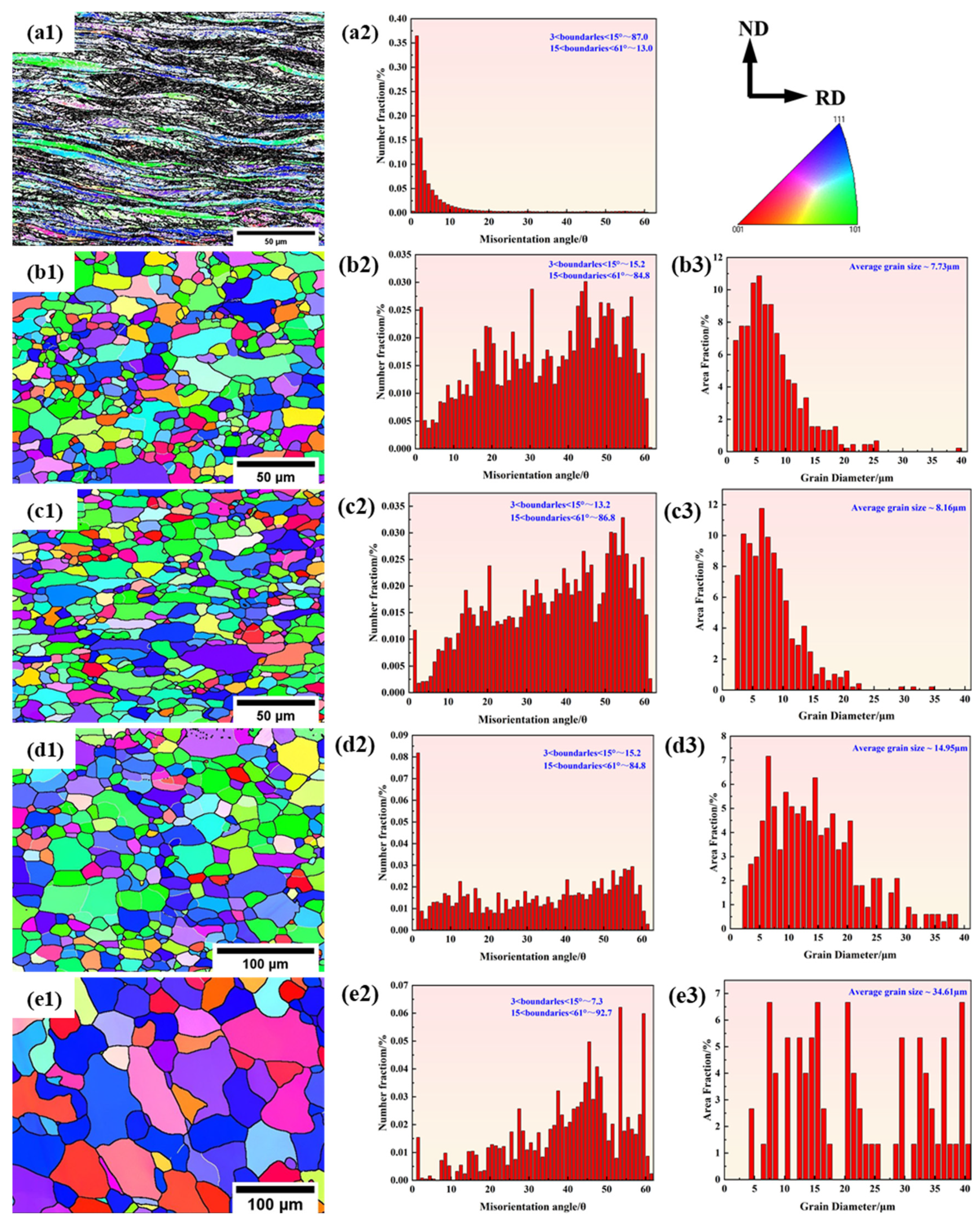
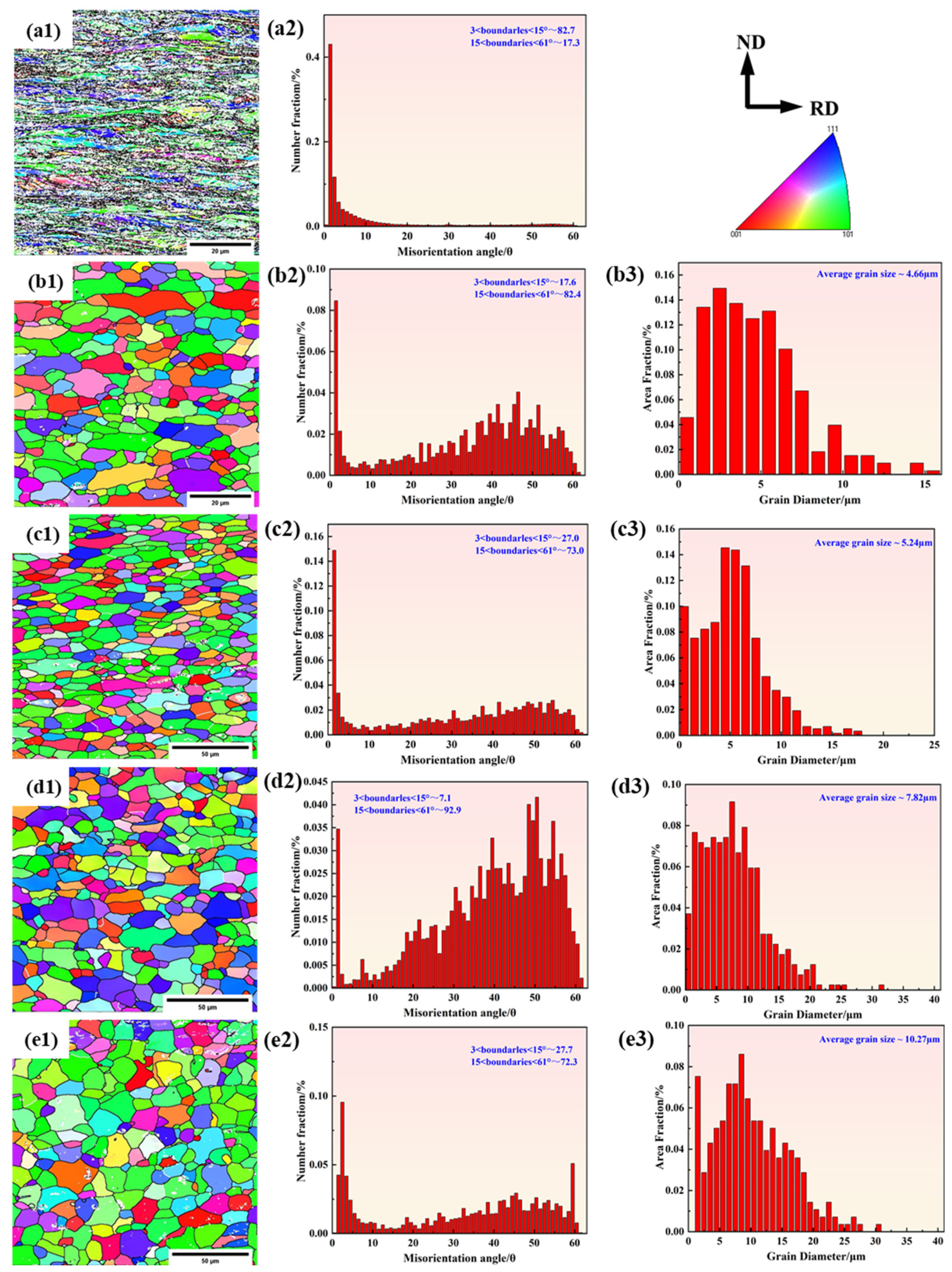
3.1.2. EBSD Analysis
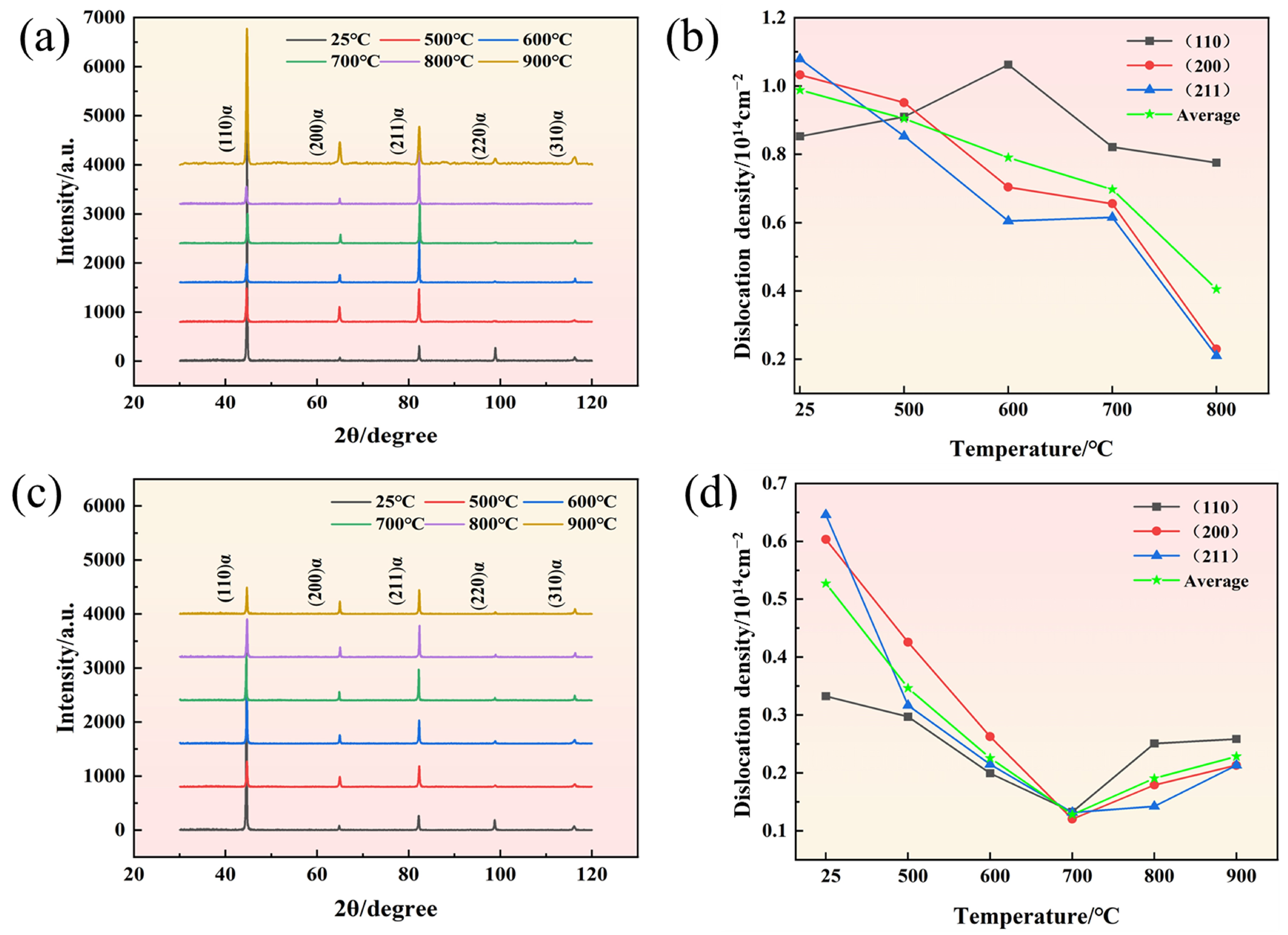
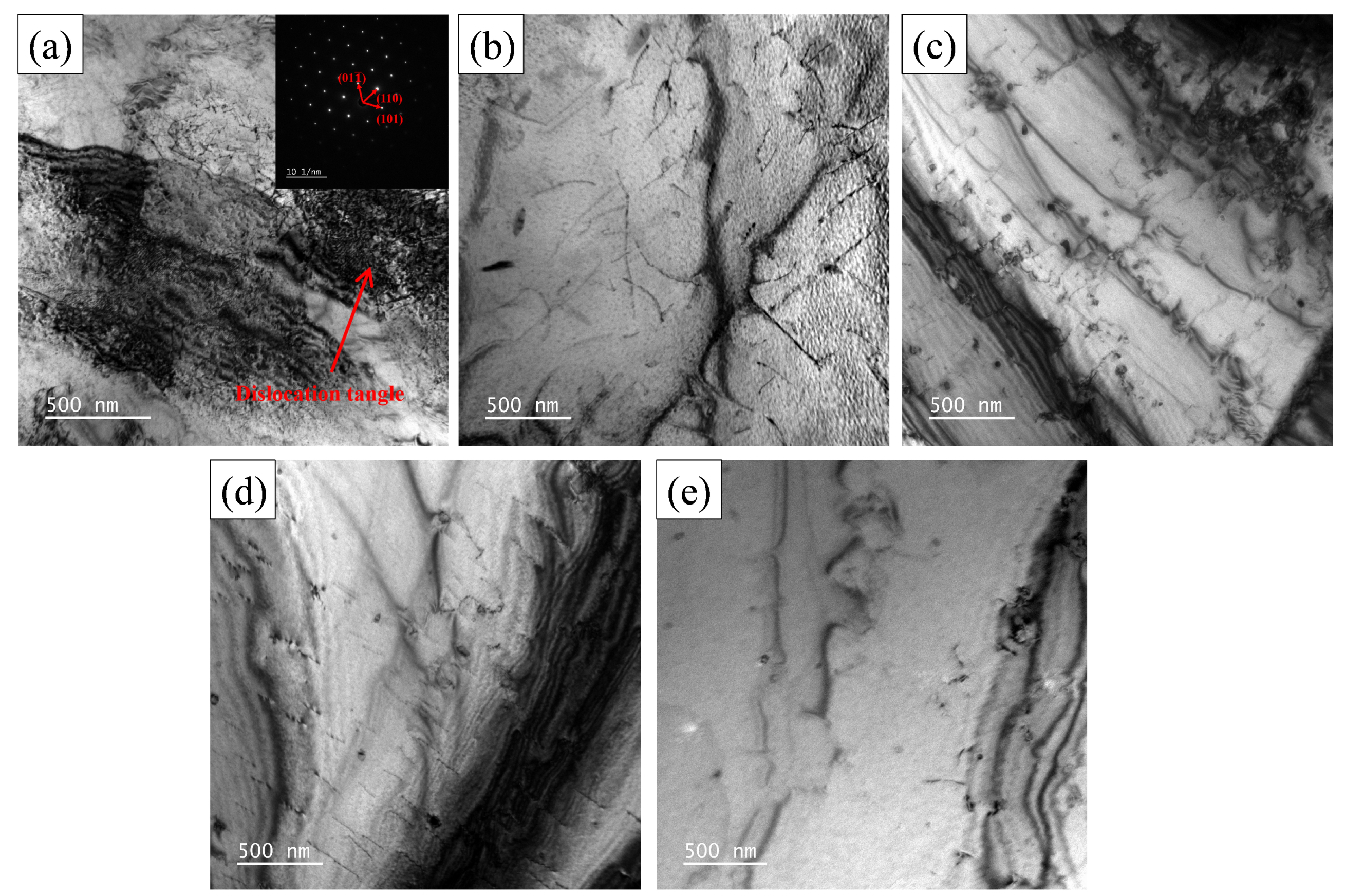
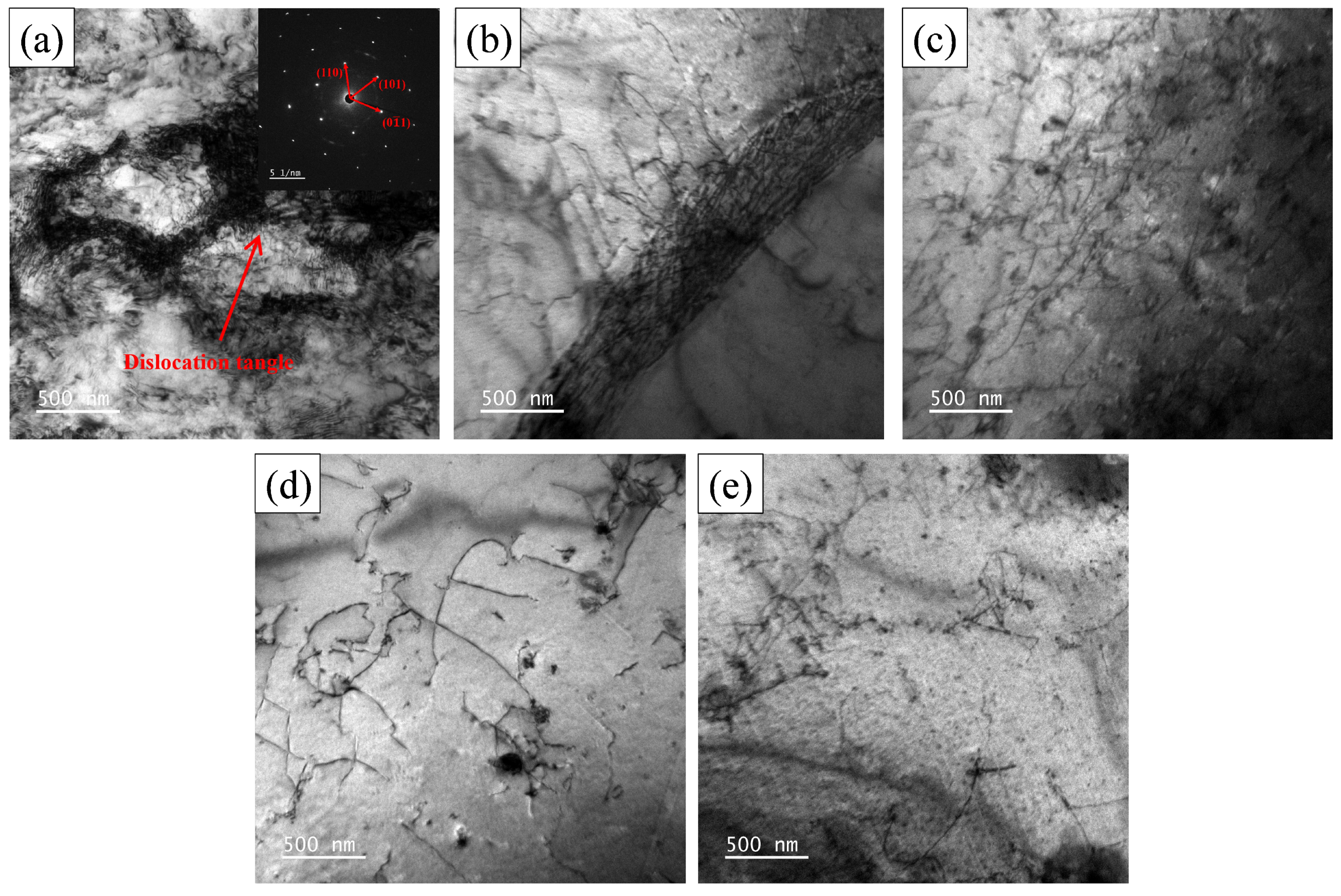
3.1.3. Dislocation Analysis
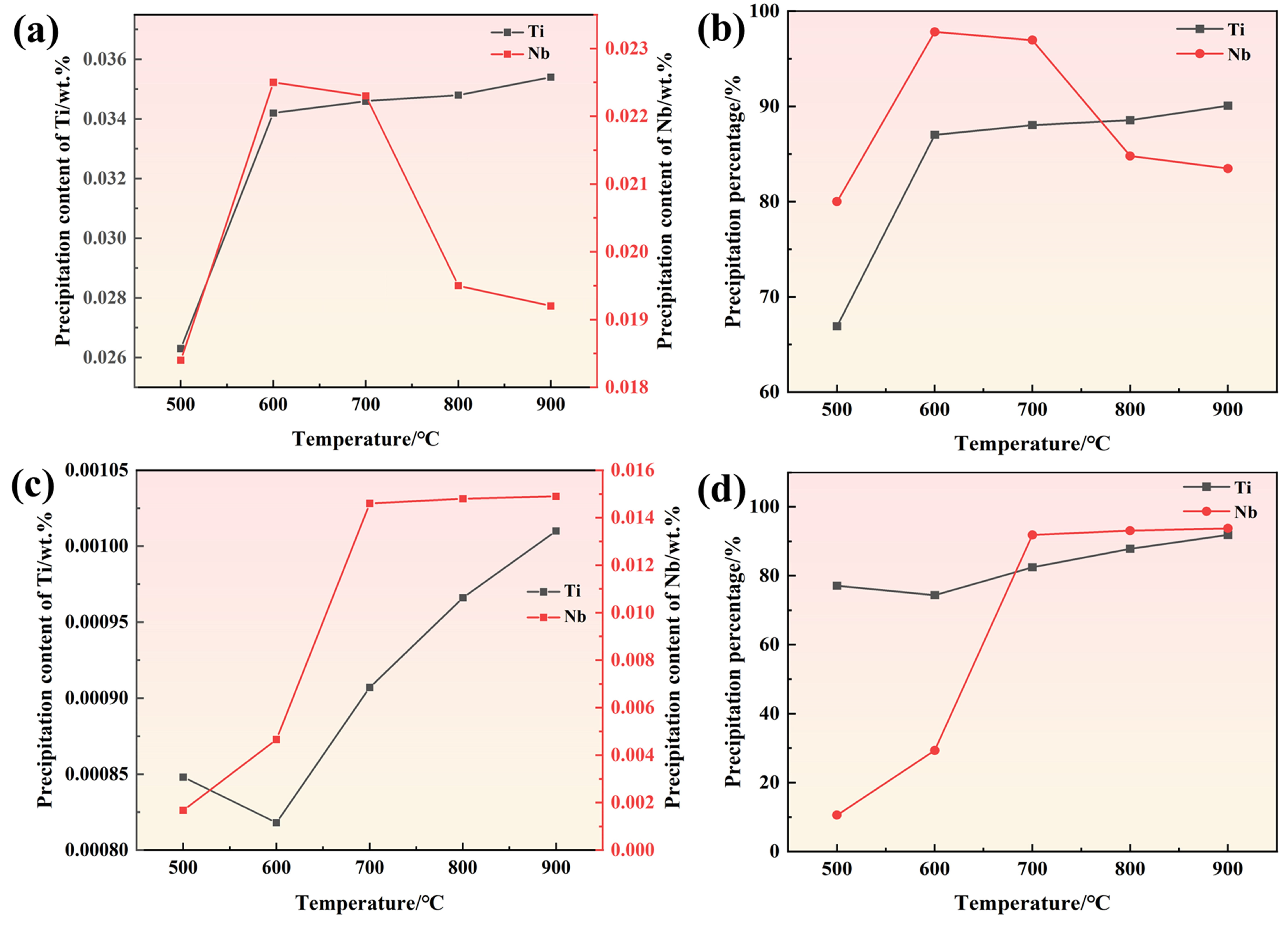
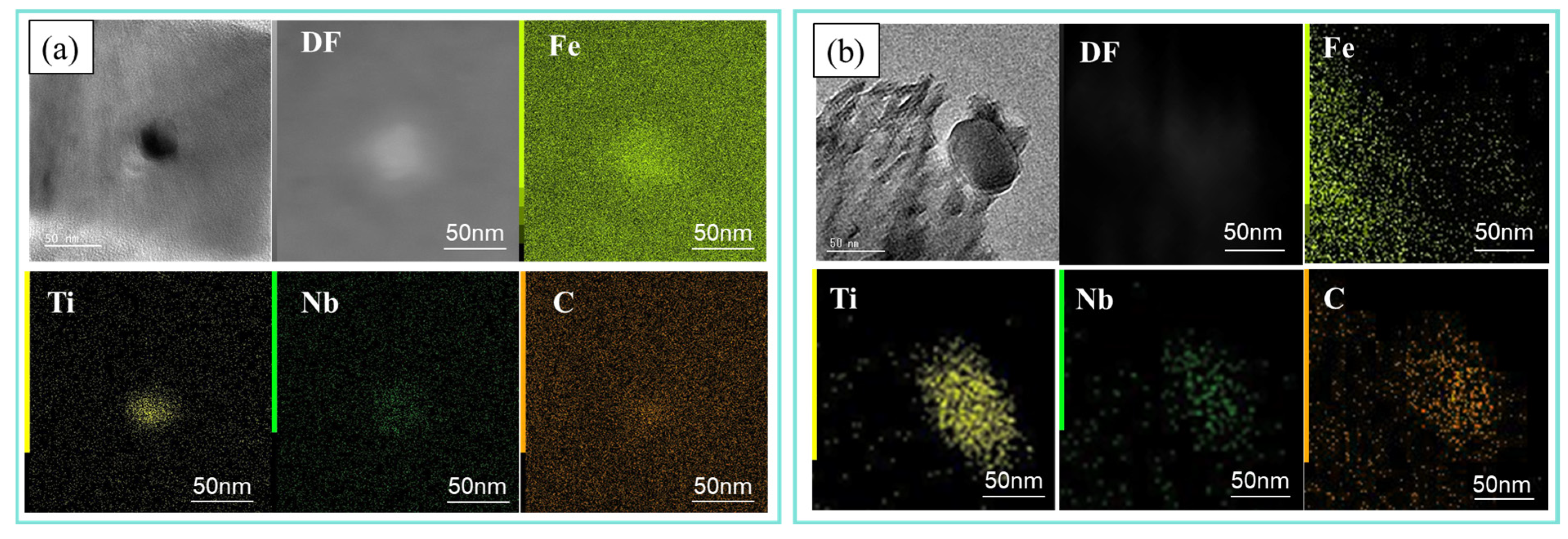
3.1.4. Precipitation Analysis
3.2. Comprehensive Performance
3.2.1. Mechanical Property
3.2.2. Conductivity
3.2.3. Phase Transition
4. Discussion
4.1. Strengthening Mechanism
4.2. Influence Mechanism of Conductivity
5. Conclusions
- (1)
- Two experimental steels underwent rapid recrystallization at an annealing temperature of 600 °C. The grain size increases as the annealing temperature increases. Compared to 1# steel, the average grain size of 2# steel is smaller. The dislocation density of 1# steel decreases with increasing annealing temperature, while 2# steel shows a trend of first decreasing and then increasing. This is mainly due to the formation of more carbon-containing precipitates with higher carbon content, which effectively fix the dislocations and hinder their movement.
- (2)
- The yield ratio of both experimental steels exhibited rapid decline with increasing annealing temperature. The 1# steel sample demonstrated superior comprehensive performance at 700 °C annealing, achieving a minimum resistivity of 13.75 μΩ/cm. Conversely, the 2# steel sample attained optimal electrical conductivity (14.66 μΩ/cm) and the best overall performance when annealed at 800 °C.
- (3)
- According to our calculations, without considering intrinsic strengthening, fine grain enhancing and precipitation enhancing make significant contributions to the yield strength of 1# steel, whereas for 2# steel, the primary contributions come from precipitation strengthening and solid solution strengthening. Based on the resistivity contribution formula and known parameters, the resistivity calculation formula was derived. The resistivity contribution of samples with small grain sizes was then calculated, and we concluded that the precipitated phase is beneficial to electrical conductivity within a certain precipitate size range.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Fu, H.; Wang, J.; Zhang, H.; Li, W.; Xie, J. Enhanced combination properties of Cu-0.8Cr alloy by Fe and P additions. J. Nucl. Mater. 2019, 526, 151753. [Google Scholar] [CrossRef]
- Pospelov, I.D.; Matveeva, D.V. Effect of Isothermal Annealing Before Cold Rolling on the Mechanical Properties of Hypereutectoid Steel for High-Strength Cold-Rolled Strips. Met. Sci. Heat Treat. 2025, 66, 606–610. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Wang, R.H. Manipulating nanostructure to simultaneously improve the electrical conductivity and strength in microalloyed Al-Zr conductors. Sci. Rep. 2018, 8, 6202. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, Z.D. Interphase precipitation behaviors of nanometer-sized carbides in a Nb-Ti-bearing Low-carbon microalloyed steel. Acta Metal. Sin. 2015, 51, 417–424. [Google Scholar]
- Singh, N.; Casillas, G.; Wexler, D.; Killmore, C.; Pereloma, E. Application of advanced experimental techniques to elucidate the strengthening mechanisms operating in microalloyed ferritic steels with interphase precipitation. Acta Mater. 2021, 201, 386–402. [Google Scholar] [CrossRef]
- Chenna Krishna, S.; Karthick, N.K.; Sudarshan Rao, G.; Jha, A.K.; Pant, B.; Cherian, R.M. High Strength, Utilizable Ductility and Electrical Conductivity in Cold Rolled Sheets of Cu-Cr-Zr-Ti Alloy. J. Mater. Eng. Perform. 2018, 27, 787–793. [Google Scholar] [CrossRef]
- Sun, L.; Liu, X.; Xu, X.; Lei, S.; Li, H.; Zhai, Q. Review on niobium application in microalloyed steel. J. Iron. Steel Res. Int. 2022, 29, 1513–1525. [Google Scholar] [CrossRef]
- Kang, S.E.; Tuling, A.; Lau, I.; Banerjee, J.R.; Mintz, B. The hot ductility of Nb/V containing high Al, TWIP steels. Mater. Sci. Tech. 2011, 27, 909–915. [Google Scholar] [CrossRef]
- Xie, Z.J.; Ma, X.P.; Shang, C.J.; Wang, X.M.; Subramanian, S.V. Nano-sized precipitation and properties of a low carbon niobium micro-alloyed bainitic steel. Mater. Sci. Eng. A 2015, 641, 37–44. [Google Scholar] [CrossRef]
- Liu, S.; Challa, V.S.A.; Natarajan, V.V.; Misra, R.D.K.; Sidorenko, D.M.; Mulholland, M.D.; Manohar, M. Significant influence of carbon and niobium on the precipitation behavior and microstructural evolution and their consequent impact on mechanical properties in microalloyed steels. Mater. Sci. Eng. A 2017, 683, 70–82. [Google Scholar] [CrossRef]
- Du, Y.; Li, S.; Ai, X.; Xiao, Q. Research progress of strengthened iron and steel with second phase particle. J. Iron Steel Res. 2020, 32, 683–689. [Google Scholar]
- Miura, H.; Kobayashi, M.; Todaka, Y.; Watanabe, C.; Aoyagi, Y. Influences of Heterogeneous Nano-Structure Developed in Heavily Cold-Rolled Austenitic Stainless Steel on Texture and Ductility. J. Jpn. Inst. Met. Mater. 2017, 81, 536–541. [Google Scholar]
- Opiela, M.; Fojt-Dymara, G.; Grajcar, A.; Borek, W. Effect of Grain Size on the Microstructure and Strain Hardening Behavior of Solution Heat-Treated Low-C High-Mn Steel. Materials 2020, 13, 1489. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Mukhopadhyay, A.; Chatterjee, S.; Chandra, S. Modelling of microstructure and heat transfer during controlled cooling of low carbon wire rod. Trans. Iron Steel Inst. Jpn. 2007, 44, 1217–1223. [Google Scholar] [CrossRef]
- Zavdoveev, A.; Baudin, T.; Pashinska, E.; Kim, H.S.; Brisset, F.; Heaton, M.; Poznyakov, V.; Rogante, M.; Tkachenko, V.; Klochkov, I.; et al. Continuous Severe Plastic Deformation of Low-Carbon Steel: Physical–Mechanical Properties and Multiscale Structure Analysis. Steel Res. Int. 2021, 92, 2000482. [Google Scholar] [CrossRef]
- Erden, M.A.; Erer, A.M.; Odabasi, C.G.S. The investigation of the effect of cu addition on the nb-v microalloyed steel produced by powder metallurgy. Sci. Sinter. 2022, 54, 153–167. [Google Scholar] [CrossRef]
- Gay, P.; Hirsch, P.B.; Kelly, A. The estimation of dislocation densities in metals from X-ray data. Acta. Metall. 1953, 1, 315–319. [Google Scholar] [CrossRef]
- Cheng, G.; Tang, X.; Zhang, Z.; Zhou, W.; Hou, Y.; Shen, Y. Effect of uniaxial compression on the microstructural evolution and magnetic properties of 20Mn23AlV non-magnetic structural steel. Mater. Today Commun. 2024, 39, 108935. [Google Scholar] [CrossRef]
- Olmsted, D.L.; Holm, E.A.; Foiles, S.M. Survey of computed grain boundary properties in face-centered cubic metals-II: Grain boundary mobility. Acta Mater. 2009, 57, 3704–3713. [Google Scholar] [CrossRef]
- Chesser, I.; Holm, E. Understanding the anomalous thermal behavior of Σ3 grain boundaries in a variety of FCC metals. Sci. Mater. 2018, 157, 19–23. [Google Scholar] [CrossRef]
- Zhang, H.M.; Chen, R.; Jia, H.B.; Li, Y.; Jiang, Z.Y. Effect of solid-solution second-phase particles on the austenite grain growth behavior in Nb-Ti high-strength if steel. Strength Mater. 2020, 52, 539–547. [Google Scholar] [CrossRef]
- Zaitsev, A.I.; Dagman, A.I.; Stepanov, A.B.; Koldaev, A.V.; Rodionova, I.G.; Orekhov, M.E. Creation of an Effective Technology for the Production of Cold-Rolled High-Strength Low-Alloy Steels with High and Stable Properties. Part 2. Cold-rolled Products. Metallurgist 2022, 66, 359–367. [Google Scholar] [CrossRef]
- Tanaka, Y.; Masumura, T.; Tsuchiyama, T.; Takaki, S. Effect of dislocation distribution on the yield stress in ferritic steel under identical dislocation density conditions. Sci. Mater. 2020, 177, 176–180. [Google Scholar] [CrossRef]
- Sato, K.; Sakakibara, Y.; Nomura, K. Effect of Cold Rolling on the Creep Rupture S rength of 12Cr-5.4W Ferritic Steel with δ-ferrite. Tetsu Hagane—J. Iron Steel Inst. Jpn. 2022, 108, 131–140. [Google Scholar] [CrossRef]
- Raabe, D.; Springer, H.; Gutiérrez-Urrutia, I.; Roters, F.; Bausch, M.; Seol, J.B.; Koyama, M. Alloy Design, Combinatorial Synthesis, and Microstructure–Property Relations for Low-Density Fe-Mn-Al-C Austenitic Steels. Jom 2014, 66, 1845–1856. [Google Scholar] [CrossRef]
- Li, X.L.; Lei, C.S.; Deng, X.T.; Li, Y.M.; Tian, Y.; Wang, Z.D.; Wang, G.D. Carbide precipitation in ferrite in Nb–V-bearing low-carbon steel during isothermal quenching process. Acta Metall. Sin. 2017, 30, 1067–1079. [Google Scholar] [CrossRef]
- Jung, J.G.; Park, J.S.; Kim, J.; Lee, Y.K. Carbide precipitation kinetics in austenite of a Nb-Ti-V microalloyed steel. Mater. Sci. Eng. A 2011, 528, 5529–5535. [Google Scholar] [CrossRef]
- Tang, X.; Cheng, G.; Liu, Y.; Wang, C.; Meng, Z.; Wang, Y.; Liu, Y.; Zhang, Z.; Huang, J.; Yu, X. Microstructure and properties evolution during annealing in low-carbon Nb containing steel with high strength and electrical conductivity: An experimental and theoretical study. J. Mater. Res. Technol. 2023, 27, 3054–3066. [Google Scholar] [CrossRef]
- Sauvage, X.; Bobruk, E.V.; Murashkin, M.Y.; Valiev, Z.R.; Enikeev, A.N. Optimization of electrical conductivity and strength combination by structure design at the nanoscale in Al–Mg–Si alloys. Acta Mater. 2015, 98, 355–366. [Google Scholar] [CrossRef]
- Dastur, P.; Zarei-Hanzaki, A.; Pishbin, M.H.; Moallemi, M.; Abedi, H.R. Transformation and twinning induced plasticity in an advanced high Mn austenitic steel processed by martensite reversion treatment. Mater. Sci. Eng. A 2017, 696, 511–519. [Google Scholar] [CrossRef]
- Yang, J.; Bu, K.; Song, K.; Zhou, Y.; Huang, T.; Niu, L.; Guo, H.; Du, Y.; Kang, J. Influence of low-temperature annealing temperature on the evolution of the microstructure and mechanical properties of Cu-Cr-Ti-Si alloy strips. Mater. Sci. Eng. A 2020, 798, 140120. [Google Scholar] [CrossRef]
- Nakagaito, T.; Yamashita, T.; Funakawa, Y.; Kajihara, M. Partitioning of Solute Elements and Microstructural Changes during Heat-treatment of Cold-rolled High Strength Steel with Composite Microstructure. ISIJ Int. 2020, 60, 1784–1795. [Google Scholar] [CrossRef]
- Wang, L.; Cao, T.; Liu, X.; Wang, B.; Xue, Y. A novel stress-induced martensitic transformation in a single-phase refractory high-entropy alloy. Sci. Mater. 2020, 189, 129–134. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Z.D.; Sun, X.J.; Yong, Q.L.; Yang, J.W.; Li, Y.M.; Zhao, P.L. Development of Ti-V-Mo complex microalloyed hot-rolled 900-MPa-grade high-strength steel. Acta Metall. Sin. 2015, 5, 8. [Google Scholar] [CrossRef]
- Yen, H.W.; Chen, P.Y.; Huang, C.Y.; Yang, J.R. Interphase precipitation of nanometer-sized carbides in a titanium–molybdenum-bearing low-carbon steel. Acta Mater. 2011, 59, 6264–6274. [Google Scholar] [CrossRef]
- Agrawal, S.; Avadhani, G.S.; Suwas, S. Satyam. Deformation behaviour of additively manufactured Hastelloy X at high temperatures: The role of concurrent carbide precipitation. J. Alloys Compd. 2025, 1021, 179636. [Google Scholar] [CrossRef]
- Mao, X.; Huo, X.; Sun, X.; Chai, Y. Strengthening mechanisms of a new 700 MPa hot rolled Ti-microalloyed steel produced by compact strip production. J. Mater. Process. Technol. 2010, 210, 1660–1666. [Google Scholar] [CrossRef]
- Morales, E.V.; Betancourt, G.; Fernandes, J.R.; Batista, G.Z.; Bott, I.S. Hardening mechanisms in a high wall thickness sour service pipe steel API 5L X65 before and after post-welding heat treatments. Mater. Sci. Eng. A 2022, 851, 143612. [Google Scholar] [CrossRef]
- Tang, X.; Kuang, C.; Zhou, W.; Chen, K.; Huang, J.; Yv, X.; Wang, C.; La, P. Effect of annealing process on microstructure and electrical conductivity of cold-rolled Ti microalloyed conductive steel. Mater. Charact. 2023, 201, 112930. [Google Scholar] [CrossRef]
- Taylor, S.; Masters, I.; Li, Z.; Kotadia, H.R. Direct observation via in situ heated stage EBSD analysis of recrystallization of phosphorous deoxidised copper in unstrained and strained conditions. Met. Mater. Int. 2020, 26, 1030–1035. [Google Scholar] [CrossRef]
- Li, J.; Ding, H.; Li, B. Study on the variation of properties of Cu–Cr–Zr alloy by different rolling and aging sequence. Mater. Sci. Eng. A 2021, 802, 140413. [Google Scholar] [CrossRef]
- Sun, P.F.; Zhang, P.L.; Hou, J.P.; Wang, Q.; Zhang, Z.F. Quantitative mechanisms behind the synchronous increase of strength and electrical conductivity of cold-drawing oxygen-free Cu wires. J. Alloy. Compd. 2021, 863, 158759. [Google Scholar] [CrossRef]
- Gladman, T. Precipitation hardening in metals. Mater. Sci. Tech. 1999, 15, 30–36. [Google Scholar] [CrossRef]
- Tian, L.; Anderson, I.; Riedemann, T.; Russell, A. Modeling the electrical resistivity of deformation processed metal–metal composites. Acta. Mater. 2014, 77, 151–161. [Google Scholar] [CrossRef]
- Scales, M.; Kornuta, J.A.; Switzner, N.; Veloo, P. Automated Calculation of Strain Hardening Parameters from Tensile Stress vs. Strain Data for Low Carbon Steel Exhibiting Yield Point Elongation. Exp. Tech. 2023, 47, 1311–1322. [Google Scholar] [CrossRef]
- Murashkin, M.Y.; Enikeev, N.A.; Sauvage, X. Potency of severe plastic deformation processes for optimizing combinations of strength and electrical conductivity of lightweight Al-based conductor alloys. Mater. Trans. 2023, 64, 1833–1843. [Google Scholar] [CrossRef]
- Zeng, W.; Xie, J.; Zhou, D.; Fu, Z.; Zhang, D.; Lavernia, E.J. Bulk Cu-NbC nanocomposites with high strength and high electrical conductivity. J. Alloy. Compd. 2018, 745, 55–62. [Google Scholar] [CrossRef]







| C | Si | Mn | P | S | Al | Nb | Ti | B | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1# | 0.0025 | 0.0120 | 0.3330 | 0.0294 | 0.0042 | 0.0452 | 0.0230 | 0.0393 | 0.0001 | Bal. |
| 2# | 0.0579 | 0.0370 | 0.7130 | 0.0170 | 0.0039 | 0.0300 | 0.0159 | 0.0011 | 0.0003 | Bal. |
| 1#-500 °C | 1#-600 | 1#-700 | 1#-800 | 1#-900 | 2#-500 | 2#-600 | 2#-700 | 2#-800 | 2#-900 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Recrystallized | 4.2 | 98.2 | 99.2 | 89.6 | 96.1 | 16.1 | 95.1 | 87.1 | 66.4 | 73.7 |
| In transit | 0.6 | 1.7 | 0.7 | 10.1 | 3.9 | 8.1 | 4.6 | 11.9 | 33.5 | 24.6 |
| Uncrystallized | 95.1 | 0.06 | 0.02 | 0.2 | 0.006 | 75.8 | 0.3 | 1.0 | 0.1 | 1.7 |
| 1-600 | 1-700 | 1-800 | 1-900 | 2-600 | 2-700 | 2-800 | 2-900 | |
|---|---|---|---|---|---|---|---|---|
| Test value | 13.99 | 13.75 | 15.02 | 13.77 | 15.16 | 15.14 | 14.66 | 15.40 |
| Calculated value | 13.99 | 13.74 | 12.25 | 10.99 | 15.89 | 14.76 | 13.19 | 12.43 |
| Error | 0 | 0 | 18% | 20.18% | 4.8% | 2.5% | 10% | 19% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, S.; Zhao, X.; Zhou, W.; Xu, X.; Tang, X.; Ren, J.; Zhang, J.; Yi, Y. The Influence of Annealing Temperature on the Microstructure and Performance of Cold-Rolled High-Conductivity and High-Strength Steel. Crystals 2025, 15, 469. https://doi.org/10.3390/cryst15050469
Ge S, Zhao X, Zhou W, Xu X, Tang X, Ren J, Zhang J, Yi Y. The Influence of Annealing Temperature on the Microstructure and Performance of Cold-Rolled High-Conductivity and High-Strength Steel. Crystals. 2025; 15(5):469. https://doi.org/10.3390/cryst15050469
Chicago/Turabian StyleGe, Shuhai, Xiaolong Zhao, Weilian Zhou, Xueming Xu, Xingchang Tang, Junqiang Ren, Jiahe Zhang, and Yaoxian Yi. 2025. "The Influence of Annealing Temperature on the Microstructure and Performance of Cold-Rolled High-Conductivity and High-Strength Steel" Crystals 15, no. 5: 469. https://doi.org/10.3390/cryst15050469
APA StyleGe, S., Zhao, X., Zhou, W., Xu, X., Tang, X., Ren, J., Zhang, J., & Yi, Y. (2025). The Influence of Annealing Temperature on the Microstructure and Performance of Cold-Rolled High-Conductivity and High-Strength Steel. Crystals, 15(5), 469. https://doi.org/10.3390/cryst15050469







