Abstract
Traditional methods of synthesizing nanoparticles often rely on physical and chemical processes using synthetic hazardous chemicals. In contrast, the rise in green chemistry emphasizes using bioactive compounds from plants for the eco-friendly synthesis of nanostructures. These green synthesis techniques are increasingly recognized for their simplicity, cost-effectiveness, and ability to yield non-toxic by-products, an approach that aligns with sustainable practices. In this research, a straightforward, cheap, environmentally friendly, and sustainable procedure was developed to fabricate Zinc oxide nanoparticles (ZnO-NPs) employing three different pulp extracts: Agave (Agave americana), Chiku (Manilkara zapota), and Soursop (Annona muricata) to serve in the synthesis as capping, reduction, or stabilization agent. Analytical characterization techniques confirmed the successful phytosynthesis of ZnO-NPs, evidenced by significant absorbance peaks of UV-Vis spectra at 362 nm, and the chemical composition of ZnO without noticeable traces of phytochemical residues by carrying out ATR-FTIR analysis. SEM, STEM microscopies, and XRD analysis verified that the ZnO nanoparticles possess spherical geometries and hexagonal crystal structures. The average size of these nanoparticles was around 15.94, 18.08, and 23.32 nm for Agave, Chiku, and Soursop extract-based synthesis, respectively. Additionally, the in vitro antibacterial activity of phytosynthetized ZnO-NPs was evaluated against E. coli and S. aureus, confirming effective bacterial growth inhibition and demonstrating their significant antimicrobial potential.
1. Introduction
Nanotechnology is poised to drive the industrial revolution of this century thanks to its rapid advancements and ability to foster a wide range of scientific and technological breakthroughs, particularly in the medical sciences []. Its applications span numerous fields, including information technology, electronics, physics, biology, agriculture, chemistry, and medicine []. The exceptional physicochemical and biological properties of nanomaterials, especially when compared to bulk counterparts, make them highly valuable across these disciplines []. For instance, nanoparticles (NPs), with their remarkably high surface-to-volume ratio [], are particularly effective for application-driven innovations such as photocatalysis, energy storage, gas sensing, packaging, electronics, and environmental remediation. This versatility positions nanotechnology as a key player in developing profitable inventions and enhancing biotechnological and biomedical applications [].
Metal oxide (MO) nanoparticles (NPs) are considered the most promising of the many types of NPs currently on the market because of their unique chemical, physical, and biological characteristics, such as their solubility, chemical stability, and adhesiveness [,]. However, while effective for MO nanoparticle synthesis, certain chemical compounds used as solvents, reducing agents, or stabilizers may pose environmental and biological risks. For example, commonly used substances such as ethanol, methanol, and acetone [,,]; sodium borohydride and hydrazine hydrate [,]; as well as stabilizers like cetyltrimethylammonium bromide (CTAB) [], Triton X-100 [] and sodium dodecyl sulfate (SDS) [] have been reported to potentially cause adverse effects depending on their concentration, persistence, and disposal [,,,,].
The increasing need to reduce chemical usage and accomplish sustainable development goals through the application of green chemistry principles has brought about an urgent requirement for alternative, environmentally friendly approaches in the synthesis of nanoparticles []. Due to severe traditional chemical and physical processes that are linked to environmental problems across the globe, the integration of green methods in many technologies has become increasingly prevalent and vital [].
Nanotechnology is receiving increased attention because of its innovative features and broad range of applications in all scientific and technological domains, including the biological sciences []. Traditionally, nanomaterials are synthesized by many physical and chemical procedures, which are not only expensive but also a threat to our environment and life on Earth [,]. A more sustainable and cost-effective approach involves synthesizing nanomaterials with plant and fruit extracts. This method allows for the large-scale production of stable nanoparticles in various sizes and shapes while being environmentally friendly [,]. Natural extracts contain phytochemicals such as flavonoids, phenols, terpenes, alkaloids, tannins, and saponins, which serve as capping, stabilization, and reducing agents during synthesis. This makes using plant and fruit extracts an attractive option for nanoparticle production [].
Zinc oxide nanoparticles (ZnO-NPs) stand out among nano-metal oxides due to their exceptional properties and versatile applications, including antimicrobial [], anti-inflammatory [], and anticancer effects [], drug delivery [,], and wound healing [] applications. Also, their ability to reflect ultraviolet rays also makes ZnO-NPs an excellent option to be employed in sunscreens []. The U.S. Food and Drug Administration (FDA) has classified ZnO-NPs as a Generally Recognized as Safe (GRAS) substance [] because they have been proven to be multifunctional and biocompatible nanomaterials.
Various methodologies to produce ZnO-NPs using diverse natural extracts from plants, stems, leaves, and pulps have been explored []. However, the use of pulp from Agave (Agave americana), Chiku (Manilkara zapota), and Soursop (Annona muricata) for synthesizing ZnO-NPs has not yet been documented.
Agave (Agave americana) is a group of plants that belong to the monocot family and are naturally found in dry areas of the Americas. This genus is recognized for its xerophytic and succulent species, which often develop huge rosettes of robust, meaty leaves. A. americana, originating from tropical America, is one of the most well-known species. Some common names in Mexico used for this plant are century plant and maguey.
Chiku (Manilkara zapota), referred to as chicozapote in Mexico, is an evergreen tree that originates from Central America and southern Mexico. A natural occurrence is commonly observed in the coastal region of Yucatán, which covers the mangrove ecosystem sited in southern Mexico along the Gulf of Mexico coast, where this plant species is subordinate []. It is extensively cultivated in Mexico and tropical regions of Asia, such as Pakistan, Malaysia, Indonesia, Vietnam, and Bangladesh.
Soursop (Annona muricata) is a common fruit in Mexico and locally famous as guanabana. This is the fruit of the Annona muricata family, a broadleaf, blooming, evergreen tree. This plant species is indigenous to the representative areas of the Americas and the Caribbean, where it is commonly cultivated.
The evaluation of extracts from natural sources has confirmed the presence of various phytochemicals, including alkaloids, steroids, glycosides, polyphenols, terpenoids, flavonoids, aromatic hydrocarbons, and resins [,]. Multiple studies have shown that these phytochemicals play a significant role in the synthesis of NPs, as they facilitate stabilization and reduction reactions. Figure 1 illustrates the potential reaction mechanism to produce ZnO-NPs via phytofabrication. In this process, the aromatic hydroxyl and polyphenolic groups derived from phytochemicals can bind to Zn2+ ions of the precursor salt, forming a stable zinc catecholate complex. Initially, zinc acetate dihydrate dissociates in water, liberating Zn2+ ions and acetate groups according to the following reaction:
Zn(CH3COO)2 2H2O → Zn2+ + 2CH3COO− + 2H2O
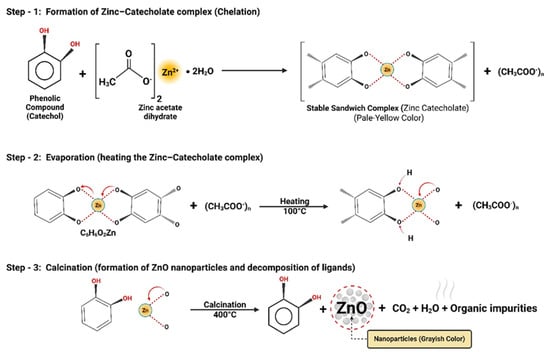
Figure 1.
Illustration of the reaction between precursor salt and extract for phytofabrication of ZnO-NPs.
Catechol groups (C6H6O2) then chelate the Zn2+ ions through their adjacent –OH groups, resulting in the formation of Zn(catechol)₂ complexes while acetate ions remain in the solution. Upon moderate heating at 100 °C, water molecules are removed, partial decomposition of acetate occurs, and organic ligands rearrange around Zn2+, creating a more open structure still coordinated by oxygen atoms. This intermediate preparation is crucial for the final transformation. Subsequently, during calcination at high temperature, complete thermal decomposition of organic ligands occurs. The Zn2+ centers combine with oxygen atoms derived both from the decomposition of oxygen-containing ligands and, potentially, from atmospheric oxygen available during the calcination process, leading to the crystallization of ZnO nanoparticles. Meanwhile, catechol and acetate groups decompose into CO2, H2O, and other organic vapors, as inferred from the well-established thermal degradation behavior of organic compounds. Thus, through centrifugation and calcination, the initially formed zinc–catecholate complex successfully yields ZnO-NPs [,].
This study introduces a novel, safe, cost-effective, and environmentally friendly method for producing ZnO-NPs. In this work, we have successfully used pulp extracts from three distinct fruits—Agave americana, Manilkara zapota, and Annona muricata—in this straightforward and safe process. The features of the as-synthesized NPs, such as optical properties, presence of functional groups, morphology, crystalline quality, and chemical composition, have been analyzed in depth through different characterization techniques such as UV-Vis, ATR-FTIR, XRD, SEM, STEM, and EDS. The primary objective of this work is to evaluate the capability of these indigenous and widespread fruit pulp extracts to produce ZnO-NPs through phytofabrication. We compare the characteristics of the synthesized materials from each extract to determine which one yields the most effective nanoparticles.
2. Materials and Methods
2.1. Chemicals
Reagent-grade (purity ≥ 98%) zinc acetate dihydrate [Zn(CH3COO)2·2H2O] was purchased from Sigma-Aldrich and used directly without any further treatment.
2.2. Collection and Preparation of Fruit Pulp
Plant specimens were collected under national and international guidelines [] from their natural habitats. Agave (Agave americana) was collected from fields of the city of Tequila, which is located in the state of Jalisco in central Mexico (Latitude 20°52′46″, Longitude 103°50′08″ W). This city is famous for producing tequila in Mexico. Chiku (Manilkara zapota) was obtained from Uruapan, which is a municipality of Michoacan in Mexico (19°25′16″ N 102°03′47″ W), coinciding with a peak season of the fruit in this region. Soursop (Annona muricata) was collected from fields in Las Varas, Compostela, in the southwest of Nayarit, Mexico (21°10′46″ N, 105°08′07″ O).
The fruit samples collected for the extract were thoroughly cleansed using tap water and then with deionized water to eliminate any dust or soil particles. Subsequently, the samples were dehydrated at room temperature (25 °C). The pulp of Agave (Agave americana), Chiku (Manilkara zapota), and Soursop (Annona muricata) was obtained, labeled, and preserved in polyethylene bags at a temperature of −20 °C until further experimentation.
2.3. Preparation of Fruit Extract
In total, 10 g of each fruit pulp, Agave (Agave americana), Chiku (Manilkara zapota), and Soursop (Annona muricata) were taken into three different beakers by adding 100 mL of deionized water, with continuous stirring and heating at 70 °C for 2 h. The prepared extract was allowed to cool to room temperature and then filtered two times using filter paper (Whatman No.1). The extracts were labeled and kept at 4 °C for the subsequent experiments.
2.4. Preparation of Precursor Salt
For each obtained extract, 4 g of [Zn(CH3COO)2·2H2O] salt was added to 100 mL of deionized water with continuous stirring at 600 rpm and heated at 70 °C for 1 h to obtain a homogenous solution. The resultant homogenous mixture was filtered twice and kept at 4 °C for further experimentation.
2.5. Phytosynthesis of ZnO Nanoparticles
100 mL of the prepared precursor salt solution was mixed with 50 mL of the prepared extract of Agave (Agave americana), Chiku (Manilkara zapota), and Soursop (Annona muricata) individually, making three different resultant mixtures. Each solution was allowed to react at a temperature of 70 °C and stirred at 600 rpm for 2 h. The aqueous solutions were changed from a cream color to a pale lemonish paste. This paste was then collected in different Petri dishes and kept in a hot-air oven at a temperature of 100 °C for 3 h to evaporate the remaining water residue. As a result, the powder of ZnO-NPs was successfully produced. To obtain a finer product, the obtained powder of ZnO-NPs was crushed mechanically using a mortar and pestle. To remove further organic impurities that may be present, ZnO-NPs powder was kept in ceramic crucibles and heated in a furnace for 3 h at a temperature of 400 °C. This whole procedure is schematically depicted in Figure 2. After the calcination, the color of ZnO-NPs turned from pale yellow to grayish. These obtained samples were stored in airtight containers at room temperature, avoiding direct sunlight, for further analytical characterizations.
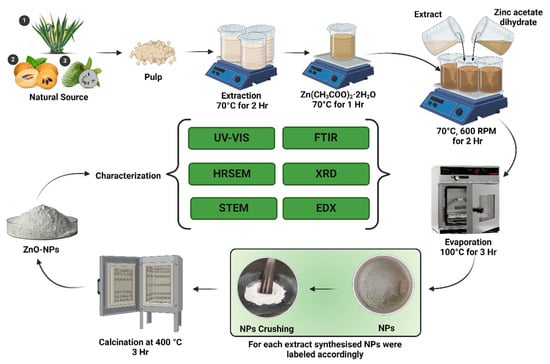
Figure 2.
Procedure of the phytofabrication of ZnO-NPs from Agave (Agave americana), Chiku (Manilkara zapota), and Soursop (Annona muricata) extract.
2.6. Materials Characterization
A noticeable color change was observed for Agave (Agave americana), Chiku (Manilkara zapota), and Soursop (Annona muricata) individually when the extract was allowed to react with zinc acetate dihydrate solution. This change in color indicated the occurrence of a reduction reaction between the extract and the precursor salt. Various characterization techniques confirmed the successful synthesis of ZnO-NPs. UV–Vis, ATR-FTIR, XRD, SEM, STEM, and EDS analytical methods were used to characterize the phytofabricated ZnO-NPs, to evaluate their optical properties, the presence of functional groups, the nanoparticles’ morphology, the crystalline structure, and the elemental composition to verify the purity of the material. The absorbance of the ZnO-NPs powder was measured on a Cary-5000 UV–Vis-NIR spectrometer (Agilent Technologies, Santa Clara, CA, USA) equipped with an integration sphere of polytetrafluoroethylene (PTFE) throughout a 200–800 nm wavelength range. The presence of organic matter and phytoconstituents that contribute to the reduction and stabilization of the structure of the ZnO-NPs was evaluated by Fourier-transform infrared spectroscopy (FTIR) using an attenuated total reflection accessory (ATR). The ATR-FTIR spectra were obtained using an IR Affinity-1S spectrometer (Shimadzu, Kyoto, Japan) between the 4000–400 cm−1 range of wavenumber.
To examine the phytofabricated ZnO-NPs crystalline nature and crystallite size, the powdered samples were taken to an Empyrean model X-ray diffractometer of Malvern Panalytical, using Cu radiation (λ = 1.5405 Å) operating at 45 kV and 40 mA. The diffraction scan was adjusted between 2θ angles from 5° to 80° at a step size of 0.02° and a scan time of 6 min. Samples were microstructurally analyzed using a JSM-IT700HR SEM (JEOL, Tokyo, Japan) with an energy dispersive spectrometer (EDS) (JEOL, Tokyo, Japan) for elemental analysis. The conditions for the semiquantitative composition analysis were adjusted to 15 kV, a working distance of 13.4 mm, and 80,000 magnification to compare. Ten zones on each sample were analyzed with punctual composition analyses, and the media and the standard deviation of element values were reported. Scanning-Transmission Electron Microscopy (STEM) was performed in a field-emission microscope, model JSM-7401-F (JEOL, Tokyo, Japan), operated in the STEM mode, with an acceleration voltage of 30 KV and at a working distance of 6 mm.
2.7. Assessment of Antibacterial Activity
In vitro bactericidal effects of phytofabricated ZnO-AG, ZnO-CH, and ZnO-SS nanomaterials were investigated against the bacterial strains of Escherichia coli (ATCC11229), Gram-negative, and Staphylococcus aureus (ATCC6538), Gram-positive. Bacterial cultures were activated in the nutrient broth at 37 °C for 24 h. The antibacterial activity was conducted using a double-layer nutrient agar medium. The bottom layer consisted of nutrient agar, while the top layer included 10 mL of a 7.5 g/L nutrient agar mixed with 100 μL of the overnight culture. After solidification, 5 μL of nanoparticles were prepared using a 50% (v/v) aqueous solution of glycerol and placed on the surface at concentrations of 2.5, 5, 10, 20, 30, and 50 mg/mL for each ZnO-AG, ZnO-CH, and ZnO-SS nanoparticle. Additionally, 5 μL of Kanamycin was added as a positive control, and 5 μL of sterile glycerol (50%) served as a negative control. The drops were allowed to dry, and the Petri dishes were incubated at 37 °C for 48 h. The experiment was conducted in triplicate for each treatment, and the inhibition zone (IZ) was recorded in millimeters. Statistical analysis of the experimental results was performed using ANOVA with a post hoc Tukey HSD test at a 95% confidence level. Statistical significance was marked with asterisks depending on the p-value: * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
3. Results and Discussion
The UV-Vis absorption spectra of the phyto-fabricated ZnO-NPs are shown in Figure 3a. The spectra for ZnO-AG (Agave), ZnO-CH (Chiku), and ZnO-SS (Soursop) display a pronounced absorption edge between 345 and 380 nm of wavelength, characteristic of the semiconductor behavior of ZnO. These results are consistent with previous reports for ZnO-NPs synthesized by both conventional chemical methods and green synthesis approaches [,,,,,,,]. Tauc’s equation was used to calculate the band gap (Eg) energies shown in Figure 3b. The obtained Eg values were 3.08, 3.11, and 3.17 eV for ZnO-AG, ZnO-CH, and ZnO-SS, respectively. The energies of the valence band (EVB) and the conduction band (ECB) were calculated from the obtained Eg values (see Figure 3c). For ZnO-AG, ZnO-CH, and ZnO-SS, the energy values of the valence band in eV were found to be +2.93, +2.94, and +2.97, whereas the energy values of the conduction band in eV were −0.15, −0.16, and −0.20, respectively.
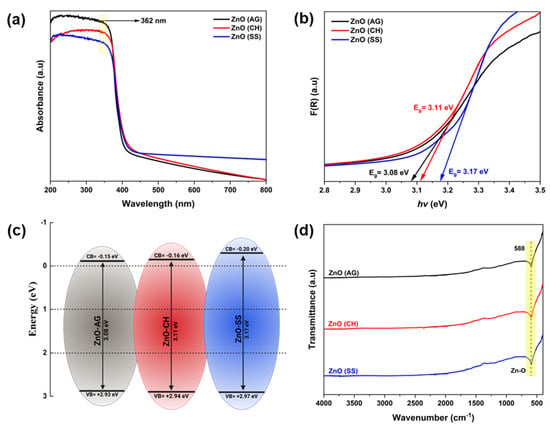
Figure 3.
(a) UV–Vis spectra, (b) bandgap energies, (c) valence and conduction bands energy positions, and (d) FTIR spectra of ZnO-AG, ZnO-CH, and ZnO-SS samples.
ATR-FTIR spectroscopy analysis was employed to identify phytochemical residues after synthesizing ZnO-NPs. The powdered ZnO-AG, ZnO-CH, and ZnO-SS samples were analyzed to detect functional groups or phytochemicals in the synthesized ZnO-NPs samples. These phytochemicals may comprise phenols, carboxylic acids, amines, and ethers. ATR-FTIR spectra of ZnO-AG, ZnO-CH, and ZnO-SS samples can be seen in Figure 3d. A strong absorbance peak at 588 cm−1, which is characteristic of a metal-oxygen bond, confirms the presence of ZnO [,]. In the FTIR spectra, no other absorbance peaks are observed that noticeably reveal the presence of functional groups.
The XRD patterns of the phytofabricated ZnO-NPs showed their highly crystalline structure (see Figure 4). The patterns of ZnO-AG, ZnO-CH, and ZnO-SS samples show nine prominent sharp diffraction peaks appearing at 2θ angles of 31.84°, 34.49°, 36.33°, 47.58°, 56.64°, 62.91°, 66.44°, 68.03°, 69.15°, 72.62°, and 77.00°, corresponding to the lattice planes (100), (002), (101), (102), (110), (103), (200), (112), (201), (004) and (202), respectively. The peaks agree with the JCPDS Card No. 96-210-7060, confirming the structure of ZnO-NPs as hexagonal wurtzite with a specific space group P63mc. The XRD patterns are also quite comparable with previous reports. In addition, the average crystallite size of ZnO-NPs was calculated by using Debye–Scherrer’s equation (Equation (1)):
where K is a shape factor commonly assumed to be 0.9, and λ is the wavelength of the X-ray source CuKα (1.5406 Å). The full width at half-maximum (FWHM) of the most intense diffraction peak is represented as (β) in radians. The Bragg’s diffraction angle is denoted by θ. The phytofabricated ZnO-NPs synthesized with the three different fruit extracts were calcined at a temperature of 400 °C for 3 h. The crystallite size (D) was calculated to be 26.73 nm, 15.47 nm, and 28.06 nm for the ZnO-AG, ZnO-CH, and ZnO-SS, respectively.
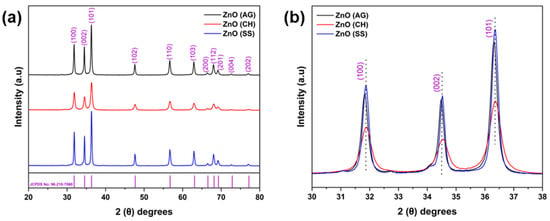
Figure 4.
(a) XRD patterns of the ZnO-AG, ZnO-CH, and ZnO-SS powdered samples of ZnO nanoparticles. The diffraction patterns are consistent with the wurtzite-like hexagonal structure of ZnO. (b) An enlarged view of the most intense diffraction peaks for the three samples reveals no peak shifting and indicates lower crystallinity in the ZnO-CH sample, as evidenced by the reduced peak intensities. Miller indices for each family of crystallographic planes are shown above their corresponding diffraction peaks.
High-resolution scanning electron microscopy (HRSEM) was used to analyze the surface morphologies of the prepared samples. The HRSEM micrographs in Figure 5 illustrate that the ZnO-NPs are well formed, with the bulk having a quasi-spherical shape. Interestingly, some particles persist in a clear hexagon shape that can be easily seen from the micrographs. These images also revealed the presence of agglomeration. The HRSEM study revealed key information about the surface morphology and structure of the phytofabricated ZnO-NPs. The morphology of nanoparticles significantly impacts their efficacy in various applications, as spherical nanoparticles exhibit high potency in antibacterial activity due to their capacity to effortlessly penetrate the cell wall of pathogens. Synthesized ZnO-NPs from Agave, Chiku, and Soursop extracts can be of immense significance in treating clinical pathogens.
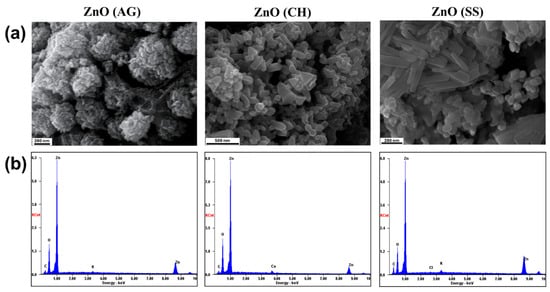
Figure 5.
(a) Surface morphology observed by HRSEM. The images reveal a predominantly quasi-spherical particle morphology across all three samples, with some rod-like and a few triangular-shaped particles also observed. (b) EDS results showing the elemental composition of the synthesized ZnO-AG, ZnO-CH, and ZnO-SS samples. It is noticed that Zn and O are the main constituents in all cases.
The EDS analysis was used to determine the elemental composition of the synthesized ZnO-NPs. As shown in Figure 5b, the presence of zinc (Zn) and oxygen (O) in all obtained spectra confirmed this for all phytofabricated ZnO-NPs. The ZnO-NPs element characterization revealed atomic percentages of 34.72% zinc and 36.34% oxygen, 42.18% zinc and 42.75% oxygen as well as 36.27% zinc and 37.32% oxygen for ZnO-AG, ZnO-CH, and ZnO-SS, respectively, which for all samples is a close 1:1 atomic ratio between zinc and oxygen, thus confirming the formation of ZnO. The samples were attached to carbon tape for analysis, so the carbon content detected by EDS was considered for the total atomic concentration. The EDS graph also showed trace concentrations of Potassium (K = 0.85%) in ZnO-AG, calcium (Ca = 2.36%) in ZnO-CH, and chlorine (Cl = 0.21%) in ZnO-SS. These elements are likely introduced by the various phytochemicals present in the fruit extracts of Agave, Chiku, and Soursop [,,] and might be present as residues from the precursors. However, their concentrations are too low to significantly affect the crystal structure or semiconductor properties of ZnO, as confirmed by XRD analysis, which shows no alteration in the ZnO phase composition, and UV-Vis results that show the light absorption in the typical region for ZnO, as discussed previously.
STEM analysis was used for a deeper study of the nanoparticle’s morphology, aiming to reveal a more detailed shape and size of the phytofabricated ZnO-NPs. STEM micrographs support the formation of particles mostly with quasi-spherical shape that tend to form agglomerates, as previously observed by HRSEM. However, STEM also reveals the formation of some elongated rod-shaped or triangular-shaped particles. This opens the possibility that the natural extracts used in this work for the phytofabrication of ZnO-NPs also allow the formation of particles with a particular morphology where some crystallographic planes are exposed on the surface. This is important since, as mentioned above, not only the size of the nanoparticles but also their morphology directly affects the nanomaterial’s properties and their performance in potential applications, for instance, applications where an antibacterial activity is required. Achieving the formation of rod-shaped or triangular-shaped particles as the primary morphology requires further investigation of the synthesis conditions, which were not explored in the present study but are proposed as a future research direction. For example, parameters such as pH, reaction time, and calcination temperature have been reported as key factors that influence the size and shape of ZnO nanoparticles [,,,,].
To determine the average nanoparticle size for the three fabricated samples, STEM images were used, measuring the particle size directly using the microscope software during observation. At least 40 quasi-spherical particles were counted in each sample, as this was the predominant shape in all three samples. It is important to mention that particles with triangular morphology were excluded from this count, as these were observed to be considerably larger than the quasi-spherical particles. Bar-shaped particles were not counted, as these present two possible measurement dimensions: length and width. Thus, by considering only particles with a quasi-spherical morphology, a single measurement is obtained for each sample, which would be the diameter. The same particle morphology is contemplated for all three synthesized samples, thus allowing for a more objective comparison of the effect of each extract on nanoparticle size. Based on the above, it was determined that the average size of the nanoparticles measured from the STEM images was 15.94 nm for the ZnO-AG samples, 18.08 nm for the ZnO-CH, and 23.32 nm for the ZnO-SS samples, as shown in the scheme in Figure 6d.
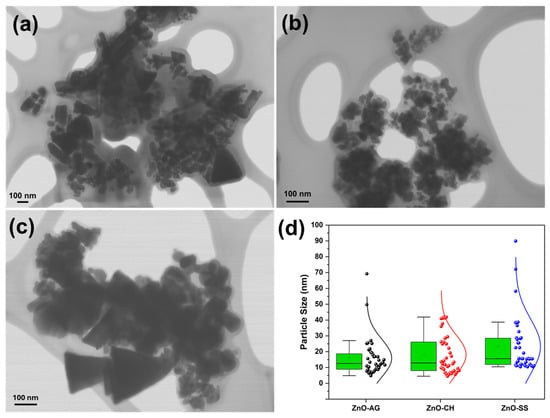
Figure 6.
Micrographies from STEM for (a) ZnO-AG, (b) ZnO-CH, and (c) ZnO-SS. The scale in all images is 100 nm. (d) NPs size distribution for the three samples, in which an asymmetric normal distribution is observed. Inside each green box, the black line shows the median, while the small square represents the average.
This diagram of Figure 6d shows an asymmetric normal distribution for samples prepared with three different extracts, exhibiting positive skewness, with a longer tail on the right (indicating considerably larger values than the mean). Indeed, particle measurements significantly larger than the average were recorded, particularly for the Soursop and Agave extracts. Due to this skew caused by extreme values, the median is a more representative measure of the typical particle size for each extract. The median values recorded were 12.53 nm, 12.87 nm, and 15.59 nm for the Agave, Chiku, and Soursop extracts, respectively, as indicated by the horizontal black line within each colored box Since electron microscopy is a semi-quantitative technique to measure particle size, we will take into account, for subsequent discussion of results, the average particle size (represented by the square inside the colored boxes), which accounts for extreme values and can reflect the overall size distribution. This is particularly relevant for the Agave extract, where the narrower vertical bars indicate a more homogeneous size range for the quasi-spherical particles measured.
Several studies report on the phytosynthesis of ZnO nanoparticles using a wide variety of plants. The use of plants is not focused on just one specific part of them, as the studies have included the use of stems, fruits, in some cases the aerial parts of the plants have been used, and in the great majority of the studies, special attention is paid to the use of the leaves, as shown in the comparative graph in Figure 7. This summarized analysis shows that the size of the ZnO nanoparticles obtained by phytosynthesis varies in a range of about 30 to 100 nm. In this work, the average size of nanoparticles obtained by using the extracts of Agave, Chiku, and Soursop falls in a range of 15–24 nm, which represents an important advantage of the method used here. As previously mentioned, the fundamental part of the development of nanotechnology lies in the interesting and remarkable properties that nanomaterials show at the nanoscale, so that the size of these becomes a relevant parameter for their potential applications.
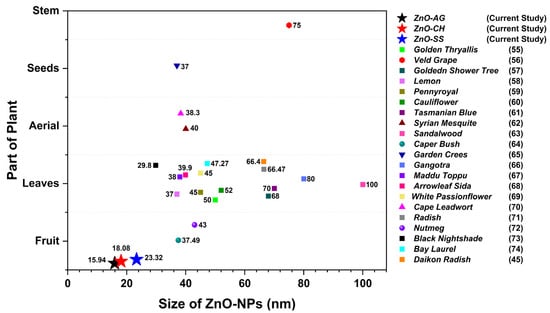
Figure 7.
Comparative graph of phyto-assisted ZnO-NPs and their size using different natural extracts as reported in the literature [,,,,,,,,,,,,,,,,,,,,]. For the results of the present work, average particle size values are considered.
Given these results, the use of the natural extracts reported here from Agave, Chiku, and Soursop is presented as a promising method for the synthesis of ZnO-NPs, not only with nanometric size but with the possibility of synthesizing nanoparticles with a particular rod-shaped or triangular-shaped morphology. Although achieving these particular morphologies requires a more in-depth study of the synthesis conditions, it is clear that the results achieved so far show the production of ZnO nanoparticles with high crystallinity and smaller particle size than reported in the literature, including other green methods, such as the assisted egg white biogenic synthesis through which hexagonal, parallelepiped, asymmetric, or rhomboid particles were obtained with sizes between 0.1 and 3.0 µm []. Hence, our approach achieves important features like high crystallinity and small particle size of ZnO nanoparticles by implementing a methodology that is environmentally friendly, straightforward, and quick to perform.
3.1. Antibacterial Activity
After an incubation period of 24 h at 37 °C, the IZ were measured, and the results are summarized in Table 1. Kanamycin served as a positive control for the bacterial strains in each Petri dish, creating a distinct IZ against both selected strains of the bacteria with varying IZ diameters, whereas the negative control showed no inhibition. The promising results for all phytofabricated NPs were observed at the highest concentration (50 μg/mL) of ZnO-AG, ZnO-CH, and ZnO-SS, respectively, against bacterial strain S. aureus and E. coli, as shown in Figure 8. All three phyto-synthesized ZnO-NPs showed remarkable bactericidal properties against the bacterial strains investigated at various concentrations.

Table 1.
Inhibition zones of ZnO-AG, ZnO-CH, and ZnO-SS against the selected bacterial species with different concentrations.
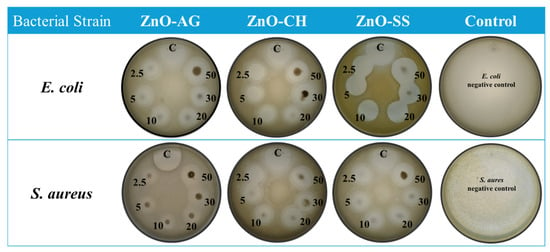
Figure 8.
Antibacterial activity of phyto-assisted nanomaterials against the E. coli and S. aureus species of bacteria.
3.1.1. Antibacterial Activity of ZnO-AG NPs
The ZnO-AG NPs exhibited excellent antibacterial activity against both E. coli and S. aureus. The IZ increased with increasing NPs concentration, showing a dose-dependent antibacterial effect. At the highest concentration (50 µg/mL), ZnO-AG showed maximum IZ with 22.03 ± 0.10 mm for E. coli and 19.06 ± 0.75 mm for S. aureus, while at the lowest concentration (2.5 µg/mL), the IZ for E. coli and S. aureus were 19.71 ± 0.27 mm and 14.58 ± 0.22 mm, respectively.
The remarkable antibacterial efficiency of ZnO-AG can be attributed to its unique physicochemical properties, particularly its high surface area, which is indirectly evidenced by the smaller particle size obtained among the three extracts used in this work. The reduced size of the nanoparticles enhances the ZnO reactivity by improving their interaction with bacterial cells, which disrupts cell membranes and leads to oxidative stress and cell death [,,]. The strong antibacterial activity, especially against E. coli, suggests its potential as a viable alternative to conventional antibiotics. Furthermore, the ability of ZnO-AG to maintain effective inhibition against S. aureus highlights its broad-spectrum antibacterial efficacy.
3.1.2. Antibacterial Activity of ZnO-CH NPs
Similar to ZnO-AG, ZnO-CH also exhibited antibacterial properties against E. coli and S. aureus, showing a concentration-dependent increase in IZ. The IZ for E. coli increased from 17.67 ± 0.17 mm at 2.5 µg/mL to 20.02 ± 1.41 mm at 50 µg/mL, while for S. aureus, the IZ ranged from 13.72 ± 0.14 mm at the lowest concentration to 17.43 ± 0.56 mm at the highest concentration. The antibacterial activity of ZnO-CH was lower than that of ZnO-AG but remained effective in inhibiting bacterial growth. ZnO-CH exhibited a higher IZ against E. coli than S. aureus, consistent with the trend observed for ZnO-AG.
Compared to ZnO-AG, ZnO-CH exhibited slightly lower antibacterial efficacy, although the observed reduction in antibacterial activity compared to ZnO-AG may be due to differences in NPs size, morphology, surface charge, and interactions with bacterial membranes []. Additionally, ZnO-CH may exhibit a slower release of Zn2+ ions [] and reduced reactive oxygen species (ROS) generation [], which are critical factors in bacterial cell membrane disruption and cytotoxicity []. Despite this, ZnO-CH displayed significant antibacterial potential, highlighting its possible application in biomedical and antimicrobial treatments.
3.1.3. Antibacterial Activity of ZnO-SS NPs
ZnO-SS displayed consistent antibacterial activity, with IZ for E. coli ranging from 16.86 ± 0.07 mm at 2.5 μg/mL to 19.85 ± 0.49 mm at 50 μg/mL, while for S. aureus, IZ varied from 12.46 ± 0.93 mm to 17.23 ± 0.86 mm. The lower efficacy of ZnO-SS as compared to ZnO-AG and ZnO-CH may also be attributed to differences in NPs size and aggregation behavior, which could limit its interaction with bacterial cells and reduce ROS production []. The observed trend aligns with previous reports indicating that Gram-positive bacteria tend to be more resistant to ZnO NPs due to their thicker peptidoglycan layer [,]. Although ZnO-SS exhibited increased IZ with an increase in concentration, its efficacy against both strains and its reliable antimicrobial activity highlight its potential for widespread applications.
Based on the results from the antibacterial activity experiments, Figure 9 graphically illustrates the efficacy of ZnO-AG, ZnO-CH, and ZnO-SS concentrations against both bacterial strains. As mentioned before, all synthesized ZnO-NPs effectively inhibited bacterial growth, demonstrating strong antimicrobial potential. Significant differences were primarily observed in the inhibition of E. coli, where the ZnO sample synthesized using the agave extract exhibited consistently larger zones of inhibition compared to the other ZnO samples across all tested concentrations. These differences are likely attributable to variations in the structural properties of the nanoparticles, influenced by the distinct phytochemical compositions of each plant extract. As previously discussed in the microscopy results, these factors are key in determining nanoparticle morphology.
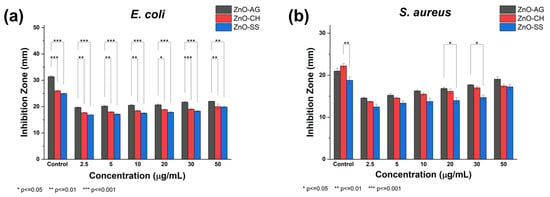
Figure 9.
Antibacterial activity of ZnO-AG, ZnO-CH, and ZnO-SS in the inhibition growth of the following bacterial strains: (a) E. coli and (b) S. aureus when the concentration of ZnO-NPs is varied. The data are presented as mean ± standard deviation (SD) for six different concentrations and the positive control (kanamycin). * p < 0.05, ** p < 0.01, and *** p < 0.001.
Nonetheless, the overall antimicrobial activity of the phytosynthesized materials reported here can be primarily attributed to their ability to generate reactive oxygen species (ROS) and release Zn2+ ions, both of which exert cytotoxic effects on bacterial cells. Zn2+ ions interact with bacterial cell membranes, disrupt critical cellular functions, and induce programmed cell death (apoptosis) through oxidative stress pathways, as has been widely reported for ZnO nanomaterials [,,,,,,,]. The dose-dependent increase in inhibition zones suggests that higher concentrations of ZnO-NPs enhance bacterial membrane disruption and oxidative stress induction. E. coli generally showed higher susceptibility than S. aureus, likely due to structural differences between Gram-positive and Gram-negative bacterial cell walls.
Kanamycin, an aminoglycoside antibiotic, exerts its antimicrobial action by inhibiting bacterial protein synthesis by binding to the 30S ribosomal subunit. Recently, its ability to inhibit cyclopropane fatty acid (CFA) synthesis in the cell membrane of Halomonas socia has also been reported [,], reinforcing its efficacy as a microbial growth inhibitor. In this study, the presence of well-defined inhibition zones in both control strains confirms adequate diffusion of kanamycin into the culture medium, which is essential for an accurate assessment of its antibacterial potency. Regarding ZnO-AG, ZnO-CH, and ZnO-SS treatments, broad inhibition zones were observed, reaching maximum diameters at a 50 μg/mL concentration. The effect was more pronounced in Escherichia coli than in Staphylococcus aureus, which may be attributed to differences in the cell wall structure of S. aureus and to intrinsic or adaptive resistance mechanisms that could decrease the efficacy of antimicrobial agents []. Although the nanoparticles demonstrated inhibitory activity against bacterial growth, this was lower than that of kanamycin. This difference may be explained by the greater aqueous solubility of the antibiotic, which favors its diffusion in the medium, in contrast to the limited mobility of the nanoparticles. Consequently, nanoparticles present a smaller effective contact area with the bacteria, without necessarily implying a decrease in their antimicrobial potential.
The escalating threat of multidrug-resistant bacterial strains has created an urgent demand for developing the next-generation, highly potent antibacterial agents. ZnO-NPs have distinguished themselves as remarkably stable and highly efficacious antimicrobial agents capable of dismantling vital bacterial cell components through many powerful, targeted mechanisms and still exhibiting negligible cytotoxicity to humans and animals [].
The plant-mediated synthesis of ZnO-NPs using extracts from Agave (Agave americana), Chiku (Manilkara zapota), and Soursop (Annona muricata) offers significant advantages over conventional chemical methods. This environmentally friendly approach eliminates the need for toxic agents, minimizes environmental hazards, and is cost-effective due to the abundance and low cost of plant resources. Moreover, it supports sustainability by utilizing renewable bioresources and enhancing biocompatibility, making these NPs highly suitable for biomedical and pharmaceutical applications.
Furthermore, plant-mediated synthesis can potentially enhance the fabricated NPs’ therapeutic efficacy by incorporating bioactive phytochemicals with intrinsic medicinal properties. However, several challenges are associated with phytosynthesis approaches. The inherent variability in the phytochemical composition of plant extracts can lead to inconsistencies in the physicochemical properties of the resulting NPs, thereby affecting reproducibility and limiting the ability to precisely control NPs size, morphology, and surface characteristics. Additionally, phyto-assisted methods may result in lower NPs yields and slower reaction kinetics compared to conventional chemical synthesis. Despite these limitations, reaction optimization through carefully regulating reaction parameters such as temperature, pH, concentration of precursor salt, and plant extract composition can significantly improve the consistency, yield, and functional properties of phytosynthesized NPs.
4. Conclusions
The present work shows a green-synthesis route to obtain ZnO-NPs from pulp extracts of Agave (Agave americana), Chiku (Manilkara zapota), and Soursop (Annona muricata). The results exhibited that the natural extracts were effective as reduction, capping, and stabilizing agents, which resulted in the successful synthesis of ZnO nanoparticles from the precursor salt, zinc acetate. According to XRD analysis, all phytosynthetized materials were formed with a highly crystalline hexagonal structure. HRSEM evidenced that the ZnO-NPs primarily exhibit a quasi-spherical shape with sizes that range from 15 to 24 nm, having the smallest average size in the synthesis in which the agave extract was used. Furthermore, the use of STEM revealed the existence of some particles with rod-like or triangular-like morphology, which suggests that the extracts used here not only enable the synthesis of ZnO in nanometric sizes smaller than those reported with other phyto-assisted methods, but also that the particles can be fabricated with a particular morphology, which could provide them with different interesting properties. All synthesized ZnO-NPs effectively inhibited bacterial growth against E. coli and S. aureus. Their antibacterial activity varied with the type of plant extract used for phytofabrication and the concentration of NPs. The agave-derived phytosynthesized material (ZnO-AG) exhibited the highest antibacterial performance, likely due to its geometric characteristics, namely size and shape. Optimizing synthesis conditions could further enhance its antibacterial activity. Given the urgent call to care for the environment, the synthesis reported here represents a sustainable and environmentally friendly method for the manufacture of ZnO-NPs that eliminates the use of hazardous chemicals while still producing a high-quality material that meets the basic requirements sought in nanomaterials, specifically size, shape, and crystallinity, with potential applications as antimicrobial agents.
Author Contributions
Conceptualization, L.M.L.; methodology, G.M.C., A.B. and J.E.-S.; validation, D.E.N.-L., E.R.L.-M. and A.L.S.-L.; formal analysis, G.M.C., D.E.N.-L., E.R.L.-M., A.L.S.-L. and L.M.L.; investigation, G.M.C., J.I.-E., A.O.S., D.V.M.-M., A.B., J.E.-S. and D.E.N.-L.; resources, A.L.S.-L. and L.M.L.; writing—original draft preparation, G.M.C.; writing—review and editing, D.E.N.-L., E.R.L.-M. and L.M.L.; visualization, G.M.C. and L.M.L.; supervision, A.L.S.-L. and L.M.L.; project administration, L.M.L.; funding acquisition, L.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been partially funded by the Challenge-Based Research Funding Program of Tecnológico de Monterrey, grant number E090-EIC-GI04-A-T3-E.
Data Availability Statement
Data are contained within the article.
Acknowledgments
G.M.C.: A.B., and J.E.-S. thank (CONAHCYT) and Tecnologico de Monterrey for the graduate student fellowships.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bhardwaj, A.; Sharma, G.; Gupta, S. Nanotechnology Applications and Synthesis of Graphene as Nanomaterial for Nanoelectronics; Springer: Cham, Switzerland, 2020; pp. 251–269. [Google Scholar]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M.J.A. Green synthesis and characterization of ZnO nanoparticles using Pelargonium odoratissimum (L.) aqueous leaf extract and their antioxidant, antibacterial and anti-inflammatory activities. Antioxid. J. Abbr. 2022, 11, 1444. [Google Scholar] [CrossRef]
- Nilavukkarasi, M.; Vijayakumar, S.; Prathipkumar, S. Capparis zeylanica mediated bio-synthesized ZnO nanoparticles as antimicrobial, photocatalytic and anti-cancer applications. Mater. Sci. Energy Technol. 2020, 3, 335–343. [Google Scholar] [CrossRef]
- Bekele, S.G.; Ganta, D.D.; Endashaw, M. Green synthesis and characterization of zinc oxide nanoparticles using Monoon longifolium leave extract for biological applications. Discov. Chem. 2024, 1, 5. [Google Scholar] [CrossRef]
- Arshad, R.; Hassan, D.; Sani, A.; Mustafa, G.; Rahdar, A.; Fathi-karkan, S.; Kharaba, Z.; Medina, D.I.; Pandey, S. Nano-engineered solutions for ibuprofen therapy: Unveiling advanced co-delivery strategies and nanoparticle systems. J. Drug. Deliv. Sci. Technol. 2024, 98, 105815. [Google Scholar] [CrossRef]
- Ahmad, W.; Kalra, D. Green synthesis, characterization and anti microbial activities of ZnO nanoparticles using Euphorbia hirta leaf extract. J. King Saud Univ. Sci. 2020, 32, 2358–2364. [Google Scholar] [CrossRef]
- Mustafa, G.; Hassan, D.; Ruiz-Pulido, G.; Pourmadadi, M.; Eshaghi, M.M.; Behzadmehr, R.; Tehrani, F.S.; Rahdar, A.; Medina, D.I.; Pandey, S.; et al. Nanoscale drug delivery systems for cancer therapy using paclitaxel—A review of challenges and latest progressions. J. Drug Deliv. Sci. Technol. 2023, 84, 104494. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Hayat, Z.; Zhang, D.-D.; Li, M.-Y.; Hu, S.; Wu, Q.; Cao, Y.-F.; Yuan, Y.J.P. Zinc oxide nanoparticles: Synthesis, characterization, modification, and applications in food and agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- PD, D.A.; Plashintania, D.R.; Putri, R.M.; Wibowo, I.; Ramli, Y.; Herdianto, S.; Indarto, A. Synthesis of zinc oxide nanoparticles using methanol propolis extract (Pro-ZnO NPs) as antidiabetic and antioxidant. PLoS ONE 2023, 18, e0289125. [Google Scholar]
- Khoza, P.B.; Moloto, M.J.; Sikhwivhilu, L. The effect of solvents, acetone, water, and ethanol, on the morphological and optical properties of ZnO nanoparticles prepared by microwave. J. Nanotechnol. 2012, 2012, 195106. [Google Scholar] [CrossRef]
- Pham, T.A.T.; Tran, V.A.; Le, V.D.; Nguyen, M.V.; Truong, D.D.; Do, X.T.; Vu, A.-T. Facile preparation of ZnO nanoparticles and Ag/ZnO nanocomposite and their photocatalytic activities under visible light. Int. J. Photoenergy 2020, 2020, 8897667. [Google Scholar] [CrossRef]
- Fei, X.Y.; Liu, T.Z.; Zhang, H.; Xu, X.M.; Duo, S.W. Synthesis of microstructured ZnO in hydrazine hydrate using a hydrothermal method and its optical properties. Adv. Mat. Res. 2014, 1015, 684–687. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, U.; Awasthi, R.; Shukla, R.; Kumar, I.; Naik, R.M.; Mishra, D. CTAB mediated synthesis of ZnO nanoparticles: Structural, optical and enhanced blue-green optical emission. Mater. Today. Proc. 2021, 46, 2229–2234. [Google Scholar] [CrossRef]
- Pusuwan, P.; Siripinyanond, A. Observing zinc oxide nanoparticles suspension stability in various media by using single particle inductively coupled plasma mass spectrometry (SP-ICP-MS). Microchem. J. 2024, 196, 109705. [Google Scholar] [CrossRef]
- Swathi, S.; Yuvakkumar, R.; Ravi, G.; Shanthini, M.; Al-Sehemi, A.G.; Thambidurai, M.; Nguyen, H.D.; Velauthapillai, D. Effect of sodium dodecyl sulfate surfactant concentrations on the novel strontium copper oxide nanostructures for enriching hydrogen evolution reaction electrochemical activity in alkaline solution. J. Alloys. Compd. 2022, 928, 167001. [Google Scholar] [CrossRef]
- Pati, P.; McGinnis, S.; Vikesland, P. Life cycle assessment of “green” nanoparticle synthesis methods. Environ. Eng. Sci. 2014, 31, 410–420. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Chenoweth, J.A.; Bebarta, V.S.; Albertson, T.E.; Nowadly, C.D. The Toxicity, pathophysiology, and treatment of acute hydrazine propellant exposure: A systematic review. Mil. Med. 2021, 186, e319–e326. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Environmental behaviour and ecotoxicity of cationic surfactants towards marine organisms. J. Hazard. Mater. 2020, 392, 122299. [Google Scholar] [CrossRef]
- Feng, M.; Xu, Z.; Yin, D.; Zhao, Z.; Zhou, X.; Song, L. Toxic effects of sodium dodecyl sulfate on planarian Dugesia japonica. Peer J. 2023, 11, e15660. [Google Scholar] [CrossRef]
- Jho, E.H.; Yun, S.H.; Thapa, P.; Nam, J.-W. Changes in the aquatic ecotoxicological effects of Triton X-100 after UV photodegradation. Environ. Sci. Pollut. Res. 2021, 28, 11224–11232. [Google Scholar] [CrossRef]
- Rahman, F.; Majed Patwary, M.A.; Bakar Siddique, M.A.; Bashar, M.S.; Haque, M.A.; Akter, B.; Rashid, R.; Haque, M.A.; Royhan Uddin, A. Green synthesis of zinc oxide nanoparticles using Cocos nucifera leaf extract: Characterization, antimicrobial, antioxidant and photocatalytic activity. R. Soc. Open Sci. 2022, 9, 220858. [Google Scholar] [CrossRef]
- Ramesh, P.; Saravanan, K.; Manogar, P.; Johnson, J.; Vinoth, E.; Mayakannan, M. Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sens. Biosens. Res. 2021, 31, 100399. [Google Scholar] [CrossRef]
- Zeghoud, S.; Hemmami, H.; Seghir, B.B.; Amor, I.B.; Kouadri, I.; Rebiai, A.; Messaoudi, M.; Ahmed, S.; Pohl, P.; Simal-Gandara, J. A review on biogenic green synthesis of ZnO nanoparticles by plant biomass and their applications. Mater. Today Commun. 2022, 33, 104747. [Google Scholar] [CrossRef]
- Salahuddin, N.; Awad, S. Optimization delivery of 5-fluorouracil onto different morphologies of ZnO NPs: Release and functional effects against colorectal cancer cell lines. Chem Papers 2021, 75, 4113–4127. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Anwar, M.M.; Saeed, H. Nanomaterials for application in wound Healing: Current state-of-the-art and future perspectives. J. Polym. Res. 2022, 29, 91. [Google Scholar] [CrossRef]
- Hameed, H.; Waheed, A.; Sharif, M.S.; Saleem, M.; Afreen, A.; Tariq, M.; Kamal, A.; Al-Onazi, W.A.; Al Farraj, D.A.; Ahmad, S. Green synthesis of zinc oxide (ZnO) nanoparticles from green algae and their assessment in various biological applications. Micromachines 2023, 14, 928. [Google Scholar] [CrossRef]
- Naiel, B.; Fawzy, M.; Halmy, M.W.A.; Mahmoud, A.E.D. Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz.) extract: Characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci. Rep. 2022, 12, 20370. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, S.; Sheraz, S.; Shams, D.F.; Rehman, G.; Nayab, S.; Shah, M.I.A.; Ateeq, M.; Shah, S.K.; Ahmad, T.; Shams, S. Biosynthesis and Anti-inflammatory Activity of Zinc Oxide Nanoparticles Using Leaf Extract of Senecio chrysanthemoides. Biomed. Res. Int. 2023, 2023, 3280708. [Google Scholar] [CrossRef]
- Al-Ajmi, M.F.; Hussain, A.; Ahmed, F. Novel synthesis of ZnO nanoparticles and their enhanced anticancer activity: Role of ZnO as a drug carrier. Ceram. Int. 2016, 42, 4462–4469. [Google Scholar] [CrossRef]
- Chao, S.; Zhang, Y.; Cheng, S.; Shao, X.; Liu, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Ibuprofen-loaded ZnO nanoparticle/polyacrylonitrile nanofibers for dual-stimulus sustained release of drugs. ACS Appl. Nano Mater. 2023, 6, 5535–5544. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Shamsabadipour, A.; Bhatti, A.; Forouzanfar, M.; Rajabnejad, M.; Behzadmehr, R.; Rahdar, A.; Medina, D.I.; Díez-Pascual, A.M. Therapeutic performance of temozolomide-loaded nanomaterials: A state-of-the-art. J. Drug Deliv. Sci. Technol. 2023, 85, 104568. [Google Scholar] [CrossRef]
- Blinov, A.V.; Kachanov, M.D.; Gvozdenko, A.A.; Nagdalian, A.A.; Blinova, A.A.; Rekhman, Z.A.; Golik, A.B.; Vakalov, D.S.; Maglakelidze, D.G.; Nagapetova, A.G. Synthesis and characterization of zinc oxide nanoparticles stabilized with biopolymers for application in wound-healing mixed gels. Gels 2023, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.J.; Mccall, M. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef]
- Rivas-Gastelum, M.F.; Garcia-Amezquita, L.E.; Garcia-Varela, R.; Sánchez-López, A.L. Manilkara zapota “chicozapote” as a fruit source of health-beneficial bioactive compounds and its effects on chronic degenerative and infectious diseases, a review. Front. Nutr. 2023, 10, 1194283. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, I.; Pradita, R.Y.; Nurfalinda, A. Plant extract mediated of ZnO nanoparticles by using ethanol extract of Mimosa pudica leaves and coffee powder. Procedia Eng. 2016, 148, 43–48. [Google Scholar] [CrossRef]
- Faye, G.; Jebessa, T.; Wubalem, T. Biosynthesis, characterisation and antimicrobial activity of zinc oxide and nickel doped zinc oxide nanoparticles using Euphorbia abyssinica bark extract. IET Nanobiotechnol. 2022, 16, 25–32. [Google Scholar] [CrossRef]
- Fagier, M.A. Plant-mediated biosynthesis and photocatalysis activities of zinc oxide nanoparticles: A prospect towards dyes mineralization. J. Nanotechnol. 2021, 2021, 6629180. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Sivakumar, T. Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci. 2014, 6, 461–465. [Google Scholar]
- Commission. IUCN Red List Categories and Criteria; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2001. [Google Scholar]
- Muhammad, W.; Ullah, N.; Haroon, M. Optical morphological biological analysis of zinc oxide nanoparticles (ZnONPs) using Papaver somniferum L. RSC Adv. 2019, 9, 29541–29548. [Google Scholar] [CrossRef]
- Song, Z.; Kelf, T.A.; Sanchez, W.H.; Roberts, M.S.; Rička, J.; Frenz, M.; Zvyagin, A.V. Characterization of optical properties of ZnO nanoparticles for quantitative imaging of transdermal transport. Biomed. Opt. Express 2011, 2, 3321–3333. [Google Scholar] [CrossRef]
- Singh, D.; Pandey, D.; Yadav, R.; Singh, D. A study of nanosized zinc oxide and its nanofluid. Pramana J. Phys. 2012, 78, 759–766. [Google Scholar] [CrossRef]
- Singh, S.; Gade, J.V.; Verma, D.K.; Elyor, B.; Jain, B. Exploring ZnO nanoparticles: UV–visible analysis and different size estimation methods. Opt. Mater. (Amst.) 2024, 152, 115422. [Google Scholar] [CrossRef]
- Jayachandran, A.; Aswathy, T.; Nair, A.S. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef]
- Umamaheswari, A.; Prabu, S.L.; John, S.A.; Puratchikody, A. Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. Longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol. Rep. (Amst.) 2021, 29, e00595. [Google Scholar]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; HM Abd El-Azim, M. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, G.M.; Ahmed, A.B.; Al-Zubaidi, A.B. Synthesis, characterization, and the influence of energy of irradiation on optical properties of ZnO nanostructures. Sci. Rep. 2022, 12, 20016. [Google Scholar] [CrossRef]
- Arreola Tostado, J.M.; Montoya Jasso, V.M.; Arreola Nava, J.M.; Castillo Valdez, X.; Olivares Arreola, E.A.; Báez Pérez, A. Efecto de la aplicación de levasa (mosto de caña de azúcar) en la producción y calidad de Agave Tequilana Weber. Rev. Mexicana Cienc. Agric. 2020, 11, 1311–1324. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Asghar, A.; Shah, Y.A.; Ikram, A.; Ateeq, H.; Hussain, M.; Ofoedu, C.E.; Chacha, J.S. Nutritional and therapeutic potential of soursop. J. Food Qual. 2022, 2022, 8828358. [Google Scholar] [CrossRef]
- Gherbi, B.; Laouini, S.E.; Meneceur, S.; Bouafia, A.; Hemmami, H.; Tedjani, M.L.; Thiripuranathar, G.; Barhoum, A.; Menaa, F. Effect of pH value on the bandgap energy and particles size for biosynthesis of ZnO nanoparticles: Efficiency for photocatalytic adsorption of methyl orange. Sustainability 2022, 14, 11300. [Google Scholar] [CrossRef]
- Manzoor, U.; Tuz Zahra, F.; Rafique, S.; Moin, M.T.; Mujahid, M. Effect of synthesis temperature, nucleation time, and postsynthesis heat treatment of ZnO nanoparticles and its sensing properties. J. Nanomater. 2015, 2015, 189058. [Google Scholar] [CrossRef]
- Toscano, M.A.P.; Peña, R.S.; Velasco, M.R.; Montalvo, J.J.I.; Guzmán, J.A.A.; Cuenca, S.L. Effect of reaction conditions on particle size of ZNO nanoparticles via controlled precipitation method and in-vitro antibacterial capacity. Quim. Nova 2022, 45, 901–905. [Google Scholar] [CrossRef]
- Sani, A.; Murad, A.; Hassan, D.; Channa, G.M.; El-Mallul, A.; Medina, D.I. Photo-catalytic and biomedical applications of one-step, plant extract-mediated green-synthesized cobalt oxide nanoparticles. Environ. Sci. Pollut. Res. Int. 2023, 30, 20736–20745. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D.; Sani, A.; Antonio Pérez, A.; Ehsan, M.; Hernández-Varela, J.D.; Chanona-Pérez, J.J.; Torres Huerta, A.L. The Impact of Nickel–Zinc Ferrite Nanoparticles on the Mechanical and Barrier Properties of Green-Synthesized Chitosan Films Produced Using Natural Juices. Polymers 2024, 16, 3455. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; lochan Mohanty, D.; Divya, N.; Bakshi, V.; Mohanty, A.; Rath, D.; Das, S.; Mondal, A.; Roy, S.; Sabui, R. A critical review on zinc oxide nanoparticles: Synthesis, properties and biomedical applications. Intell. Pharm. 2024, 3, 53–70. [Google Scholar] [CrossRef]
- Sathappan, S.; Kirubakaran, N.; Gunasekaran, D.; Gupta, P.K.; Verma, R.S.; Sundaram, J. Green synthesis of zinc oxide nanoparticles (ZnO NPs) using Cissus quadrangularis: Characterization, antimicrobial and anticancer studies. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 289–296. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Hossain, A.; Abdallah, Y.; Ali, M.A.; Masum, M.M.I.; Li, B.; Sun, G.; Meng, Y.; Wang, Y.; An, Q. Lemon-fruit-based green synthesis of zinc oxide nanoparticles and titanium dioxide nanoparticles against soft rot bacterial pathogen Dickeya dadantii. Biomolecules 2019, 9, 863. [Google Scholar] [CrossRef]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar] [CrossRef]
- Manojkumar, U.; Kaliannan, D.; Srinivasan, V.; Balasubramanian, B.; Kamyab, H.; Mussa, Z.H.; Palaniyappan, J.; Mesbah, M.; Chelliapan, S.; Palaninaicker, S. Green synthesis of zinc oxide nanoparticles using Brassica oleracea var. botrytis leaf extract: Photocatalytic, antimicrobial and larvicidal activity. Chemosphere 2023, 323, 138263. [Google Scholar] [CrossRef]
- Shahid, M.; Ijaz, N.; Shahid, B.; Tufail, T.; Ain, H.B.U.; Hussain, M.; Basharat, S.; Ikram, A.; Al Jbawi, E. Eucalyptus globulus Labill. Mediated synthesis of ZnO nanoparticles, their Optimization and characterization. Food. Sci. Technol. 2024, 10, 2293332. [Google Scholar] [CrossRef]
- Miri, A.; Khatami, M.; Ebrahimy, O.; Sarani, M. Cytotoxic and antifungal studies of biosynthesized zinc oxide nanoparticles using extract of Prosopis farcta fruit. Green Chem. Lett. Rev. 2020, 13, 27–33. [Google Scholar] [CrossRef]
- Kavithaa, K.; Paulpandi, M.; Ponraj, T.; Murugan, K.; Sumathi, S. Induction of intrinsic apoptotic pathway in human breast cancer (MCF-7) cells through facile biosynthesized zinc oxide nanorods. Karbala Int. J. Mod. Sci. 2016, 2, 46–55. [Google Scholar] [CrossRef]
- Neamah, S.A.; Albukhaty, S.; Falih, I.Q.; Dewir, Y.H.; Mahood, H.B. Biosynthesis of zinc oxide nanoparticles using Capparis spinosa L. fruit extract: Characterization, biocompatibility, and antioxidant activity. Appl. Sci. 2023, 13, 6604. [Google Scholar] [CrossRef]
- Efati, Z.; Shahangian, S.S.; Darroudi, M.; Amiri, H.; Hashemy, S.I.; Aghamaali, M.R. Green chemistry synthesized zinc oxide nanoparticles in Lepidium sativum L. seed extract and evaluation of their anticancer activity in human colorectal cancer cells. Ceram. Int. 2023, 49, 32568–32576. [Google Scholar] [CrossRef]
- Bhagat, T.; Lokhande, R.; Khadke-Lokhande, L.; Chandorkar, J. Green synthesis, characterization, application and study of antimicrobial properties of zinc oxide nano particles using Cyathocline purpurea phytoextract. Pharma Innov. 2023, 12, 2627–2633. [Google Scholar]
- Kumar, N.H.; Andia, J.D.; Manjunatha, S.; Murali, M.; Amruthesh, K.; Jagannath, S. Antimitotic and DNA-binding potential of biosynthesized ZnO-NPs from leaf extract of Justicia wynaadensis (Nees) Heyne-A medicinal herb. Biocatal. Agric. Biotechnol. 2019, 18, 101024. [Google Scholar]
- Kavya, J.; Murali, M.; Manjula, S.; Basavaraj, G.; Prathibha, M.; Jayaramu, S.; Amruthesh, K. Genotoxic and antibacterial nature of biofabricated zinc oxide nanoparticles from Sida rhombifolia Linn. J. Drug. Deliv. Sci. Technol. 2020, 60, 101982. [Google Scholar] [CrossRef]
- Chunchegowda, U.A.; Shivaram, A.B.; Mahadevamurthy, M.; Ramachndrappa, L.T.; Lalitha, S.G.; Krishnappa, H.K.N.; Anandan, S.; Sudarshana, B.S.; Chanappa, E.G.; Ramachandrappa, N.S. Biosynthesis of Zinc oxide nanoparticles using leaf extract of Passiflora subpeltata: Characterization and antibacterial activity against Escherichia coli isolated from poultry faeces. J. Clust. Sci. 2021, 32, 1663–1672. [Google Scholar] [CrossRef]
- Melk, M.M.; El-Hawary, S.S.; Melek, F.R.; Saleh, D.O.; Ali, O.M.; El Raey, M.A.; Selim, N.M. Nano zinc oxide green-synthesized from Plumbago auriculata lam. alcoholic extract. Plants 2021, 10, 2447. [Google Scholar] [CrossRef]
- Al Awadh, A.A.; Shet, A.R.; Patil, L.R.; Shaikh, I.A.; Alshahrani, M.M.; Nadaf, R.; Mahnashi, M.H.; Desai, S.V.; Muddapur, U.M.; Achappa, S.; et al. Sustainable synthesis and characterization of zinc oxide nanoparticles using Raphanus sativus extract and its biomedical applications. Crystals 2022, 12, 1142. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N. Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: Their characterizations and biological and environmental applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Shobiya, M. Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomed. Pharmacother. 2016, 84, 1213–1222. [Google Scholar] [CrossRef]
- Vijan, E.A.; Modan, E.M.; Moga, S.G.; Negrea, D.A.; Schiopu, A.-G.; Oproescu, M.; Istrate, D. Assisted Egg White Biogenic Synthesis for Elaboration of ZnO Nanoparticles. Crystals 2025, 15, 71. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.J.; Nayef, U.M.; Mutlak, F.A.-H.; Jabir, M.S. Laser-ablated zinc oxide nanoparticles and evaluation of their antibacterial and anticancer activity against an ovarian cancer cell line: In vitro study. Plasmonics 2023, 18, 2091–2101. [Google Scholar] [CrossRef]
- Mendes, A.R.; Granadeiro, C.M.; Leite, A.; Pereira, E.; Teixeira, P.; Poças, F. Optimizing Antimicrobial Efficacy: Investigating the Impact of Zinc Oxide Nanoparticle Shape and Size. Nanomaterials 2024, 14, 638. [Google Scholar] [CrossRef]
- Caron, A.J.; Ali, I.J.; Delgado, M.J.; Johnson, D.; Reeks, J.M.; Strzhemechny, Y.M.; McGillivray, S.M. Zinc oxide nanoparticles mediate bacterial toxicity in Mueller-Hinton Broth via Zn2+. Front. Microbiol. 2024, 15, 1394078. [Google Scholar] [CrossRef]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the mechanism of antibacterial activity of ZnO: Surface defects mediated reactive oxygen species even in the dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Al-Momani, H.; Massadeh, M.I.; Almasri, M.; Al Balawi, D.; Aolymat, I.; Hamed, S.; Albiss, B.A.; Ibrahim, L.; Balawi, H.A.; Al Haj Mahmoud, S. Anti-Bacterial Activity of Green Synthesised Silver and Zinc Oxide Nanoparticles against Propionibacterium acnes. Pharmaceuticals 2024, 17, 255. [Google Scholar] [CrossRef]
- Puspasari, V.; Ridhova, A.; Hermawan, A.; Amal, M.I.; Khan, M.M. ZnO-based antimicrobial coatings for biomedical applications. Bioprocess Biosyst. Eng. 2022, 45, 1421–1445. [Google Scholar] [CrossRef]
- Lundstedt, E.; Kahne, D.; Ruiz, N. Assembly and maintenance of lipids at the bacterial outer membrane. Chem. Rev. 2020, 121, 5098–5123. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver nanoparticles: Bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lin, K.; Cai, M. ZnO nanomaterials: Current advancements in antibacterial mechanisms and applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef]
- Johnson, D.; Reeks, J.M.; Caron, A.; Tzoka, I.; Ali, I.; McGillivray, S.M.; Strzhemechny, Y.M. Influence of surface properties and microbial growth media on antibacterial action of ZnO. Coatings 2022, 12, 1648. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Gigliobianco, M.R.; Di Martino, P. Biogenic zinc oxide nanoparticles as a promising antibacterial agent: Synthesis and characterization. Int. J. Mol. Sci. 2024, 25, 9500. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Wu, S.; Zhou, R.; Zhang, K.; Zhang, Z.; Liu, J.; Qin, S.; Shi, J. Nanomaterial-based zinc ion interference therapy to combat bacterial infections. Front. Inmunol. 2022, 13, 899992. [Google Scholar] [CrossRef]
- Suzuki, J.; Kunimoto, T.; Hori, M. Effects of kanamycin on protein synthesis: Inhibition of elongation of peptide chains. J. Antibiot. 1970, 23, 99–101. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, H.-J.; Kim, B.; Kim, S.-H.; Cho, D.-H.; Jung, H.-J.; Bhatia, S.K.; Choi, K.-Y.; Kim, W.; Lee, J.; et al. Inhibition of cyclopropane fatty acid synthesis in the membrane of halophilic Halomonas socia CKY01 by kanamycin. Biotechnol. Bioprocess Eng. 2022, 27, 788–796. [Google Scholar] [CrossRef]
- Rajput, P.; Nahar, K.S.; Rahman, K.M. Evaluation of antibiotic resistance mechanisms in Gram-positive bacteria. Antibiotics 2024, 13, 1197. [Google Scholar] [CrossRef]
- Akbar, N.; Aslam, Z.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Zinc oxide nanoparticles conjugated with clinically-approved medicines as potential antibacterial molecules. AMB Express 2021, 11, 104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).