Abstract
Solar energy-absorbing and -storing integrated ceramics are a new type of material that absorbs sunlight and stores it as heat energy, with properties such as high absorptivity, high thermal storage density, and high temperature stability. In this study, forsterite ceramics were prepared from fused magnesia, quartz, α-Al2O3, and Sm2O3, and concurrently, two additives of Fe2O3 and CuO were doped to improve the absorptivity, and the effects of the composite additives on the performance of forsterite ceramics were investigated. The results showed that the optimal Fe2O3/CuO content ratio was 8:2, at which time the apparent porosity, bulk density, and thermal storage density of the sample were 0.21%, 3.08 g/cm3, and 1516.71 kJ/kg (1000 °C), respectively. After 30 thermal shock cycles, the precipitation of samarium silicate in the samples resulted in a tighter grain bonding, increased the bending strength by 70.6%, and exhibited excellent thermal shock resistance. The solar absorptivity reached 93.80% in the 0.3–2.5 μm wavelength range. Fe2O3 doping replaced part of the positions of Al3+ in MgAl2O4 to form MgFe0.6Al1.4O4 phase. This replacement caused lattice distortion, which triggered electronic transition and augmented the intrinsic absorption capacity, thereby enhancing the sample’s absorptivity. CuO’s low reflectivity across the spectrum further reduced sample reflectivity.
1. Introduction
Since the 1950s, renewable energy sources like solar, wind, and hydro have been developed to address fossil fuel shortages and environmental issues [1,2]. Solar energy, in particular, has emerged as a promising solution, aligning with dual-carbon goals while offering energy saving and emission reduction benefits [3,4]. However, solar radiation’s low energy density and variability due to location, season, and climate pose challenges for consistent energy supply [5]. To address this, efficient solar energy-absorbing and -storing integrated technology is crucial. This technology can store solar energy when there is sufficient light and release it when needed, ensuring a stable energy supply [6,7,8]. The performance of solar energy-absorbing and -storing integrated materials, which combine thermal absorption and storage functions, directly affects the system’s efficiency and reliability [9,10]. Ideal materials should have high thermal storage density, excellent thermal shock resistance, and high solar absorptivity.
Forsterite is mainly synthesized at high temperatures from materials of magnesium and silicon sources, possessing advantages such as high strength [11] and high temperature stability [12,13,14], and is suitable for high temperature working environments. It is one of the ideal materials for solar thermal storage; for example, the low co-melting point of periclase and forsterite is at 1850 °C. Hence, it is widely used in insulating materials [15], biomaterials [16,17], insulation, and refractory industries [18,19,20,21,22]. M.M.S. Wahsh et al. [23] synthesized forsterite in situ at 1550 °C using high-purity MgO, nanoscale SiO2, and zircon as raw materials; the resulting ceramic samples exhibited high densities and excellent mechanical properties. Israfil Kucuk et al. [24] prepared aluminum titanate (Al2TiO5)-reinforced forsterite (Mg2SiO4) ceramics by the powder metallurgy route and investigated the thermal shock resistance of the samples (1000–25 °C). It was shown that the hardness value of the pure Mg2SiO4 samples decreased by about 40% after thermal shock, whereas the 20 wt% Al2TiO5-reinforced forsterite ceramic composites exhibited good resistance to thermal shock, and the hardness value only decreased by about 11% after thermal shock. Xu et al. [25] prepared forsterite–zirconia composite ceramics by pressureless sintering, using fused magnesia, quartz, and 3Y-ZrO2 as raw materials. The results showed that the sample sintered at 1600 °C exhibited optimal performance, with bending strength; specific heat capacity; and thermal storage density of 83.66 MPa, 1.09 J/(g·K), and 1062.36 kJ/kg, respectively. After 30 thermal shock cycles (1000–25 °C, air-cooled), the sample displayed a good high-temperature stability, with a strength loss of only 9.73%. Wu et al. [26] prepared MgO-Mg2SiO4 refractories using low-grade magnesite tailings as the main raw material and silica fume and quicklime as additives. It was shown that the residual compressive strength of the sample with additives calcium–silicate ratio of 5/5 increased by a maximum of 29.75%, and the residual flexural strength increased by a maximum of 11.84%, compared with the sample without additives. Based on the above discussion, this ceramic material not only has the advantage of high strength but also has good thermal shock resistance, so it is able to adapt to the challenging working environment of solar thermal power receivers and is suitable to be used as a thermal storage material for solar thermal power generation. However, few studies have investigated forsterite-based ceramics with excellent thermal shock resistance (with improved strength after thermal shock), and few in-depth discussions have been conducted on the mechanisms for improving the thermal shock resistance of forsterite-based ceramics.
The high-temperature performance of forsterite meets the requirements for thermal storage materials in solar thermal power generation technology; however, the solar energy absorptivity of forsterite is comparatively low. To enhance its absorptivity, additives must be incorporated to ultimately fulfill the performance criteria for the absorption and storage within thermal energy systems; that is, the material must possess both high solar absorptivity and high thermal storage density, as well as excellent thermal shock resistance and other desirable properties [27,28]. Regarding the enhancement of absorptivity in ceramic materials, Wu et al. [29] prepared corundum ceramics co-doped with Fe2O3 and TiO2, exhibiting excellent thermal shock resistance, through pressureless sintering at 1460 °C, utilizing bauxite as raw material. After 30 thermal shock cycles (1000–25 °C, air-cooled), the bending strength of the sample reached 222.05 MPa, and the absorptivity was 90.40%; these two measurements were 43.44% and 1.35% higher than that before thermal shock, respectively. Besisa et al. [30] prepared ZrO2/Fe2O3 and Al2O3/CuO ceramics through pressureless sintering at 1700 °C and investigated the thermal efficiency and heat transfer uniformity of both ceramics. Results indicated that both composites possessed high densities, and the Al2O3/10 wt% CuO composite exhibited higher thermal and solar-to-thermal efficiencies than the ZrO2/Fe2O3 composites, with the best thermal emissivity of 0.561 and the thermal conductivity of up to 15.4 W/m. In a follow-up study [31], Besisa et al. conducted an in-depth investigation on the optical properties of Al2O3/CuO ceramics, and their findings revealed that the alumina ceramic material doped with 40 wt% CuO achieved an absorptivity of 75% in the visible-light spectrum and exhibited excellent stress durability, as well as uniform thermal distribution. Fe2O3 and CuO, as highly efficient additives to increase the absorptivity of ceramic materials, not only confer upon the ceramic materials higher absorptivity and thermal conductivity, but they also augment the thermal shock resistance and durability of the materials, rendering them ideal additives for the preparation of high-efficiency solar absorptive materials. The forsterite, as a solar energy absorption and storage of integrated materials, has the potential to reduce the production cost of the entire system and enhance the energy conversion efficiency of solar and thermal energy. However, limited research has systematically investigated forsterite-based ceramics as absorbing and storing integrated materials, and there is a scarcity of in-depth discussion on the mechanism by which additives influence the absorptivity of forsterite-based ceramics.
Based on the above analysis, in this study, forsterite-absorption and -storage integrated ceramics were prepared by pressureless sintering method with electrofused magnesium sand, quartz, α-Al2O3, and Sm2O3 as the primary raw materials, and various ratios of Fe2O3 and CuO as additives. The phase composition, microstructure, physical properties, thermal shock resistance, absorptivity, and thermophysical properties of forsterite-absorption and -storage integrated ceramics were systematically investigated. The mechanism underlying the effects of Fe2O3 and CuO on absorptivity and thermophysical properties was investigated.
2. Experiment Procedures
2.1. Sample Preparation
In this study, forsterite was prepared from fused magnesia (Xinshi, China), quartz (Yingde, China), α-Al2O3 (Shandong Aluminum Industry, China), and Sm2O3 (AR, 99.7%) as the primary raw materials. Concurrently, two types of additives, Fe2O3 (AR, 99.7%) and CuO (AR, 99.7%), were doped, with the additive ratios of Fe2O3 to CuO being 9:1, 8:2, 7:3, 6:4, and 5:5, respectively. This study aimed to investigate the effects of these composite additives on the absorptivity of forsterite. The three chemical reagents, Sm2O3, Fe2O3, and CuO, were sourced from Sinopharm Chemical Reagent Co. The chemical composition of the experimental raw materials is detailed in Table 1, and the formula composition is detailed in Table 2.

Table 1.
Composition of experimental raw materials (wt%).

Table 2.
J-series formula composition (wt%).
The weighed raw materials were ball-milled and mixed for 30 min according to the content of each raw material in Table 2. The mixed raw material powder was granulated and aged with 5 wt% PVA (polyvinyl alcohol), after which the aged powder was pressed into cylindrical samples with a diameter of 31.0 mm and a height of 9.0 mm, and rectangular samples with dimensions of 37.0 mm × 6.5 mm × 6.5 mm, using a hydraulic press. The pressed ceramic blanks were dried at 90 °C for 24 h and then placed in a silicon molybdenum rod furnace for pressureless sintering at 1480–1560 °C to prepare the forsterite-absorption and -storage integrated ceramic samples. The sintering parameters for the ceramics were as follows: the heating rate was 5 °C per minute for temperatures below 1000 °C, and 3 °C per minute for temperatures above 1000 °C, and the holding time was 2 h when the maximum sintering temperature was attained.
After the sintering process, a solar energy-absorbing and -storing integrated ceramic sample, specialized for use in the field of solar thermal power generation, was prepared. The ceramic sample effectively absorbs solar energy during the daytime and converts it into thermal energy, which is further converted into electricity by the converter and subsequently incorporated into the power grid, while the excess thermal energy will be stored. When sunlight is not available, such as at night, the ceramic receiver, with its high-efficiency thermal storage properties, can still supply the converter with thermal energy and continue to supply power to the grid.
2.2. Characterization
The water absorption (Wa/%), apparent porosity (Pa/%), and bulk density (D/g·cm−3) of the samples were tested via the static weighing method, according to Archimedes’ principle. The bending strength of the samples was determined by the three-point bending method on an electronic universal testing machine (Shenzhen, China, Rigel, RGM-4100) under the following test conditions: uniaxial test with a span of 28 mm and a loading rate of 0.5 mm/min. The phase composition of the samples was analyzed by using a rotary-target X-ray diffractometer (D/MAX-RB) of Rigaku Corporation (Tokyo, Japan), and the test conditions were as follows: Cu target, scanning speed of 0.02°/s, operating voltage of 40 kV, operating current of 30 mA, and scanning range of 5–80°. The microstructure of the sample section was observed using a JSM-5610LV scanning electron microscope manufactured by Nihon Electronics Corporation (Beijing, China), with an operating voltage of 20 kV. The micro-area composition of the samples was analyzed using a Model Quanta FEG450 field-emission scanning electron microscope (FE-SEM) from FEI (Hillsboro, USA). The thermophysical properties (thermal conductivity, thermal diffusion coefficient, and specific heat capacity) of the samples were determined using a laser thermal conductivity meter and a microcalorimeter (C80 microcalorimeter, Setaram, France). For solid sensible thermal storage materials, the thermal storage density per unit mass is calculated as shown in Equation (1).
where Q is the thermal storage density (kJ/kg), C is the specific heat capacity (J/g·K), T0 is the room temperature (°C), and T1 is the maximum temperature at the time of testing (°C).
Thermal shock tests on ceramic samples were carried out in a box-type resistance furnace (Model SX2-5-12, Yingshan Jianli Electric Furnace Manufacturing Co., Ltd., Huanggang, China). The process of the thermal shock resistance test is as follows: (1) The box-type resistance furnace is heated up to 1100 °C at a rate of 5 °C per minute. (2) The sintered ceramic samples are put into the box-type resistance furnace to hold for 15 min. (3) After 15 min, the samples are taken out of the box-type resistance furnace, and then the samples are cooled down to the room temperature by means of air cooling. This is a thermal shock cycling process, and then steps (2) and (3) are repeated. After repeated thermal shock cycles, the bending strength of the samples was tested, and the rate of strength loss was used to characterize the thermal shock resistance of the samples.
The solar absorptivity of the samples was measured by a UV-Vis-NIR spectrophotometer (Shimadzu Corporation, Kyoto, Japan, UV-3600 plus), and the sample absorptivity was calculated using Equation (2).
where α is the sample solar absorptivity (%), λ is the wavelength (μm), R(λ) is the sample solar reflectivity (%), and Es(λ) is the solar radiation intensity (w/m2).
3. Results and Discussion
3.1. Physical Properties and Solar Absorptivity Analysis
To investigate the effects of varying ratios of Fe2O3 and CuO on the solar absorptivity of forsterite-based ceramics, the physical properties and solar absorptivity of the J-series samples were tested in the temperature range of 1480–1560 °C, and the absorptivity of the samples was calculated according to Equation (2). The temperature dependence curves of the physical properties of the J-series samples sintered at 1480–1560 °C are shown in Figure 1, and the temperature dependence of the solar absorptivity of the J-series samples and the photographs of the samples are shown in Figure 2.
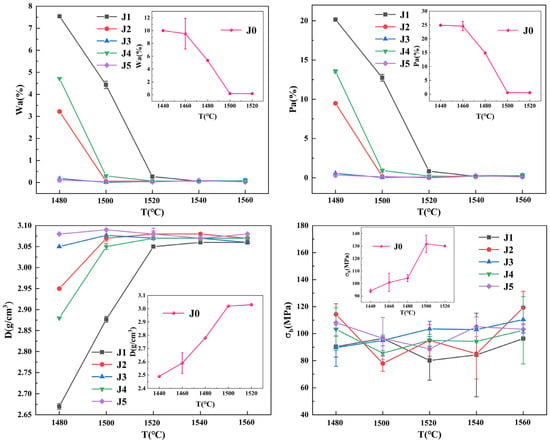
Figure 1.
Water absorption (Wa), porosity (Pa), bulk density (D), and bending strength (MPa) versus sintering temperature for J-series samples.
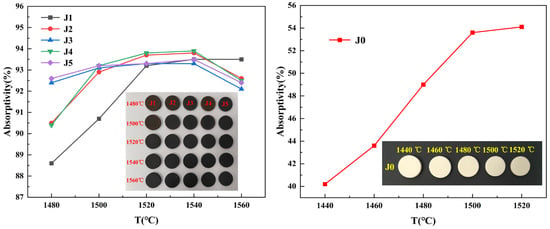
Figure 2.
Solar absorptivity profiles and photographs of the J-series samples sintered at different temperatures.
Photographs of the samples in Figure 2 show that the J-series samples shrink as the temperature increases, and the samples become progressively darker in color. As can be seen from Figure 1 and Figure 2, the temperature has a large effect on the physical properties and absorptivity of the samples, basically increasing them at higher temperatures. With the addition of Fe2O3 and CuO composite additives, the absorptivity of the samples was significantly increased, which can be attributed to the additives imparting a black color to the samples, thereby facilitating sunlight absorption [29,30]. From 1520 °C, the physical properties of the J-series samples tend to stabilize. When the sintering temperature is 1520 °C, the water absorption rate of the J-series samples is lower, 0.5%, and the samples have undergone sintering. At this temperature, the bulk density of the J-series samples is within the range of 3.05–3.08 g/cm3, indicating that the J-series samples are denser. Upon further increases in temperature, the physical properties of the J-series samples remain largely unchanged. After sintering at 1540 °C, the J-series samples exhibited their peak solar absorptivity, and the absorptivity of the J4 sample was 93.90%; at this sintering temperature, the apparent porosity, bulk density, and bending strength of the sample were 0.17%, 3.07 g/cm3, and 94.22 MPa, respectively. However, the solar absorptivity of the J2 sample sintered at 1540 °C is comparable to that of the J4 sample, with an absorptivity of 93.80%; the apparent porosity, bulk density, and flexural strength of the sample were 0.21%, 3.08 g/cm3, and 84.09 MPa, respectively. Upon increasing the sintering temperature to 1560 °C, the solar absorptivity was marginally decreased, and the bending strength of the J-series samples was enhanced, while other physical properties remained essentially unchanged. For inorganic materials such as ceramics, when sunlight irradiates the material, the atoms and valence electrons inside the material absorb energy, which thereby causes them to vibrate and migrate [32]. With an increase in sintering temperature, the samples exhibit higher bulk density, more densified structure, regular grain arrangement, and enhanced atomic vibration and energy transfer; thus, the solar absorptivity of the samples increases [33]. At the optimum sintering temperature of 1540 °C, it is observed that the ratio of Fe2O3 to CuO has a greater influence on the absorptivity of the samples, with a maximum absorptivity of 93.90% achieved for Fe2O3/CuO of 6:4. The absorptivity is 93.80% for Fe2O3/CuO of 8:2, which is marginally lower than that of the J4 sample. The absorptivity of the samples with other Fe2O3/CuO ratios varied between 93.30% and 93.50%.
The wavelength range of sunlight is mainly divided into three bands of ultraviolet, visible, and near-infrared light, accounting for 7%, 50%, and 43% of sunlight, respectively. The wavelength ranges of UV, visible, and NIR light are 0.3–0.4 μm, 0.4–0.76 μm, and 0.76–2.5 μm, respectively [33]. In order to investigate the effects of Fe2O3 and CuO composite additives on the absorptivity of forsterite-based ceramics, UV–visible–near-infrared reflectance light tests were performed on the J-series samples sintered at the optimum sintering temperature in the wavelength range of 300–2500 nm, and the profiles are shown in Figure 3. Within the wavelength range of 300–2500 nm, the reflectance of the J1–J5 samples is low, and the reflectivity of the samples is in the range of 0–20%. The reflectance of the J1–J5 samples is low in the visible range, and it varies slightly in the near-infrared wavelength range, resulting in higher absorptivity for the samples. The absorptivity of the J1–J5 samples sintered at 1540 °C reached 93.50%, 93.80%, 93.30%, 93.90%, and 93.50%, respectively. Among them, the J4 sample had the highest absorptivity, followed by the J2 sample.
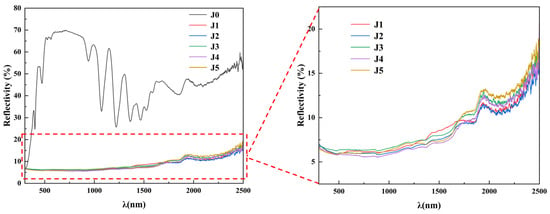
Figure 3.
UV-Vis-NIR reflection spectra of the J0 sample sintered at 1500 °C and the J1–J5 samples sintered at 1540 °C.
As reported by Xu et al. [34], doping of Fe2O3 allows Fe3+ to enter the mullite lattice, thus increasing the absorptivity of the sample. In conjunction with this study, it can be seen that the addition of Fe2O3 does increase the absorptivity and decrease the reflectivity of the J-series samples. As the sintering temperature of the sample increases, the Fe3+ provided by Fe2O3 is doped into the magnesium–aluminum spinel lattice, inducing lattice distortions, which in turn trigger electronic transitions and enhance the intrinsic absorption capacity, thus increasing the sample’s absorptivity. Meanwhile, CuO is an excellent infrared material with a narrow forbidden bandwidth, so it can be used as an additive to improve the absorptivity of ceramic materials [31]. This is because CuO can absorb photons in the visible and near-infrared light region, thus reducing the intensity of reflected light [35]. Recently, scholars have applied CuO in thin-film technology, and it was found that CuO films have good solar selective absorption properties and significantly reduced reflectivity [36,37]. Consequently, the simultaneous doping of Fe2O3 and CuO can significantly reduce the reflectivity of the sample in the UV–visible–near-infrared wavelength range and increase the absorptivity of the sample, making it an ideal candidate as an efficient solar thermal-absorbing material.
3.2. Phase Composition Analysis
In the temperature range of 1480–1560 °C, the highest value of absorptivity was reached for the J1–J5 samples sintered at 1540 °C. Therefore, the J1–J5 samples at 1540 °C were tested and analyzed by XRD, and Figure 4 shows the XRD patterns of the J-series samples. The crystalline phases of the J-series are Mg2SiO4, MgAl2O4, and MgFe0.6Al1.4O4. As is evident from Figure 4, following doping with the Fe2O3/CuO composite additives, the MgAl2O4 of the samples was transformed into MgFe0.6Al1.4O4, and its diffraction peak was shifted toward the low-angle direction. At high temperatures, Fe2O3 reacts with magnesium–aluminum spinel (MgAl2O4) in a solid-phase reaction, and Fe3+ ions diffuse into the lattice of MgAl2O4, replacing some of the Al3+ ions and forming a solid solution [38]. This is because point defects such as cationic vacancies or interstitials are thermally activated; meanwhile, Fe3+ has a higher charge number than Mg3+, and its activation energy for diffusion is lower, so Fe3+ may preferentially migrate through these defects. Meanwhile, Fe3+ has a higher charge density compared to Al3+, which enhances its interaction with the oxygen anion, facilitates localized bond reorganization, and makes it easier for Fe3+ to occupy the octahedral sites. In addition, the ionic radius of Fe3+ (0.645 Å) is larger than that of Al3+ (0.535 Å). When Fe3+ replaces Al3+ in the MgAl2O4 spinel lattice, it introduces localized lattice distortions and creates a strain field, which puts the lattice in an activated state, and therefore the chemical reaction is easier to carry out, thus promoting the rearrangement and diffusion of atoms [39]. The diffraction peak intensity of MgFe0.6Al1.4O4 was gradually weakened as the Fe2O3/CuO ratio decreased, indicating that the generation of MgFe0.6Al1.4O4 was related to the addition of Fe2O3.
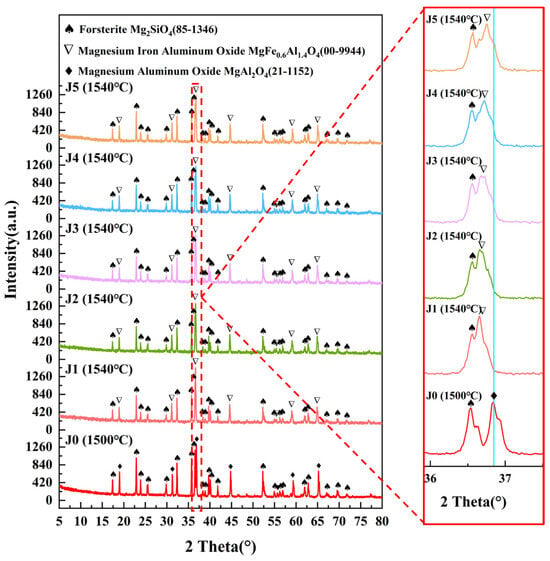
Figure 4.
XRD patterns of the J-series samples sintered at different temperatures.
As can be seen from Table 3, the lattice constant and cell volume of magnesium–(iron)–aluminum spinel gradually increase with the increase in Fe2O3 content in the sample. This result is consistent with the analytical results of the corresponding diffraction peaks slightly shifted to the left in Figure 4. The lattice distortion induced by the formation of the MgFe0.6Al1.4O4 solid solution enhances the non-simple harmonic vibration of the lattice, making the sample more susceptible to absorbing the external energy, thus increasing its absorption rate [34].

Table 3.
Crystal structure parameters of spinel in J-series samples.
3.3. Microstructure Analysis
SEM analysis was carried out on the cross-sectional views of the J1–J5 samples sintered at 1540 °C. Figure 5 shows that the microstructure of the J1 sample has less glassy phase content, probably because of the lower content of CuO. It is observed from Figure 5a that there are aggregated growing grains in the pores of the J-series samples, and the number of pores is reduced. From Figure 5c,d, it can be seen that the grain boundaries of the J-series samples are wrapped by the glassy phase, which is uniformly distributed. At elevated temperatures, the liquid phase infiltrates the pores and promotes the rearrangement of the grains, thus facilitating the discharge of pores and promoting sintering. Large grains and octahedral grains are observed in the J-series samples, and the glassy phase connects the two to form the entire cross-sectional microstructure. Combined with Figure 4, it can be seen that Fe3+ replaces part of Al3+ to form the MgFe0.6Al1.4O4 phase, with the large grains being Mg2SiO4 and the octahedral grains being MgFe0.6Al1.4O4. Therefore, at high temperatures, the liquid phase is mainly formed by Sm2O3 and CuO to fill the whole microstructure, and Fe3+ is mainly solidly dissolved in MgAl2O4. The CuO content of the J5 sample is 5 wt%; thus, the J5 sample has the largest amount of glassy phase, and the grain edges are blurred. When there is too much high-temperature liquid phase, the sample forms a large amount of glassy phase at a low temperature, which increases the reflectivity of the sample; thus, the absorptivity of the J5 sample decreases to 93.50%.
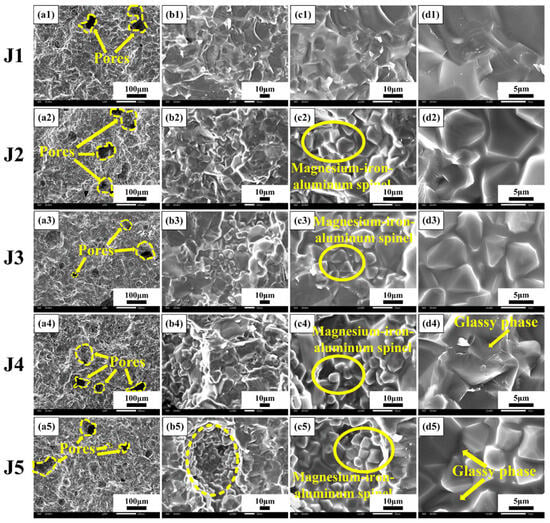
Figure 5.
SEM images of fractured surface of the J1–J5 samples sintered at 1540 °C: (a1–d1) is the J1 sample; (a2–d2) is the J2 sample; (a3–d3) is the J3 sample; (a4–d5) is the J4 sample; (a5–d5) is the J5 sample.
3.4. Thermal Shock Resistance Analysis
The J2 and J4 samples sintered at 1540 °C have high solar absorptivity of 93.80% and 93.90%, respectively, and the difference in absorptivity between the two samples is minimal, with their physical properties being essentially identical. Therefore, thermal shock experiments were conducted on the J2 and J4 samples sintered at 1540 °C, and the test results of the J2 and J4 samples after 30 thermal shock cycles are shown in Figure 6 and Figure 7. With an increasing number of thermal shock cycles, the water absorption and apparent porosity of the J2 and J4 samples increased, while the bulk density remained largely unchanged. The bending strength of the J2 and J4 samples increased after 30 thermal shock cycles. The bending strength of the J2 sample was 143.46 MPa, which was an increase of 70.60%, while the bending strength of the J4 sample increased to 132.78 MPa, which was an increase of 40.93%.
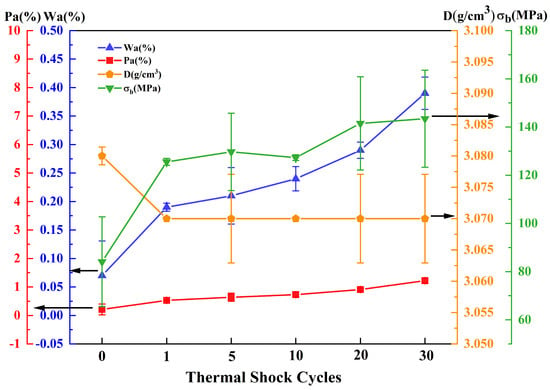
Figure 6.
Curve of relationship between bending strength and thermal shock times of the J2 sample sintered at 1540 °C.
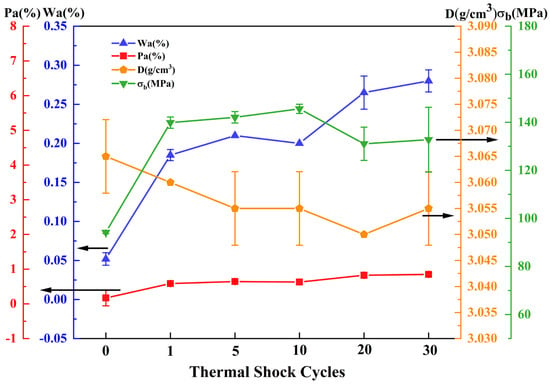
Figure 7.
Curve of relationship between bending strength and thermal shock times of the J4 sample sintered at 1540 °C.
The XRD patterns of the J2 and J4 samples sintered at 1540 °C before and after 30 thermal shock cycles are shown in Figure 8. There was no change in the crystalline phase of the J2 sample before and after 30 thermal shock cycles, and the crystalline phases were Mg2SiO4 and MgFe0.6Al1.4O4. After 30 thermal shock cycles, the intensity of diffraction peaks of the MgFe0.6Al1.4O4 phase is increased, suggesting that the process of thermal shock promotes the growth and development of this phase. The crystalline phases of the J4 sample before the thermal shock were Mg2SiO4 and MgFe0.6Al1.4O4. Subsequent to 30 thermal shock cycles, the crystalline phases of the J4 sample changed, and new phases appeared, and the crystalline phases of the J4 sample were Mg2SiO4, MgFe0.6Al1.9O4, and Sm4(SiO4)3. During the process of repeated thermal shock cycling, crystal boundaries in the sample precipitated Sm4(SiO4)3, which has a high melting point. The precipitation of Sm4(SiO4)3 prevents the nucleation of cavities generated by grain boundary slip and inhibits their growth, thus improving the bending strength of the sample [40]. The enhanced intensity of the diffraction peak of the MgFe0.6Al1.9O4 crystalline phase of the J4 sample indicates the continuous development and growth of this phase during the continuous heating process.
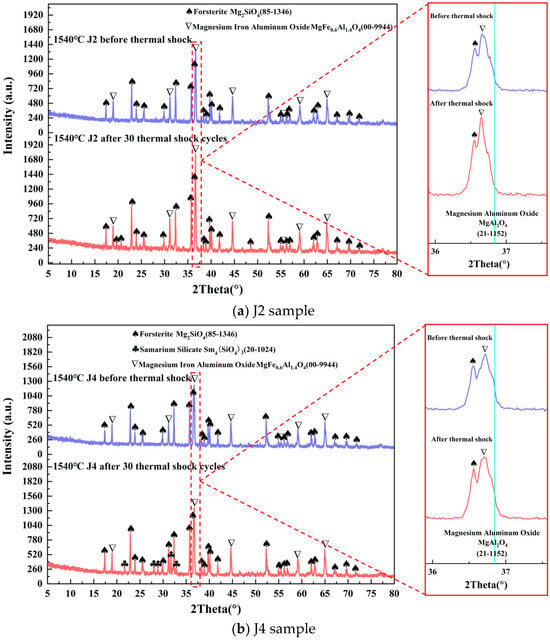
Figure 8.
XRD patterns of the J2 and J4 samples before and after thermal shock.
SEM images of the J2 and J4 samples sintered at 1540 °C before and after 30 thermal shock cycles are shown in Figure 9. After 30 thermal shock cycles, the amount of glassy phase of the J2 sample decreases, and the small grains are octahedral in shape and grow in aggregation with staggered distribution. The grain size is uneven, and there are large plate-like grains and octahedral grains. Combined with Figure 8a, it can be seen that after thermal shock, the intensity of MgFe0.6Al1.4O4 diffraction peaks increases, and MgFe0.6Al1.4O4 develops and grows, indicating that the small octahedral grains in the figure may be MgFe0.6Al1.4O4, and the large grains are forsterite, and the XRD result is in agreement with the microstructure. After 30 thermal shock cycles, the amount of glassy phase of the samples decreases, the grain boundaries become clear, there is almost no glassy phase between the grains, and the grains are tightly adhered to each other. In addition, a combination of large and octahedral grains grows, and diffuse grains appear at the grain boundaries. As shown in Figure 8b, the large grains are probably forsterite, the octahedral grains are MgFe0.6Al1.4O4, and the dendritic grains are Sm4(SiO4)3. This may be due to the fact that the crystals were exposed to rapid cooling and heating, so that Sm4(SiO4)3 exists in the form of dendrites [41]. The second-phase Sm4(SiO4)3 exists in the grain boundaries, which can resist and retard the crack extension, and thus can improve the bending strength of the samples [42]. After 30 thermal shock cycles, the bending strength of the J2 sample increased up to 143.46 MPa, and that of the J4 sample increased to 132.78 MPa. The intergranular bonding in the J2 sample is tighter than that in the J4 sample, and the microstructure densification is enhanced, so the strength of the J4 sample is slightly lower than that of the J2 sample after thermal shock.
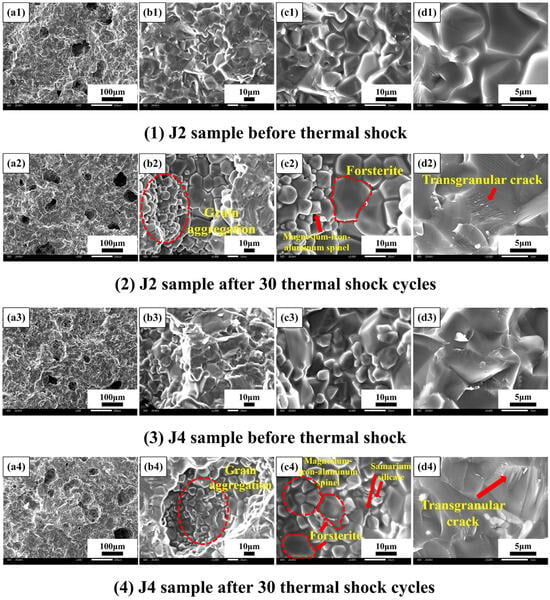
Figure 9.
Comparison of SEM microphotos of the fractured surface of the J2 and J4 samples sintered at 1540 °C before and after thermal shock (1–4).
After thermal shock, the bending strength of both J2 and J4 samples is not lost, the bending strength of the J2 sample increases by 70.60%, and the J2 sample is denser than the J4 sample; therefore, the J2 sample before and after thermal shock is selected for the EPMA testing, and the results before and after the thermal shock are shown in Figure 10/Table 4 and Figure 11/Table 5. As observed in Figure 10, Si elements overlap with some regions of Mg and O elements, and Al elements overlap with some regions of Mg, Fe, and O elements. A small amount of Sm elements is concentrated on the grain boundaries, and a larger amount of high-brightness Sm elements overlap with Si elements. O, Ca, Na, and Cu elements are basically uniformly distributed in the whole cross-section. Combined with Figure 10 and Table 4, it can be seen that spot 1 consists of magnesium–iron–aluminum spinel and samarium silicate, and spot 5 is identified as forsterite. Spot 2, spot 3, and spot 4 are also composed of magnesium–iron–aluminum spinel, indicating that the octahedral grains in the section are magnesium–iron–aluminum spinel. The associated phases are not detected in the XRD analysis due to the low content of Sm2O3.
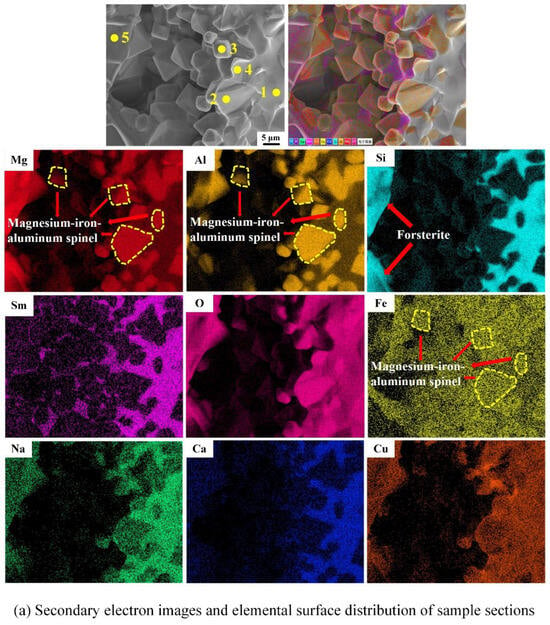
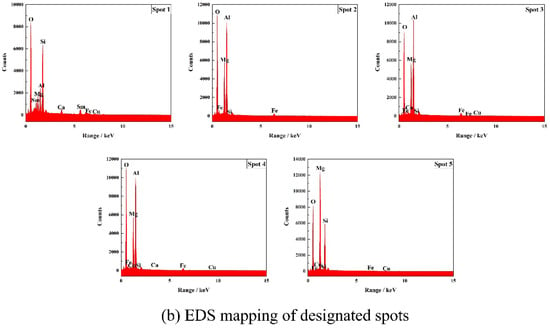
Figure 10.
Microcomponent analysis of the fractured surface of the J2 sample sintered at 1540 °C.

Table 4.
Component analysis of the given spot element in Figure 10b (wt%).
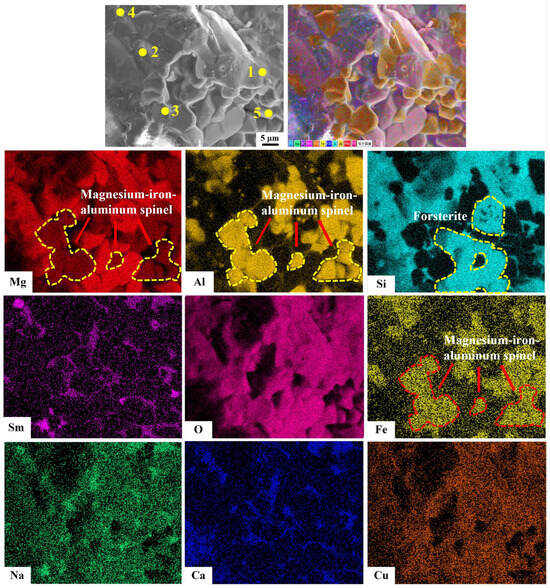
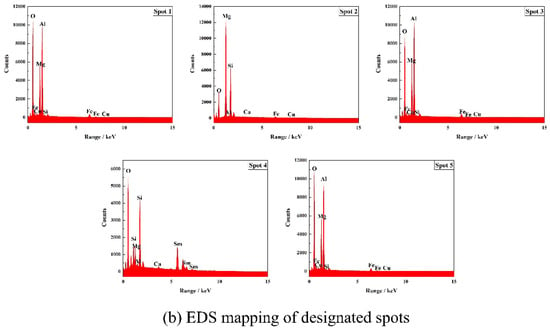
Figure 11.
Microcomponent analysis of the fractured surface of the J2 sample sintered at 1540 °C after 30 thermal shock cycles.

Table 5.
Component analysis of the given points element in Figure 11b (wt%).
As shown in Figure 11, the Si element overlaps with some areas of the Mg and O elements, and the Al and Fe elements highly overlap with some areas of the Mg and O. The Sm element is mainly concentrated on the grain boundaries of the section and exists in the form of a glassy phase in the section. In contrast, the elements O, Ca, Na, and Cu are uniformly distributed throughout the section. From Figure 11 and Table 5, it can be seen that spot 1, spot, 3 and spot 5 are identified as magnesium–iron–aluminum spinel; spot 2 is forsterite; and spot 4 has a combination of forsterite and samarium silicate. Combined with Figure 8 and Figure 9, it can be seen that the octahedral grains are magnesium–iron–aluminum spinel. The EPMA test results corroborate those from XRD and SEM analyses.
3.5. Thermophysical Properties Analysis
After 30 thermal shock cycles of the J2 and J4 samples sintered at 1540 °C, the bending strength increased, and the bending strength of the J2 sample increased by 70.60%, indicating that the J2 sample possessed good resistance to thermal shock, so the thermophysical property tests were conducted on the J2 sample, and the results are shown in Table 6.

Table 6.
Thermophysical properties of the J2 sample.
In the temperature range of 25–1000 °C, the increase in temperature intensifies the thermal motion of atoms and ions of forsterite-based ceramics, resulting in an increase in the entropy value, and consequently, the specific heat capacity of the sample increases. The specific heat capacity and thermal storage density at 1000 °C are 1.56 J/(g·K) and 1516.71 kJ/kg, respectively. The addition of Fe2O3/CuO composite additives results in a change in the phase composition, generating a MgFe0.6Al1.4O4 solid solution. This causes lattice distortion, generation of defects, and alteration of the microstructure of the sample, thereby causing phonon scattering. After the generation of phonon scattering, the phonon mean free path diminishes, resulting in a decrease in the thermal conductivity of forsterite-based ceramics at 1000 °C relative to that at 25 °C [42]. However, the J2 sample maintains a high thermal storage density, demonstrating that it possesses favorable thermophysical properties and is appropriate for use as a thermal storage material in solar thermal power generation systems.
4. Conclusions
In this study, we investigated the effects of the ratio of Fe2O3/CuO composite additives on the physical properties, microstructure, and solar absorptivity of forsterite-based composite ceramics, which reflected the potential application of forsterite-based ceramics in the field of solar energy-absorbing and -storing integrated materials. The specific conclusions are as follows:
(1) The J2 sample sintered at 1540 °C exhibits the optimal overall performance, featuring water absorption and apparent porosity values as low as 0.07% and 0.21%, respectively, and bulk density and bending strength values as high as 3.08 g/cm3 and 84.09 MPa, respectively. The J2 sample consists of crystalline phases, including Mg2SiO4 and MgFe0.6Al1.4O4. Fe3+ replaces some of the Al3+ in MgAl2O4 to form MgFe0.6Al1.4O4 solid solution, which distorts the lattice, activates the lattice, makes chemical reactions easier, and promotes the sintering of the sample. In the J2 sample, large grains and octahedral grains are present, and the glassy phase serves to connect these two phases, comprising the entire microstructure of the cross-section.
(2) After thermal shock cycles, there was no significant difference in water absorption, apparent porosity, and bulk density between the J2 and J4 samples, yet the enhancement in bending strength of the J2 sample surpassed that of the J4 sample. The bending strength of the J2 sample rose to 143.46 MPa, representing a 70.60% increase, while that of the J4 sample increased by 40.93%, amounting to 132.78 MPa. After the thermal shock, the J2 sample exhibits a reduced glassy phase, distinct grain boundaries, virtually no glassy phase between grains, and strongly bonded grains. The J4 sample reveals the presence of the Sm4(SiO4)3 crystal phase, with the secondary phase, Sm4(SiO4)3, localized at grain boundaries, capable of resisting and retarding crack extension, thereby enhancing the bending strength. The intergranular bonding in the J2 sample is more intimate than in the J4 sample, with a decreased glassy phase, enhanced microstructural densification, and expanded loaded area, resulting in a greater rate of increase in bending strength compared to the J4 sample.
(3) The thermal storage density of the J2 sample at 1000 °C is 1516.71 kJ/kg. The addition of the Fe2O3/CuO composite additive leads to the formation of the MgFe0.6Al1.4O4 solid solution, inducing lattice defects and prompting phonon scattering. Following phonon scattering, the mean free path of phonons diminishes, resulting in a decrease in the thermal conductivity of the forsterite-based ceramics at 1000 °C relative to that at 25 °C. Lattice vibrations and migration occur within the samples at elevated temperatures, thus increasing sample absorptivity. Within the 0.3–2.5 μm band range, the absorptivity of the J2 sample sintered at 1540 °C attained 93.80%. After the addition of Fe2O3, the J2 sample forms the MgFe0.6Al1.4O4 phase, where Fe3+ replaces some of the Al3+ positions, distorting the crystal lattice, causing electrons to leap, and augmenting the intrinsic absorption capacity, thereby boosting sample absorptivity. CuO exhibits low reflectivity across the entire spectrum, thereby reducing sample reflectivity. The presence of CuO as a liquid phase in samples at high temperatures expedites the sintering process and increases the synthesis rate of forsterite. The synergistic effects of Fe2O3 and CuO can increase the solar absorptivity of forsterite-based ceramics.
Author Contributions
Conceptualization, Y.L. and T.C.; methodology, Y.L. and T.C.; software, Y.L., T.C. and Y.S.; validation, Y.L., X.X. and J.W.; formal analysis, S.Q. and J.Y.; investigation, Y.L.; resources, X.X.; data curation, Y.L. and T.C.; writing—original draft preparation, Y.L. and T.C.; writing—review and editing, Y.L.; visualization, Y.L. and T.C.; supervision, S.Q., J.W. and J.Y.; project administration, J.W.; funding acquisition, X.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Technology Research and Development Program of the Ministry of Science and Technology of China (No. 2023YFB4204303).
Data Availability Statement
Due to the confidentiality requirements of the National Key Research and Development Program of China (2023YFB4204303), the dataset generated and analyzed during the current research period is not publicly available. However, upon reasonable request, relevant authors may provide aggregated or anonymous data to support the survey results and undergo review by relevant ethics committees or institutions.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ju, X.; Xu, C.; Hu, Y.; Han, X.; Wei, G.; Du, X. A review on the development of photovoltaic/concentrated solar power (PV-CSP) hybrid systems. Sol. Energy Mater. Sol. Cells 2017, 161, 305–327. [Google Scholar] [CrossRef]
- Li, J.; Lu, T.; Yi, X.; Hao, R.; Ai, Q.; Guo, Y.; An, M.; Wang, S.; He, X.; Li, Y. Concentrated solar power for a reliable expansion of energy systems with high renewable penetration considering seasonal balance. Renew. Energy 2024, 226, 120089. [Google Scholar] [CrossRef]
- Liu, H.; Wang, W.; Zhang, Y. Performance gap between thermochemical energy storage systems based on salt hydrates and materials. J. Clean. Prod. 2021, 313, 127908. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, Z.; Zhao, Y.; Yang, Q.; Liu, G.; Li, L. Performance comparison of three supercritical CO2 solar thermal power systems with compressed fluid and molten salt energy storage. Energy 2023, 282, 128807. [Google Scholar] [CrossRef]
- Guo, R.; Lei, D.; Liu, H.; Guo, Y.; Yin, H.; Lv, Y.; Wang, Z. Capacity configuration and economic analysis of integrated wind–solar–thermal–storage generation system based on concentrated solar power plant. Case Stud. Therm. Eng. 2024, 59, 104469. [Google Scholar] [CrossRef]
- Dinker, A.; Agarwal, M.; Agarwal, G. Heat storage materials, geometry and applications: A review. J. Energy Inst. 2017, 90, 1–11. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, X.; Luo, Q.; Tian, Y.; Dang, C.; Yao, H.; Song, C.; Xuan, Y.; Zhao, J.; Ding, Y. Loofah-derived eco-friendly SiC ceramics for high-performance sunlight capture, thermal transport, and energy storage. Energy Storage Mater. 2022, 45, 786–795. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Xu, Q.; Gao, K.; Dang, C.; Li, P.; Luo, Q.; Zheng, H.; Song, C.; Tian, Y.; et al. Bamboo derived SiC ceramics-phase change composites for efficient, rapid, and compact solar thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 240, 111726. [Google Scholar] [CrossRef]
- Wu, J.; Yu, J.; Xu, X.; Liu, Y.; Zhang, Z.; Wei, P. Preparation and thermal shock resistance of anorthite solar thermal energy storage ceramics from magnesium slag. Ceram. Int. 2022, 48, 33604–33614. [Google Scholar] [CrossRef]
- Dong, H.; Liang, Y.; Nie, J.; Cai, M.; Ju, M.; Li, Z.; Wen, L.; Zhou, Y. Synthesis of forsterite with high strength and low acid solubility using magnesite tailings. Ceram. Int. 2023, 49, 13258–13264. [Google Scholar] [CrossRef]
- Jung, I.-H.; Decterov, S.A.; Pelton, A.D. Critical thermodynamic evaluation and optimization of the CaO–MgO–SiO2 system. J. Eur. Ceram. Soc. 2004, 25, 313–333. [Google Scholar] [CrossRef]
- Qi, X.; Qi, D.; Luo, X.; Wang, S.; Zhang, L.; Zhao, J.; You, J.; Liu, Y.; Zhou, Y.; Pan, Z. Fabrication and thermal shock behavior of periclase-forsterite aggregates with micro-nanometer dual-pore-size structures. Ceram. Int. 2023, 49, 1811–1819. [Google Scholar] [CrossRef]
- Laziri, K.; Djemli, A.; Redaoui, D.; Sahnoune, F.; Dhahri, E.; Hassan, S.; Saheb, N. Kinetics of formation, microstructure, and properties of monolithic forsterite (Mg2SiO4) produced through solid-state reaction of nano-powders of MgO and SiO2. Ceram. Int. 2024, 50, 45179–45188. [Google Scholar] [CrossRef]
- Sasikala, T.; Suma, M.; Mohanan, P.; Pavithran, C.; Sebastian, M. Forsterite-based ceramic–glass composites for substrate applications in microwave and millimeter wave communications. J. Alloys Compd. 2007, 461, 555–559. [Google Scholar] [CrossRef]
- Zhu, T.; Zhu, M.; Zhu, Y. Fabrication of forsterite scaffolds with photothermal-induced antibacterial activity by 3D printing and polymer-derived ceramics strategy. Ceram. Int. 2020, 46, 13607–13614. [Google Scholar] [CrossRef]
- Mohagheghiyan, K.; Mokhtari, H.; Kharaziha, M. Gelatin-coated mesoporous forsterite scaffold for bone tissue engineering. Ceram. Int. 2024, 50, 13526–13535. [Google Scholar] [CrossRef]
- Ramezani, A.; Emami, S.; Nemat, S. Effect of waste serpentine on the properties of basic insulating refractories. Ceram. Int. 2018, 44, 9269–9275. [Google Scholar] [CrossRef]
- Gu, F.; Peng, Z.; Zhang, Y.; Tang, H.; Ye, L.; Tian, W.; Liang, G.; Rao, M.; Li, G.; Jiang, T. Facile Route for Preparing Refractory Materials from Ferronickel Slag with Addition of Magnesia. ACS Sustain. Chem. Eng. 2018, 6, 4880–4889. [Google Scholar] [CrossRef]
- Acar, I. Sintering properties of olivine and its utilization potential as a refractory raw material: Mineralogical and microstructural investigations. Ceram. Int. 2020, 46, 28025–28034. [Google Scholar] [CrossRef]
- Nguyen, M.; Sokolář, R. Corrosion Resistance of Novel Fly Ash-Based Forsterite-Spinel Refractory Ceramics. Materials 2022, 15, 1363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.-H.; Wang, L.; Sun, W. Mg recovery from salt lake brine into forsterite refractory materials via precipitation–calcination process. Trans. Nonferrous Met. Soc. China 2024, 34, 694–708. [Google Scholar] [CrossRef]
- Wahsh, M.; Othman, A.; El-Aleem, S.A. The influence of nano-silica and zircon additions on the sintering and mechanical properties of in situ formed forsterite. J. Ind. Eng. Chem. 2014, 20, 3984–3988. [Google Scholar] [CrossRef]
- Kucuk, I.; Boyraz, T.; Gökçe, H.; Öveçoğlu, M.L. Thermomechanical properties of aluminium titanate (Al2TiO5)-reinforced forsterite (Mg2SiO4) ceramic composites. Ceram. Int. 2018, 44, 8277–8282. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, T.; Wu, J.; Shen, Y.; Yu, J.; Shi, X. Microstructure and properties of forsterite-zirconia composite ceramics for solar thermal storage. Ceram. Int. 2024, 50, 25282–25292. [Google Scholar] [CrossRef]
- Wu, S.; Hou, Q.; Yu, J.; Wang, C.; Zhao, J.-L.; Wang, S.; Luo, X.; Qi, X. Sintering behavior and thermal shock resistance of MgO-Mg2SiO4 refractories by co-doped silica fume and quicklime. Ceram. Int. 2024, 50, 56060–56069. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Zhou, Y.; Xu, X.; Tian, K.; Liu, Y. Thermal shock resistance and oxidation resistance of MgAl2O4–Si3N4 ceramics for solar thermal absorber: The effects of TiO2 additive content. Ceram. Int. 2021, 47, 25081–25088. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Xu, X.; Shen, Y.; Qiu, S.; Zhang, D. Effects of Sm2O3 and TiO2 on the performance of MgAl2O4-Si3N4 ceramics for solar thermal absorber in concentrating solar power. Ceram. Int. 2024, 50, 44843–44851. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Xu, X.; Liu, S.; Li, M.; Yin, Y. The microstructures and properties of Fe2O3 and TiO2 co-doped corundum ceramics for solar thermal absorbing materials. Ceram. Int. 2023, 49, 10765–10773. [Google Scholar] [CrossRef]
- Besisa, D.H.; Ewais, E.M.; Ahmed, Y.M. A comparative study of thermal conductivity and thermal emissivity of high temperature solar absorber of ZrO2/Fe2O3 and Al2O3/CuO ceramics. Ceram. Int. 2021, 47, 28252–28259. [Google Scholar] [CrossRef]
- Besisa, D.H.; Ewais, E.M.; Mohamed, H.H.; Besisa, N.; Mohamed, E.A. Thermal stress durability and optical characteristics of a promising solar air receiver based black alumina ceramics. Ceram. Int. 2023, 49, 20429–20436. [Google Scholar] [CrossRef]
- Pan, H.; Luo, F.; Feng, X.-Y.; Qing, Y.; Chen, Q.; Wang, C.-H.; Ren, Z.; Nan, H.; Wang, S.; Duan, S. Construction of compound interface in SiCf/mullite ceramic-matrix composites for enhanced mechanical and microwave absorbing performance. J. Eur. Ceram. Soc. 2023, 43, 4916–4926. [Google Scholar] [CrossRef]
- Chen, J.; Riaz, A.; Taheri, M.; Kumar, A.; Coventry, J.; Lipiński, W. Optical and radiative characterisation of alumina–silica based ceramic materials for high-temperature solar thermal applications. J. Quant. Spectrosc. Radiat. Transf. 2021, 272, 107754. [Google Scholar] [CrossRef]
- Xu, X.; Shen, Y.; Zhang, Z.; Wu, J.; Yu, J.; Zhou, Y. Influence of Fe2O3 on the absorptivity of in situ synthesized solar high-temperature absorbing and storing integrated mullite-based ceramics. Ceram. Int. 2024, 50, 27339–27348. [Google Scholar] [CrossRef]
- Welegergs, G.; Akoba, R.; Sacky, J.; Nuru, Z. Structural and optical properties of copper oxide (CuO) nanocoatings as selective solar absorber. Mater. Today Proc. 2021, 36, 509–513. [Google Scholar] [CrossRef]
- Ot, H.; Ozel, K.; Kutlu-Narin, E.; Serin, T.; Yildiz, A. Tailored key parameters of CuO thin films for emerging solar cells. J. Mater. Sci. Mater. Electron. 2024, 35, 1942. [Google Scholar] [CrossRef]
- El Aakib, H.; Rochdi, N.; Tchenka, A.; Pierson, J.-F.; Outzourhit, A. Copper oxide coatings deposited by reactive radio-frequency sputtering for solar absorber applications. Mater. Chem. Phys. 2023, 296, 127196. [Google Scholar] [CrossRef]
- Kiryakov, A.; Zatsepin, A.; Osipov, V. Optical properties of polyvalent iron ions and anti-site defects in transparent MgAl2O4 ceramics. J. Lumin. 2021, 239, 118390. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Huang, M.; Zheng, P.; Hou, Q.; Qi, X.; Li, R.; Chen, L.; Luo, X. Effects of different additives on properties of magnesium aluminate Spinel–Periclase castable. Ceram. Int. 2023, 49, 4412–4421. [Google Scholar] [CrossRef]
- Quan, Z.; Wang, Z.; Wang, X.; Liu, H.; Ma, Y. Effects of Sm2O3 addition on sintering behavior of pre-synthesized magnesia-rich magnesium aluminate spinel. J. Rare Earths 2021, 39, 1450–1454. [Google Scholar] [CrossRef]
- Xu, X.; Xu, X.; Wu, J.; Lao, X.; Zhang, Y.; Li, K. Effect of Sm2O3 on microstructure, thermal shock resistance and thermal conductivity of cordierite-mullite-corundum composite ceramics for solar heat transmission pipeline. Ceram. Int. 2016, 42, 13525–13534. [Google Scholar] [CrossRef]
- Zhao, K.; Ye, F.; Cheng, L.; Yang, J.; Chen, X. An overview of ultra-high temperature ceramic for thermal insulation: Structure and composition design with thermal conductivity regulation. J. Eur. Ceram. Soc. 2023, 43, 7241–7262. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).