Energy and Resource Efficient Continuous Cooling Crystallization with Modular Lab-Scale Equipment
Abstract
1. Introduction
2. Modular and Smart Equipment and Plants
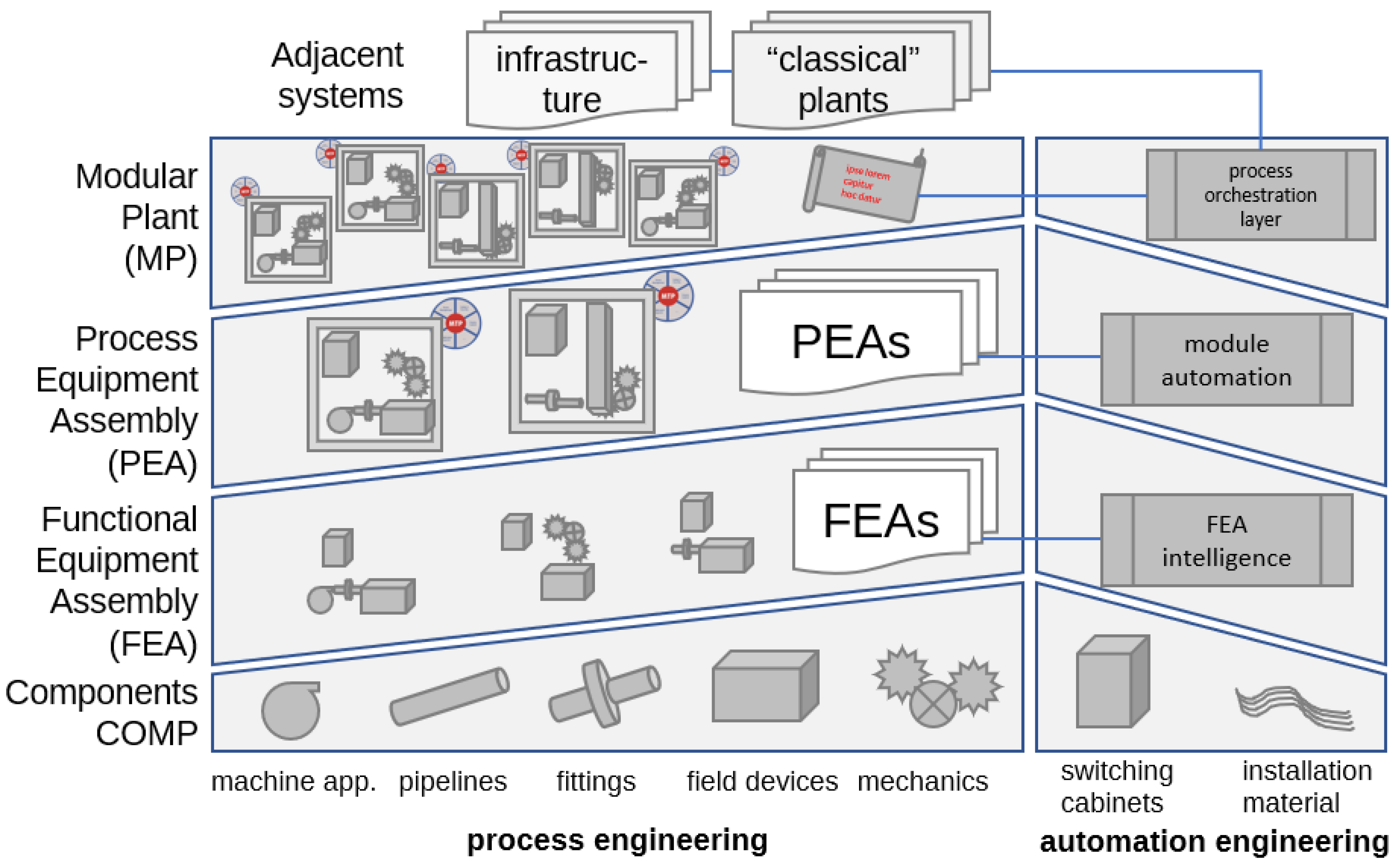
2.1. Modular Equipment and Automation
2.2. Sensors and Automation
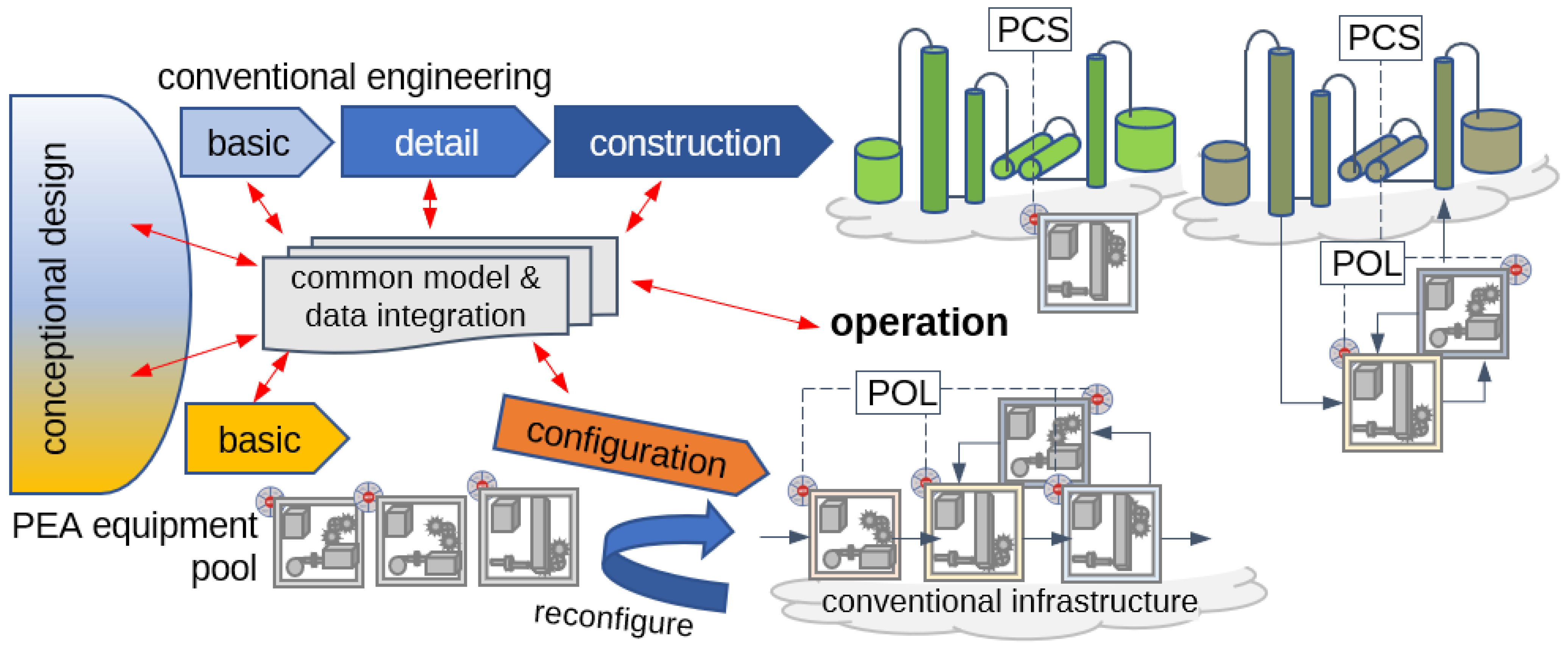
2.3. Modular Process Development and Transfer to Production
3. Modular Crystallizers
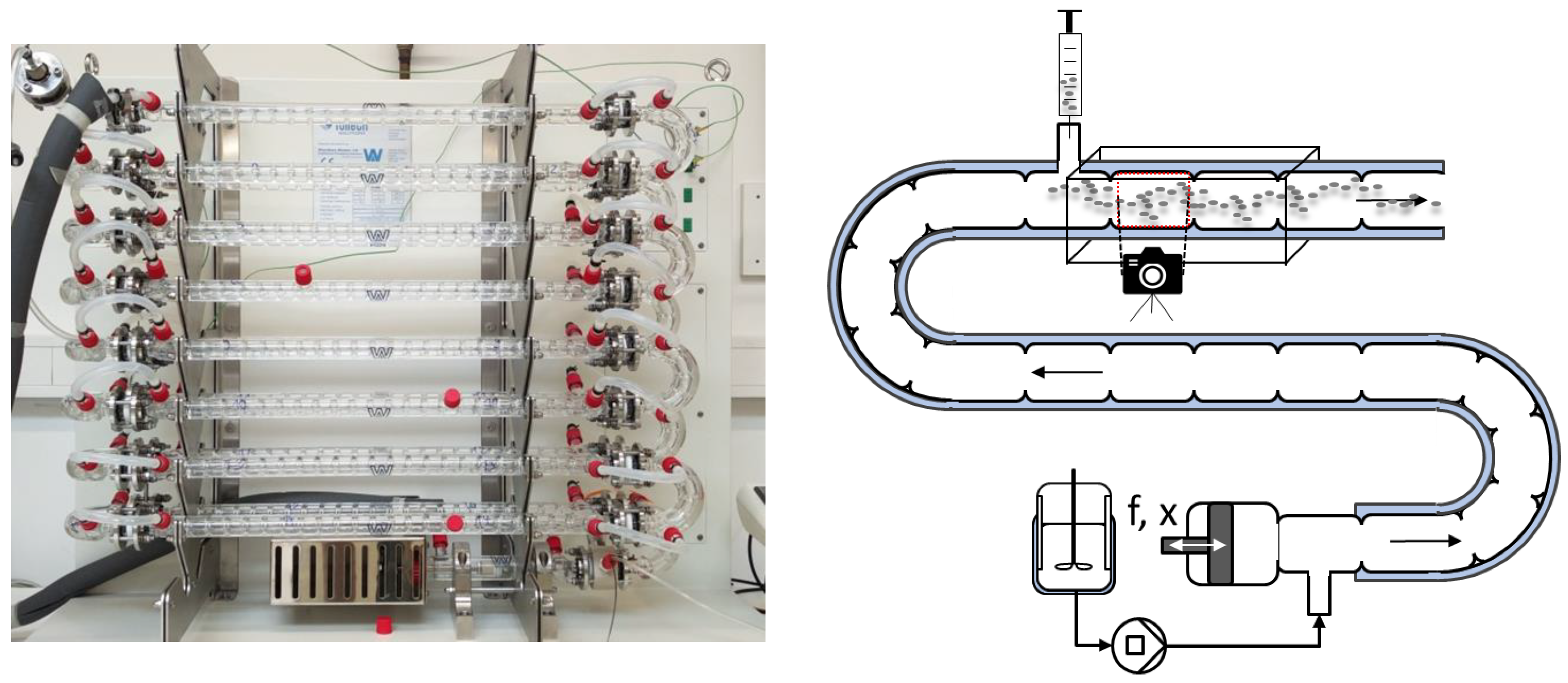
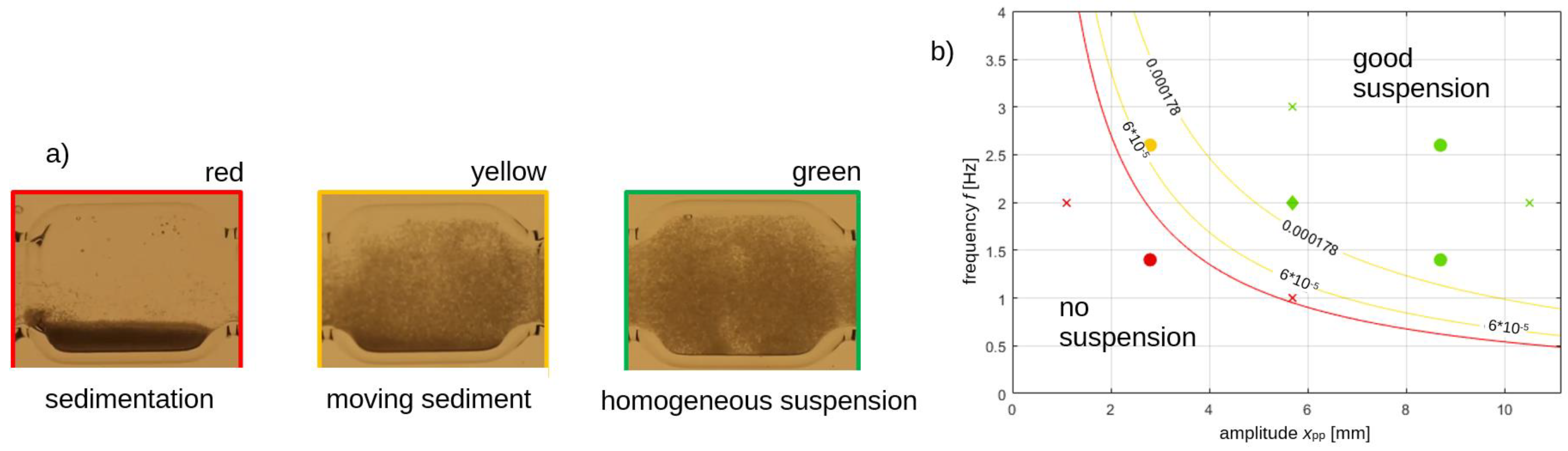
3.1. Continuous Oscillatory Baffled Crystallizer (COBC)
3.2. Coiled Flow Inverter Crystallizer (CFIC)
3.3. Mixed Suspension Mixed Product Removal (MSMPR) Cascade
3.4. Draft Tube Baffle (DTB) Crystallizer
4. Demonstration of Energy and Resource Savings
4.1. Potential Savings in the Crystallization Step
4.2. Industrial Demonstrators
4.3. Potential Energy and Raw Material Savings in the Entire Process
- -
- Product yield: By increasing the yield, the total amount of CO2 equivalents was reduced, i.e., that of energy input and raw material consumption. In studies on typical industrial product groups, yield increases of 5 to 20% were achieved. To calculate the savings, a yield increase of 10% was used, and thus, the savings in CO2 equivalents was calculated.
- -
- Raw material/auxiliary material consumption: By using continuous modular systems that have all the basic operations required and are equipped with internal and cross-module recirculation, the amount of solvent required and the amount of waste produced can be reduced. The need for auxiliary materials can also be reduced or even eliminated by intensifying heat transfer and mass transfer and using new technologies. For the typical industrial products investigated, savings in auxiliary materials of 10 to 70% were achieved. To calculate the savings, an excipient savings of 40% was estimated, and thus, a savings in CO2 equivalents was calculated. Assuming that the thermal supply, the product quantity to be produced, and the running time are very similar, a reduction in power demand of 85% was expected through the use of a continuous modular system. Measurements and calculations based on pilot plants have shown that reductions of up to 90% are possible.
- -
- Hold-up reduction: The internal volume of a system was determined to be 20 to 50% lower on a pilot plant scale. For further calculation, 30% was assumed, from which a reduction in CO2 equivalents was determined.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANSYS | CFD simulation program package |
| ATR-FTIR | Attenuated total reflection-Fourier-transformed IR |
| CFI | Coiled flow inverter |
| CIP | Cleaning in place |
| COBC | Continuous oscillatory baffled crystallizer |
| COMSOL | process simulation program |
| CU | crystallization units |
| DoE | Design of experiments |
| DTB | Draft Tube Baffle |
| DWSIM | Process simulation program (dwsim.org, last access on 30 March 2025) |
| EIT | Electrical impedance tomography |
| ENPRO | Energieeffizienz und Prozessbeschleunigung—Energy efficiency and process acceleration |
| FEA | Functional Equipment Assembly |
| FEP | Fluorinated polyethylene propylene |
| FMCW | Frequency Modulated Continuous Wave |
| gPROMS | numerical simulation program |
| IR | Infra-red |
| MATLAB | numerical simulation program |
| MSMPR | Mixed suspension mixed product removal |
| MSZW | metastable zone width |
| MTP | Modular Type Package |
| ORCA | ENPRO project Orchestration of modular plants |
| P&ID | Pipe & Instrumentation Diagram |
| PCS | Process control system |
| PEA | Process Equipment Assembly |
| PNT | Primary nucleation threshold |
| POL | Process Orchestration Layer |
| PSD | Particle size distribution |
| SL | solid–liquid |
| SMekT | ENPRO project Smart miniplant with continuous separation steps |
| USU | ultrasonic nucleation unit |
| VoPa | ENPRO project Fully-integrated particle generation |
References
- Ciriminna, R.; Della Pina, C.; Luque, R.; Pagliaro, M. Reshoring fine chemical and pharmaceutical productions. Org. Process Res. Dev. 2024, 28, 3026–3034. [Google Scholar] [CrossRef]
- Testa, C.J.; Shvedova, K.; Hu, C.; Wu, W.; Born, S.C.; Takizawa, B.; Mascia, S. Heterogeneous crystallization as a process intensification technology in an integrated continuous manufacturing process for pharmaceuticals. Org. Process Res. Dev. 2021, 25, 225–238. [Google Scholar] [CrossRef]
- Silva, T.C.; Eppink, M.; Ottens, M. Automation and miniaturization: Enabling tools for fast, high-throughput process development in integrated continuous biomanufacturing. J. Chem. Technol. Biotechnol. 2022, 97, 2365–2375. [Google Scholar] [CrossRef]
- Bramsiepe, C.; Sievers, S.; Seifert, T.; Stefanidis, G.D.; Vlachos, D.G.; Schnitzer, H.; Muster, B.; Brunner, C.; Sanders, J.P.M.; Bruins, M.E.; et al. Low-cost small scale processing technologies for production applications in various environments—Mass produced factories. Chem. Eng. Process. Process Intensif. 2012, 51, 32–52. [Google Scholar] [CrossRef]
- Dechema. Modular Plants Flexible Chemical Production by Modularization and Standardization—Status Quo and Future Trends. 2016. Available online: https://dechema.de/dechema_media/modularplants.pdf (accessed on 30 March 2025).
- Hessel, V.; Escriba-Gelonch, M.; Bricout, J.; Tran, N.N.; Anastasopoulou, A.; Ferlin, F.; Valentini, F.; Lanari, D.; Vaccaro, L. Quantitative sustainability assessment of flow chemistry–from simple metrics to holistic assessment. ACS Sustain. Chem. Eng. 2021, 9, 9508–9540. [Google Scholar] [CrossRef]
- Cherkasov, N.; Adams, S.J.; Bainbridge, E.G.; Thornton, J.A. Continuous stirred tank reactors in fine chemical synthesis for efficient mixing, solids-handling, and rapid scale-up. React. Chem. Eng. 2023, 8, 266–277. [Google Scholar] [CrossRef]
- Schrimpf, M.; Esteban, J.; Warmeling, H.; Färber, T.; Behr, A.; Vorholt, A.J. Taylor-Couette reactor: Principles, design, and applications. AIChE J. 2021, 67, e17228. [Google Scholar] [CrossRef]
- Orehek, J.; Teslic, D.; Likozar, B. Continuous crystallization processes in pharmaceutical manufacturing: A review. Org. Process Res. Dev. 2020, 25, 16–42. [Google Scholar]
- Vaidya, S.N.; Agrawal, S.; Lambole, V.; Pimpale, A.; Telrandhe, U. Pharmaceutical manufacturing continuous crystallization procedures: A review. Asian J. Pharm. 2023, 17, 344–354. [Google Scholar]
- Temmel, E.; Lorenz, H. Advances in Industrial Crystallization. Crystals 2020, 10, 997. [Google Scholar] [CrossRef]
- Hessel, V.; Löb, P.; Löwe, H.; Kolb, G. Microreactor concepts and processing. In Micro Instrumentation: For High Throughput Experimentation and Process Intensification—A Tool for PAT; Koch, M.V., VandenBussche, K.M., Chrisman, R.W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 85–129. [Google Scholar]
- Neugebauer, P.; Soritz, S.; Khinast, J.G.; Gruber-Woelfler, H. Enhanced sustainability with crystallization in continuous flow. Curr. Opin. Green Sustain. Chem. 2024, 48, 100937. [Google Scholar] [CrossRef]
- Wu, K.; Kuhn, S. Strategies for solids handling in microreactors. Chim. Oggi 2014, 32, 62. [Google Scholar]
- Hartman, R.L. Managing solids in microreactors for the upstream continuous processing of fine chemicals. Org. Process Res. Dev. 2012, 16, 870–887. [Google Scholar] [CrossRef]
- Bernshausen, J.; Haller, A.; Bloch, H.; Hoernicke, M.; Hensel, S.; Menschner, A.; Stutz, A.; Maurmaier, M.; Holm, T.; Schäfer, C.; et al. Plug & Produce auf dem Sprung in den Markt: Neuerungen in Spezifikation und Implementierung des MTP. Atp Mag. 2019, 61, 56–69. [Google Scholar]
- Grundemann, L.; Schoenitz, M.; Scholl, S. Shorter Time-to-Market with Micro-Conti Processes. Chem. Ing. Tech. 2012, 84, 685–693. [Google Scholar] [CrossRef]
- Zentel, K.M.; Fassbender, M.; Pauer, W.; Luinstra, G.A. 3D printing as chemical reaction engineering booster. In Advances in Chemical Engineering; Academic Press: Cambridge, MA, USA, 2020; Volume 56, pp. 97–137. [Google Scholar]
- Schembecker, G.; Bott, T. Die 50%-Idee-Vom Produkt zur Produktionsanlage in der halben Zeit. Chem. Ing. Tech. 2009, 81, 1094–1095. [Google Scholar] [CrossRef]
- Available online: http://enpro-initiative.de/ (accessed on 30 March 2025).
- Hohmann, L.; Kössl, K.; Kockmann, N.; Schembecker, G.; Bramsiepe, C. Modules in process industry—A life cycle definition. Chem. Eng. Process. Process Intensif. 2017, 111, 115–126. [Google Scholar] [CrossRef]
- Seifert, T.; Sievers, S.; Bramsiepe, C.; Schembecker, G. Small scale, modular and continuous: A new approach in plant design. Chem. Eng. Process. Process Intensif. 2012, 52, 140–150. [Google Scholar] [CrossRef]
- Baldea, M.; Edgar, T.F.; Stanley, B.L.; Kiss, A.A. Modular manufacturing processes: Status, challenges, and opportunities. AIChE J. 2017, 63, 4262–4272. [Google Scholar] [CrossRef]
- Kockmann, N. Modular equipment for chemical process development and small-scale production in multipurpose plants. ChemBioEng Rev. 2016, 3, 5–15. [Google Scholar] [CrossRef]
- Bamberg, A.; Urbas, L.; Bröcker, S.; Bortz, M.; Kockmann, N. The digital twin–your ingenious companion for process engineering and smart production. Chem. Eng. Technol. 2021, 44, 954–961. [Google Scholar] [CrossRef]
- Blumenstein, M.; Henkel, V.; Fay, A.; Stutz, A.; Scheuren, S.; Austermann, N. Integration of flexible transport systems into modular production-related logistics areas. In Proceedings of the 2023 IEEE 21st International Conference on Industrial Informatics (INDIN), Lemgo, Germany, 18–20 July 2023; pp. 1–8. [Google Scholar]
- Becker, T.; Lier, S.; Werners, B. Value of modular production concepts in future chemical industry production networks. Eur. J. Oper. Res. 2019, 276, 957–970. [Google Scholar] [CrossRef]
- Bielenberg, J.; Palou-Rivera, I. The RAPID Manufacturing Institute–Reenergizing US efforts in process intensification and modular chemical processing. Chem. Eng. Process. Process Intensif. 2019, 138, 49–54. [Google Scholar] [CrossRef]
- Dobler, T.; Buchheiser, S.; Gleiß, M.; Nirschl, H. Development and commissioning of a small-scale, modular and integrated plant for the quasi-continuous production of crystalline particles. Processes 2021, 9, 663. [Google Scholar] [CrossRef]
- Pollak, P. Fine Chemicals: The Industry and the Business; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ma, Y.; Wu, S.; Macaringue, E.G.J.; Gong, J.; Wang, J. Recent Progress in Continuous Crystallization of Pharmaceutical Products: Precise Preparation and Control. Org. Process Res. Dev. 2020, 24, 1785–1801. [Google Scholar] [CrossRef]
- Wood, B.; Girard, K.P.; Polster, C.S.; Croker, D.M. Progress to date in the design and operation of continuous crystallization processes for pharmaceutical applications. Org. Process Res. Dev. 2019, 23, 122–144. [Google Scholar] [CrossRef]
- Kumar, R.; Thakur, A.K.; Banerjee, N.; Kumar, A.; Gaurav, G.K.; Arya, R.K. Liquid antisolvent crystallization of pharmaceutical compounds: Current status and future perspectives. Drug Deliv. Transl. Res. 2023, 13, 400–418. [Google Scholar] [CrossRef]
- Bennett, R.C. Crystallization Apparatus and Method. US Patent 3,873,275, 25 March 1975. [Google Scholar]
- Rogers, L.; Briggs, N.; Achermann, R.; Adamo, A.; Azad, M.; Brancazio, D.; Capellades, G.; Hammersmith, G.; Hart, T.; Imbrogno, J.; et al. Continuous production of five active pharmaceutical ingredients in flexible plug-and-play modules: A demonstration campaign. Org. Process Res. Dev. 2020, 24, 2183–2196. [Google Scholar] [CrossRef]
- Kadam, S.S.; Vissers, J.A.; Forgione, M.; Geertman, R.M.; Daudey, P.J.; Stankiewicz, A.I.; Kramer, H.J. Rapid crystallization process development strategy from lab to industrial scale with PAT tools in skid configuration. Org. Process Res. Dev. 2012, 16, 769–780. [Google Scholar] [CrossRef]
- Chen, X.; Kang, Q.; Li, Q.; Ye, F.; Liang, J.; Wang, H.; Yu, H.; Li, X.; Yang, C. Direct Optical Measurement for Particle Dynamics in Multiphase Reactors: Hardware Composition, Image Processing and Uncertainties. Ind. Eng. Chem. Res. 2024, 63, 22232–22252. [Google Scholar] [CrossRef]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-flow technology—A tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef] [PubMed]
- Tumanov, N.A.; Boldyreva, E.V.; Kolesov, B.A.; Kurnosov, R.A.V.; Quesada Cabrera, R. Pressure-induced phase transitions in L-alanine, revisited. Struct. Sci. 2010, 66, 458–471. [Google Scholar]
- Yu, Y.; Robertson, P.K.; Ranade, V.V. Continuous antisolvent crystallization using fluidic devices: Fluidic oscillator, helical coil, and coiled flow inverter. Ind. Eng. Chem. Res. 2022, 61, 15000–15013. [Google Scholar] [CrossRef]
- Dechema. Modular Plants: Paradigm Shift—Describing Process Functionalities Instead of Specifying Equipment; Progress Report; Dechema: Frankfurt, Germany, 2022. [Google Scholar]
- Dechema. Cost Engineering for Modular Plants, Position Paper for the Evaluation of Different Plant Setups, Construction, and Operational Concepts; Dechema: Frankfurt, Germany, 2022. [Google Scholar]
- Grahl, L. Development and Automation of a Smart Distillation Unit for Modular Plants. Ph.D. Dissertation, TU Dortmund University, Dortmund, Germany, 2025. [Google Scholar]
- Schmalenberg, M.; Frede, T.A.; Mathias, C.; Kockmann, N. Efficient Shortcut Method for Determining the Process Window in Stirred-Pulsed Extraction Columns. Chem. Ing. Tech. 2021, 93, 466–472. [Google Scholar] [CrossRef]
- van Delden, M.; Westerdick, S.; Musch, T. Investigations on Foam Detection Utilizing Ultra-Broadband Millimeter Wave FMCW Radar. In Proceedings of the IEEE MTT-S International Microwave Workshop Series on Advanced Materials and Processes for RF and THz Applications (IMWS-AMP), Bochum, Germany, 16–18 July 2019; pp. 103–105. [Google Scholar]
- Gevers, M.; Gebhardt, P.; Westerdick, S.; Vogt, M.; Musch, T. Fast electrical impedance tomography based on code-division-multiplexing using orthogonal codes. IEEE Trans. Instrum. Meas. 2015, 64, 1188–1195. [Google Scholar] [CrossRef]
- Karsch, N.; Westerdick, S.; Musch, T.; Kaufhold, L.; Dittmann, M.; Mallach, M.; Tebrügge, J.; Förster, J.; Vogt, M. Electrical impedance spectroscopy: Machine learning in crystallization processes. Atp Magazin, 21 September 2021; pp. 88–93. [Google Scholar]
- Mallach, M.; Gevers, M.; Gebhardt, P.; Musch, T. Fast and precise soft-field electromagnetic tomography systems for multiphase flow imaging. Energies 2018, 11, 1199. [Google Scholar] [CrossRef]
- Vogt, M.; Hidalgo, S.; Musch, T.; Mallach, M.; Lange, T.; Förster, J. 3 Concepts for accurate electrical conductivity measurement of liquids in industrial process analytics. In Ama Science; Tagungsband: Nuremberg, Germany, 2019; Chapter 1.4; pp. 105–112. [Google Scholar]
- Kockmann, N.; Bittorf, L.; Krieger, W.; Reichmann, F.; Schmalenberg, M.; Soboll, S. Smart Equipment—A Perspective Paper. Chem. Ing. Tech. 2018, 90, 1806–1822. [Google Scholar] [CrossRef]
- Hohmann, L.; Löbnitz, L.; Menke, C.; Santhirakumaran, B.; Stier, P.; Stenger, F.; Dufour, F.; Wiese, G.; zur Horst-Meyer, S.; Kusserow, B.; et al. Continuous Downstream Processing of Amino Acids in a Modular Miniplant. Chem. Eng. Technol. 2018, 41, 1152–1164. [Google Scholar] [CrossRef]
- Dobler, T.; Höving, S.; Dreiser, C.; Gleiß, M.; Gröschen, M.; Henkel, A.; Hörne, M.; Schäfer, M.; Sonnenschein, J.; Wiese, G.; et al. From Lab to Pilot Scale: Commissioning of an Integrated Device for the Generation of Crystals. Chem. Eng. Technol. 2023, 46, 1511–1520. [Google Scholar] [CrossRef]
- Markay, A.; Fay, A.; Kockmann, N. Definition, Characterization, and Modeling of Hybrid Modular-Monolithic Process Plants. Chem. Ing. Tech. 2022, 94, 1117–1130. [Google Scholar] [CrossRef]
- Ramyashree, M.S.; Nandy, A.; Bohari, Y.R.; Pramodh, M.; Kumar, S.H.; Priya, S.S.; Sudhakar, K. Modeling and simulation of reactors for methanol production by CO2 reduction: A comparative study. Results Eng. 2024, 23, 102306. [Google Scholar]
- McGlone, T.; Briggs, N.E.B.; Clark, C.A.; Brown, C.J.; Sefcik, J.; Florence, A.J. Oscillatory Flow Reactors (OFRs) for Continuous Manufacturing and Crystallization. Org. Process Res. Dev. 2015, 19, 1186–1202. [Google Scholar] [CrossRef]
- Ni, X.; Liao, A. Effects of mixing, seeding, material of baffles and final temperature on solution crystallization of l-glutamic acid in an oscillatory baffled crystallizer. Chem. Eng. J. 2010, 156, 226–233. [Google Scholar] [CrossRef]
- Briggs, N.E.; McGinty, J.; McCabe, C.; Raval, V.; Sefcik, J.; Florence, A.J. Heat transfer and residence time distribution in plug flow continuous oscillatory baffled crystallizers. ACS Omega 2021, 6, 18352–18363. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.R.; Phan, A.N.; Harvey, A.P. Rapid process development using oscillatory baffled mesoreactors—A state-of-the-art review. Chem. Eng. J. 2015, 265, 110–121. [Google Scholar] [CrossRef]
- Kacker, R.; Regensburg, S.I.; Kramer, H.J.M. Residence time distribution of dispersed liquid and solid phase in a continuous oscillatory flow baffled crystallizer. Chem. Eng. J. 2017, 317, 413–423. [Google Scholar] [CrossRef]
- Briggs, N.E.B.; Schacht, U.; Raval, V.; McGlone, T.; Sefcik, J.; Florence, A.J. Seeded Crystallization of β-l-Glutamic Acid in a Continuous Oscillatory Baffled Crystallizer. Org. Process Res. Dev. 2015, 19, 1903–1911. [Google Scholar] [CrossRef]
- Termühlen, M.; Strakeljahn, B.; Schembecker, G.; Wohlgemuth, K. Quantification and evaluation of operating parameters’ effect on suspension behavior for slug flow crystallization. Chem. Eng. Sci. 2021, 243, 116771. [Google Scholar] [CrossRef]
- Hohmann, L.; Schmalenberg, M.; Prasanna, M.; Matuschek, M.; Kockmann, N. Suspension flow behavior and particle residence time distribution in helical tube devices. Chem. Eng. J. 2019, 360, 1371–1389. [Google Scholar] [CrossRef]
- Lührmann, M.-C.; Timmermann, J.; Schembecker, G.; Wohlgemuth, K. Enhanced Product Quality Control through Separation of Crystallization Phenomena in a Four-Stage MSMPR Cascade. Cryst. Growth Des. 2018, 18, 7323–7334. [Google Scholar] [CrossRef]
- Orehek, J.; Teslić, D.; Likozar, B. Mechanistic modeling of a continuous multi-segment multi-addition antisolvent crystallization of benzoic acid in a coiled flow inverter (CFI) crystallizer. Sep. Purif. Technol. 2022, 298, 121571. [Google Scholar] [CrossRef]
- Benitez-Chapa, A.G.; Nigam, K.D.P.; Alvarez, A.J. Process intensification of continuous antisolvent crystallization using a coiled flow inverter. Ind. Eng. Chem. Res. 2019, 59, 3934–3942. [Google Scholar] [CrossRef]
- Gargi, M.A.A.; Trinadh, K.V.V.S.; Maharana, A.; Sarkar, D. Continuous Isothermal Antisolvent Crystallization of Pyrazinamide in a Coiled Flow Inverter Device. Ind. Eng. Chem. Res. 2024, 63, 17221–17235. [Google Scholar] [CrossRef]
- Ganjare, A.; Ranade, V. Strategies for managing clogging and encrustation in compact continuous crystallizers: Anti-solvent crystallization of paracetamol with fluidic oscillator and helical coil. Chem. Eng. Sci. 2025, 305, 121102. [Google Scholar] [CrossRef]
- Kaabipour, S.; Hemmati, S. Continuous, green, and room-temperature synthesis of silver nanowires in a helically-coiled millifluidic reactor. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130806. [Google Scholar] [CrossRef]
- Pophali, A.; Kajala, R.; Ali, H.; Verma, N.; Nigam, K.D.P. Coiled flow inverter mediated synthesis of activated carbon fiber-supported Ni nanoparticles. React. Chem. Eng. 2022, 7, 719–729. [Google Scholar] [CrossRef]
- Fan, W.; Zhao, F.; Chen, M.; Li, J.; Guo, X. Accelerated and simplified synthesis of magnetic mesoporous nanoparticles in a continuous multistep microfluidic system. Chem. Eng. Process.-Process Intensif. 2022, 181, 109104. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Weick, L.K.; Kockmann, N. Nucleation in continuous flow cooling sonocrystallization for coiled capillary crystallizers. J. Flow Chem. 2021, 11, 303–319. [Google Scholar]
- Borchert, C.; Sundmacher, K. Crystal Aggregation in a Flow Tube: Image-Based Observation. Chem. Eng. Technol. 2011, 34, 545–556. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, T.; Liu, H.; Ma, C.Y.; Wang, X.Z. In-situ crystal morphology identification using imaging analysis with application to the L-glutamic acid crystallization. Chem. Eng. Sci. 2016, 148, 126–139. [Google Scholar] [CrossRef]
- Neugebauer, P.; Zettl, M.; Moser, D.; Poms, J.; Kuchler, L.; Sacher, S. Process analytical technology in Downstream-Processing of Drug Substances–A review. Int. J. Pharm. 2024, 661, 124412. [Google Scholar] [PubMed]
- Wu, Y.; Gao, Z.; Rohani, S. Deep learning-based oriented object detection for in situ image monitoring and analysis: A process analytical technology (PAT) application for taurine crystallization. Chem. Eng. Res. Des. 2021, 170, 444–455. [Google Scholar] [CrossRef]
- Liu, J.; Kuang, W.; Liu, J.; Gao, Z.; Rohani, S.; Gong, J. In-situ multi-phase flow imaging for particle dynamic tracking and characterization: Advances and applications. Chem. Eng. J. 2022, 438, 135554. [Google Scholar]
- Adams, D.; Lee, J.H.; Kim, S.H.; Heo, S. Noninvasive inline imaging and computer vision-based quality variable estimation for continuous slug-flow crystallizers. Comput. Chem. Eng. 2025, 197, 109067. [Google Scholar] [CrossRef]
- Rossi, D.; Jamshidi, R.; Saffari, N.; Kuhn, S.; Gavriilidis, A.; Mazzei, L. Continuous-flow sonocrystallization in droplet-based microfluidics. Cryst. Growth Des. 2015, 15, 5519–5529. [Google Scholar] [CrossRef]
- Jamshidi, R.; Rossi, D.; Saffari, N.; Gavriilidis, A.; Mazzei, L. Investigation of the effect of ultrasound parameters on continuous sonocrystallization in a millifluidic device. Cryst. Growth Des. 2016, 16, 4607–4619. [Google Scholar] [CrossRef]
- Jordens, J.; Canini, E.; Gielen, B.; Van Gerven, T.; Braeken, L. Ultrasound assisted particle size control by continuous seed generation and batch growth. Crystals 2017, 7, 195. [Google Scholar] [CrossRef]
- Devos, C.; Brozzi, E.; Van Gerven, T.; Kuhn, S. Characterization of a modular microfluidic section for seeded nucleation in multiphase flow. Org. Process Res. Dev. 2023, 27, 311–321. [Google Scholar] [CrossRef]
- Zhang, B.; Stefanidis, G.D.; Van Gerven, T. Can Ultrasound Replace Seeding in Flow Reactive Crystallization of an Aromatic Amine? Org. Process Res. Dev. 2024, 28, 4431–4443. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Kreis, S.; Weick, L.-K.; Haas, C.; Sallamon, F.; Kockmann, N. Continuous Cooling Crystallization in a Coiled Flow Inverter Crystallizer Technology—Design, Characterization, and Hurdles. Processes 2021, 9, 1537. [Google Scholar] [CrossRef]
- Wiedmeyer, V.; Anker, F.; Bartsch, C.; Voigt, A.; John, V.; Sundmacher, K. Continuous Crystallization in a Helically Coiled Flow Tube: Analysis of Flow Field, Residence Time Behavior, and Crystal Growth. Ind. Eng. Chem. Res. 2017, 56, 3699–3712. [Google Scholar] [CrossRef]
- Hohmann, L. Equipment and Process Design for Small-Scale Continuous Crystallization. Ph.D. Dissertation, TU Dortmund University, Dortmund, Germany, 2019. [Google Scholar]
- Cui, Y.; O’Mahony, M.; Jaramillo, J.J.; Stelzer, T.; Myerson, A.S. Custom-built miniature continuous crystallization system with pressure-driven suspension transfer. Org. Process Res. Dev. 2016, 20, 1276–1282. [Google Scholar] [CrossRef]
- Alvarez, A.J.; Singh, A.; Myerson, A.S. Crystallization of cyclosporine in a multistage continuous MSMPR crystallizer. Cryst. Growth Des. 2011, 11, 4392–4400. [Google Scholar] [CrossRef]
- Lührmann, M.-C.; Termühlen, M.; Timmermann, J.; Schembecker, G.; Wohlgemuth, K. Induced nucleation by gassing and its monitoring for the design and operation of an MSMPR cascade. Chem. Eng. Sci. 2018, 192, 840–849. [Google Scholar] [CrossRef]
- Melches, C.; Plate, H.; Schürhoff, J.; Buchfink, R. The steps from batchwise to continuous crystallization for a fine chemical: A case study. Crystals 2020, 10, 542. [Google Scholar] [CrossRef]
- ÓMeadhra, R.; Van Rosmalen, G.M. Scale-up of ammonium sulphate crystallization in a DTB crystallizer. Chem. Eng. Sci. 1996, 51, 3943–3950. [Google Scholar] [CrossRef]
- Oh, D.H.; Jeon, R.Y.; Kim, J.H.; Lee, C.H.; Oh, M.; Kim, K.J. Scale-up of a semi-batch draft tube baffled crystallizer for hexanitrohexaazaisowurtzitane based on experiments and computational fluid dynamics simulation. Cryst. Growth Des. 2018, 19, 658–671. [Google Scholar] [CrossRef]
- Wantha, W.; Flood, A.E. Numerical simulation and analysis of flow in a DTB crystallizer. Chem. Eng. Commun. 2008, 195, 1345–1370. [Google Scholar] [CrossRef]
- Rane, C.V.; Ganguli, A.A.; Kalekudithi, E.; Patil, R.N.; Joshi, J.B.; Ramkrishna, D. CFD simulation and comparison of industrial crystallizers. Can. J. Chem. Eng. 2014, 92, 2138–2156. [Google Scholar] [CrossRef]
- Ma, J.; Wang, W.; Huang, W.; Liu, W.; Yao, X.; Li, T.; Ren, B. Numerically investigation of particle distribution in industrial-scale DTB crystallizer based on CFD modelling. Particuology 2024, 93, 186–202. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Jin, Y.; Yang, B.; Li, X. Numerical investigation of the effect of bottom shape on the flow field and particle suspension in a DTB crystallizer. Int. J. Chem. Eng. 2016, 2016, 6862152. [Google Scholar] [CrossRef]
- Wong, S.Y.; Bund, R.K.; Connelly, R.K.; Hartel, R.W. Designing a lactose crystallization process based on dynamic metastable limit. J. Food Eng. 2012, 111, 642–654. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Mensing, L.; Lindemann, S.; Krell, T.; Kockmann, N. Miniaturized Draft Tube Baffle Crystallizer for Continuous Cooling Crystallization. Chem. Eng. Res. Des. 2021, 178, 232–250. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Nocon, A.-K.; Kockmann, N. Design and Hydrodynamic Characterization of a Draft Tube Baffle Tank for Lab-Scale. Chem. Ing. Tech. 2020, 92, 288–294. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Krell, T.; Mathias, C.; Kockmann, N. Continuous Miniaturized Draft Tube Baffle Crystallizer with Particle Screw for Supportive Suspension Discharge. Ind. Eng. Chem. Res. 2021, 60, 18094–18105. [Google Scholar] [CrossRef]
- Höving, S.; Bobers, J.; Kockmann, N. Open-source multi-purpose sensor for measurements in continuous capillary flow. J. Flow Chem. 2022, 12, 185–196. [Google Scholar] [CrossRef]
- Besenhard, M.O.; Neugebauer, P.; Ho, C.D.; Khinast, J.G. Crystal Size Control in a Continuous Tubular Crystallizer. Cryst. Growth Des. 2015, 15, 1683–1691. [Google Scholar] [CrossRef]
- Rahm, J.; Theißen, M.; Klose, A.; Soemers, M.; Temmen, H.; Schäfer, C.; Urbas, L. Efficient automation engineering of modular process equipment assemblies using the digital twin. Chem. Ing. Tech. 2021, 93, 2081–2091. [Google Scholar] [CrossRef]
- Dechema Press Release 2022. Available online: https://dechema.de/%C3%9Cber+uns/Presse/Pressemitteilungen/Archiv/2022/19_2022+Enpro_Studie-p-20229803.html?highlight=enpro (accessed on 30 March 2025).
- Etmanski, M.M.; Breloer, M.; Weber, M.; Schembecker, G.; Wohlgemuth, K. Interplay of Particle Suspension and Residence Time Distribution in a Taylor–Couette Crystallizer. Crystals 2022, 12, 1845. [Google Scholar] [CrossRef]
- Steenweg, C.; Seifert, A.I.; Schembecker, G.; Wohlgemuth, K. Characterization of a modular continuous vacuum screw filter for small-scale solid–liquid separation of suspensions. Org. Process Res. Dev. 2021, 25, 926–940. [Google Scholar] [CrossRef]
- Termühlen, M.; Etmanski, M.M.; Kryschewski, I.; Kufner, A.C.; Schembecker, G.; Wohlgemuth, K. Continuous slug flow crystallization: Impact of design and operating parameters on product quality. Chem. Eng. Res. Des. 2021, 170, 290–303. [Google Scholar] [CrossRef]
- Rasche, M.L.; Jiang, M.; Braatz, R.D. Mathematical modeling and optimal design of multi-stage slug-flow crystallization. Comput. Chem. Eng. 2016, 95, 240–248. [Google Scholar] [CrossRef]
- Liao, H.; Huang, W.; Zhou, L.; Fang, L.; Gao, Z.; Yin, Q. Ultrasound-assisted continuous crystallization of metastable polymorphic pharmaceutical in a slug-flow tubular crystallizer. Ultrason. Sonochemistry 2023, 100, 106627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, Q.; Yao, C.; Chen, G. Investigation of anti-clogging mechanism of ultrasound-driven oscillating slugs/bubbles and its application on continuous crystallization process. Chem. Eng. Sci. 2024, 290, 119898. [Google Scholar] [CrossRef]
- Mondal, M.; Roy, S.; Mukhopadhyay, M. Process intensification of cooling crystallization of cholesterol from acetone solution using CO2 gas bubbles: Experiments and modeling. Chem. Eng. Process. Process Intensif. 2022, 172, 108794. [Google Scholar] [CrossRef]

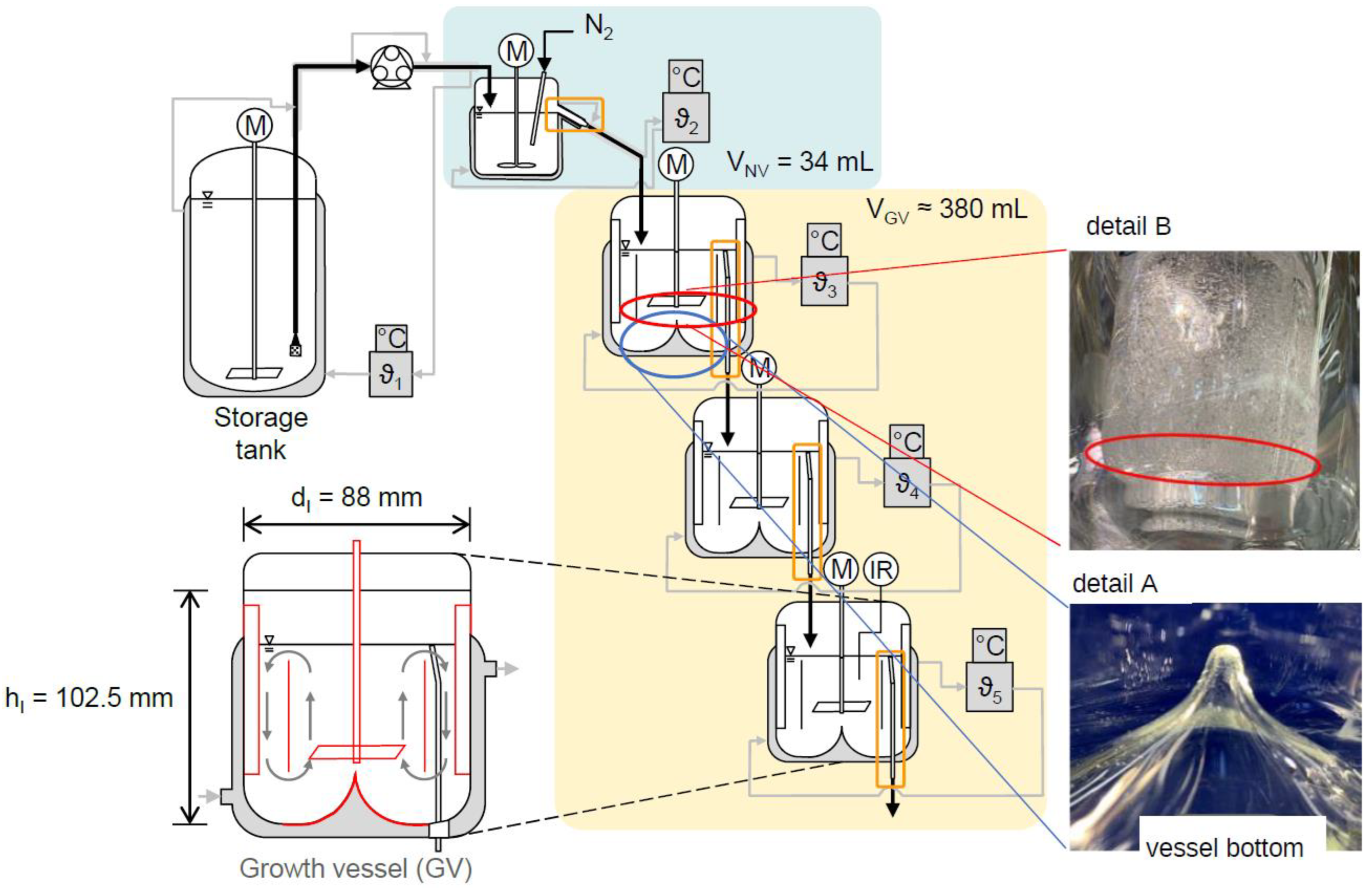
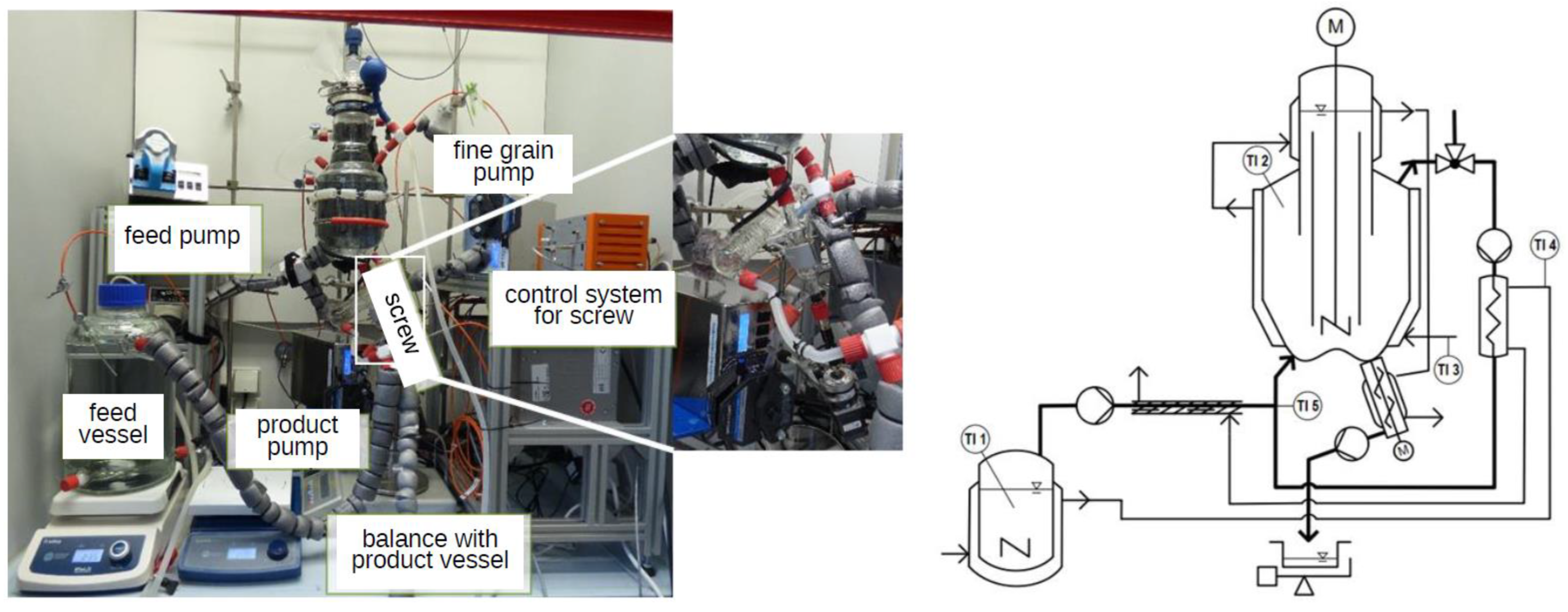
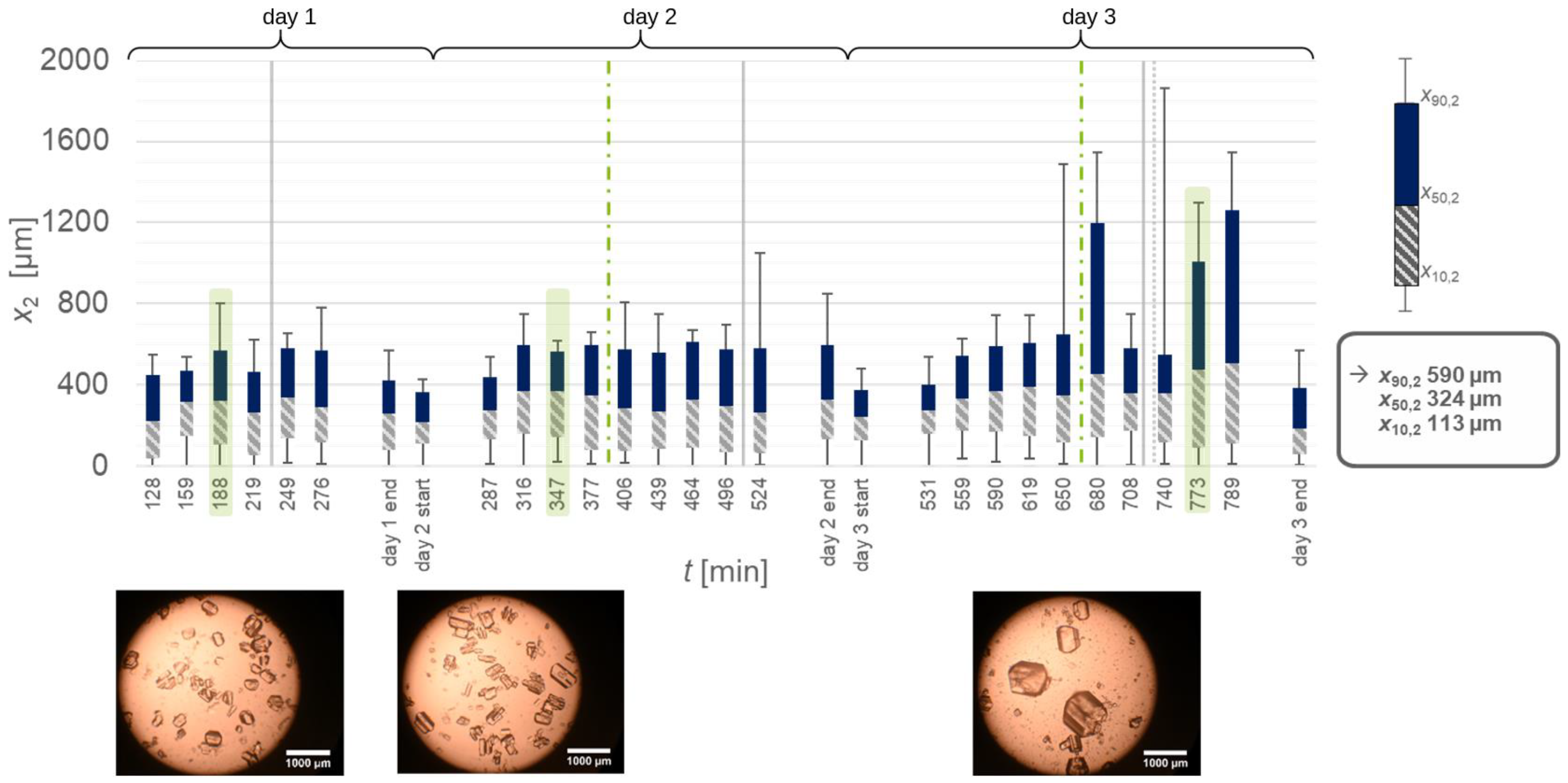

| Material System | Mass Flow Rate [g min−1] | Equipment Used | Seed Crystal Sieve Fraction [µm] | Solid Content of Seed Crystals [w.%] | Growth Rate [µm s−1] | Δx50.3 [µm] |
|---|---|---|---|---|---|---|
| l-alanine/water [72] | 15.4–19.8 | 4 CUs | 90–125 125–180 90–180 | 0.1; 1 1 1 | up to 0.294 | up to 71.5 |
| glycine/water [72] | 26 | 4 CUs | 90–125 | 1 | 0.248 | 39.9 |
| Substance system B/ethanol and n-heptane | 16.1 | USU without ultrasound | 90–125 | 1 | 0.458 | 18 |
| Characteristic | COBC, di = 9/16 mm | CFI, di = 1.6 and 4 mm | MSMPR, 3 × 380 mL | DTB, 2100 mL |
|---|---|---|---|---|
| throughput lab-scale | 10–50 mL min−1, 2–10 w.-% solids, up to 540 gsolids h−1 | 16–50 mL min−1, 2–5 w.-% solids, up to 270 gsolids h−1 | 5–30 mL min−1, 2–3 w.-% solids, up to 100 gsolids h−1 | 5–20 mL min−1, 2–4.5 w.-% solids, up to 100 gsolids h−1 |
| scale-up | difficult due to mechanical and fluidization limits | based on dimensionless numbers feasible, 1.6 to 10 mm realized | based on residence time and throughput | feasible in principle, but only limited experience from lab results |
| typical crystal size | 0.2 to 0.5 mm, depends on crystal density and viscosity | 0.3 to 0.8 (1.5) mm, becoming larger with inner tube diameter | 0.5 to 1.5 mm, depends on inner diameter of transfer line between vessels | 0.5 to 2.5 mm, depends on fluidization degree and SL density difference |
| residence time (RT) characteristics | RT is independent of suspension from oscillating flow | RT depends on flow rate and suspension characteristics, nearly plug flow behavior | RT is independent of suspension from internal agitation | RT is independent of suspension from agitation and internal classification |
| temperature profile and cooling characteristics | linear, progressive, and adaptable to flow rate and temperature range | linear, progressive, and adaptable to flow rate and temperature range | stepwise asymptotic and adaptable to flow rate and temperature range | batch-wise linear and progressive profile, has to be tested with the material system |
| clogging potential | medium due to particle sedimentation close to the outlet | low due to regular flushing | critical in transfer lines, particles sedimentation due to high SL density difference | bypass with fine grain dissolution is sensitive to plugging |
| operational handling | high mechanical load on glass as material from vibrations | quite narrow operational window, which has to be adapted to the material system | long startup period and slow reaction on changes | only few experimental runs of experience, data base has to be enlarged |
| open issues | mechanical stability and scale-up | more experience with other material systems and scale-up | scale-up comparison with existing plants | more experimental experience and scale-up runs are missing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kockmann, N.; Schmalenberg, M.; Strakeljahn, B.; Wohlgemuth, K. Energy and Resource Efficient Continuous Cooling Crystallization with Modular Lab-Scale Equipment. Crystals 2025, 15, 421. https://doi.org/10.3390/cryst15050421
Kockmann N, Schmalenberg M, Strakeljahn B, Wohlgemuth K. Energy and Resource Efficient Continuous Cooling Crystallization with Modular Lab-Scale Equipment. Crystals. 2025; 15(5):421. https://doi.org/10.3390/cryst15050421
Chicago/Turabian StyleKockmann, Norbert, Mira Schmalenberg, Benedikt Strakeljahn, and Kerstin Wohlgemuth. 2025. "Energy and Resource Efficient Continuous Cooling Crystallization with Modular Lab-Scale Equipment" Crystals 15, no. 5: 421. https://doi.org/10.3390/cryst15050421
APA StyleKockmann, N., Schmalenberg, M., Strakeljahn, B., & Wohlgemuth, K. (2025). Energy and Resource Efficient Continuous Cooling Crystallization with Modular Lab-Scale Equipment. Crystals, 15(5), 421. https://doi.org/10.3390/cryst15050421







