Ion Substitution Behavior and Chromatographic Study of “Ya’an Green” Seal Stone
Abstract
1. Introduction
2. Materials and Methods
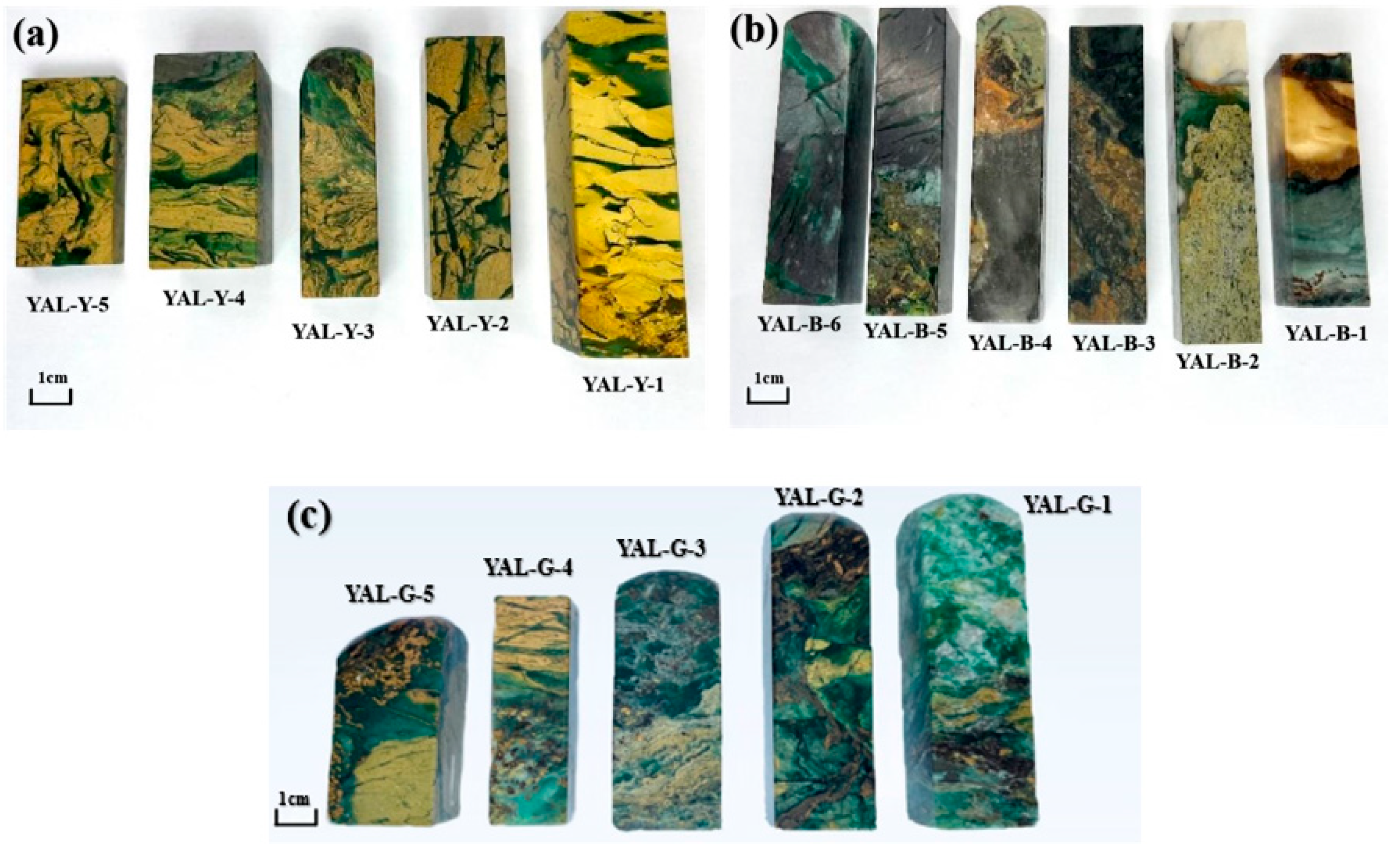
2.1. Materials
2.2. Methods
3. Results
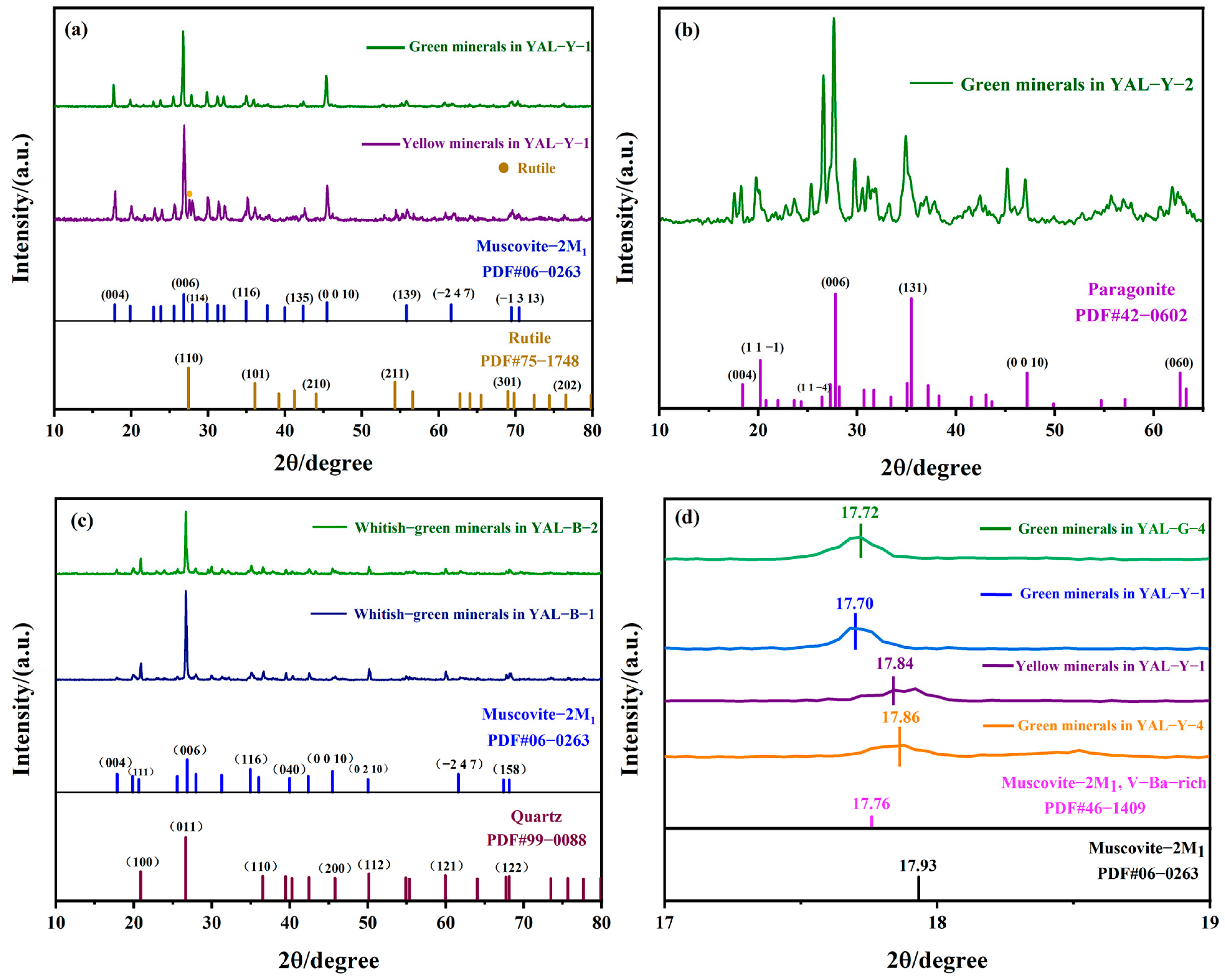
3.1. X-ray Powder Diffraction Test
3.2. Electron Probe Microanalysis Test
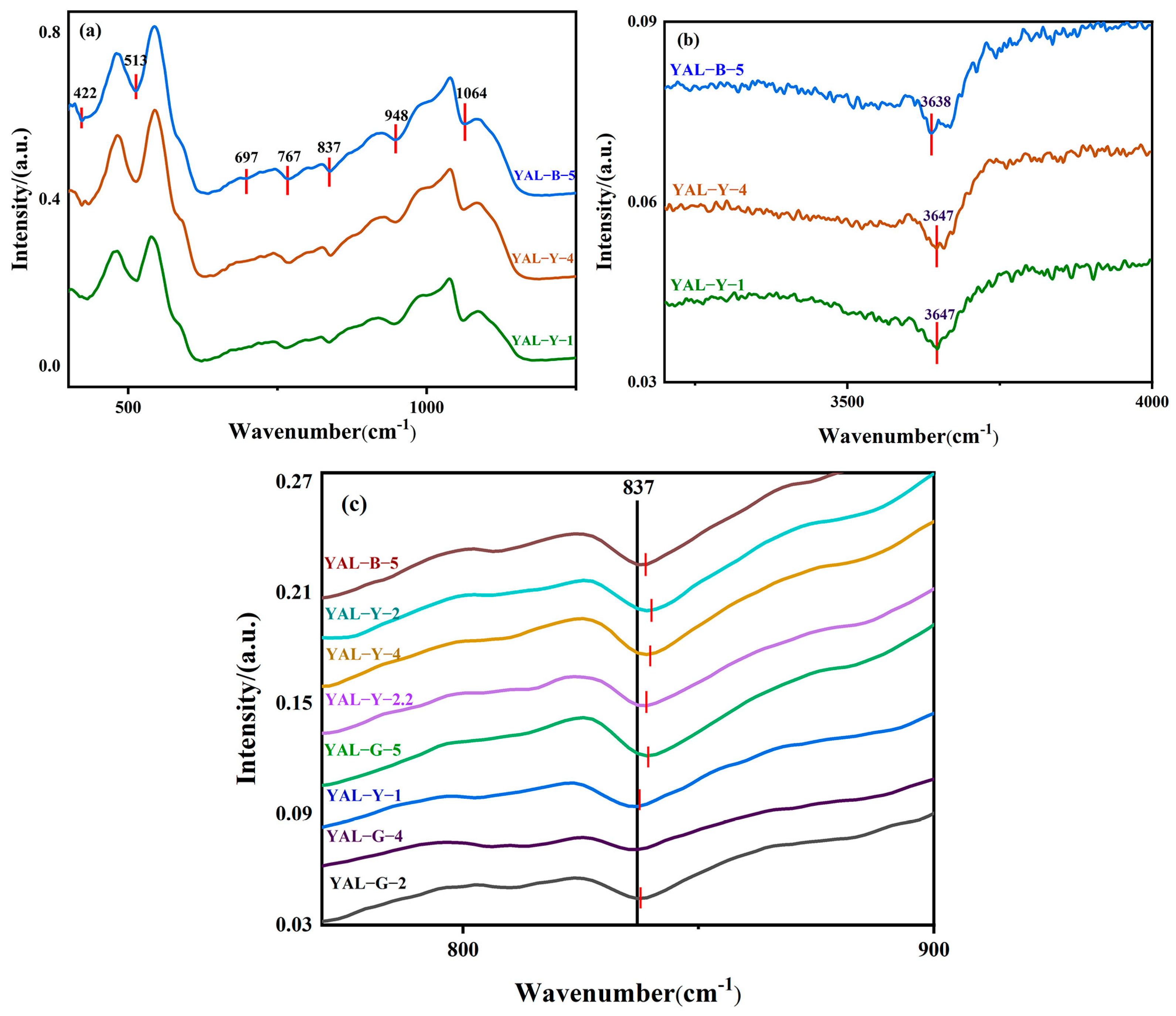
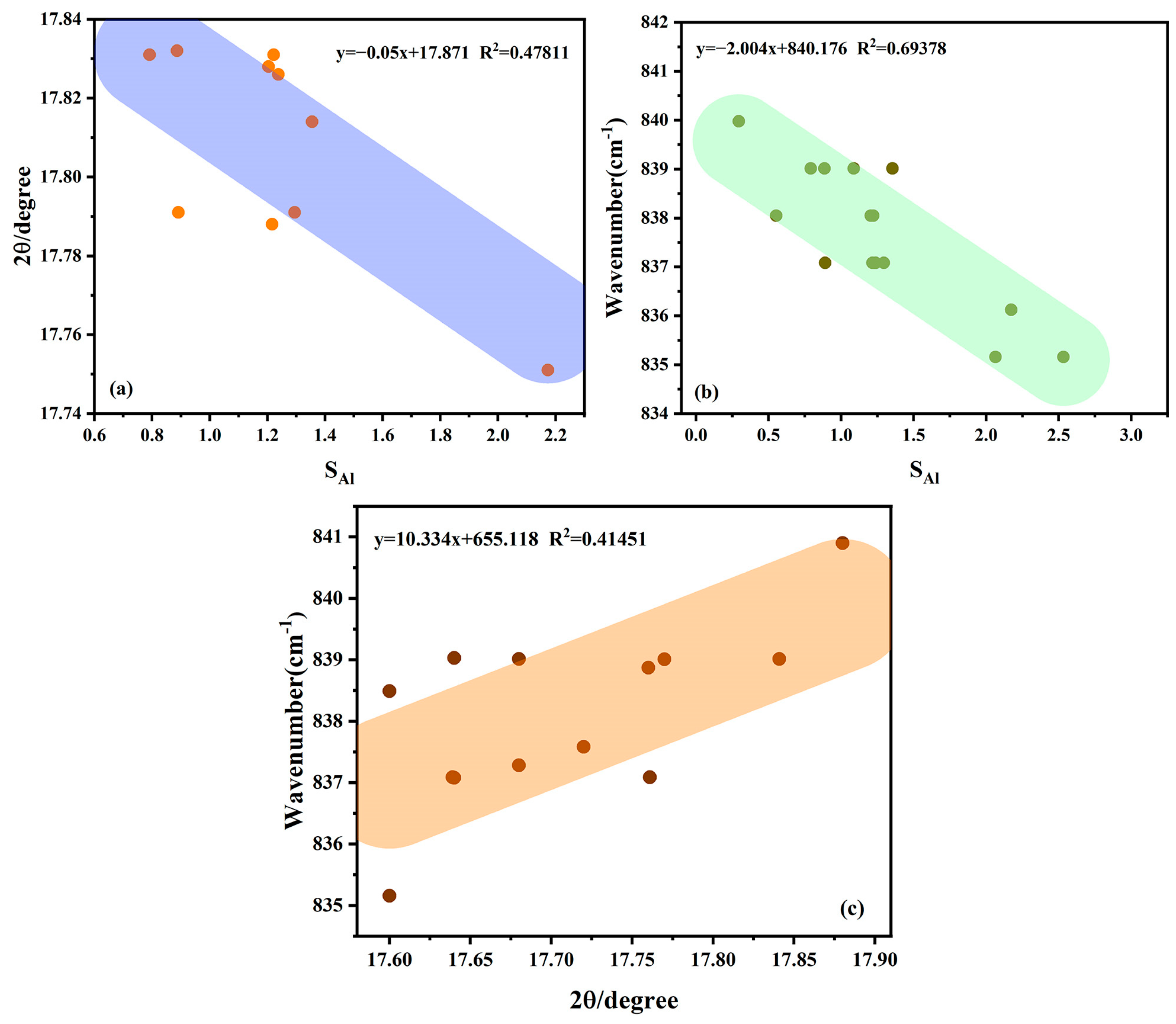
3.3. Infrared Spectroscopic Testing
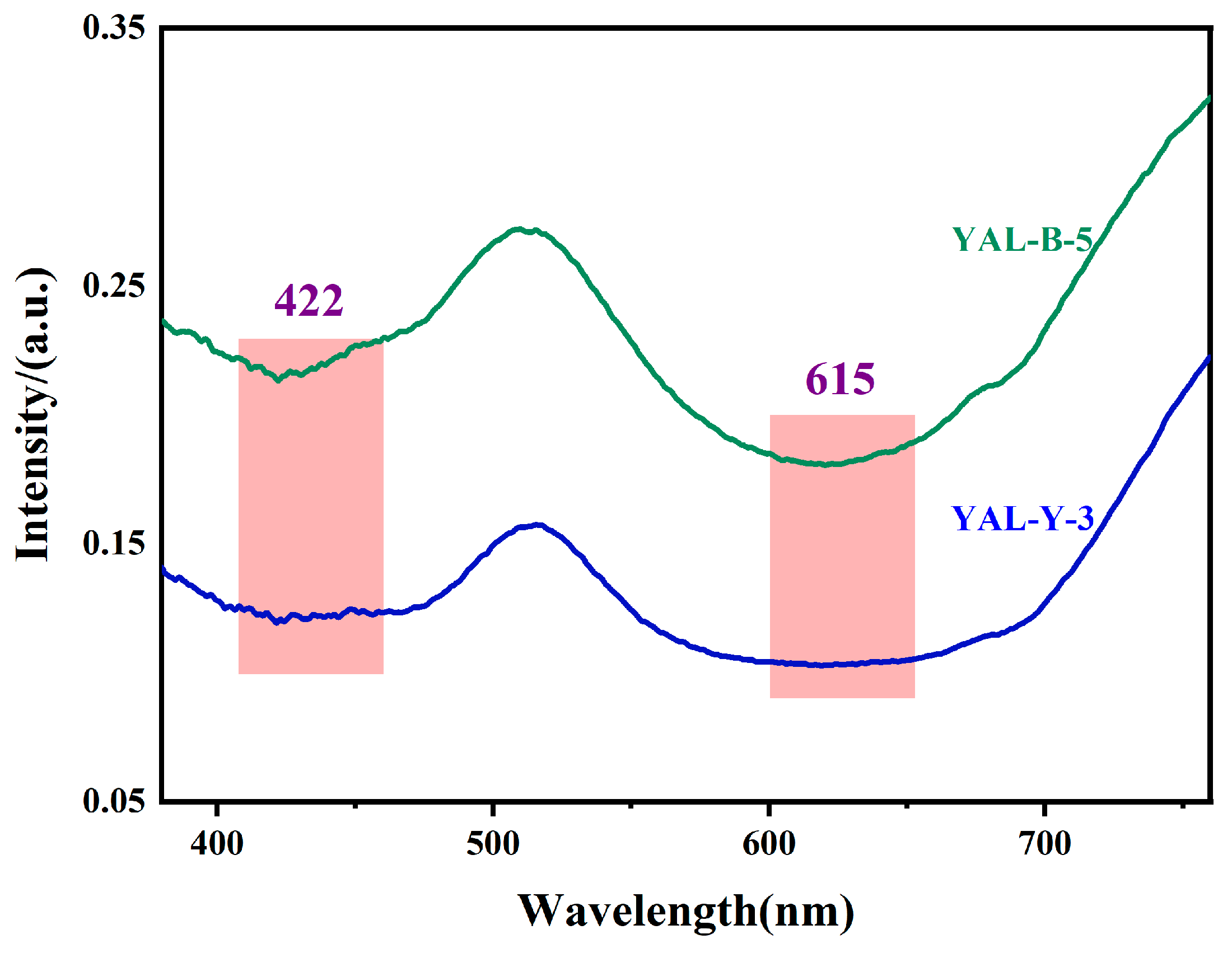
3.4. Ultraviolet–Visible Spectroscopy Tests
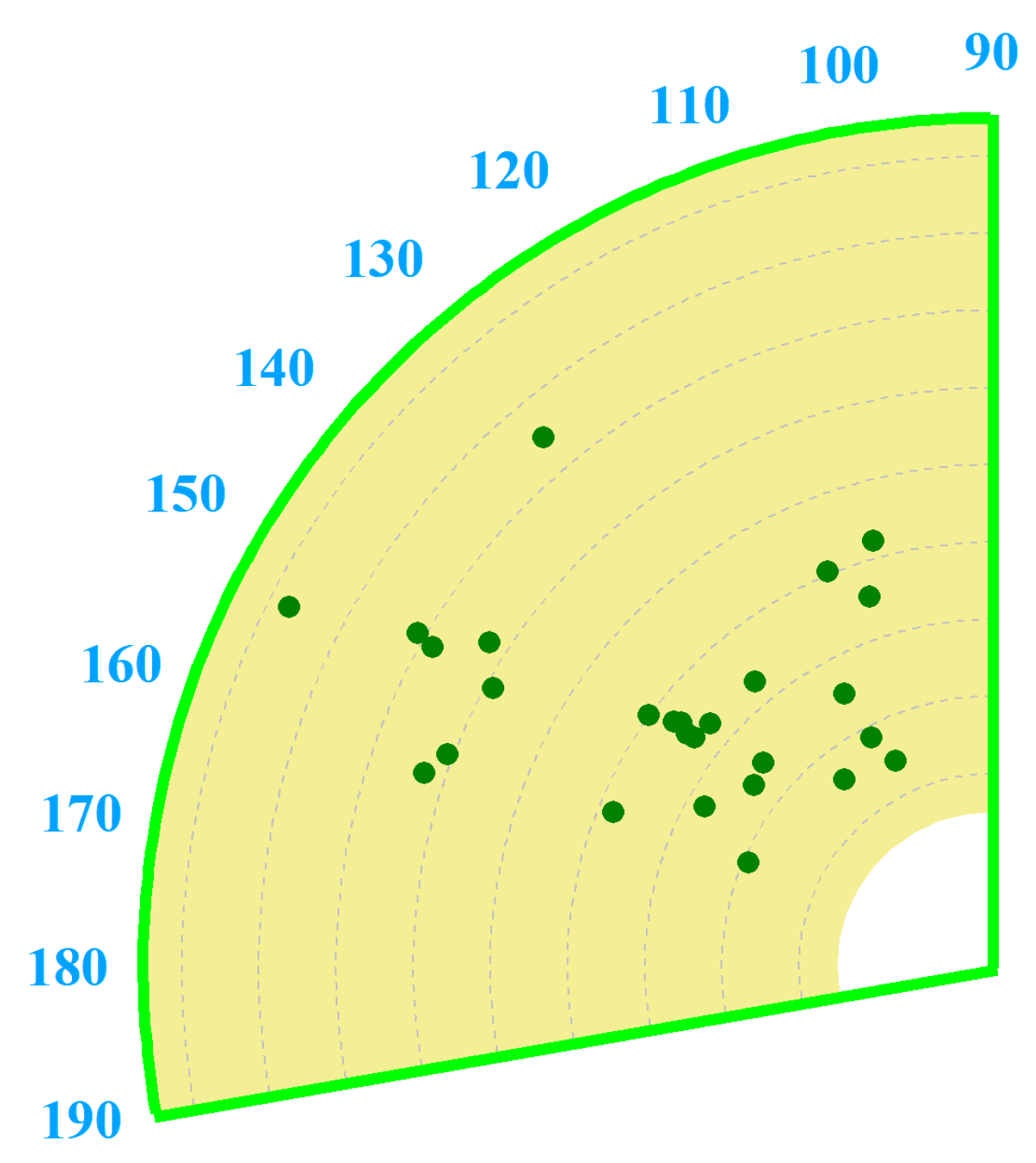
3.5. Chromaticity Test
4. Discussion
4.1. Physical Phase and Spectroscopic Discussion
4.2. Chromaticity Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.M. Gemological and Mineralogical Study of “Xi’an Green” and “Ya’an Green”. Fujian Anal. Test. 2021, 30, 45–49. [Google Scholar]
- Li, J.; Liu, Y. Mineralogical Characteristics of Muscovite-Rutile Seal Stones. Mineral. Petrol. 2023, 43, 11–17. [Google Scholar]
- Zhao, Z.; Liu, K.; Zheng, H.; Chen, T. Gemological and Mineralogical Characteristics of “Ya’an Green” Seal Stones. J. Gems Gemol. 2024, 26, 47–56. [Google Scholar]
- Yuan, Y.; Shi, G.; He, M.; Liu, Y. Application of Spectroscopic Studies in Seal Stones: A Case Study of Changhua Stone. Mineral. Petrol. 2011, 31, 18–24. [Google Scholar]
- Chen, T.; Han, W.; Li, T.; Zhou, Q. Mineralogical Characteristics of Muscovite in Three Types of Green Seal Stones from China. Earth Sci. J. China Univ. Geosci. 2012, 37, 981–988. [Google Scholar]
- Chen, X.; Chen, T. Gemological and Mineralogical Characteristics of Qingtian “Longdan Stone”. In Proceedings of the International Academic Exchange Conference on Jewelry and Precious Stones of China, Beijing, China, 13–14 November 2019. [Google Scholar]
- Brigatti, F.M.; Frigieri, P.; Poppi, L. Crystal Chemistry of Mg-, Fe-bearing Muscovites-2M1. Am. Mineral. 2015, 83, 775–785. [Google Scholar] [CrossRef]
- Giovanni, F.; Gabriella, I. Structural Features of Micas. Rev. Mineral. Geochem. 2002, 46, 117–153. [Google Scholar]
- Zhang, Z.; Dai, J.; Wang, X.; Hu, Z.; Wan, X.; Peng, B.; Fu, M. Application of Spectroscopic Characteristics of Muscovite in Porphyry Tungsten Deposits: A Case Study Involving the Shimensi Deposit in Northern Jiangxi. Minerals 2023, 13, 256. [Google Scholar] [CrossRef]
- Zhou, W.B.; Qin, D.M.; Wang, H.T.; Chen, T.; Wang, Z.W. Gemological, Mineralogical and Spectroscopic Study of Indian “Longdan Stone”. Spectrosc. Spectr. Anal. 2023, 43, 1895–1899. [Google Scholar]
- Jin, X.; Li, G.; Liao, B.; Li, X.; Long, C. Gemological and vibrational spectroscopic characterization of a mica-quartz jade ‘strawberry crystal’. J. Gems Gemol. 2021, 23, 19–28. [Google Scholar]
- Williams, Q.; Knittle, E.; Scott, H.P.; Liu, Z. The High-Pressure Behavior of Micas: Vibrational Spectra of Muscovite, Biotite, and Phlogopite to 30 GPa. Am. Mineral. 2015, 97, 241–252. [Google Scholar] [CrossRef]
- Zhang, M.; Redfern, S.A.T.; Salje, E.K.H.; Carpenter, M.A.; Hayward, C.L. Thermal Behavior of Vibrational Phonons and Hydroxyls of Muscovite in Dehydroxylation: In Situ High-Temperature Infrared Spectroscopic Investigations. Am. Mineral. 2010, 95, 1444–1457. [Google Scholar] [CrossRef]
- Maksimovic, Z.; White, J.L.; Logar, M. Chromium-bearing Dickite and Chromium-bearing Kaolinite from Teslic, Yugoslavia. Clays Clay Miner. 1981, 29, 213–218. [Google Scholar] [CrossRef]
- Dyar, M.D.; Schaefer, M.W.; Sklute, E.C.; Bishop, J.L. Mössbauer Spectroscopy of Phyllosilicates: Effects of Fitting Models on Recoil-free Fractions and Redox Ratios. Clay Miner. J. Clay Miner. Group Mineral. Soc. 2008, 43, 3–33. [Google Scholar] [CrossRef]
- Yan, X.; Yue, S.; Lv, X. Gemological Characteristics and Color Origin Analysis of Green Kyanite. Laser Optoelectron. Prog. 2021, 58, 1516029. [Google Scholar]
- Xu, M.; Zu, E.; Zhan, J. Colorimetric Study of Nanhong Agate. J. Kunming Univ. Sci. Technol. Nat. Sci. Ed. 2017, 42, 13–18. [Google Scholar]

| Sample | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | Cr2O3 | FeO |
|---|---|---|---|---|---|---|---|---|---|
| YAL-Y-1 | 0.044 | 0.019 | 44.530 | 43.522 | 9.731 | 0.000 | 0.302 | 0.016 | 1.836 |
| YAL-Y-1.2 | 1.007 | 0.330 | 41.649 | 43.374 | 11.437 | 0.060 | 0.059 | 0.046 | 2.039 |
| YAL-G-1 | 0.026 | 0.018 | 46.184 | 41.780 | 9.945 | 0.000 | 0.744 | 0.037 | 1.266 |
| YAL-G-5 | 0.035 | 0.022 | 44.594 | 44.586 | 9.548 | 0.000 | 0.910 | 0.013 | 0.294 |
| YAL-G-5.2 | 0.234 | 0.010 | 50.067 | 44.066 | 4.429 | 0.002 | 0.926 | 0.010 | 0.256 |
| YAL-Y-4.2 | 0.061 | 0.015 | 46.558 | 43.096 | 9.399 | 0.000 | 0.680 | 0.006 | 0.185 |
| YAL-Y-4 | 0.032 | 0.029 | 43.900 | 44.629 | 10.217 | 0.000 | 0.905 | 0.022 | 0.266 |
| YAL-Y-5 | 0.034 | 0.017 | 46.291 | 42.677 | 10.207 | 0.006 | 0.545 | 0.013 | 0.210 |
| YAL-Y-5.2 | 0.017 | 0.012 | 46.684 | 41.492 | 10.512 | 0.000 | 1.086 | 0.021 | 0.176 |
| YAL-G-4.2 | 0.087 | 0.022 | 46.207 | 42.900 | 9.719 | 0.000 | 0.852 | 0.004 | 0.209 |
| YAL-Y-2 | 8.760 | 0.178 | 43.500 | 41.664 | 4.927 | 0.250 | 0.083 | 0.150 | 0.489 |
| YAL-Y-3.2 | 0.071 | 0.013 | 45.069 | 44.176 | 10.390 | 0.001 | 0.042 | 0.003 | 0.236 |
| YAL-Y-3 | 0.014 | 0.024 | 43.691 | 44.844 | 10.561 | 0.000 | 0.507 | 0.022 | 0.339 |
| YAL-G-4 | 0.073 | 0.016 | 45.937 | 43.068 | 9.705 | 0.000 | 0.983 | 0.002 | 0.216 |
| YAL-G-1 | 0.028 | 0.019 | 47.755 | 40.862 | 10.000 | 0.000 | 0.302 | 0.004 | 1.030 |
| YAL-B-5.2 | 0.157 | 0.008 | 47.111 | 45.574 | 6.605 | 0.004 | 0.185 | 0.018 | 0.338 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, Y.; Wang, Z.; Zhang, Z.; Xie, M.; Peng, Z.; Meng, B.; Yang, S.; Zu, E. Ion Substitution Behavior and Chromatographic Study of “Ya’an Green” Seal Stone. Crystals 2025, 15, 420. https://doi.org/10.3390/cryst15050420
Sun Y, Wang Y, Wang Z, Zhang Z, Xie M, Peng Z, Meng B, Yang S, Zu E. Ion Substitution Behavior and Chromatographic Study of “Ya’an Green” Seal Stone. Crystals. 2025; 15(5):420. https://doi.org/10.3390/cryst15050420
Chicago/Turabian StyleSun, Yicong, Yigeng Wang, Zixuan Wang, Zheng Zhang, Mingming Xie, Zhuchun Peng, Bin Meng, Siqi Yang, and Endong Zu. 2025. "Ion Substitution Behavior and Chromatographic Study of “Ya’an Green” Seal Stone" Crystals 15, no. 5: 420. https://doi.org/10.3390/cryst15050420
APA StyleSun, Y., Wang, Y., Wang, Z., Zhang, Z., Xie, M., Peng, Z., Meng, B., Yang, S., & Zu, E. (2025). Ion Substitution Behavior and Chromatographic Study of “Ya’an Green” Seal Stone. Crystals, 15(5), 420. https://doi.org/10.3390/cryst15050420






