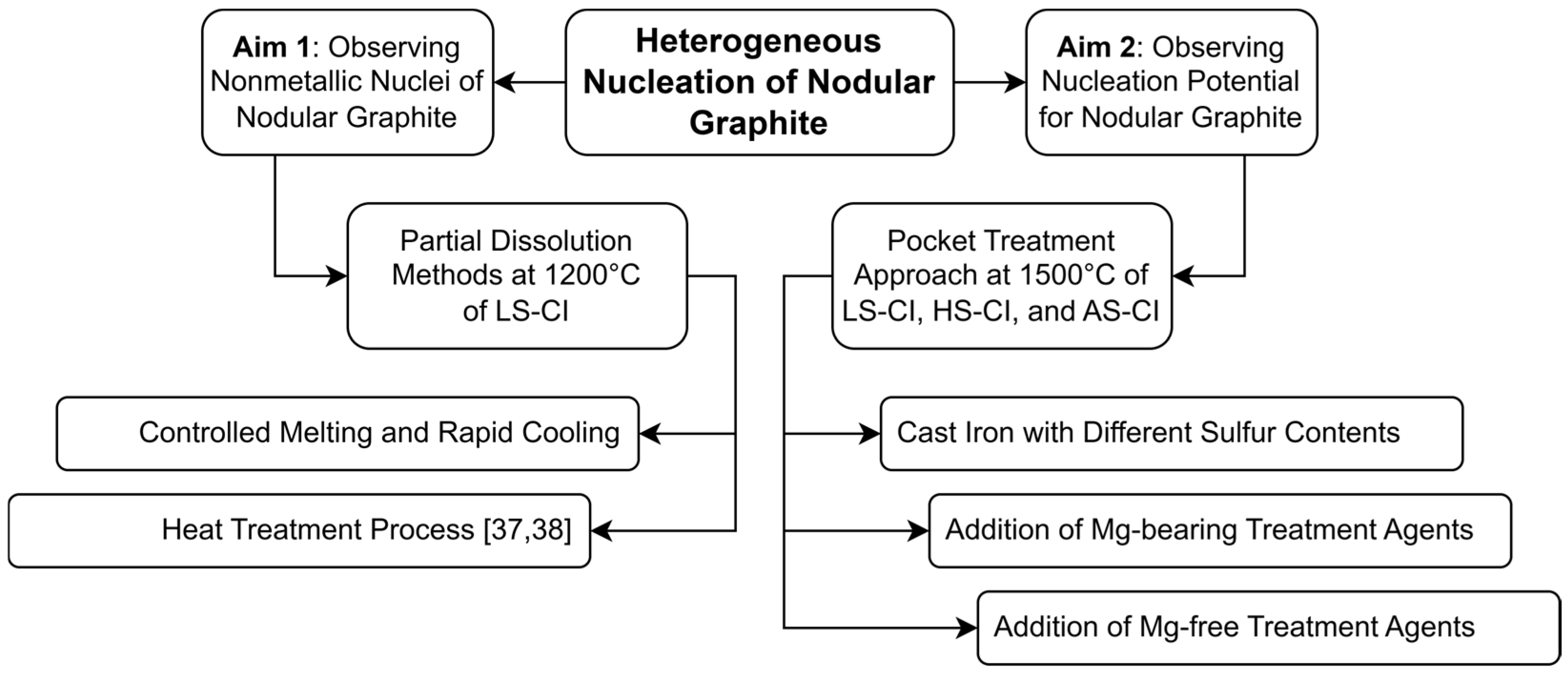
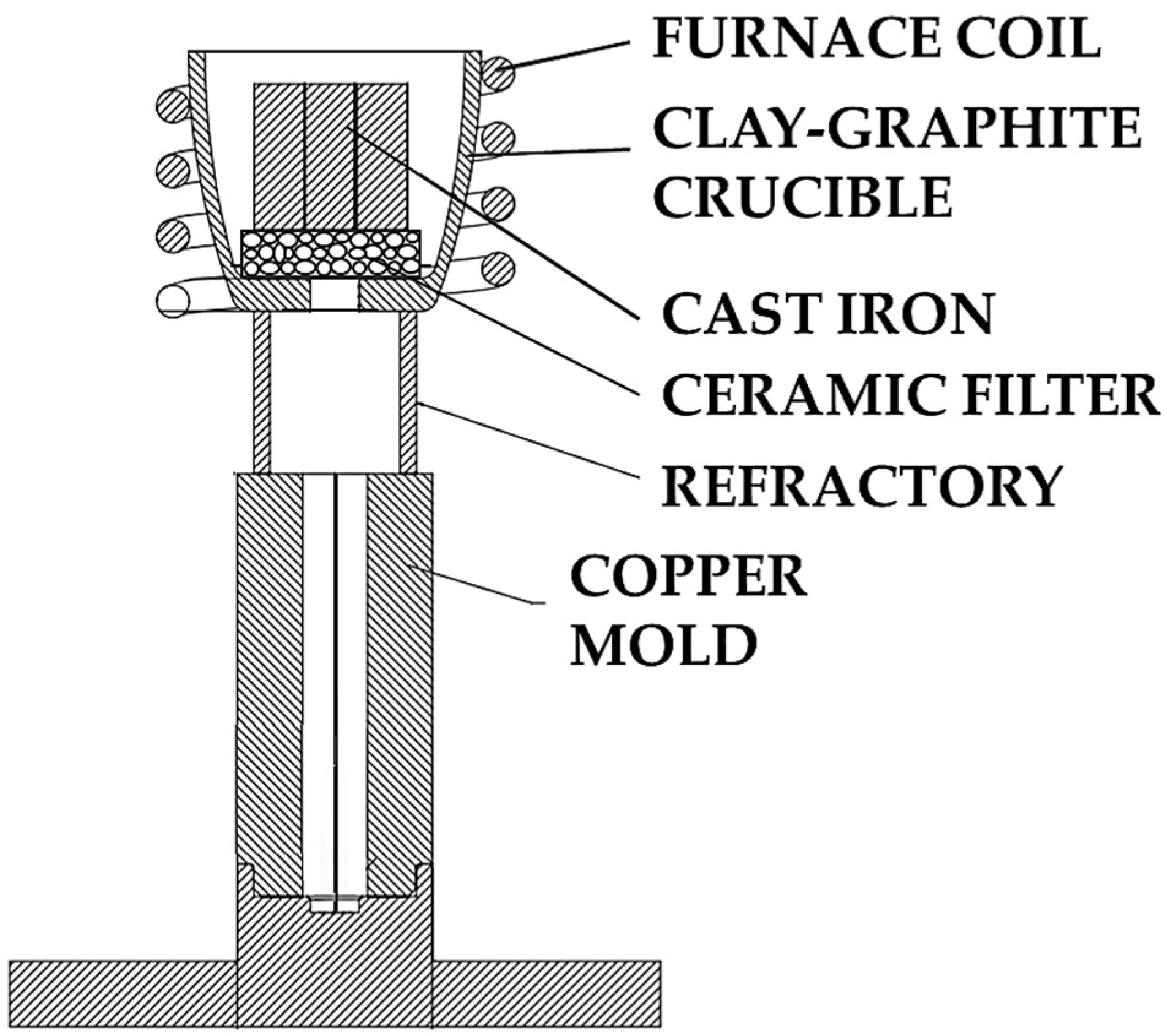
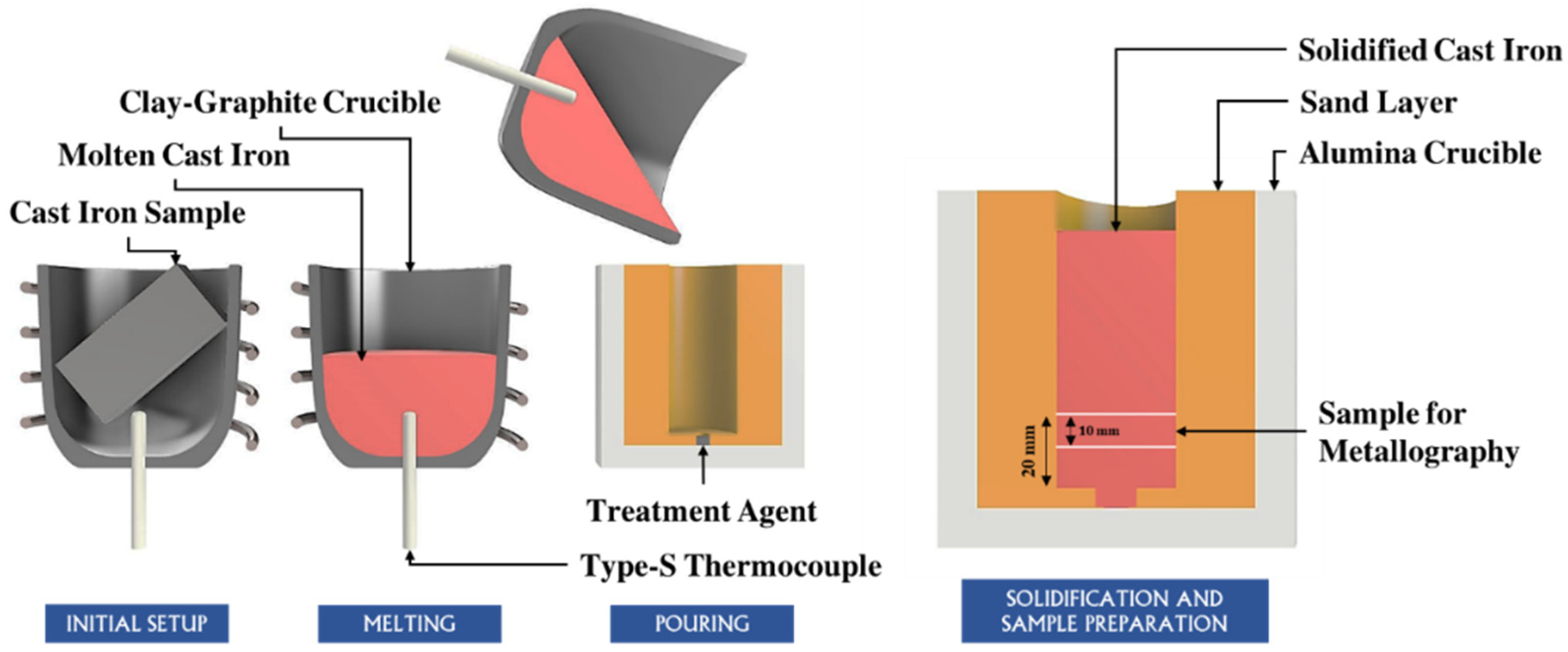
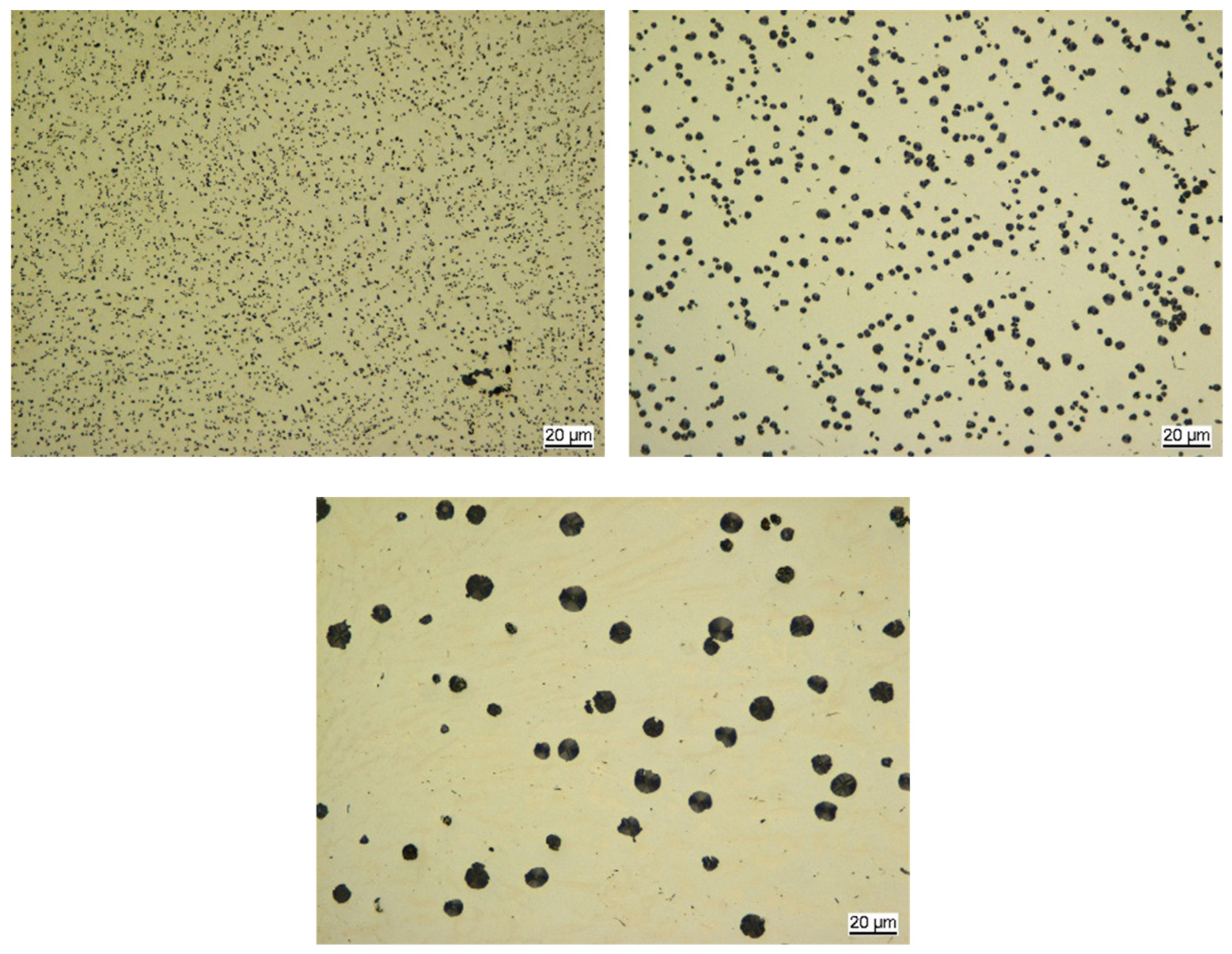
4.1. The Interplay Between Inclusions, Liquid Composition, and Graphite Development
As shown in
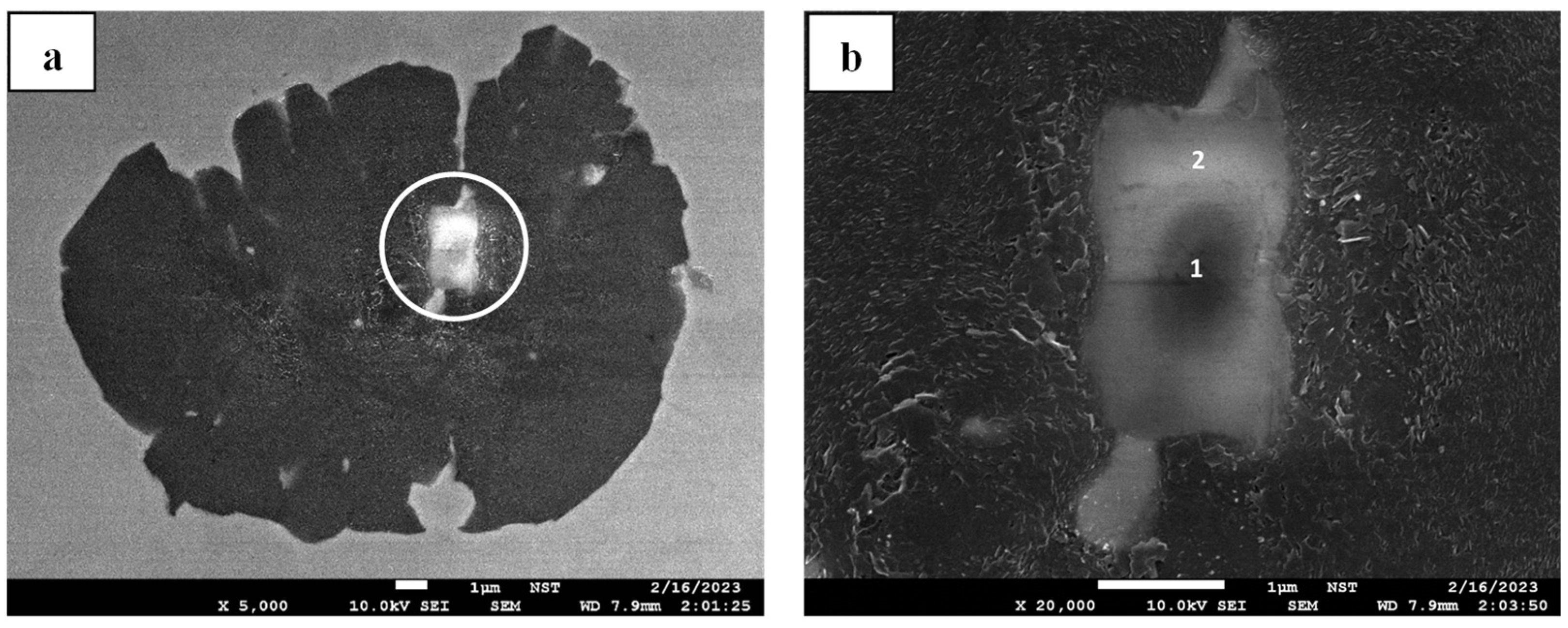
Figure 8, detecting an Mg-O-based system in the center of a well-preserved graphite nodule suggests that this nodular graphite nucleated heterogeneously. However, this establishment of Mg-O alone does not guarantee that the nucleated graphite grows as nodular graphite. As captured in
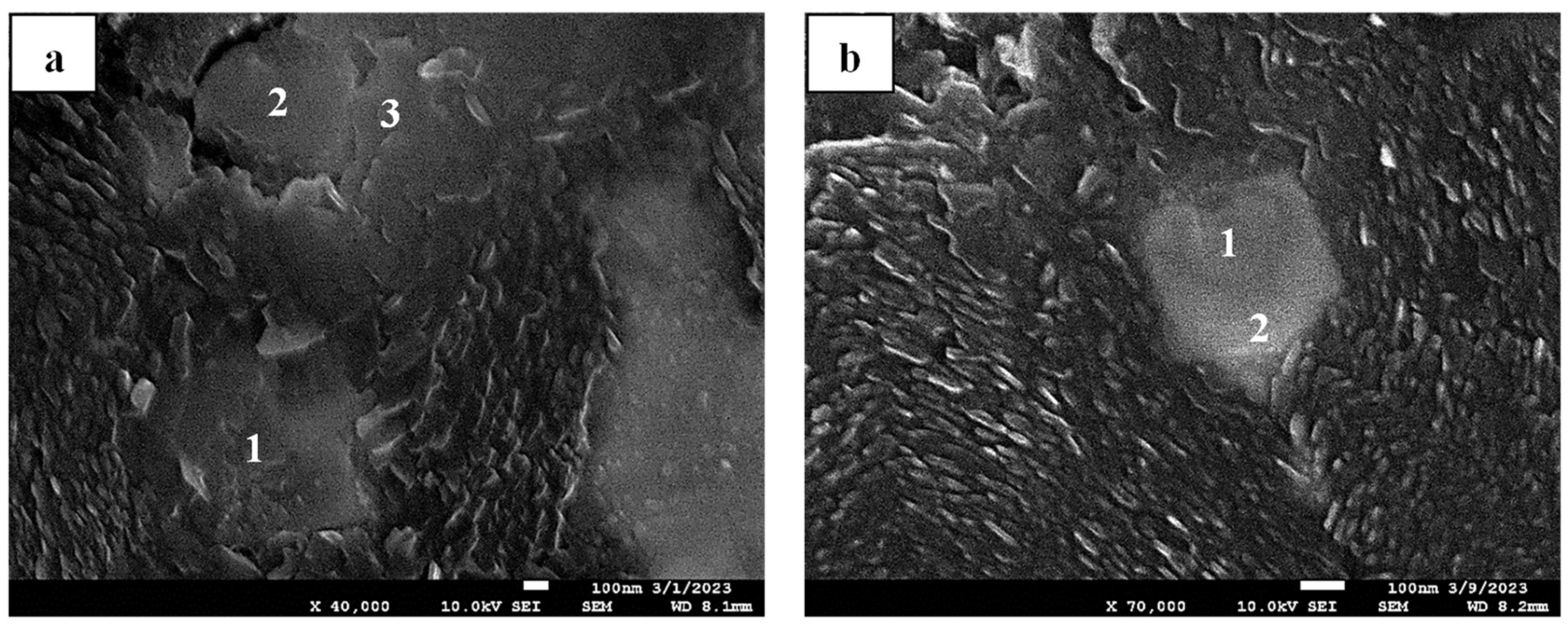
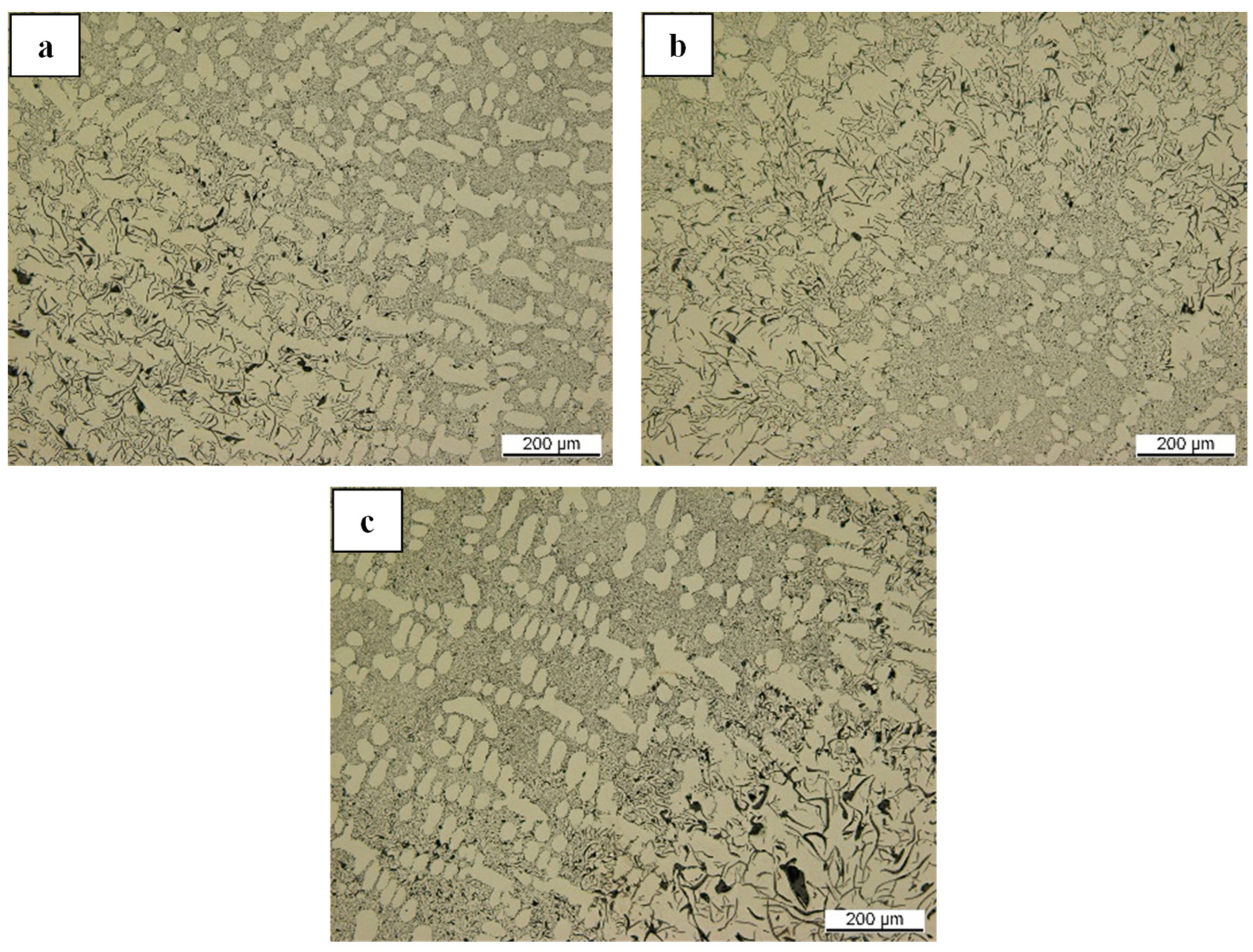
Figure 10b and
Figure 21a, distinct graphite morphologies of nodular and lamellar graphite are captured, respectively, despite possessing a comparable composition of nonmetallic compounds as their heterogeneous nucleation sites by exhibiting a certain extent of Mg-O and Ti-N configurations. In this context, despite originating from a similar LS-CI sample, the examination in
Figure 21a resulted from the re-melting process at 1500 °C (in
Figure 15a) instead of at 1200 °C for controlled dissolution (as in
Figure 10b); hence, significant fading of the remaining magnesium (measured at less than 30 ppm) is highly anticipated. Based on these findings, it is suggested that graphite nucleation should not always immediately start with the availability of the Mg-O nonmetallic system since it can also provide a nucleation site for another compound before (or parallel with) graphite nucleation.
Moreover, although nonmetallic inclusions might induce or trigger graphite nucleation, the abovementioned results reaffirm that the concentration of magnesium in molten iron has a more pivotal role in determining the nucleation and growth of nodular graphite, as further amplified in
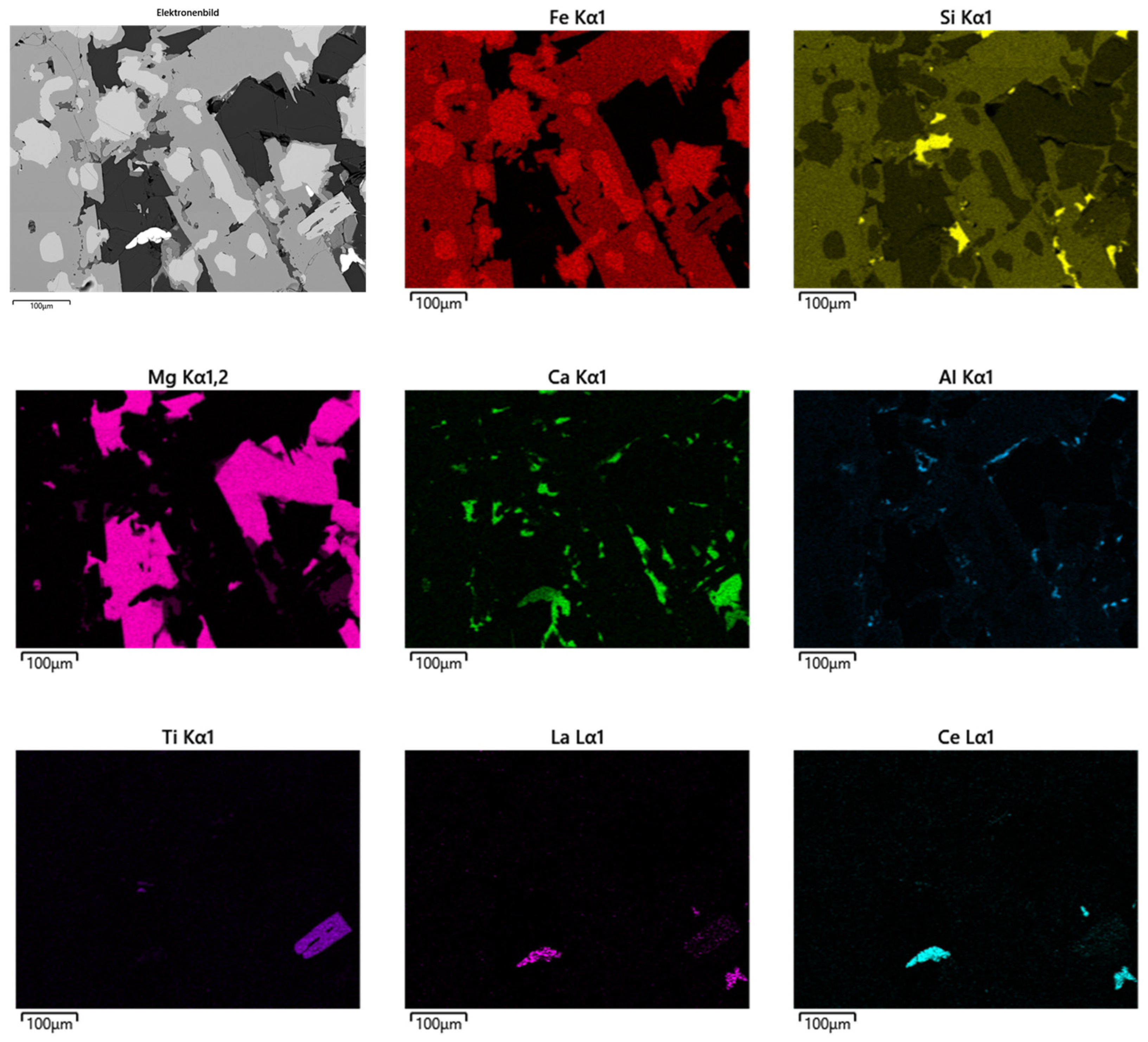
Figure 16, which aligns well with [
41,
42]. On the other hand, it is also essential to note that the residual dissolved magnesium concentration is related to the dissolved oxygen content in molten cast iron [
43]; thus, the presence of Mg-O should be attributed to the oxidation of magnesium. This formation is crucial because only the Mg-O system established during the process can preferably serve as an effective graphite nucleation site since the direct introduction of MgO particles has been industrially proven to be a disadvantage [
44].
The residual sulfur concentration could also influence the remaining dissolved magnesium in molten cast iron [
45]. However, it is worth noting that the desulfurization reaction to MgS can proceed just after a specific low level of dissolved oxygen has been attained [
46]. In the context of nucleation, this reaction will produce the Mg-S inclusion system, which could serve as an effective nucleation site for graphite as either a dominant compound (
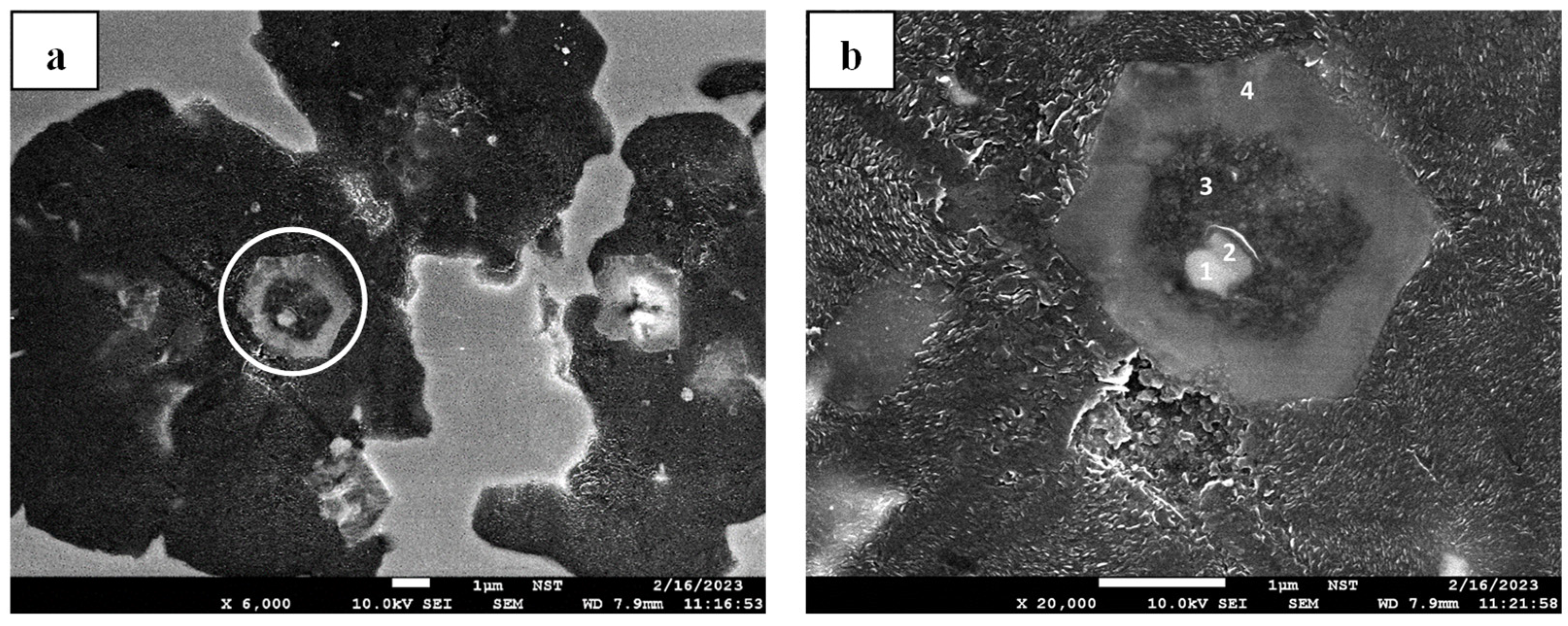
Figure 22b) or coexist with Mg-O (
Figure 9). As already explored in the Mg-O system, solely establishing the Mg-S inclusion system could not determine whether the graphite would nucleate on its surface. Concurrently, assuming that graphite nucleation has started, it does not necessarily mean that a nodular graphite structure will develop, as shown in
Figure 21b, where a lamellar graphite structure grows instead.
Given the circumstances of the abovementioned observed sample (HS-CI with 0.1 wt.% S with 10 g of FeSiMg6 as a pocket treatment agent captured in
Figure 17c), the formation of the Mg-S system is expected to occur early during solidification [
47]. However, no graphite nucleation was directly started, and sulfide formation consisting of Mg-Mn was preferred because of the limited amount of magnesium compared to sulfur content. This sulfide facilitates the nucleation and growth of lamellar graphite, amplifying the results documented in [
48]. Furthermore, this sulfide system also provides a nucleation site for an oxy-sulfide containing Ce and La originating from FeSiMg6, as suggested in [
36]. Hence, as depicted in
Figure 17, enhancing the previous results documented in [
49,
50], achieving certain low levels of sulfur and oxygen is crucial for promoting the growth of nodular graphite.
Despite the anticipated low solubility limit in molten cast iron [
51,
52], the introduction of magnesium-bearing materials should be followed by a dissolution process if the dissolved oxygen and sulfur concentrations are maintained at lower levels than the critical thresholds. Considering that FeSiMg6 was utilized, the introduction of Mg involves the dissolution of Mg
2Si, which has a melting point of ca. 1100 °C [
53]. In this instance, the graphite is expected to be able to start its nucleation at an early stage of dissolution, as indicated in
Figure 22c, where high Mg and Si contents are detected.
On the other hand, this inhomogeneous dispersion could also induce nitridation of Mg
2Si, producing the core structure shown in
Figure 6b. This nitrogen interaction arises from the decreasing solubility with increasing concentrations of carbon and silicon [
54,
55], which could be triggered by segregation associated with FeSi dissolution. Moreover, since the silicon content increases, the solubility of carbon decreases [
56] and might be, to a certain extent, favorable for graphite nucleation on MgSiN
2, forming the structure depicted in
Figure 12. Similarly, since FeSi has no direct nodularization effect, as shown in
Figure 20, which strengthens previous findings in [
23,
57], the nucleated graphite could only eventually develop into a nodular structure because of the low oxygen and sulfur concentrations coupled with a sufficient dissolved magnesium concentration in molten cast iron.
4.2. The Importance of Inclusion and Magnesium to the Growth of Nodular Graphite
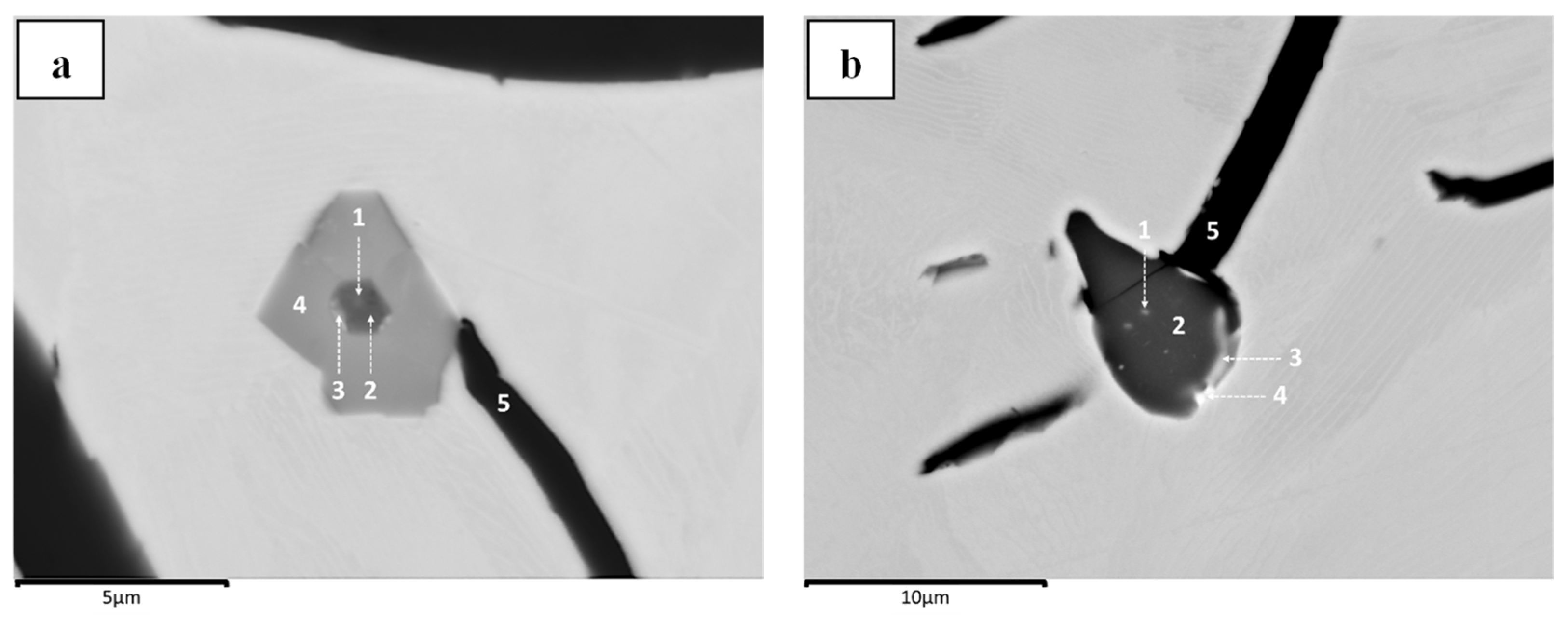
Since the composition of inclusions varies despite originating from one iron sample, it is suggested that no definite format is required for graphite nucleation. This case is supported by the fact that the graphite structure can nucleate on a single system based on magnesium (
Figure 8), silicon (
Figure 11a), and cerium (
Figure 6c), as well as start congruently with the nucleation chain as in staged (
Figure 13), compound (
Figure 10a) and complex (
Figure 14) configurations. The formation of this variance also suggests that either inhomogeneity or segregation was involved in inclusion formation, as supported by the results of
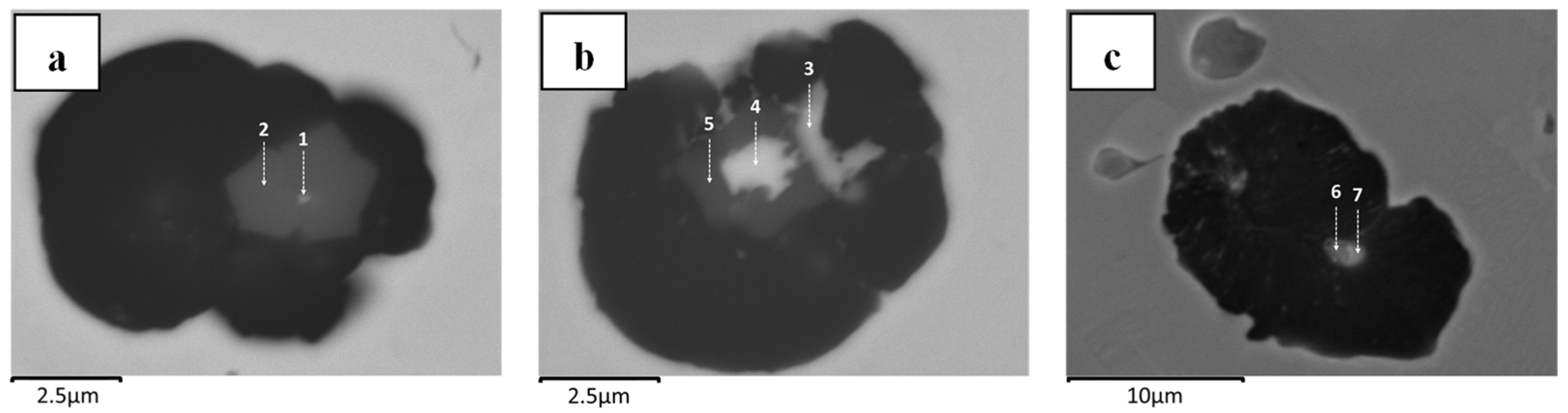
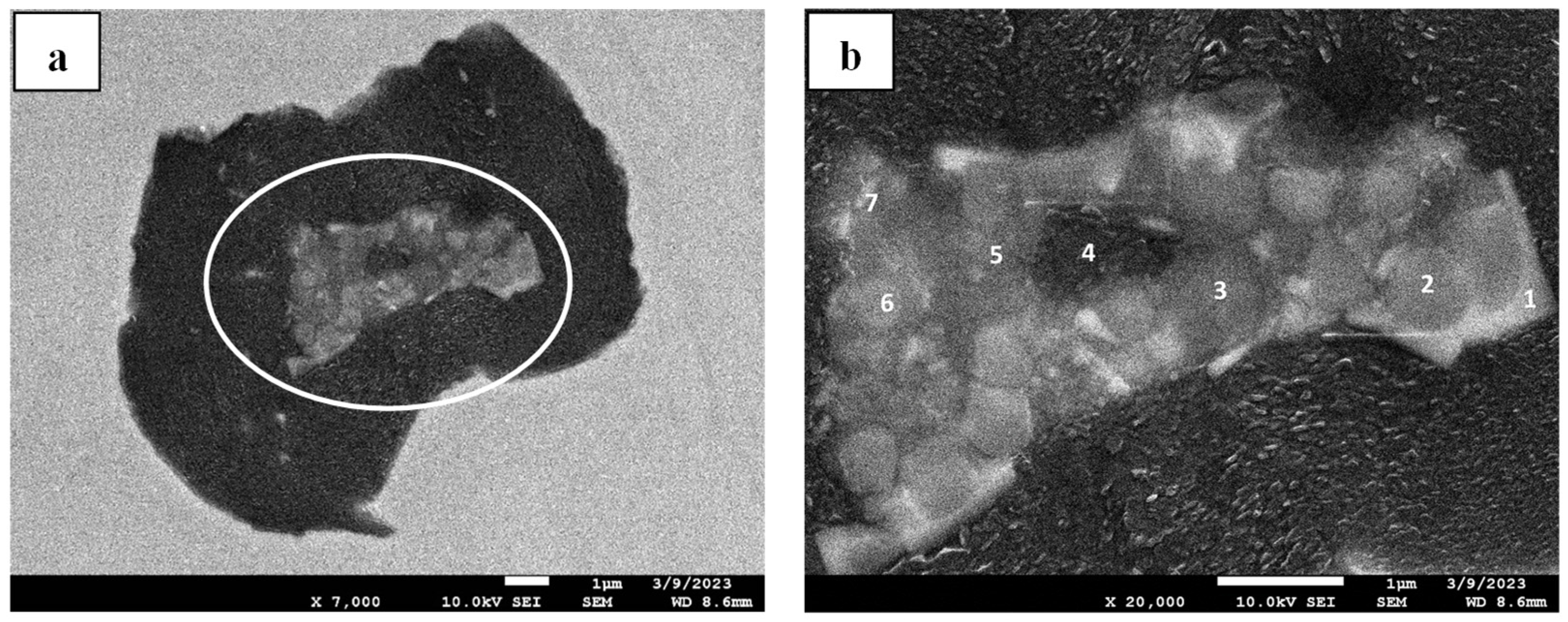
Figure 22. In this case, three different inclusions were found in one similar sample of HS-CI in
Figure 17b. These chemical compositions should be thermodynamically chronological (A1: Mg-O system first—then B2: Mg-S format—and eventually C3: Mg-Si) yet detected as a coexistence.
The findings of this study also substantiate that there is no direct relationship between the composition or morphology of inclusions and the growth of nodular graphite. In addition to the case explored earlier, despite being revealed as a nucleus in
Figure 11a, the utilization of SiC as a pocket treatment agent showed no nodularization effect, which is analogous to the direct involvement of MgO. In the case of morphology, as highlighted in
Figure 23, despite the similar nucleus shape as a predominant Al-O system was established, the associated graphite developed toward a different structure (coexists in one similar HS-CI in
Figure 17b). Accordingly, it is suggested that the (local) liquid composition plays a pivotal role in the nucleation and growth of nodular graphite, which might be associated with the availability of dissolved magnesium, sulfur, and oxygen.
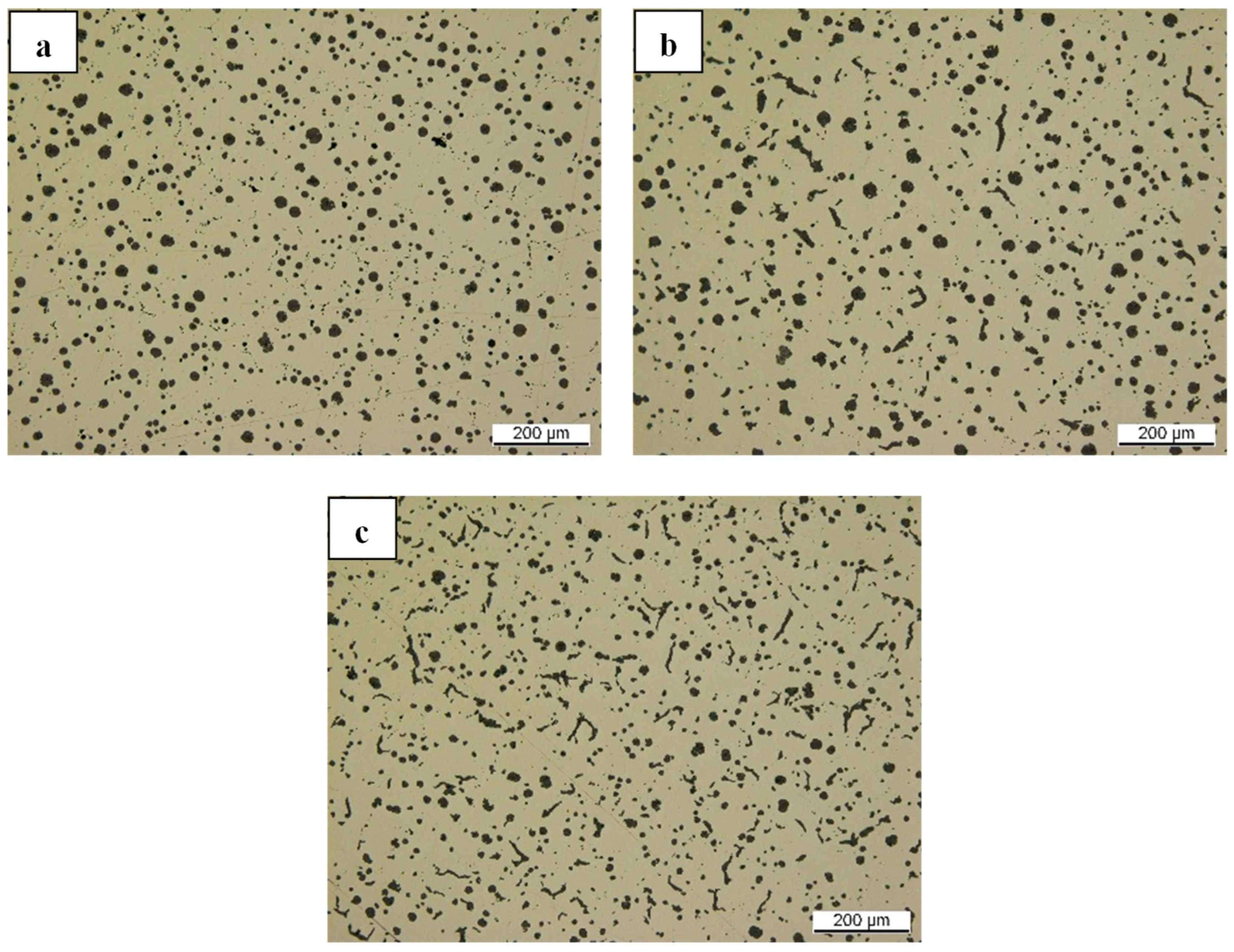
The role of magnesium in promoting the nucleation and growth of nodular graphite can also be observed as other Mg-bearing materials have been utilized. As indicated in
Figure 18a,b, where Mg-AlSn and NiMg15 were employed, respectively, nodular graphite was established despite having a white iron matrix structure, as shown in
Figure 24a,b. This carbide formation could be associated with Mg-overtreatment, as demonstrated in [
58]. In this case, it is induced by either excessive addition or more efficient dissolution than FeSiMg6, as delineated in [
59]. However, magnesium is not the only available alternative to produce nodular graphite, as revealed in
Figure 18c. In this case, FeSi-CeLa (ca. 17 wt.% Ce and 14 wt.% La) was utilized as a pocket treatment agent without the involvement of magnesium. Compared with Mg-AlSn and NiMg15, the cast iron matrix also indicates a carbide system, as depicted in
Figure 24c. These instances are congruently related to the possible excessive addition of Ce and La, which is also reported to be responsible for the observed deteriorated graphite structures, as provided in [
60].
Calcium is also assumed as an element that promotes the nodularization of graphite in cast iron. Compared with magnesium, calcium has a high affinity for oxygen and sulfur and is thus commonly employed in deep desulfurization in the iron industry [
61,
62,
63]. The solubilities of both magnesium and calcium in iron at 1600 °C are significantly low, ranging from 0.023 to 0.059 wt.% [
51,
52] and 0.024 to 0.046 wt.% [
64,
65,
66], respectively. However, despite the resemblance in characteristics, no convincing nodularization effect can be observed, as shown in
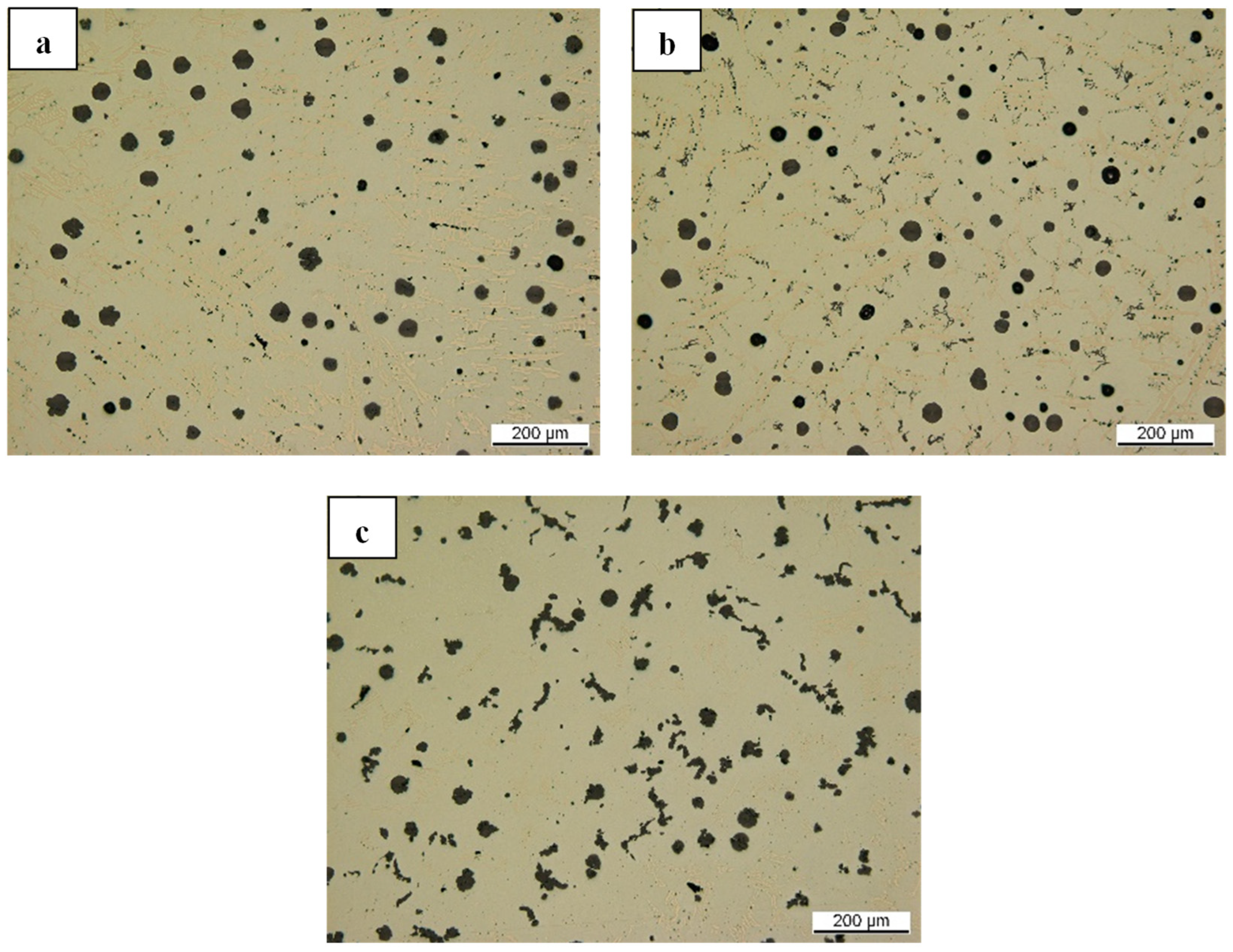
Figure 19, including by employing Ca and CaC
2 in a similar trial setup (melting at 1500 °C and a pocket treatment).
In fact, there are only two published reports related to the nodularization process of SGI using CaSi [
67,
68] and one for Ca [
69]. Based on the reported utilization of CaSi, a certain amount of Mg was measured in the CaSi employed in the studies; thus, distinguishing the effects of Mg or Ca given the total amount of approximately 5 to 10 wt.% introduced into the molten iron is unclear. Considering only the Ca-metal reported in [
69], the expected quality of graphite nodularity was proven unattained. Moreover, the charge materials used during the investigation were pure Fe, metallic Si, and graphite, thus questioning the role of Ca since a similar approach was able to produce a treatment-free nodular graphite structure in cast iron [
70], which will be explored in the next section.
4.3. Theoretical Dynamics in the Nucleation and Growth of Nodular Graphites
Based on the current results, two prerequisites are needed to produce commercial SGI: a low concentration of sulfur and oxygen coupled with the addition of magnesium or cerium-lanthanum. Since Mg, Ce, and La react with sulfur and oxygen during the treatment process, it is relatively easy to conclude that these metals act only as scavengers of surface-active elements, as proposed by Jung et al. [
71]. In this context, the results of the present study suggest that they might not be utterly true since the use of Ca-bearing materials did not result in positive nodularity. Accordingly, a direct role of Mg, Ce, and La should also be expected during the growth of nodular graphite. Notably, heterogeneous nucleation cannot be perceived as indirect, as once proven by Dhindaw and Verhoeven [
72] in the vacuum melting of synthetic Fe-C-Si alloys. Based on their report, nodular graphite can form at a specific cooling rate if high-purity iron is used, but this is not the case when ultrahigh-purity iron is utilized.
Consequently, it is convincing that the cause of nodular graphite establishment in synthetic Fe-C-Si alloys is, to a certain extent, comparable with that in commercial SGI, as revealed in the present study, particularly in explaining the role of Mg, Ce, and La. As key findings provided in [
70,
71,
72], a high cooling rate and superheating are necessary to ensure the formation of nodular graphite in a carbide-containing cast iron matrix. These two factors are also known in foundry practice to be correlated with high undercooling, thus increasing the chill tendency, carbide formation, and carbon supersaturation degree of molten iron [
73,
74]. This carbon supersaturation as a determining factor in the growth of nodular graphite, as reported in [
75], could be the intersection between synthetic and commercial SGI. As indicated in
Figure 24, spheroidal graphite within a carbide-containing matrix associated with an excessive introduction of Mg, Ce, and La is also identified. It is correlated with the characteristics of those elements as carbide former, and their addition increases the degree of undercooling [
58,
76], contrasting with Ca, which tends to act as a graphitizer [
69]. Accordingly, the supplementary role of Mg, Ca, and La in commercial SGI might be associated with increasing the degree of carbon supersaturation coupled with low sulfur and oxygen concentrations.
Despite the rational resonance between both phenomena in synthetic and commercial SGI regarding carbon supersaturation, further empirical investigations are necessary to describe precisely the mechanism of this complex and dynamic interaction. The hypothetical explanation might be related to the carbon solubility and activity since those factors are correlated with the dissolved sulfur and oxygen concentrations [
77,
78]. Furthermore, since carbide formation is involved, it is also conventionally known that the carbide stabilizers reduce the activity of carbon [
79] as well as carbon equivalent (CE) [
80] in molten iron, which might not be the case for Mg, Ce, and La. Specifically for Mg, the shift in the CE value and saturation degree is substantiated by a review of several experimental results provided in [
81], which suggests that adding Mg during the treatment of molten iron displaces the liquidus line to the left. This shift would change the measured liquidus temperature, which is typically associated with an increasing CE. Since the solubility of Mg in austenite is negligible, the lowering liquidus temperature at the hypoeutectic composition indicates that the bulk of molten cast iron solidifies in supercooled circumstances, known as constitutional undercooling.