An Insight into Synthesis, Optical Properties, and Applications of Green Fluorescent Carbon Dots
Abstract
1. Introduction
2. Challenges of Conventional Synthesis Methods for Nanoparticles
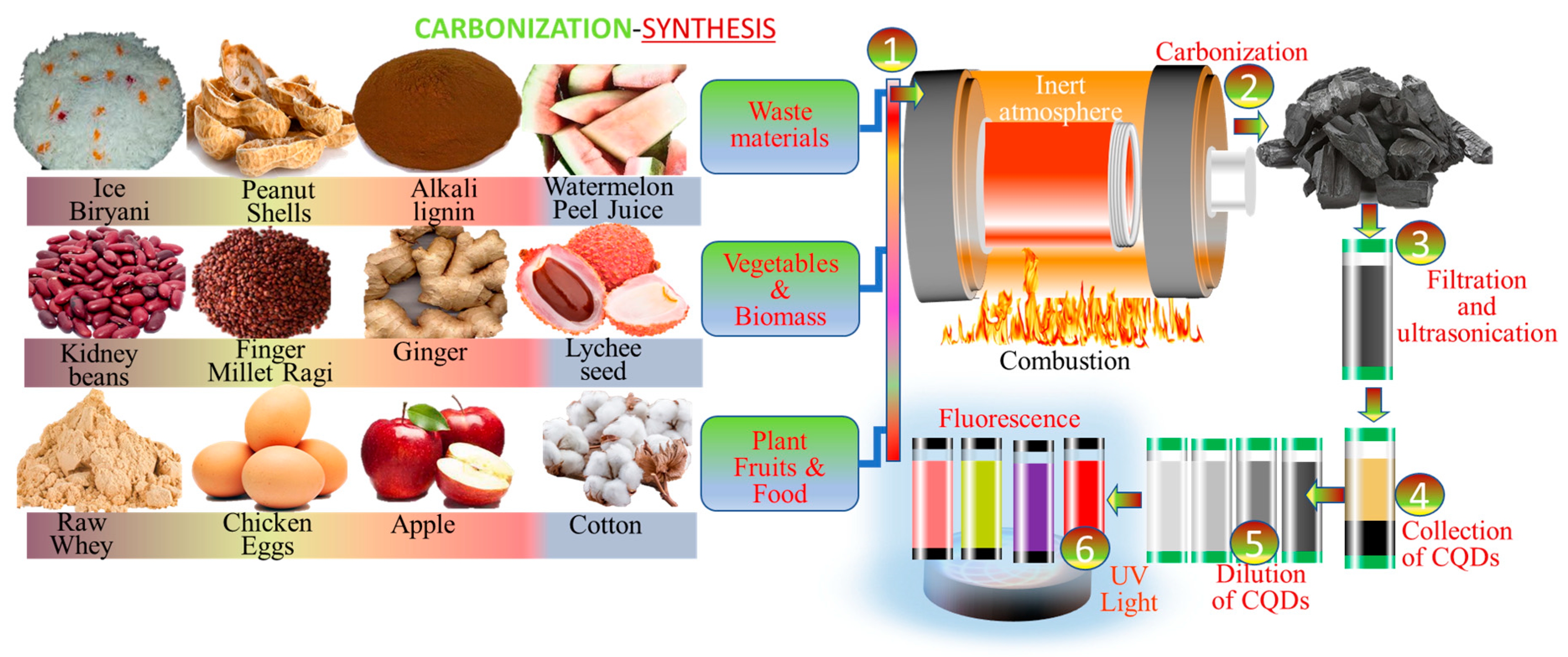
3. Green Synthesis Method of Carbon Dots
3.1. Hydrothermal Assisted Synthesis of CDs
3.2. Microwave-Assisted Synthesis of CDs
3.3. Pyrolysis Treatment Assisted Synthesis of CDs
| Precursor Type | Precursors Used | Molar Mass/Molar Ratio | Crystal Size/Particle Size (nm) | Optimum Parameters | Applications | Ref. |
|---|---|---|---|---|---|---|
| Waste polyolefins | MM 0.033 g/mL | PS 70 | PT120 °C for 12 h, S-700 W for 2 h and Dr-72 h | Sensing and live cell imaging | [104] | |
| Tea and peanut shells | MR 0.6 | PS 7–9 | PT 200 °C for 4 h, S-15 min, and Dr-24 h | Biomarkers, ion detection, and photocatalysis | [105] | |
| Peanut shells | MM 0.00025 g/mL | PS 4.26 | PT 200 °C for 15 min, Dr-24 h, and P-65 °C for 22 h | Sensing | [106] | |
| Tea leaf residue | MM 0.2 g/mL | PS 2 | CT 350 °C for 2 h, Cen-4000 rpm, and pH-7 | Bioimaging | [107] | |
| Biomass residue | MM 0.2 g/mL | PT 300 °C to 500 °C for 5 °C/min, and Cen-19,000 rpm | Efficient surfactants | [108] | ||
| Durian peel waste | MM 0.1 g/mL | PT-250 °C for 5 h, S-30 min, and C-10,000 rpm for 15 min | Supercapacitor | [109] | ||
| Gram peel | -- | 5.5 for C1 20 nm for C2 | PT 200 °C and 450 °C for 8 h | Ultrafast response humidity sensor | [110] | |
| Mango peels | MM 0.03 g/mL | PS 3 | CT 300 °C for 2 h | Detection of ferrous succinate, biological imaging | [111] | |
| Vegetables | Allium sativum (garlic) | MM 0.1 g/mL | PS 2 | PT 315 °C for 3 h and S-15 min | Photobleaching, solar conversion, and in vitro cell imaging | [112] |
| Zingiberis rhizome | -- | CS 0.2 | PT 300, 350, and 400 °C for 0.5, 1, and 1.5 h, Dr-72 h, and Cen-11,000 rpm for 30 min | Drug delivery | [113] | |
| Biomass | Konjac flour | MM 0.05 g/mL | PS 3.37 | PT 470 °C for 1.5 h | Bioimaging | [102] |
| Finger millet ragi (Eleusine Coracana) | MM 0.01 g/mL | PS 6 | PT 80 °C for 5 h T-300 °C for 5 °C /min | Detection of Cu2+ ions | [114] | |
| Seed | Kidney beans | -- | PS 20–30 | PT 450 °C for 2 h and pH-7 | Biological cell imaging | [103] |
| Fennel seeds | -- | PS 0.22 | PT500 °C for 3 h, S-5 min, and Cen-15,000 rpm for 10 min | LED, bio-sensing, and cellular imaging | [115] | |
| Leaf | Plant leaf | MM 001 g/mL | PS 3.7 | PT 250, 300, 350 and 400 °C for 2 h at 5 °C/min and Cen-12,000 rpm for 10 min | Coding, bioimaging, and drug delivery | [116] |
| Prosopis juliflora leaves | -- | PS 5.8 | PT 200 °C for 30 min followed by grinding to powder and heating at 200 °C for about 1 h. | Sensitive, selective, label-free, and reproducible off–on sensing assay | [117] | |
| Plant | Cotton | MM 0.1 g/mL | PS 4.9 | PT 300 °C for 2 h, Cen-4000 rpm for 10 min, and Dr-2 days | Multi-color imaging, patterning, and sensing | [118] |
| Fruit | Malus domestica (apple) | -- | PS 3 | PT 300 °C for 1 h, S-10 min, Cen-8000 rpm for 5 min, and Dr-24 h | Biosensing and cell imaging | [119] |
| Food | Chicken egg | -- | PS 2.15 | PT 230 °C for 19 min | Printing ink | [120] |
| Raw material | Raw whey | 20 mL | PS 4 | PT180–225 °C for 10 to 40 min | Photocatalysis, biosensing, and drug delivery | [121] |
3.4. Carbonization-Assisted Synthesis of CDs
| Precursor Type | Precursors | M Mass/M Ratio | CS, PS Size (nm) | Optimum Parameters | Applications | Reference |
|---|---|---|---|---|---|---|
| Seed | Lychee seed | MM 0.01 g/mL | PS 1.12 | CT 300 °C for 2 h at 10 °C min−1 | Microbiology, surgery, and in the diagnostic field | [122] |
| Waste material | Alkali lignin | 11.8 g | PS 8 | CT 300 °C for 30 min and RE-55 °C | Biomedicine | [124] |
| Ice-biryani | 1 g | PS 41 | CT 250 °C for 24 h, ST-1200 rpm for 36 h, and pH 1–10 | Bioimaging | [125] | |
| Peanut shell | MM-0.1 g/mL | PS 1.62 | CT 250 °C for 2 h at 10 °C min−1, and pH 3–12 | Cell imaging | [126] | |
| Lorhange | -- | PS 5.72 | CT 220 °C for 2 h, Cen-18,000 rpm for 20 min, and Dr-48 h | Cell imaging | [127] | |
| Fresh oranges and lemons peels | -- | PS 6.5 and 4.5 nm for citrus sinensis and citrus limon | CT 180 °C for 2 h, Cen-8000 rpm for 5 min | Iron and tartrazine sensing and cell imaging | [128] | |
| Leaf | Water hyacinth | -- | PS 5.22 | MT-48 h CT 160 °C at 10 °C min−1, S-30 min, and pH 7 | Sensors | [129] |
| Fruits | Date palm | -- | PS 50 nm | CT 300 °C for couple of hours at a heating rate of 10 °C/min | -- | [123] |
| Mango | MM-0.1 g/mL | S-5 min CT 100 °C for 60 min, and 72 h | Bioimaging | [130] | ||
| Litchi peel | MM-0.0033 g/mL | PS 3.1 | CT 140 °C for 12 h, 24 h, and Cen-16,000 rpm for 15 min | Colorimetric determination of ascorbic acid | [131] |
3.5. Laser Ablation-Assisted Synthesis of CDs
3.6. Ultrasonic Assisted Synthesis of CDs
4. Structure and Properties of Carbon Dots
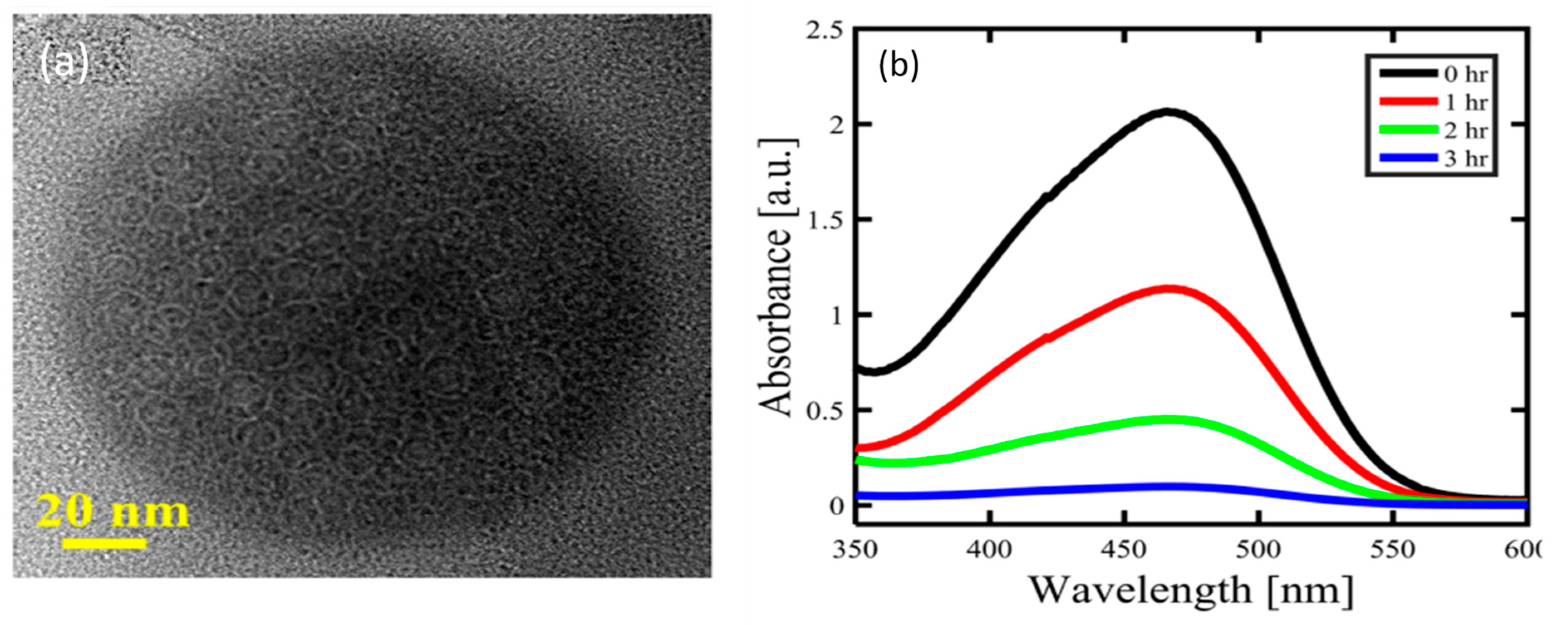
4.1. Structure of Carbon Dots
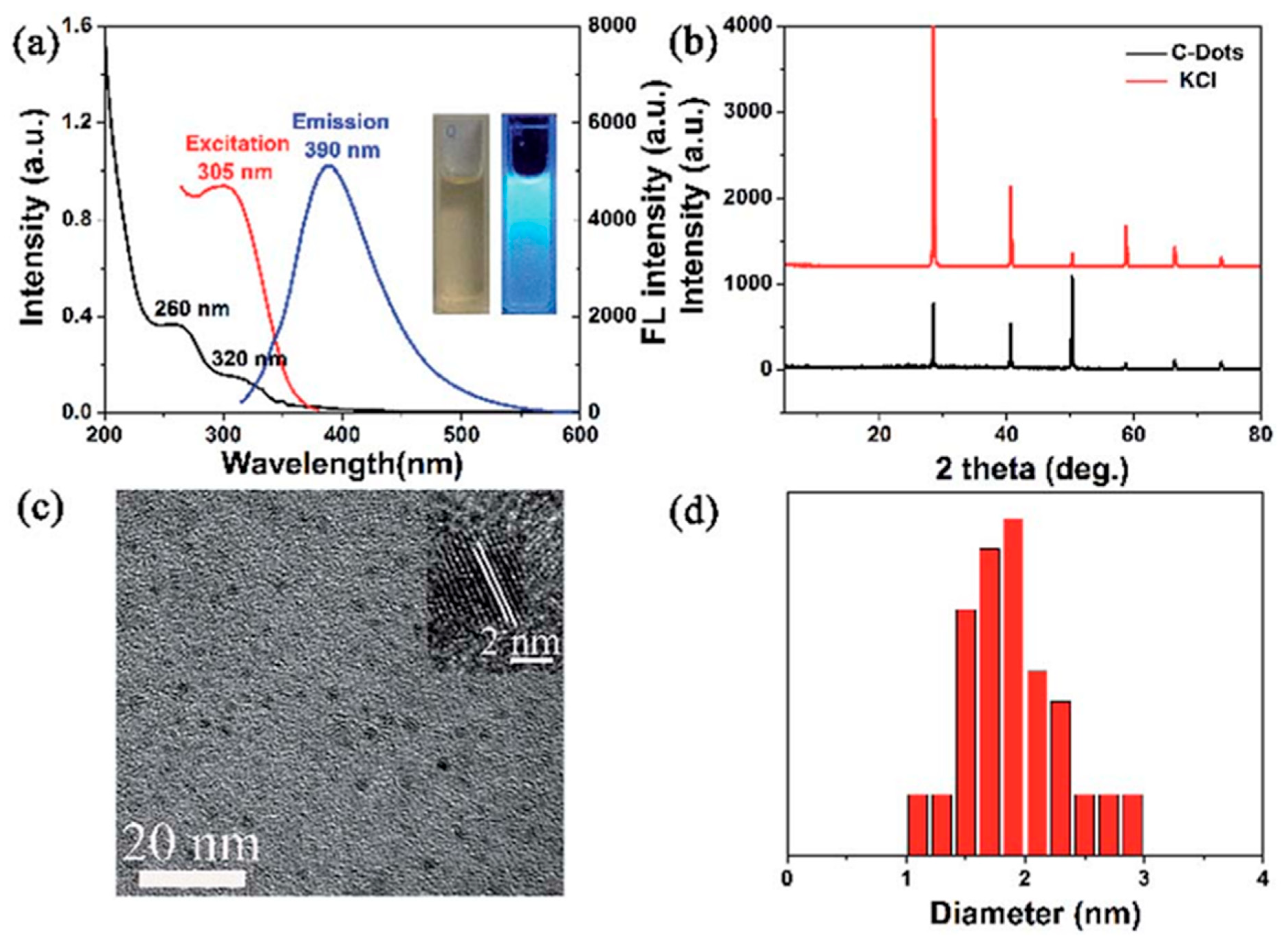
4.2. Luminescence Properties
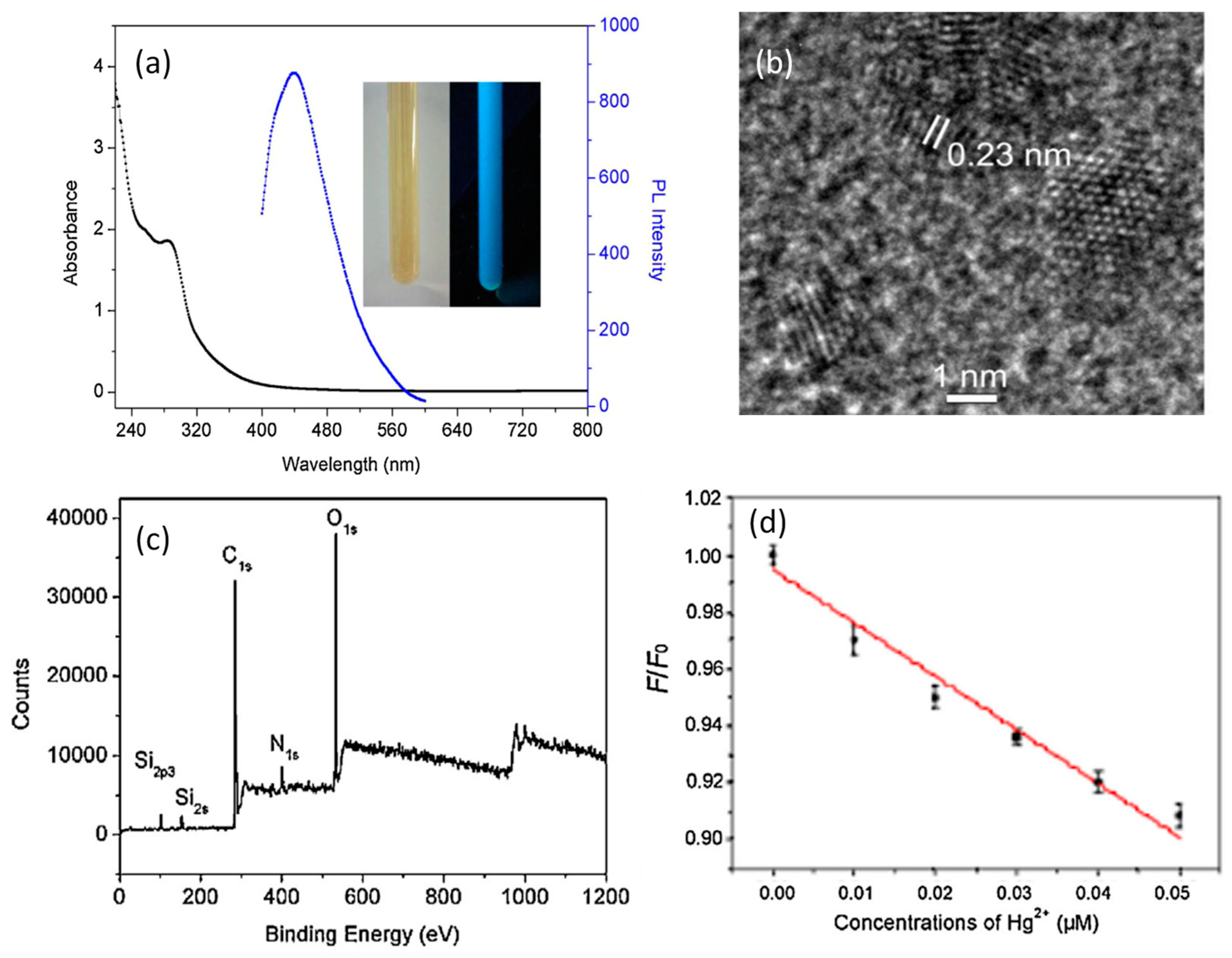
4.3. Optical Properties
4.3.1. UV-Absorption Properties
4.3.2. Emission Property
4.3.3. Toxicity
4.3.4. Biocatalyst
5. Applications
5.1. Catalysis
5.1.1. Photocatalysis
5.1.2. Other Catalysis
5.2. Sensors
5.2.1. Metal Ion Sensors
5.2.2. Biosensors
5.3. Bioimaging
5.4. Drug Delivery
5.5. Hybridization of CDs with Other Functional Materials like Liquid Crystals
6. Outlook and Summary
Funding
Data Availability Statement
Conflicts of Interest
References
- Cohen, I.B. A History of Luminescence From the Earliest Times Until 1900. By E. Newton Harvey. [Memoirs of the American Philosophical Society, Volume 44]. (Philadelphia: The Society. 1957. Pp. xxiv, 692. $6.00.). Am. Hist. Rev. 1958, 63, 937–939. [Google Scholar] [CrossRef][Green Version]
- Rahman, A. Solid State Luminescent Materials: Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Francis, P.S.; Hogan, C.F. Chapter 13—Luminescence. In Comprehensive Analytical Chemistry; Kolev, S.D., McKelvie, I.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 54, pp. 343–373. [Google Scholar]
- Murthy, K.V.R.; Virk, H. Luminescence Phenomena: An Introduction. Defect. Diffus. Forum 2013, 347, 1–34. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Gayen, B.; Palchoudhury, S.; Chowdhury, J. Carbon Dots: A Mystic Star in the World of Nanoscience. J. Nanomater. 2019, 2019, 3451307. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Microchim. Acta 2018, 185, 424. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.; Lee, N.Y. Green synthesis of carbon quantum dots and their environmental applications. Environ. Res. 2022, 212, 113283. [Google Scholar] [CrossRef]
- Ganesh, S.S.; Anushikaa, R.; Swetha Victoria, V.S.; Lavanya, K.; Shanmugavadivu, A.; Selvamurugan, N. Recent Advancements in Electrospun Chitin and Chitosan Nanofibers for Bone Tissue Engineering Applications. J. Funct. Biomater. 2023, 14, 288. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, P.; Wen, F.; Xu, Q.; Guo, Q.; Su, W. Green synthesis of carbon quantum dots from plant turmeric holds promise as novel photosensitizer for in vitro photodynamic antimicrobial activity. J. Mater. Res. Technol. 2023, 22, 17–34. [Google Scholar] [CrossRef]
- Portela, C.I.; Vieira, N.C.S.; Brazil, T.R.; Giroto, A.S.; Gabriel Filho, J.B.; Gonçalves, M. Utilization of biodiesel residue through efficient microwave-assisted synthesis of carbon quantum dots: A versatile nanomaterial for environmental remediation. Environ. Res. 2025, 264, 120311. [Google Scholar] [CrossRef] [PubMed]
- Parambil, A.M.; Rajan, S.; Huang, P.-C.; Shashikumar, U.; Tsai, P.-C.; Rajamani, P.; Lin, Y.-C.; Ponnusamy, V.K. Carbon and graphene quantum dots based architectonics for efficient aqueous decontamination by adsorption chromatography technique—Current state and prospects. Environ. Res. 2024, 251, 118541. [Google Scholar] [CrossRef] [PubMed]
- Gedda, G.; Sankaranarayanan, S.A.; Putta, C.L.; Gudimella, K.K.; Rengan, A.K.; Girma, W.M. Green synthesis of multi-functional carbon dots from medicinal plant leaves for antimicrobial, antioxidant, and bioimaging applications. Sci. Rep. 2023, 13, 6371. [Google Scholar] [CrossRef]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. Environ. Sci. Health. Part C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Hegde, K.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Current understandings of toxicity, risks and regulations of engineered nanoparticles with respect to environmental microorganisms. Nanotechnol. Environ. Eng. 2016, 1, 5. [Google Scholar] [CrossRef]
- Yang, H.-L.; Bai, L.-F.; Geng, Z.-R.; Chen, H.; Xu, L.-T.; Xie, Y.-C.; Wang, D.-J.; Gu, H.-W.; Wang, X.-M. Carbon quantum dots: Preparation, optical properties, and biomedical applications. Mater. Today Adv. 2023, 18, 100376. [Google Scholar] [CrossRef]
- Kaushal, S.; Kumari, V.; Singh, P.P. Sunlight-driven photocatalytic degradation of ciprofloxacin and organic dyes by biosynthesized rGO–ZrO2 nanocomposites. Environ. Sci. Pollut. Res. 2023, 30, 65602–65617. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Devatha, C.P.; Thalla, A.K. Green Synthesis of Nanomaterials. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 169–184. [Google Scholar]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, R.; Kumar, K.; Thakur, N. Sustainable applications of biowaste-derived carbon dots in eco-friendly technological advancements: A review. Mater. Sci. Eng. B 2024, 305, 117414. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Shabbir, H.; Tokarski, T.; Ungor, D.; Wojnicki, M. Eco Friendly Synthesis of Carbon Dot by Hydrothermal Method for Metal Ions Salt Identification. Materials 2021, 14, 7604. [Google Scholar] [CrossRef]
- Ferjani, H.; Abdalla, S.; Oyewo, O.A.; Onwudiwe, D.C. Facile synthesis of carbon dots by the hydrothermal carbonization of avocado peels and evaluation of the photocatalytic property. Inorg. Chem. Commun. 2024, 160, 111866. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef]
- Sachdev, A.; Gopinath, P. Green synthesis of multifunctional carbon dots from coriander leaves and their potential application as antioxidants, sensors and bioimaging agents. Analyst 2015, 140, 4260–4269. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Sethuraman, M.G.; Lee, Y.R. Efficient synthesis of highly fluorescent nitrogen-doped carbon dots for cell imaging using unripe fruit extract of Prunus mume. Appl. Surf. Sci. 2016, 384, 432–441. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.-J.; Lee, Y.R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B Chem. 2017, 246, 497–509. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Nitrogen-doped carbon dots originating from unripe peach for fluorescent bioimaging and electrocatalytic oxygen reduction reaction. J. Colloid Interface Sci. 2016, 482, 8–18. [Google Scholar] [CrossRef]
- Lai, Z.; Guo, X.; Cheng, Z.; Ruan, G.; Du, F. Green Synthesis of Fluorescent Carbon Dots from Cherry Tomatoes for Highly Effective Detection of Trifluralin Herbicide in Soil Samples. ChemistrySelect 2020, 5, 1956–1960. [Google Scholar] [CrossRef]
- Carvalho, J.; Santos, L.; Germino, J.; Terezo, A.; Moreto, J.; Quites, F.; Freitas, R. Hydrothermal Synthesis to Water-stable Luminescent Carbon Dots from Acerola Fruit for Photoluminescent Composites Preparation and its Application as Sensors. Mater. Res. 2019, 22, e20180920. [Google Scholar] [CrossRef]
- Hoan, B.T.; Tam, P.D.; Pham, V.-H. Green Synthesis of Highly Luminescent Carbon Quantum Dots from Lemon Juice. J. Nanotechnol. 2019, 2019, 2852816. [Google Scholar] [CrossRef]
- Kasibabu, B.S.; D’Souza, S.L.; Jha, S.; Kailasa, S.K. Imaging of Bacterial and Fungal Cells Using Fluorescent Carbon Dots Prepared from Carica papaya Juice. J. Fluoresc. 2015, 25, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.N.; Jha, S.; Kailasa, S.K. One-pot green synthesis of carbon dots by using Saccharum officinarum juice for fluorescent imaging of bacteria (Escherichia coli) and yeast (Saccharomyces cerevisiae) cells. Mater. Sci. Engineering. C Mater. Biol. Appl. 2014, 38, 20–27. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Basu, H.; Singhal, R.K.; Kailasa, S.K. One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sens. Actuators B Chem. 2015, 213, 434–443. [Google Scholar] [CrossRef]
- Fatahi, Z.; Esfandiari, N.; Ehtesabi, H.; Bagheri, Z.; Tavana, H.; Ranjbar, Z.; Latifi, H. Physicochemical and cytotoxicity analysis of green synthesis carbon dots for cell imaging. EXCLI J. 2019, 18, 454–466. [Google Scholar] [CrossRef]
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6, 28633–28639. [Google Scholar] [CrossRef]
- Cheng, C.; Shi, Y.; Li, M.; Xing, M.; Wu, Q. Carbon quantum dots from carbonized walnut shells: Structural evolution, fluorescence characteristics, and intracellular bioimaging. Mater. Sci. Eng. C 2017, 79, 473–480. [Google Scholar] [CrossRef]
- Huang, G.; Chen, X.; Wang, C.; Zheng, H.; Huang, Z.; Chen, D.; Xie, H. Photoluminescent carbon dots derived from sugarcane molasses: Synthesis, properties, and applications. RSC Adv. 2017, 7, 47840–47847. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable synthesis of carbon quantum dots from banana peel waste using hydrothermal process for in vivo bioimaging. Phys. E Low-Dimens. Syst. Nanostructures 2021, 126, 114417. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, F.; Liao, Y.; Wang, F.; Liu, H. Carbon Quantum Dots from Pomelo Peel as Fluorescence Probes for “Turn-Off-On” High-Sensitivity Detection of Fe3+ and L-Cysteine. Molecules 2022, 27, 4099. [Google Scholar] [CrossRef]
- Vandarkuzhali, S.A.A.; Natarajan, S.; Jeyabalan, S.; Sivaraman, G.; Singaravadivel, S.; Muthusubramanian, S.; Viswanathan, B. Pineapple Peel-Derived Carbon Dots: Applications as Sensor, Molecular Keypad Lock, and Memory Device. ACS Omega 2018, 3, 12584–12592. [Google Scholar] [CrossRef]
- Qin, X.; Lu, W.; Asiri, A.M.; Al-youbi, A.; Sun, X. Green, low-cost synthesis of photoluminescent carbon dots by hydrothermal treatment of willow bark and their application as an effective photocatalyst for fabricating Au nanoparticles–reduced graphene oxide nanocomposites for glucose detection. Catal. Sci. Technol. 2013, 3, 1027–1035. [Google Scholar] [CrossRef]
- Raja, D.; Sundaramurthy, D. Facile synthesis of fluorescent carbon quantum dots from Betel leafs (Piper betle) for Fe3+sensing. Mater. Today Proc. 2020, 34, 488–492. [Google Scholar] [CrossRef]
- Atchudan, R.; Gangadaran, P.; Edison, T.N.J.I.; Perumal, S.; Sundramoorthy, A.K.; Vinodh, R.; Rajendran, R.L.; Ahn, B.-C.; Lee, Y.R. Betel leaf derived multicolor emitting carbon dots as a fluorescent probe for imaging mouse normal fibroblast and human thyroid cancer cells. Phys. E Low-Dimens. Syst. Nanostructures 2022, 136, 115010. [Google Scholar] [CrossRef]
- Amer, W.A.; Rehab, A.F.; Abdelghafar, M.E.; Torad, N.L.; Atlam, A.S.; Ayad, M.M. Green synthesis of carbon quantum dots from purslane leaves for the detection of formaldehyde using quartz crystal microbalance. Carbon 2021, 179, 159–171. [Google Scholar] [CrossRef]
- Chellasamy, G.; Arumugasamy, S.K.; Govindaraju, S.; Yun, K. Green synthesized carbon quantum dots from maple tree leaves for biosensing of Cesium and electrocatalytic oxidation of glycerol. Chemosphere 2022, 287, 131915. [Google Scholar] [CrossRef] [PubMed]
- Arumugham, T.; Alagumuthu, M.; Amimodu, R.G.; Munusamy, S.; Iyer, S.K. A sustainable synthesis of green carbon quantum dot (CQD) from Catharanthus roseus (white flowering plant) leaves and investigation of its dual fluorescence responsive behavior in multi-ion detection and biological applications. Sustain. Mater. Technol. 2020, 23, e00138. [Google Scholar] [CrossRef]
- Bano, D.; Kumar, V.; Singh, V.K.; Hasan, S.H. Green synthesis of fluorescent carbon quantum dots for the detection of mercury(ii) and glutathione. N. J. Chem. 2018, 42, 5814–5821. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green synthesis of carbon dots from Ocimum sanctum for effective fluorescent sensing of Pb2+ ions and live cell imaging. Sens. Actuators B Chem. 2017, 242, 679–686. [Google Scholar] [CrossRef]
- Bhatt, S.; Bhatt, M.; Kumar, A.; Vyas, G.; Gajaria, T.; Paul, P. Green route for synthesis of multifunctional fluorescent carbon dots from Tulsi leaves and its application as Cr(VI) sensors, bio-imaging and patterning agents. Colloids Surf. B Biointerfaces 2018, 167, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Asha Jhonsi, M.; Kathiravan, A. Photoinduced interaction of arylamine dye with carbon quantum dots ensued from Centella asiatica. J. Lumin. 2017, 192, 321–327. [Google Scholar] [CrossRef]
- Shahshahanipour, M.; Rezaei, B.; Ensafi, A.A.; Etemadifar, Z. An ancient plant for the synthesis of a novel carbon dot and its applications as an antibacterial agent and probe for sensing of an anti-cancer drug. Mater. Sci. Eng. C 2019, 98, 826–833. [Google Scholar] [CrossRef]
- Komalavalli, L.; Amutha, P.; Monisha, S. A facile approach for the synthesis of carbon dots from Hibiscus sabdariffa & its application as bio-imaging agent and Cr (VI) sensor. Mater. Today Proc. 2020, 33, 2279–2285. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, Y.; Zhao, J.; Miao, M.; Cao, S.; Fang, J.; Shi, L. Easy synthesis of photoluminescent N-doped carbon dots from winter melon for bio-imaging. RSC Adv. 2015, 5, 31250–31254. [Google Scholar] [CrossRef]
- Zhao, S.; Lan, M.; Zhu, X.; Xue, H.; Ng, T.-W.; Meng, X.; Lee, C.-S.; Wang, P.; Zhang, W. Green Synthesis of Bifunctional Fluorescent Carbon Dots from Garlic for Cellular Imaging and Free Radical Scavenging. ACS Appl. Mater. Interfaces 2015, 7, 17054–17060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, Y.; Liang, G.; Yu, S.-H. Scale-Up Synthesis of Fragrant Nitrogen-Doped Carbon Dots from Bee Pollens for Bioimaging and Catalysis. Adv. Sci. 2015, 2, 1500002. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Afkhami, A.; Hosseinzadeh, L.; Madrakian, T. Green and cost-effective synthesis of carbon dots from date kernel and their application as a novel switchable fluorescence probe for sensitive assay of Zoledronic acid drug in human serum and cellular imaging. Anal. Chim. Acta 2018, 1030, 183–193. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, V.; Tiwari, P.; Saini, A.K.; Mobin, S.M. “Vigna radiata” based green C-dots: Photo-triggered theranostics, fluorescent sensor for extracellular and intracellular iron (III) and multicolor live cell imaging probe. Sens. Actuators B Chem. 2019, 291, 275–286. [Google Scholar] [CrossRef]
- Vandarkuzhali, S.A.A.; Jeyalakshmi, V.; Sivaraman, G.; Singaravadivel, S.; Krishnamurthy, K.R.; Viswanathan, B. Highly fluorescent carbon dots from Pseudo-stem of banana plant: Applications as nanosensor and bio-imaging agents. Sens. Actuators B Chem. 2017, 252, 894–900. [Google Scholar] [CrossRef]
- Shen, J.; Shang, S.; Chen, X.; Wang, D.; Cai, Y. Facile synthesis of fluorescence carbon dots from sweet potato for Fe3+ sensing and cell imaging. Mater. Sci. Eng. C 2017, 76, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Guo, X.; Jia, L.; Zhang, Y.; Zhao, Z.; Lonshakov, F. Green preparation of carbon dots with mangosteen pulp for the selective detection of Fe3+ ions and cell imaging. Appl. Surf. Sci. 2017, 423, 426–432. [Google Scholar] [CrossRef]
- D’souza, S.L.; Chettiar, S.S.; Koduru, J.R.; Kailasa, S.K. Synthesis of fluorescent carbon dots using Daucus carota subsp. sativus roots for mitomycin drug delivery. Optik 2018, 158, 893–900. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Leng, J.; Liu, H.; Zhang, Y.; Wang, C.; An, W.; Bao, C.; Lei, H. The green synthesis of carbon quantum dots and applications for sulcotrione detection and anti-pathogen activities. J. Saudi Chem. Soc. 2021, 25, 101373. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Li, X.; Liu, R.; Lin, L.; Zhao, S. Green Preparation of S and N Co-Doped Carbon Dots from Water Chestnut and Onion as Well as Their Use as an Off–On Fluorescent Probe for the Quantification and Imaging of Coenzyme A. ACS Sustain. Chem. Eng. 2017, 5, 4992–5000. [Google Scholar] [CrossRef]
- Tai, J.Y.; Leong, K.H.; Saravanan, P.; Tan, S.T.; Chong, W.C.; Sim, L.C. Facile green synthesis of fingernails derived carbon quantum dots for Cu2+ sensing and photodegradation of 2,4-dichlorophenol. J. Environ. Chem. Eng. 2021, 9, 104622. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Q.; Cao, J.; Qian, C.; Ye, J.; Xu, S.; Zhang, Y.; Li, Y. Facile and Green Synthesis of Highly Fluorescent Carbon Quantum Dots from Water Hyacinth for the Detection of Ferric Iron and Cellular Imaging. Nanomaterials 2022, 12, 1528. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Gao, S.; Xu, C.; Fang, Y.; Xu, L.; Zhang, W. Carbon quantum dots derived from waste acorn cups and its application as an ultraviolet absorbent for polyvinyl alcohol film. Appl. Surf. Sci. 2021, 556, 149774. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. Facile synthesis of novel carbon quantum dots from biomass waste for highly sensitive detection of iron ions. Mater. Res. Bull. 2020, 124, 110730. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Architha, N.; Ragupathi, M.; Shobana, C.; Selvankumar, T.; Kumar, P.; Lee, Y.S.; Kalai Selvan, R. Microwave-assisted green synthesis of fluorescent carbon quantum dots from Mexican Mint extract for Fe3+ detection and bio-imaging applications. Environ. Res. 2021, 199, 111263. [Google Scholar] [CrossRef]
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Xu, Y.; Gan, Z.; Zou, X.; Shi, J.; Huang, X.; Li, Z.; Li, Y. Green one-step synthesis of carbon quantum dots from orange peel for fluorescent detection of Escherichia coli in milk. Food Chem. 2021, 339, 127775. [Google Scholar] [CrossRef] [PubMed]
- Başoğlu, A.; Ocak, Ü.; Gümrükçüoğlu, A. Synthesis of Microwave-Assisted Fluorescence Carbon Quantum Dots Using Roasted-Chickpeas and its Applications for Sensitive and Selective Detection of Fe3+ Ions. J. Fluoresc. 2020, 30, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Suhail, B.; Bajpai, S.K.; Souza, A.D. ‘Microwave assisted facile green synthesis of carrageenan carbon dots(CDs) and their interaction with Hisbiscus Rosa sinensis leaf cells’. Int. J. Environ. Anal. Chem. 2022, 102, 2697–2713. [Google Scholar] [CrossRef]
- Dager, A.; Baliyan, A.; Kurosu, S.; Maekawa, T.; Tachibana, M. Ultrafast synthesis of carbon quantum dots from fenugreek seeds using microwave plasma enhanced decomposition: Application of C-QDs to grow fluorescent protein crystals. Sci. Rep. 2020, 10, 12333. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Kasouni, A.I.; Troganis, A.N.; Stalikas, C.D. Carbonization of Human Fingernails: Toward the Sustainable Production of Multifunctional Nitrogen and Sulfur Codoped Carbon Nanodots with Highly Luminescent Probing and Cell Proliferative/Migration Properties. ACS Appl. Mater. Interfaces 2018, 10, 16024–16032. [Google Scholar] [CrossRef]
- Sivasankaran, U.; Jesny, S.; Jose, A.R.; Girish Kumar, K. Fluorescence Determination of Glutathione Using Tissue Paper-derived Carbon Dots as Fluorophores. Anal. Sci. 2017, 33, 281–285. [Google Scholar] [CrossRef]
- Ko, N.R.; Nafiujjaman, M.; Cherukula, K.; Lee, S.J.; Hong, S.-J.; Lim, H.-N.; Park, C.H.; Park, I.-K.; Lee, Y.-K.; Kwon, I.K. Microwave-Assisted Synthesis of Biocompatible Silk Fibroin-Based Carbon Quantum Dots. Part. Part. Syst. Charact. 2018, 35, 1700300. [Google Scholar] [CrossRef]
- Asghar, K.; Qasim, M.; Das, D. One-pot green synthesis of carbon quantum dot for biological application. AIP Conf. Proc. 2017, 1832, 050117. [Google Scholar] [CrossRef]
- Qin, X.; Lu, W.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Microwave-assisted rapid green synthesis of photoluminescent carbon nanodots from flour and their applications for sensitive and selective detection of mercury(II) ions. Sens. Actuators B Chem. 2013, 184, 156–162. [Google Scholar] [CrossRef]
- Yahaya Pudza, M.; Zainal Abidin, Z.; Abdul Rashid, S.; Md Yasin, F.; Noor, A.S.M.; Issa, M.A. Eco-Friendly Sustainable Fluorescent Carbon Dots for the Adsorption of Heavy Metal Ions in Aqueous Environment. Nanomaterials 2020, 10, 315. [Google Scholar] [CrossRef]
- Monte-Filho, S.S.; Andrade, S.I.E.; Lima, M.B.; Araujo, M.C.U. Synthesis of highly fluorescent carbon dots from lemon and onion juices for determination of riboflavin in multivitamin/mineral supplements. J. Pharm. Anal. 2019, 9, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Dadhich, P.; Pal, P.; Srivas, P.K.; Bankoti, K.; Dhara, S. Carbon nanodots from date molasses: New nanolights for the in vitro scavenging of reactive oxygen species. J. Mater. Chem. B 2014, 2, 6839–6847. [Google Scholar] [CrossRef] [PubMed]
- Sargin, I.; Yanalak, G.; Arslan, G.; Patir, I.H. Green synthesized carbon quantum dots as TiO2 sensitizers for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 21781–21789. [Google Scholar] [CrossRef]
- Muktha, H.; Sharath, R.; Kottam, N.; Smrithi, S.P.; Samrat, K.; Ankitha, P. Green Synthesis of Carbon Dots and Evaluation of Its Pharmacological Activities. BioNanoScience 2020, 10, 731–744. [Google Scholar] [CrossRef]
- Ramezani, Z.; Qorbanpour, M.; Rahbar, N. Green synthesis of carbon quantum dots using quince fruit (Cydonia oblonga) powder as carbon precursor: Application in cell imaging and As3+ determination. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 58–66. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Shen, G.; Zhang, C.; Li, C.; Ji, W.; Wang, C.; Cui, D. A multifunctional ribonuclease A-conjugated carbon dot cluster nanosystem for synchronous cancer imaging and therapy. Nanoscale Res. Lett. 2014, 9, 397. [Google Scholar] [CrossRef]
- Ramanan, V.; Thiyagarajan, S.K.; Raji, K.; Suresh, R.; Sekar, R.; Ramamurthy, P. Outright Green Synthesis of Fluorescent Carbon Dots from Eutrophic Algal Blooms for In Vitro Imaging. ACS Sustain. Chem. Eng. 2016, 4, 4724–4731. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Chen, M.; Chen, W.; Zhou, X. Microwave-assisted synthesis of xylan-derived carbon quantum dots for tetracycline sensing. Opt. Mater. 2018, 85, 329–336. [Google Scholar] [CrossRef]
- Gu, D.; Shang, S.; Yu, Q.; Shen, J. Green synthesis of nitrogen-doped carbon dots from lotus root for Hg(II) ions detection and cell imaging. Appl. Surf. Sci. 2016, 390, 38–42. [Google Scholar] [CrossRef]
- Genc, M.T.; Yanalak, G.; Arslan, G.; Patir, I.H. Green preparation of Carbon Quantum dots using Gingko biloba to sensitize TiO2 for the photohydrogen production. Mater. Sci. Semicond. Process. 2020, 109, 104945. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, I.; Bera, M.K. Microwave-Assisted Green Synthesis of Carbon Quantum Dots Derived from Calotropis gigantea as a Fluorescent Probe for Bioimaging. J. Fluoresc. 2022, 32, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, Z.; Pal, K.; Yehye, W.; Suresh, S.; Shah, S.T.; Adebisi, A.; Marliana, E.; Rafique, R.; Johan, R. Pyrolysis: A Sustainable Way to Generate Energy from Waste. Pyrolysis 2017, 1, 3–6. [Google Scholar]
- Al-Rumaihi, A.; Shahbaz, M.; McKay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Teng, X.; Ma, C.; Ge, C.; Yan, M.; Yang, J.; Zhang, Y.; Morais, P.C.; Bi, H. Green synthesis of nitrogen-doped carbon dots from konjac flour with “off–on” fluorescence by Fe3+ and l-lysine for bioimaging. J. Mater. Chem. B 2014, 2, 4631–4639. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Tran, T.S.; Tung, T.T.; Losic, D.; Kim, T. Water Soluble Fluorescent Carbon Nanodots from Biosource for Cells Imaging. J. Nanomater. 2017, 2017, 7029731. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, A.; Sahu, S.K.; Kumar, S. Synthesis of green fluorescent carbon quantum dots using waste polyolefins residue for Cu2+ ion sensing and live cell imaging. Sens. Actuators B Chem. 2018, 254, 197–205. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, F.; Yue, X.; Chen, P.; Sun, Y.; Zhang, L.; Mu, D.; Ke, F. Waste Utilization of Synthetic Carbon Quantum Dots Based on Tea and Peanut Shell. J. Nanomater. 2019, 2019, 7965756. [Google Scholar] [CrossRef]
- Singh, L.; Sharma, T.; Singh, V. Study of structural and functional properties of fluorescent EDTA@CQDs synthesized from peanut shells via pyrolysis technique. Mater. Today Proc. 2021, 44, 192–198. [Google Scholar] [CrossRef]
- Hu, Z.; Jiao, X.-Y.; Xu, L. The N,S co-doped carbon dots with excellent luminescent properties from green tea leaf residue and its sensing of gefitinib. Microchem. J. 2020, 154, 104588. [Google Scholar] [CrossRef]
- Ren, R.; Zhang, Z.; Zhao, P.; Shi, J.; Han, K.; Yang, Z.; Gao, D.; Bi, F. Facile and one-step preparation carbon quantum dots from biomass residue and their applications as efficient surfactants. J. Dispers. Sci. Technol. 2019, 40, 627–633. [Google Scholar] [CrossRef]
- Praneerad, J.; Neungnoraj, K.; In, I.; Paoprasert, P. Environmentally Friendly Supercapacitor Based on Carbon Dots from Durian Peel as an Electrode. Key Eng. Mater. 2019, 803, 115–119. [Google Scholar] [CrossRef]
- Chaudhary, P.; Maurya, D.K.; Yadav, S.; Pandey, A.; Tripathi, R.K.; Yadav, B.C. Ultrafast responsive humidity sensor based on roasted gram derived carbon quantum dots: Experimental and theoretical study. Sens. Actuators B Chem. 2021, 329, 129116. [Google Scholar] [CrossRef]
- Jiao, X.-Y.; Li, L.-s.; Qin, S.; Zhang, Y.; Huang, K.; Xu, L. The synthesis of fluorescent carbon dots from mango peel and their multiple applications. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 306–314. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Maity, P.P.; Srivastava, H.K.; Bose, M.; Dhara, S.; Bandyopadhyay, S.; Das, A.K.; Banerjee, S.; Das, N.C. Converting waste Allium sativum peel to nitrogen and sulphur co-doped photoluminescence carbon dots for solar conversion, cell labeling, and photobleaching diligences: A path from discarded waste to value-added products. J. Photochem. Photobiol. B Biol. 2019, 197, 111545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cheng, J.; Zhang, Y.; Kong, H.; Wang, S.; Luo, J.; Qu, H.; Zhao, Y. Green synthesis of Zingiberis rhizoma-based carbon dots attenuates chemical and thermal stimulus pain in mice. Nanomedicine 2020, 15, 851–869. [Google Scholar] [CrossRef]
- Murugan, N.; Prakash, M.; Jayakumar, M.; Sundaramurthy, A.; Sundramoorthy, A.K. Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn-off’ sensor probe for selective detection of Cu2+. Appl. Surf. Sci. 2019, 476, 468–480. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of Mono-disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence analysis using Machine Learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef]
- Zhu, L.; Yin, Y.; Wang, C.-F.; Chen, S. Plant leaf-derived fluorescent carbon dots for sensing, patterning and coding. J. Mater. Chem. C 2013, 1, 4925–4932. [Google Scholar] [CrossRef]
- Pourreza, N.; Ghomi, M. Green synthesized carbon quantum dots from Prosopis juliflora leaves as a dual off-on fluorescence probe for sensing mercury (II) and chemet drug. Mater. Sci. Eng. C 2019, 98, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Shi, L.; Wen, G.; Li, Y.; Dong, C.; Yang, J.; Shuang, S. Green synthesis of carbon nanodots from cotton for multicolor imaging, patterning, and sensing. Sens. Actuators B Chem. 2015, 221, 769–776. [Google Scholar] [CrossRef]
- Chatzimarkou, A.; Chatzimitakos, T.G.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Stalikas, C.D. Selective FRET-based sensing of 4-nitrophenol and cell imaging capitalizing on the fluorescent properties of carbon nanodots from apple seeds. Sens. Actuators B Chem. 2018, 258, 1152–1160. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.-F.; Chen, S. Amphiphilic Egg-Derived Carbon Dots: Rapid Plasma Fabrication, Pyrolysis Process, and Multicolor Printing Patterns. Angew. Chem. Int. Ed. 2012, 51, 9297–9301. [Google Scholar] [CrossRef]
- Devi, P.; Kaur, G.; Thakur, A.; Kaur, N.; Grewal, A.; Kumar, P. Waste derivitized blue luminescent carbon quantum dots for selenite sensing in water. Talanta 2017, 170, 49–55. [Google Scholar] [CrossRef]
- Xue, M.; Zou, M.; Zhao, J.; Zhan, Z.; Zhao, S. Green preparation of fluorescent carbon dots from lychee seeds and their application for the selective detection of methylene blue and imaging in living cells. J. Mater. Chem. B 2015, 3, 6783–6789. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, T.; Kumar, S. Turning date palm fronds into biocompatible mesoporous fluorescent carbon dots. Sci. Rep. 2018, 8, 16269. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Shi, Y.; Liu, X.; Wang, M.; Song, P.; Xu, F.; Zhang, X. Synthesis of Nitrogen-Doped Lignin/DES Carbon Quantum Dots as a Fluorescent Probe for the Detection of Fe3+ Ions. Polymers 2018, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Anthony, A.M.; Murugan, R.; Subramanian, R.; Selvarangan, G.K.; Pandurangan, P.; Dhanasekaran, A.; Sohrab, A. Ultra-radiant photoluminescence of glutathione rigidified reduced carbon quantum dots (r-CQDs) derived from ice-biryani for in vitro and in vivo bioimaging applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124266. [Google Scholar] [CrossRef]
- Xue, M.; Zhan, Z.; Zou, M.; Zhang, L.; Zhao, S. Green synthesis of stable and biocompatible fluorescent carbon dots from peanut shells for multicolor living cell imaging. N. J. Chem. 2016, 40, 1698–1703. [Google Scholar] [CrossRef]
- Zhou, J.; Sheng, Z.; Han, H.; Zou, M.; Li, C. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Troganis, A.; Stalikas, C. Two of a kind but different: Luminescent carbon quantum dots from Citrus peels for iron and tartrazine sensing and cell imaging. Talanta 2017, 175, 305–312. [Google Scholar] [CrossRef]
- Deka, M.J.; Dutta, P.; Sarma, S.; Medhi, O.K.; Talukdar, N.C.; Chowdhury, D. Carbon dots derived from water hyacinth and their application as a sensor for pretilachlor. Heliyon 2019, 5, e01985. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.J.; Roy, A.K.; Kim, S.H.; Lee, J.-E.; Jeong, J.H.; In, I.; Park, S.Y. Fluorescent carbon nanoparticles derived from natural materials of mango fruit for bio-imaging probes. Nanoscale 2014, 6, 15196–15202. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Chang, Y.; Wen, H.; Yao, X.; Wang, Y. Colorimetric determination of ascorbic acid based on carbon quantum dots as peroxidase mimetic enzyme. RSC Adv. 2020, 10, 14953–14957. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem. Commun. 2011, 47, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Reyes, D.; Camacho, M.; Camacho, M.; Mayorga, M.; Weathers, D.; Salamo, G.; Wang, Z.; Neogi, A. Laser Ablated Carbon Nanodots for Light Emission. Nanoscale Res. Lett. 2016, 11, 424. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason. Sonochemistry 2020, 64, 105009. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.-T.; Kang, Z. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Khayal, A.; Dawane, V.; Amin, M.A.; Tirth, V.; Yadav, V.K.; Algahtani, A.; Khan, S.H.; Islam, S.; Yadav, K.K.; Jeon, B.-H. Advances in the Methods for the Synthesis of Carbon Dots and Their Emerging Applications. Polymers 2021, 13, 3190. [Google Scholar] [CrossRef]
- Huang, H.; Cui, Y.; Liu, M.; Chen, J.; Wan, Q.; Wen, Y.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. A one-step ultrasonic irradiation assisted strategy for the preparation of polymer-functionalized carbon quantum dots and their biological imaging. J. Colloid Interface Sci. 2018, 532, 767–773. [Google Scholar] [CrossRef]
- Saikia, M.; Hower, J.C.; Das, T.; Dutta, T.; Saikia, B.K. Feasibility study of preparation of carbon quantum dots from Pennsylvania anthracite and Kentucky bituminous coals. Fuel 2019, 243, 433–440. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.-B.; Wei, J.-S.; Xiong, H.-M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Holá, K.; Sudolská, M.; Kalytchuk, S.; Nachtigallová, D.; Rogach, A.L.; Otyepka, M.; Zbořil, R. Graphitic Nitrogen Triggers Red Fluorescence in Carbon Dots. ACS Nano 2017, 11, 12402–12410. [Google Scholar] [CrossRef]
- Kwon, W.; Rhee, S.-W. Facile synthesis of graphitic carbon quantum dots with size tunability and uniformity using reverse micelles. Chem. Commun. 2012, 48, 5256–5258. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, S.; Li, B.; Yang, K.; Lan, M.; Zeng, L. Advances and perspectives in carbon dot-based fluorescent probes: Mechanism, and application. Coord. Chem. Rev. 2021, 431, 213686. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, S. Creating high yield water soluble luminescent graphene quantum dots via exfoliating and disintegrating carbon nanotubes and graphite flakes. Chem. Commun. 2012, 48, 10177–10179. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Ahmad, K.; Dutta, D.; Chattopadhyay, A. Boron Doped Carbon Dots with Unusually High Photoluminescence Quantum Yield for Ratiometric Intracellular pH Sensing. Chemphyschem A Eur. J. Chem. Phys. Phys. Chem. 2019, 20, 1018–1027. [Google Scholar] [CrossRef]
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; von Seckendorff, M.; Susha, A.S.; Kasák, P.; Rogach, A.L. Molecular Fluorescence in Citric Acid-Based Carbon Dots. J. Phys. Chem. C 2017, 121, 2014–2022. [Google Scholar] [CrossRef]
- Jiang, Z.; Krysmann, M.J.; Kelarakis, A.; Koutnik, P.; Anzenbacher, P.; Roland, P.J.; Ellingson, R.; Sun, L. Understanding the Photoluminescence Mechanism of Carbon Dots. MRS Adv. 2017, 2, 2927–2934. [Google Scholar] [CrossRef]
- Krysmann, M.J.; Kelarakis, A.; Dallas, P.; Giannelis, E.P. Formation Mechanism of Carbogenic Nanoparticles with Dual Photoluminescence Emission. J. Am. Chem. Soc. 2012, 134, 747–750. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B. Marine Bioactive Peptides—Structure, Function and Application. Mar. Drugs 2023, 21, 275. [Google Scholar] [CrossRef]
- Singh, V.; Rawat, K.S.; Mishra, S.; Baghel, T.; Fatima, S.; John, A.A.; Kalleti, N.; Singh, D.; Nazir, A.; Rath, S.K.; et al. Biocompatible fluorescent carbon quantum dots prepared from beetroot extract for in vivo live imaging in C. elegans and BALB/c mice. J. Mater. Chem. B 2018, 6, 3366–3371. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Khatun, Z.; Huh, K.M.; Park, S.Y.; Lee, D.Y.; Cho, K.J.; Lee, Y.-k. In Vivo Biodistribution and Toxicology of Carboxylated Graphene Quantum Dots. ACS Nano 2013, 7, 6858–6867. [Google Scholar] [CrossRef] [PubMed]
- Hutton, G.A.M.; Martindale, B.C.M.; Reisner, E. Carbon dots as photosensitisers for solar-driven catalysis. Chem. Soc. Rev. 2017, 46, 6111–6123. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.H.; Tieves, F.; Choi, D.S.; Hollmann, F.; Paul, C.E.; Park, C.B. Biocatalytic C=C Bond Reduction through Carbon Nanodot-Sensitized Regeneration of NADH Analogues. Angew. Chem. Int. Ed. 2018, 57, 13825–13828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ren, X.; Zhang, J.; Zhang, W.; Chen, X.; Meng, X. Dendritic silica with carbon dots and gold nanoclusters for dual nanozymes. N. J. Chem. 2020, 44, 1988–1992. [Google Scholar] [CrossRef]
- Reale, M.; Chandra, S.; Buscarino, G.; Emanuele, A.; Cannas, M.; Ikkala, O.; Sciortino, A.; Messina, F. Photoinduced charge separation in functional carbon–silver nanohybrids. Phys. Chem. Chem. Phys. 2022, 24, 12974–12983. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2021, 406, 126848. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Ghaedi, M.; Tashkhourian, J.; Montazerozohori, M.; Nejati Biyareh, M.; Sadeghian, B. Highly selective and sensitive determination of copper ion by two novel optical sensors. Arab. J. Chem. 2017, 10, S2319–S2326. [Google Scholar] [CrossRef]
- Verwilst, P.; Sunwoo, K.; Kim, J.S. The role of copper ions in pathophysiology and fluorescent sensors for the detection thereof. Chem. Commun. 2015, 51, 5556–5571. [Google Scholar] [CrossRef]
- Kawamura, A.; Miyata, T. 4.2—Biosensors. In Biomaterials Nanoarchitectonics; Ebara, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 157–176. [Google Scholar]
- Suárez-García, S.; Solórzano, R.; Novio, F.; Alibés, R.; Busqué, F.; Ruiz-Molina, D. Coordination polymers nanoparticles for bioimaging. Coord. Chem. Rev. 2021, 432, 213716. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.L.; Huang, C.Z. Recent advances of carbon dots in imaging-guided theranostics. TrAC Trends Anal. Chem. 2021, 134, 116116. [Google Scholar] [CrossRef]
- Wang, Z.; She, M.; Chen, J.; Cheng, Z.; Li, J. Rational Modulation Strategies to Improve Bioimaging Applications for Organic NIR-II Fluorophores. Adv. Opt. Mater. 2022, 10, 2101634. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Waterhouse, G.I.N.; Qu, X.; Yang, B.; Lu, S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Sci. 2022, 2, 2200012. [Google Scholar] [CrossRef]
- Soni, N.; Singh, S.; Sharma, S.; Batra, G.; Kaushik, K.; Rao, C.; Verma, N.C.; Mondal, B.; Yadav, A.; Nandi, C.K. Absorption and emission of light in red emissive carbon nanodots. Chem. Sci. 2021, 12, 3615–3626. [Google Scholar] [CrossRef]
- Xu, G.; Bao, X.; Chen, J.; Zhang, B.; Li, D.; Zhou, D.; Wang, X.; Liu, C.; Wang, Y.; Qu, S. In Vivo Tumor Photoacoustic Imaging and Photothermal Therapy Based on Supra-(Carbon Nanodots). Adv. Healthc. Mater. 2019, 8, 1800995. [Google Scholar] [CrossRef] [PubMed]
- Ren, E.; Pang, X.; Lei, Z.; Liu, G. Vesicular antibodies for immunotherapy: The blooming intersection of nanotechnology and biotechnology. Nano Today 2020, 34, 100896. [Google Scholar] [CrossRef]
- Shen, Y.; Levin, A.; Kamada, A.; Toprakcioglu, Z.; Rodriguez-Garcia, M.; Xu, Y.; Knowles, T.P.J. From Protein Building Blocks to Functional Materials. ACS Nano 2021, 15, 5819–5837. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Liu, K.-K.; Wu, X.-Y.; Lou, Q.; Sui, L.-Z.; Dong, L.; Yuan, K.-J.; Shan, C.-X. Lifetime-Engineered Carbon Nanodots for Time Division Duplexing. Adv. Sci. 2021, 8, 2003433. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, X.; Zhang, S.; Zhu, A.; Yuan, Y.; Xu, H.; Lei, J.; Yan, C. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 2021, 184, 370–383.e13. [Google Scholar] [CrossRef]
- Dheman, N.; Mahoney, N.; Cox, E.M.; Farley, J.J.; Amini, T.; Lanthier, M.L. An Analysis of Antibacterial Drug Development Trends in the United States, 1980–2019. Clin. Infect. Dis. 2021, 73, e4444–e4450. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.A.; Cha, S. Ambient desorption/ionization mass spectrometry for direct solid material analysis. TrAC Trends Anal. Chem. 2021, 144, 116420. [Google Scholar] [CrossRef]
- Ghaffarkhah, A.; Hosseini, E.; Kamkar, M.; Sehat, A.A.; Dordanihaghighi, S.; Allahbakhsh, A.; van der Kuur, C.; Arjmand, M. Synthesis, Applications, and Prospects of Graphene Quantum Dots: A Comprehensive Review. Small 2022, 18, 2102683. [Google Scholar] [CrossRef]
- Devi, M.; Vomero, M.; Fuhrer, E.; Castagnola, E.; Gueli, C.; Nimbalkar, S.; Hirabayashi, M.; Kassegne, S.; Stieglitz, T.; Sharma, S. Carbon-based neural electrodes: Promises and challenges. J. Neural Eng. 2021, 18, 041007. [Google Scholar] [CrossRef]
- Liu, S.; Han, Z.; Hao, J.-N.; Zhang, D.; Li, X.; Cao, Y.; Huang, J.; Li, Y. Engineering of a NIR-activable hydrogel-coated mesoporous bioactive glass scaffold with dual-mode parathyroid hormone derivative release property for angiogenesis and bone regeneration. Bioact. Mater. 2023, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, N.; Tian, J.; Xin, G.; Liu, L.; Sun, X.; Li, B. Advanced approaches for improving bioavailability and controlled release of anthocyanins. J. Control. Release 2022, 341, 285–299. [Google Scholar] [CrossRef]
- De Rocco, A.G.J.S. Mesophases: The Physics of Liquid Crystals. PG de Gennes. Clarendon (Oxford University Press), New York, 1974. xii, 334 pp., illus.+ plates. $32.50. International Series of Monographs on Physics. Science 1974, 186, 1199. [Google Scholar] [CrossRef]
- Chandrasekhar, S. Liquid Crystals, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Eskalen, H. Influence of carbon quantum dots on electro–optical performance of nematic liquid crystal. Appl. Phys. A 2020, 126, 708. [Google Scholar] [CrossRef]
- Rastogi, A.; Hegde, G.; Manohar, T.; Manohar, R. Effect of oil palm leaf-based carbon quantum dot on nematic liquid crystal and its electro-optical effects. Liq. Cryst. 2021, 48, 812–831. [Google Scholar] [CrossRef]
- Rastogi, A.; Pandey, F.P.; Parmar, A.S.; Singh, S.; Hegde, G.; Manohar, R. Effect of carbonaceous oil palm leaf quantum dot dispersion in nematic liquid crystal on zeta potential, optical texture and dielectric properties. J. Nanostructure Chem. 2021, 11, 527–548. [Google Scholar] [CrossRef]




| Precursor Type | Precursors Used | Molar Mass /Molar Ratio | Crystallite Size/ Particle Size (nm) | Optimum Parameters | Applications | Ref. |
|---|---|---|---|---|---|---|
| Fruits | Prunus mume | MR-0.6 | PS-9 | HT-180 °C for 5 h pH-2.3, 5, 7, and 9 Cen-10,000 rpm for 20 min, and Dr-24 h | Cellular imaging | [31] |
| Chionanthus retusus | MR-0.5 | PS-5 | HT-180 °C for 6 h | Metal ion sensing and imaging of fungal cells | [32] | |
| Unripe peach | MR-0.5 | PS-8 | HT-180 °C for 5 h and Cen-10,000 rpm for 15 min | Cellular imaging and oxygen reduction reaction | [33] | |
| Cherry tomatoes | - | PS-7 | HT-180 °C for 6 h and pH-2–11 | Sensing and environmental monitoring | [34] | |
| Juices | Acerola juice | MM-1 g/mL | HT-100, 130, 160, and 180 °C for 12, 18, 24, and 36 h, respectively, and Cen-8500 rpm for 15 min | Sensors | [35] | |
| Lemon juice | 40 mL | PS-50 | HT-120–280 °C for 12 h | Optoelectronics and bioimaging | [36] | |
| Carica papaya juice | MR-50 mL | PS-3 | HT-125, 150 and 170 °C for 12 h pH-6 Cen-15,000 rpm for 20 min, and Dr-12 h | Cellular imaging | [37] | |
| S. Officinarum juice | MR-2.33 | PS-2.5–3.0 | HT-120 °C for 18 min Cen-5000 rpm for 20 min and 13,000 rpm for 15 min | Imaging probes | [38] | |
| Malus domestica (apple) | 1 g/mL | CS-4.5 | HT-150 °C for 12 h C-5000 rpm for 20 min D-24 h | Bioimaging of fungal cell | [39] | |
| Bitter oranges | -- | PS-1–2 | HT-120 °C for 2.5 h and 7 h, and 180 °C for 7 h pH-7, and Cen-1000 rpm for 15 min | Imaging of live cells | [40] | |
| Plant/fruit waste | Onion waste | -- | PS-15 | HT-120 °C, and pH-4–8 | Sensing of Fe3+ ion and cellular imaging | [41] |
| Walnut shells | MM-0.02 g/mL | PS-3.4 | HT-100 °C and 140 °C for 12 h, and pH-7 | Photocatalysis, photoelectric devices, phototherapy, and bioimaging | [42] | |
| Sugarcane molasses | 0.5 g/mL | PS-1.2–3.8 | HT-280 °C for 12 h Cen-6000 rpm for 10 min L-72 h | Drug delivery, bioimaging, and biosensors | [43] | |
| Lemon peel waste | MM-0.05 g/mL | PS1–3 | HT-200 °C for 12 h pH-7 Cen-10,000 rpm for 30 min | Sensing and photocatalysis | [44] | |
| Banana peel | -- | PS 4–6 | HT-200 °C for 24 h Stored at 4 °C. | Vivo bioimaging | [45] | |
| Pomelo peel | 0.01 g/mL | 180 °C for 5 h Cen-10,000 for 10 min | Detection of Fe3+ and L-cysteine | [46] | ||
| Pineapple peel | 1 mg/mL | PS 2–3 nm | 150 °C for 2 h 10,000 rpm for 15 min | Applications as sensor, molecular, and memory device | [47] | |
| Leaf | Willow bark | 0.066 g/mL | PS 0.5 | HT-200 °C for 3 h Cen-14,000 rpm for 10 min Dr-10 min | Glucose biosensor | [48] |
| Coriander leaves | MM-0.125 g/mL | PS 2.3 | HT-240 °C for 4 h | Sensing of Fe3+ ion and cellular imaging | [23] | |
| Betel leaves | -- | CS 3–7 | HT-180 °C for 24 h Cen-10,000 rpm | Detection of trace Fe3+ ions in water samples | [49] | |
| Betel leaves | -- | CS 4.5 | 200 °C at different reaction times of 12 h, 24 h, 36 h, and 48 h | Multicolour emitting carbon dots as a fluorescent probe for imaging mouse normal fibroblast, and human thyroid cancer cells | [50] | |
| Purslane leaves | 0.05 g/mL | CS 6.1 | HT-150 °C for 4 h Cen-12,000 rpm | Detection of formaldehyde using quartz crystal microbalance | [51] | |
| Maple leaves | -- | CS 2–10 | HT-190 °C for 8 h pH 7–9 | To detect cesium in environmental samples and catalytic oxidation of glycerol | [52] | |
| Catharanthus roseus (white flowering plant) | 0.01 g/mL | CS 5 | 200 °C for 4 h | Dual fluorescence responsive behavior in multi-ion detection, and biological application | [53] | |
| Tamarindus indica (T. indica) | 0.4 g/mL | CS 3.4 | 210 °C for 5 h Cen-10,000 rpm | Detection of mercury (II) and glutathione | [54] | |
| Tulsi leaves (Ocimum sanctum) | 0.33 mg/mL | CS 4–7 | 180 °C for 4 h | Sensing probe for label-free, sensitive detection of Pb2+ ions. | [55] | |
| Fresh leaves of Tulsi | 0.07 g/mL | CS 5 | 200 °C for 4 h | Selective detection of Cr(VI) in aqueous media | [56] | |
| Leaves of Centella asiatica | 1.25 g/mL | CS 2.18 | 180 °C for 8 h Cen-3000 rpm for 15 min | Development of an efficient hierarchical electron transfer cascade system for photovoltaic applications. | [57] | |
| Henna leaf powder | 0.0125 g/mL | CS 5 | 180 °C for 12 h Cen-12,000 rpm for 20 min | An antibacterial agent and probe for sensing of an anti-cancer drug | [58] | |
| Hibiscus sabdariffa leaf | 0.033 g/mL | CS 3–5 | 160 °C for 8 h Cen-8000 rpm for 15 min. | Bio-imaging agent and Cr (VI) sensor | [59] | |
| Vegetables | Winter melon | MM-0.4 | PS 4.5–5.2 | HT-180 °C for 2 h | Cell imaging | [60] |
| Garlic | MM-0.033 g/mL | PS 11 | HT-200 °C for 3 h pH-7–8 | Free radical scavenging and cellular imaging | [61] | |
| Insect | Bee pollens | MM-0.025 g/mL | PS 1–2 | HT-180 °C for 24 h | Cellular imaging and catalysis | [62] |
| Seeds | Date kernels | 0.2 g/mL | PS 2.5 | HT-200 °C for 8 h Cen-16,000 rpm for 20 min Dr-24 h | Switchable fluorescence probe for sensitive assay of zoledronic acid drug in human serum and cellular imaging | [63] |
| Yigna radiata | 0.05–0.01 mL/µL | CS 10 nm | HT-180 °C for 24 h | Photo-triggered theragnostic, fluorescent sensor for extracellular and intracellular iron (III), and multicolor live cell imaging probe | [64] | |
| Stems | Pseudo-stem of banana plant | MM-0.75 | CS 0.22 | HT-180 °C for 2 h Cen-5000 rpm for 15 min | Nano-sensor and bioimaging agents | [65] |
| Agricultural | Sweet potato | 0.285 g/mL | PS 3.39 | HT-180 °C for 18 h C-8000 rpm for 20 min Dr-48 h | Sensing of Fe3+ ions and cell imaging | [66] |
| Raw material | Mangosteen pulp | 0.5 g/mL | PS 5 | CT-room temperature for 10 min Cen-9000 rpm for 10 min and Dr-24 h | Cellular imaging | [67] |
| Roots | Daucus carota subsp. Sativus (carrot) | 0.3 g/mL | PS 2.30 | HT-170 °C for 12 h Cen-5000 rpm, and Dr-48 h | Drug delivery | [68] |
| Moringa oleifera roots | -- | CS 3.6 | Cen-10,000 rpm for 10 min | Sulcotrione detection and anti-pathogen activities | [69] | |
| Other | Water chestnut and onion | 0.1 g/mL | PS 3.5 | HT-180 °C for 4 h pH-5.8 Cen-12,000 rpm for 20 min Dr-48 h | Quantification of CoA in pig liver and imaging of CoA in living T24 cells | [70] |
| Finger nail | 0.66 g/mL | CS 1.96–4.15 | HT-200 °C for 3 h, Cen-12,000 rpm for 10 min, and vacuum dried at 60 °C for 48 h | Cu2+ sensing and photodegradation of 2,4-dichlorophenol | [71] | |
| Biomass water hyacinth | 0.1 g/mL | CS 1.2–4.2 | HT-180 °C for 12 h and Cen-8000 rpm for 20 min | Detection of ferric iron and cellular imaging | [72] | |
| Acorn cups waste | 0.01 g/mL | CS 4–7 | HT-200 °C for 8 h and Cen-10,000 for 15 min | Ultraviolet absorbent for polyvinyl alcohol film | [73] | |
| Orange peel, ginkgo Biloba leaves, Paulownia leaves, Magnolia flower | 0.01 g/mL | CS 2.6 | HT-200 °C for 8 h and Cen-10,000 rpm for 10 min | Detection of iron ions | [74] |
| Precursor Type | Precursors Used | Molar Mass/Molar Ratio | Particle Size (nm) | Optimum Parameters | Applications | Ref. |
|---|---|---|---|---|---|---|
| Seeds | Fenugreek seeds | MR 0.2 g | 4.25 | MT 500 W MPED 30 sccm for 5 min P 30 Pa, and Cen-15,000 rpm for 10 min | Bio-targeting | [81] |
| Roasted chickpeas | MM 0.04 g/mL | 3 and 9 | MT 350 W for 2 min Cen-3000 rpm for 15 min, and Cen-12,000 rpm 15 min | Detection of Fe3+ ions | [79] | |
| Waste materials | Human fingernails | MM 0.1 g/mL | 2.2 | MT 400 W for 2 min and pH-5 | Analytical and bioimaging | [82] |
| Orange peel | MM 0.1 g/mL | 3–5 | MT 900 W for 1 min and Cen-15,000 rpm | Detection of E. coli in milk | [78] | |
| Tissue paper | MM 0.01 g/mL | 4.2 | MT 800 W for 4 min and Cen 400 rpm | Spiked artificial saliva samples | [83] | |
| Insect | Silk fibroin | MM 0.005 g/mL | 5.4–6.1 | MT 200 °C for 20 min and Cen-3500 rpm for 30 min 2 times | Bioimaging, biosensing, and drug delivery | [84] |
| Honey | -- | 2–7 | MT 15–20 min and Dr-24 h | Biomedical | [85] | |
| Agri. | Flour | MR 0.08 g | 1–4 | MT 180 °C for 20 min and Cen-14,000 rpm for 10 min | Sensitive and selective detection of mercury (II) ions | [86] |
| Tapioca flour | MM 0.00625 g/mL | 3–3.99 | MT 175 °C for 1.45 h And Cen-3000 rpm for 20 min | Water pollution detection and medical bioimaging | [87] | |
| Juices | Onion and lemon juice | MR 2.05 | 6.15 | MT-1450 W for 6 min, Cen-6000 rpm for 30 min, and Dr-24 h | Determination of riboflavin in multivitamin/mineral supplement | [88] |
| Date molasses | MM 0.25 g/mL | 6.57 | pH-7 and IT-3 min | Free radical scavenging system | [89] | |
| Food | Mushrooms (Agaricus bisporus) | MM 0.1 g/mL | 20 | MT 400, 800 and 1600 W for 5 min and Cen-4500 rpm for 30 min | Hydrogen evolution | [90] |
| Carrageenan | MM 0.1 g/mL | 3–6 | MT room temperature 15 s/cycle, S-30 min, and Cen-5000 rpm 15 min | Plant-related diseases | [80] | |
| Fruit | Fresh ripe pomegranate and watermelon peel | MM 0.025 g/mL | 1–5 | MT 70 °C for 40 min and C-10,000 rpm for 10 min | Biomedical | [91] |
| Quince fruit (Cydonia oblonga) | MM 0.004 g/mL | 4.85 | MT 220 °C, 850 W for 1 min 30 s and IP-700 W | Cell imaging and As3+ determination | [92] | |
| Biomolecule residue | Ribonuclease | MM 0.215 g/mL | 25–45 | MT 700 W for 3–5 min Dr-2 days | Synchronous cancer imaging and therapy | [93] |
| Bloomed algae | MM 0.004 g/mL | 8 | Dr-48 h | In vivo imaging | [94] | |
| Biomass | Xylan | MM 0.05 g/mL | 4.44 | MT 200 W (200 °C) for 10 min and pH-5 | Cell imaging and photocatalysts | [95] |
| Roots | Lotus roots | N/A | 9.41 | pH 1–10 | Heavy metal ion detection and cellular imaging | [96] |
| Plant | Ginkgo biloba | MM 0.02, 0.06, and 0.1 g/mL | 15–20 | MT 400–800 W for 1, 3, 5, 7, and 10 min, and Cen-4500 rpm for 20 min | Photocatalyst | [97] |
| Calotropis gigantea (crown flower) leaves | MR 0.1 g/ml | 5.7 nm | MT 900 W 15 min and Cen-15,000 rpm. | Fluorescent probe for bioimaging | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Gaur, J.; Kaushal, S.; Dalal, J.; Misra, M.; Kaur, H.; Kaur, S.; Kaur, N.; Singh, G.; Singh, G. An Insight into Synthesis, Optical Properties, and Applications of Green Fluorescent Carbon Dots. Crystals 2025, 15, 320. https://doi.org/10.3390/cryst15040320
Kumar S, Gaur J, Kaushal S, Dalal J, Misra M, Kaur H, Kaur S, Kaur N, Singh G, Singh G. An Insight into Synthesis, Optical Properties, and Applications of Green Fluorescent Carbon Dots. Crystals. 2025; 15(4):320. https://doi.org/10.3390/cryst15040320
Chicago/Turabian StyleKumar, Sanjeev, Jyoti Gaur, Sandeep Kaushal, Jasvir Dalal, Mrinmoy Misra, Harpreet Kaur, Supreet Kaur, Navneet Kaur, Gautam Singh, and Gurjinder Singh. 2025. "An Insight into Synthesis, Optical Properties, and Applications of Green Fluorescent Carbon Dots" Crystals 15, no. 4: 320. https://doi.org/10.3390/cryst15040320
APA StyleKumar, S., Gaur, J., Kaushal, S., Dalal, J., Misra, M., Kaur, H., Kaur, S., Kaur, N., Singh, G., & Singh, G. (2025). An Insight into Synthesis, Optical Properties, and Applications of Green Fluorescent Carbon Dots. Crystals, 15(4), 320. https://doi.org/10.3390/cryst15040320






