Impressive 1D (Ferrocenyl⋯C6F5R⋯)n Stacking Due to Cooperative Interactions in N-(Ferrocenylmethyl)Pentafluorobenzenecarboxamide: Four Crystal Structures and Contacts Analyses in N-(Ferrocenylalkyl)Benzenecarboxamides
Abstract
1. Introduction
2. Experimental
2.1. Materials and Characterisation
2.2. Methods: Hirshfeld Surface Analysis Details
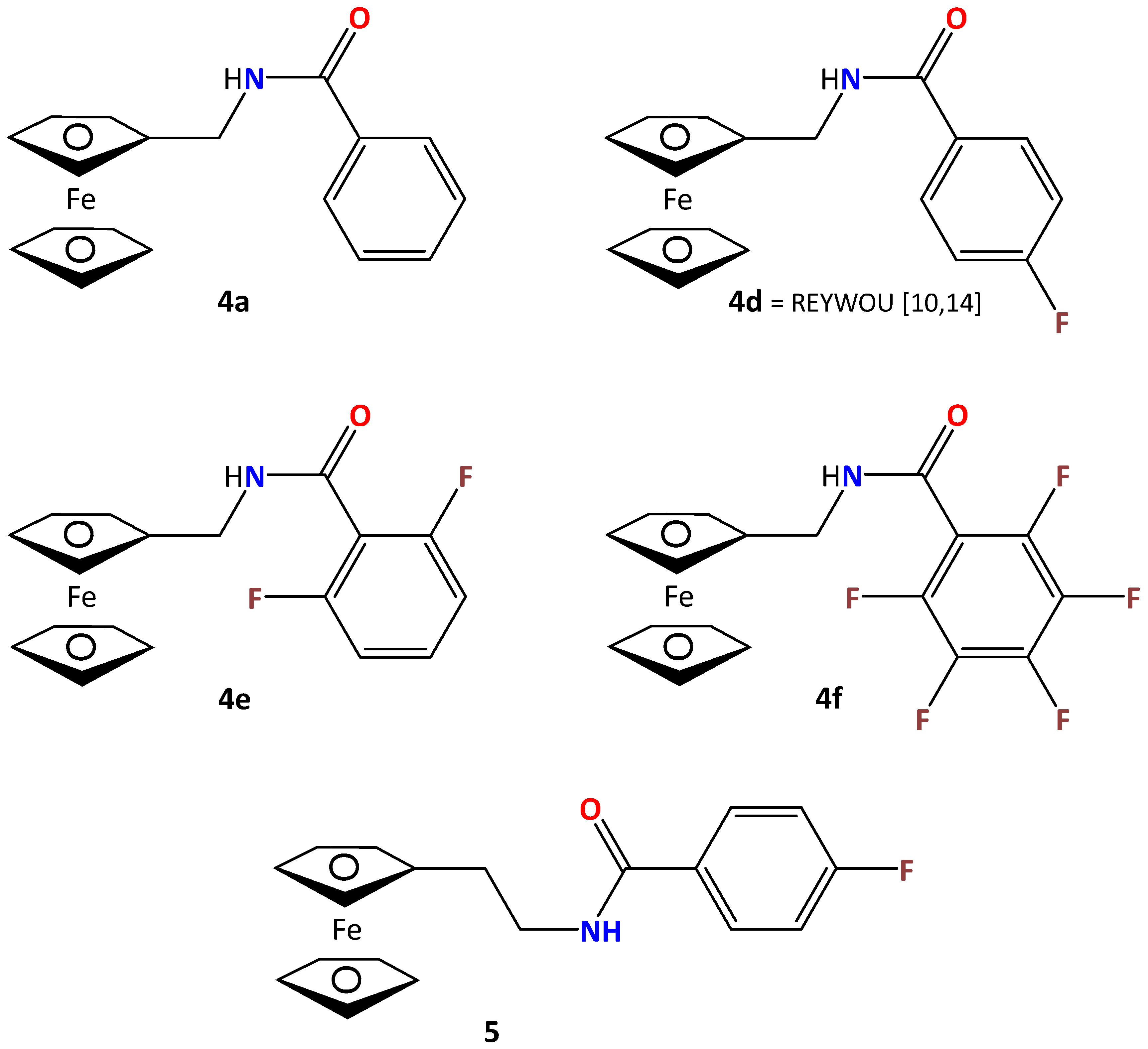
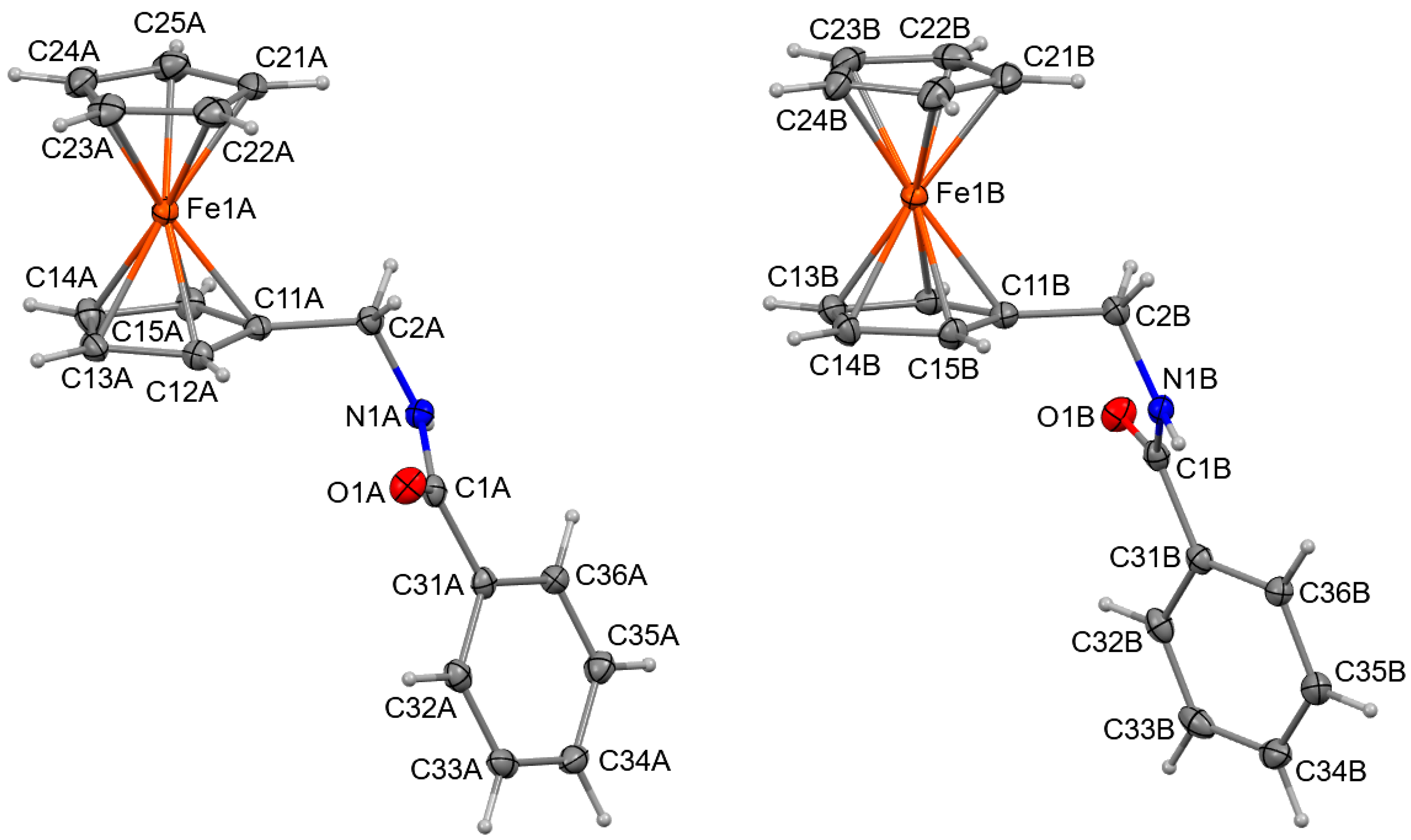
2.3. Four Crystal and Molecular Structures
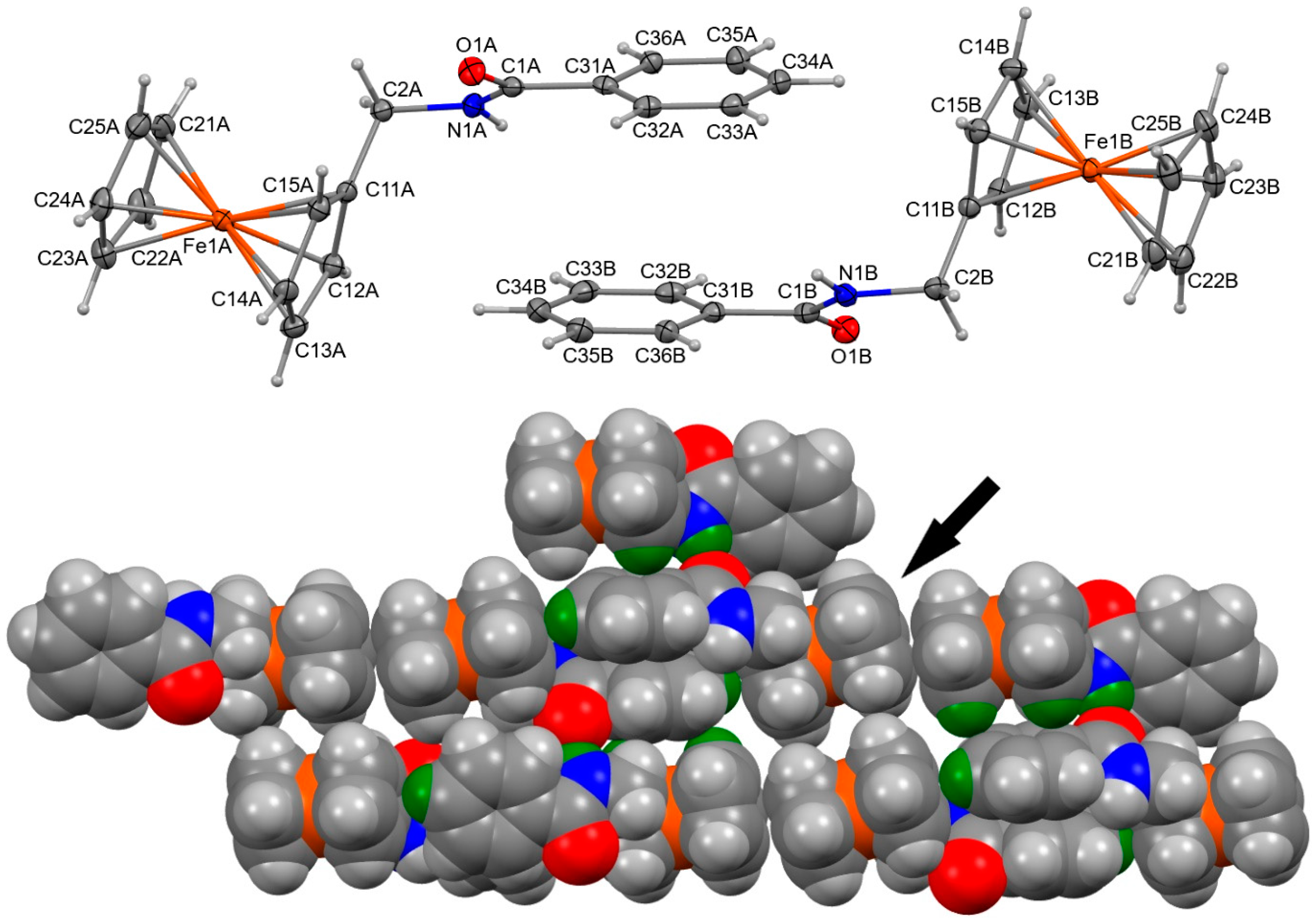
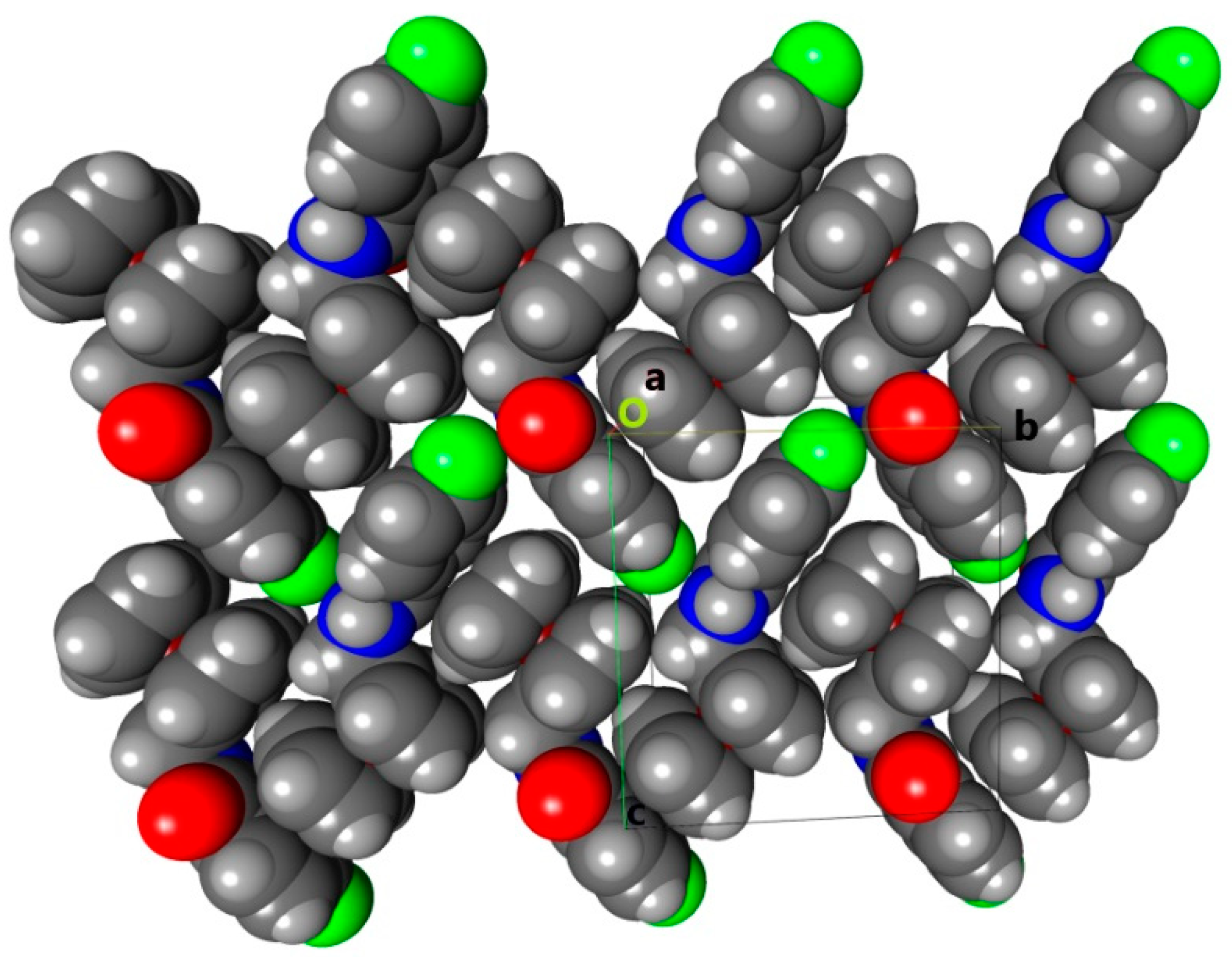
2.4. Hirshfeld Surface Analysis: Contacts Enrichment Analyses of Five Structures (4a, 4d–f, and 5)
2.5. Comparisons with Ferrocene Derivatives That Exhibit Distinct Aggregation and Packing
3. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Togni, A.; Hayashi, T. (Eds.) Ferrocenes: Homogeneous Catalysis, Organic Synthesis, Materials Science; VCH Publishers: Weinheim, Germany, 1995. [Google Scholar] [CrossRef]
- Phillips, E.S. (Ed.) Ferrocenes: Compounds, Properties & Applications; Nova Science Publishers Inc.: New York, NY, USA, 2011. [Google Scholar]
- Astruc, D. Why is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nature 2017, 1, 0066. [Google Scholar]
- Bear, J.C.; Cockcroft, J.K.; Williams, J.H. Influence of Solvent in Crystal Engineering: A Significant Change to the Order-Disorder Transition in Ferrocene. J. Am. Chem. Soc. 2020, 142, 1731–1734. [Google Scholar]
- Batsanov, A.S.; Collings, J.C.; Marder, T.B. Arene-perfluoroarene interactions in crystal engineering. XV. Ferrocene-decafluorobiphenyl (1/1). Acta Crystallogr 2006, C62, m229–m231. [Google Scholar]
- Torubaev, Y.V.; Skabitsky, I.V.; Saratov, G.; Barzolivich, P.Y. Halogen vs. ionic bonding: An unusual isomorphism between the neutral (C5Me5)2Fe/C2I2 cocrystal and ionic [(C5Me5)2Fe]Br3 crystal. Mendeleev Commun. 2021, 31, 58–61. [Google Scholar]
- Singh, A.; Torubaev, Y.; Ansari, S.N.; Singh, S.K.; Mobin, S.N.; Mathur, P. The borderline: Exploring the structural landscape of triptycene in cocrystallization with ferrocene. CrystEngComm 2020, 22, 1314–1320. [Google Scholar]
- Gallagher, J.F.; Alley, S.; Lough, A.J. A structural systematic study of semi-rigid ferrocene derivatives as a 3 × 3 metallocene isomer grid: p-/m-/o-(FcC6H4)CONH(p-/m-/o-C6H4)CO2Et, [Fc = (η5-C5H5)Fe(η5-C5H4)]. Inorg. Chim. Acta 2016, 444, 113–125. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar]
- Kraatz, H.-B.; Lusztyk, J.; Enright, G.D. Ferrocenoyl Amino Acids: A Synthetic and Structural Study. Inorg. Chem. 1997, 36, 2400–2405. [Google Scholar]
- van Staveren, D.R.; Metzler-Nolte, N. Bioorganometallic Chemistry of Ferrocene. Chem. Rev. 2004, 104, 5931–5986. [Google Scholar]
- Gallagher, J.F.; Kelly, P.N.; Kenny, P.T.M.; Lough, A.J. Intermolecular interactions in N-(ferrocenylmethyl)anthracene-9-carboxamide. Acta Crystallogr. 2003, C59, m552–m554. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.N.; Prêtre, A.; Devoy, S.; O’Rielly, I.; Devery, R.; Goel, A.; Gallagher, J.F.; Lough, A.J.; Kenny, P.T.M. Synthesis, structural characterisation and biological activity of novel N-(ferrocenylmethyl)benzene-carboxamide derivatives. J. Organomet. Chem. 2007, 692, 1327–1331. [Google Scholar]
- Hess, J.; Patra, M.; Pierroz, V.; Spingler, B.; Jabbar, A.; Ferrari, S.; Gasser, R.B.; Gasser, G. Synthesis, Characterization, and Biological Activity of Ferrocenyl Analogues of the Anthelmintic Drug Monepantel. Organometallics 2016, 35, 3369–3377. [Google Scholar] [CrossRef]
- Sharma, B.; Kumar, V. Has Ferrocene Really Delivered Its Role in Accentuating the Bioactivity of Organic Scaffolds? J. Med. Chem. 2021, 64, 16865–16921. [Google Scholar] [PubMed]
- Snegur, L.V. Modern Trends in Bio-Organometallic Ferrocene Chemistry. Inorganics 2022, 10, 226. [Google Scholar] [CrossRef]
- Gupta, P.; Madhavan, S.; Kapur, M. Synthesis of Ferrocene 1,3 derivatives by Distal C−H Activation. Angew Chem. Int. Ed. 2023, 62, e202305278. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.F.; Alley, S.; Brosnan, M.; Lough, A.J. 1,1′-Fc(4-C6H4CO2Et)2 and its unusual salt derivative with Z’ = 5, catena-[Na+]2[1,1′-Fc(4-C6H4CO2-)2].0.6H2O [1,1′-Fc = (η5-(C5H4)2Fe]. Acta Crystallogr. 2010, B66, 196–205. [Google Scholar]
- Savage, D.; Neary, N.; Malone, G.; Alley, S.R.; Gallagher, J.F.; Kenny, P.T.M. The synthesis and structural characterization of novel N-meta-ferrocenylbenzoyl amino acid esters. Inorg. Chem. Commun. 2005, 8, 429–432. [Google Scholar]
- Harry, A.G.; Murphy, J.P.; O’Donovan, N.; Crown, J.; Rai, D.K.; Kenny, P.T.M. The synthesis, structural characterization and biological evaluation of novel N-{para-(ferrocenyl)ethynylbenzoyl}amino acid and dipeptide methyl and ethyl esters as anticancer agents. J. Organomet Chem. 2017, 846, 379–388. [Google Scholar] [CrossRef]
- Enraf-Nonius Diffractometer, KappaCCD Server Software. Windows 3.11 Version; Nonius BV: Delft, The Netherlands, 1997.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Vuković, V.; Leduc, T.; Jelić-Matošević, Z.; Didierjean, C.; Favier, F.; Guillot, B.; Jelsch, C. A rush to explore protein–ligand electrostatic interaction energy with Charger. Acta Crystallogr. 2021, D77, 1292–1304. [Google Scholar] [CrossRef]
- Jelsch, C.; Ejsmont, K.; Huder, L. The enrichment ratio of atomic contacts in crystals, an indicator derived from the Hirshfeld surface analysis. IUCrJ 2014, 1, 119–128. [Google Scholar] [CrossRef]
- Vrbancich, J.; Ritchie, G.L.D. Quadrupole Moments of Benzene, hexafluorobenzene and other Non-dipolar Aromatic Molecules. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1980, 76, 648–659. [Google Scholar] [CrossRef]
- Ritchie, G.L.D.; Cooper, M.K.; Calvert, R.; Dennis, G.R.; Phillips, L.; Vrbancich, J. Molecular Quadrupole Moments, Magnetic Anisotropies and Charge Distributions of Ferrocene and Ruthenocene. J. Am. Chem. Soc. 1983, 105, 5215–5219. [Google Scholar] [CrossRef]
- Hernandez-Trujillo, J.; Vela, A. Molecular Quadrupole Moments for the Series of Fluoro- and Chlorobenzenes. J. Phys. Chem. 1996, 100, 6524–6530. [Google Scholar] [CrossRef]
- Rheingold, A.L. Ferrocene∙Decafluorophenanthrene (LUZJIB). CSD Commun. 2015, 1430306. [Google Scholar]
- Xua, G.X.; Cordes, D.B.; Slawin, A.M.Z.; Woollins, D.B. Organo Phosphorus-Sulfur-Nitrogen Heterocycles from Thionation of Schiff Bases. Z. Für Anorg. Allg. Chem. 2021, 646, 239–244. [Google Scholar]
- Senthilkumar, K.; Pizzotti, M.; Thirumoorthy, K.; DiCarlo, G.; Righetto, S.; Orbelli Biroli, A.; Haukka, M.; Palanisami, N. New Internal-Charge-Transfer Second-Order Nonlinear Optical Chromophores Based on the Donor Ferrocenylpyrazole Moiety. J. Phys. Chem. C 2016, 120, 20277–20287. [Google Scholar]
- Deck, P.A.; Kroll, C.E.; Hollis, W.G., Jr.; Fronczek, F.R. Conformational control of intramolecular arene stacking in ferrocene complexes bearing tert-butyl and pentafluorophenyl substituents. J. Organomet. Chem. 2001, 637–639, 107–115. [Google Scholar]
- Haneline, M.R.; Gabbaï, F.P. Elecrophilic Double-Sandwiches Formed by Interaction of [Cp2Fe] and [Cp2Ni] with the Tridentate Lewis Acid [(o-C6H4Hg)3]. Angew Chem. Int. Ed. 2004, 43, 5471–5475. [Google Scholar]
- Mao, Y.Q.; Maley, I.; Watson, W.H. Syntheses and structures of N-phenylmaleimidetriazoles and by-products. J. Chem. Crystallogr. 2005, 35, 385–403. [Google Scholar]
- Isaac, C.J.; Price, C.; Horrocks, B.R.; Houlton, A.; Elsegood, M.R.J.; Clegg, W. Synthesis, structure and coordination chemistry of mono- and bis-heterocyclic-ferrocenyl derivatives. J. Organomet. Chem. 2000, 598, 248–253. [Google Scholar]
- Wang, C.-H.; Chen, K.-J.; Wu, T.-H.; Chang, H.-K.; Tsuchido, Y.; Sei, Y.; Chene, P.-L.; Horie, M. Ring rotation of ferrocene in interlocked molecules in single crystals. Chem. Sci. 2021, 12, 3871–3875. [Google Scholar]
- Robertson, C.C.; Wright, J.S.; Carrington, E.J.; Perutz, R.N.; Hunter, C.A.; Brammer, L. Hydrogen bonding vs. halogen bonding: The solvent decides. Chem. Sci. 2017, 8, 5392–5398. [Google Scholar] [PubMed]
- Abeysekera, A.M.; Day, V.W.; Sinha, A.S.; Aakeröy, C.B. Mapping out the Relative Influence of Hydrogen and Halogen Bonds in Crystal Structures of a Family of Amide-Substituted Pyridines. Cryst. Growth Des. 2020, 20, 7399–7410. [Google Scholar]
- Gallagher, J.F.; Farrell, M.; Hehir, N.; Mocilac, P.; Aubert, E.; Espinosa, E.; Guillot, B.; Jelsch, C. At the Interface of Isomorphous Behavior in a 3 × 3 Isomer Grid of Monochlorobenzamides: Analyses of the Interaction Landscapes via Contact Enrichment Studies. Cryst. Growth Des. 2019, 19, 6141–6158. [Google Scholar]
- Gallagher, J.F.; Hehir, N.; Mocilac, P.; Violin, C.; O’Connor, B.F.; Aubert, E.; Espinosa, E.; Guillot, B.; Jelsch, C. Probing the Electronic Properties and Interaction Landscapes in a Series of N-(Chlorophenyl)pyridine-carboxamides. Cryst. Growth Des. 2022, 22, 3343–3358. [Google Scholar]
- Osman, I.A.; McKee, V.; Jelsch, C.; Gallagher, J. Roles of Hydrogen, Halogen Bonding and Aromatic Stacking in a series of Isophthalamides. Symmetry 2023, 15, 738. [Google Scholar] [CrossRef]
- Jaime-Adán, E.; Hernández-Ortega, S.; Toscano, R.A.; Germán-Acacio, J.M.; Sánchez-Pacheco, A.D.; Hernández-Vergara, M.; Barquera, J.E.; Valdéz-Martínez, J. Competition of Hydrogen Bonds, Halogen Bonds and π-π Interactions in Crystal Structures. Exploring the Effect of One Atom Substitution. Cryst. Growth Des. 2024, 24, 1888–1897. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner., T. The Weak Hydrogen Bond in Structural Chemistry and Biology; IUCr Monographs on Crystallography; Oxford Science Publications; Oxford University Press: Oxford, UK, 1999; ISBN 9780198509707. [Google Scholar]
- Nishio, M. CH/hydrogen bonds in crystals. CrystEngComm 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 42, 8617–8636. [Google Scholar] [CrossRef]
- Dance, I.; Scudder, M. Molecules embracing in Crystals. CrystEngComm 2009, 11, 2233–2247. [Google Scholar]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Katrusiak, A.; Rusek, M.; Dušek, M.; Petříček, V.; Szafrański, M. Dipole-Moment Modulation in New Incommensurate Ferrocene. J. Chem. Phys. Lett. 2023, 14, 3111–3119. [Google Scholar]
- Schulte, Y.; Wölper, C.; Rupf, S.M.; Malischewski, M.; SantaLucia, D.J.; Neese, F.; Haberhauer, G.; Schulz, S. Structural characterization and reactivity of a room-temperature-stable, antiaromatic cyclopentadienyl cation salt. Nat. Chem. 2024, 16, 651–657. [Google Scholar] [CrossRef]
- Köring, L.; Stepen, A.; Birenheide, B.; Barth, S.; Leskov, M.; Schoch, R.; Krämer, F.; Breher, F.; Paradies, J. Boron-Centered Lewis Superacid through Redoc-Active Ligands: Application in C-F and S-F Bond Activation. Angew Chem. Int. Ed. 2023, 62, e202216959. [Google Scholar]
- Preuss, A.; Notz, S.; Kovalski, E.; Korb, M.; Blaudeck, T.; Hu, X.; Schuster, J.; Miesel, D.; Rüffer, T.; Hildebrandt, A.; et al. Ferrocenyl-Pyrenes, Ferrocenyl-9,10-Phenanthrenediones, and Ferrocenyl-9,10-Dimethoxyphenanthrenes: Charge-Transfer Studies and SWCNT Functionalization. Chem. Eur. J. 2020, 26, 2635–2652. [Google Scholar]
- Torubaev, Y.V.; Skabitsky, I.V.; Lyssenko, K.A. Structure-defining interactions in the salt cocrystals of [(Me5C5)2Fe]+I3−–XC6H4OH (X = Cl, I): Weak noncovalent vs. strong ionic bonding. Mendeleev Comm. 2020, 30, 580–582. [Google Scholar]
- Torubaev, Y.V.; Skabitsky, I.V.; Raghuvanshi, A. The structural landscape of ferrocenyl polychalocogenides. J. Organomet. Chem. 2021, 951, 122006. [Google Scholar]
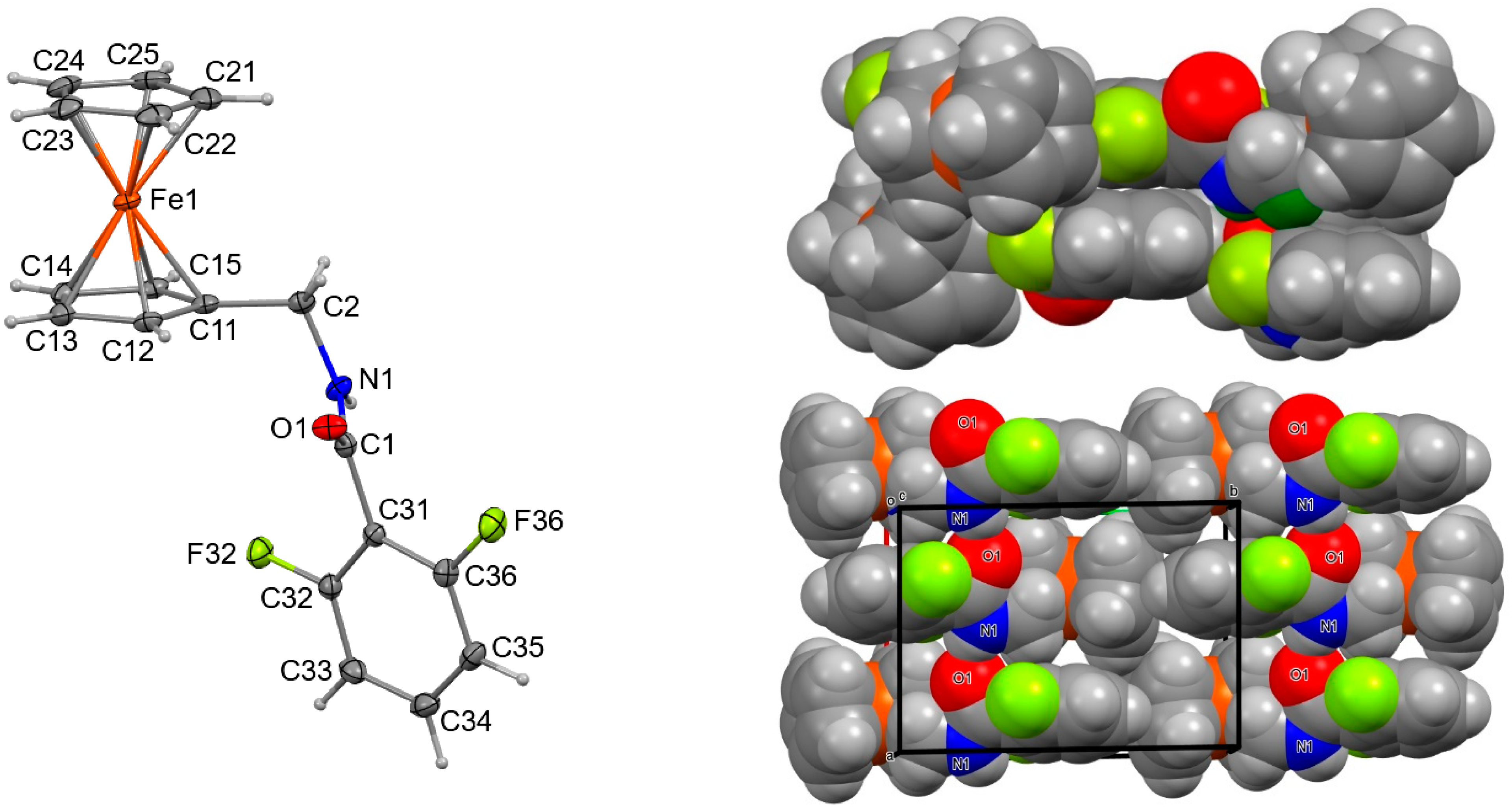

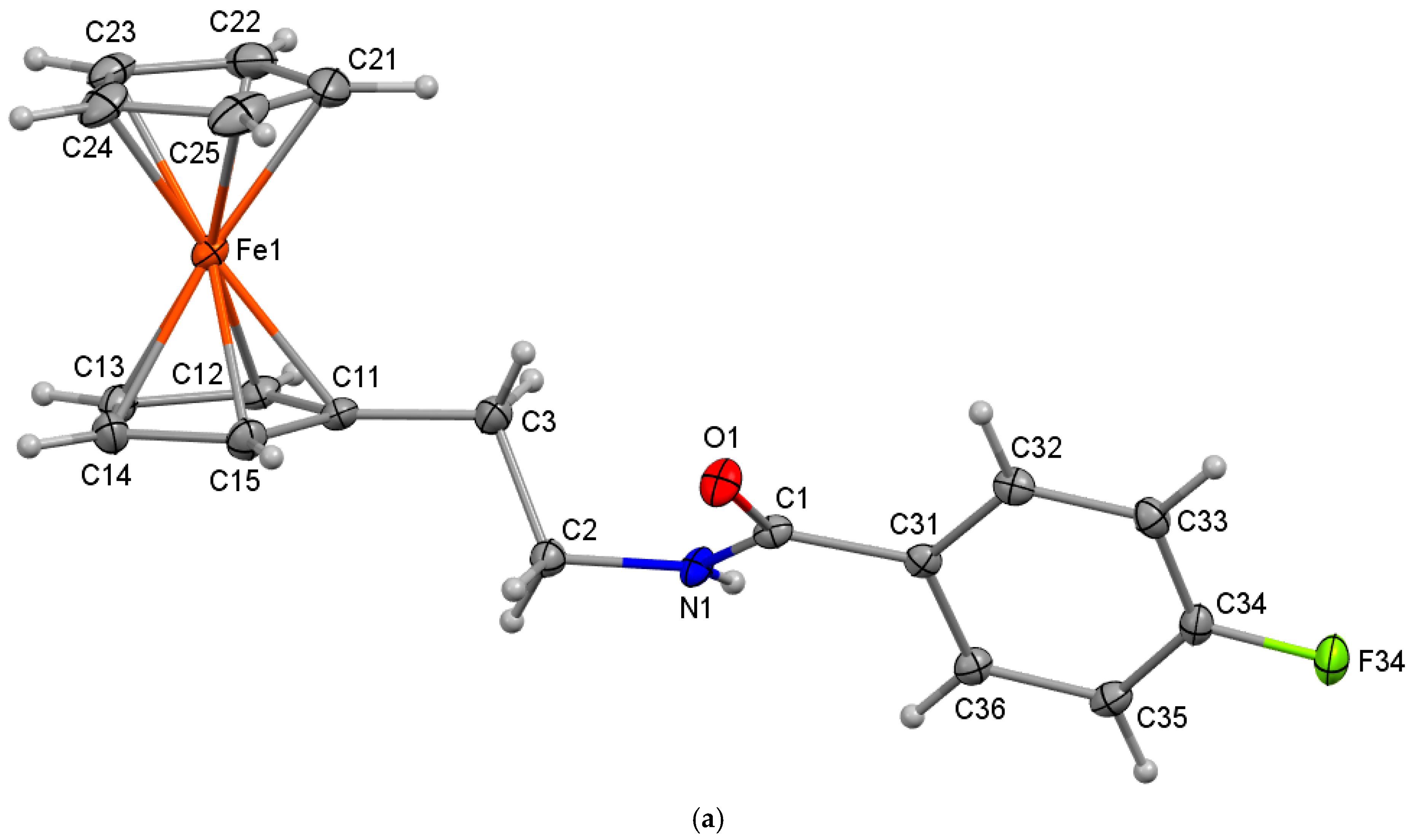
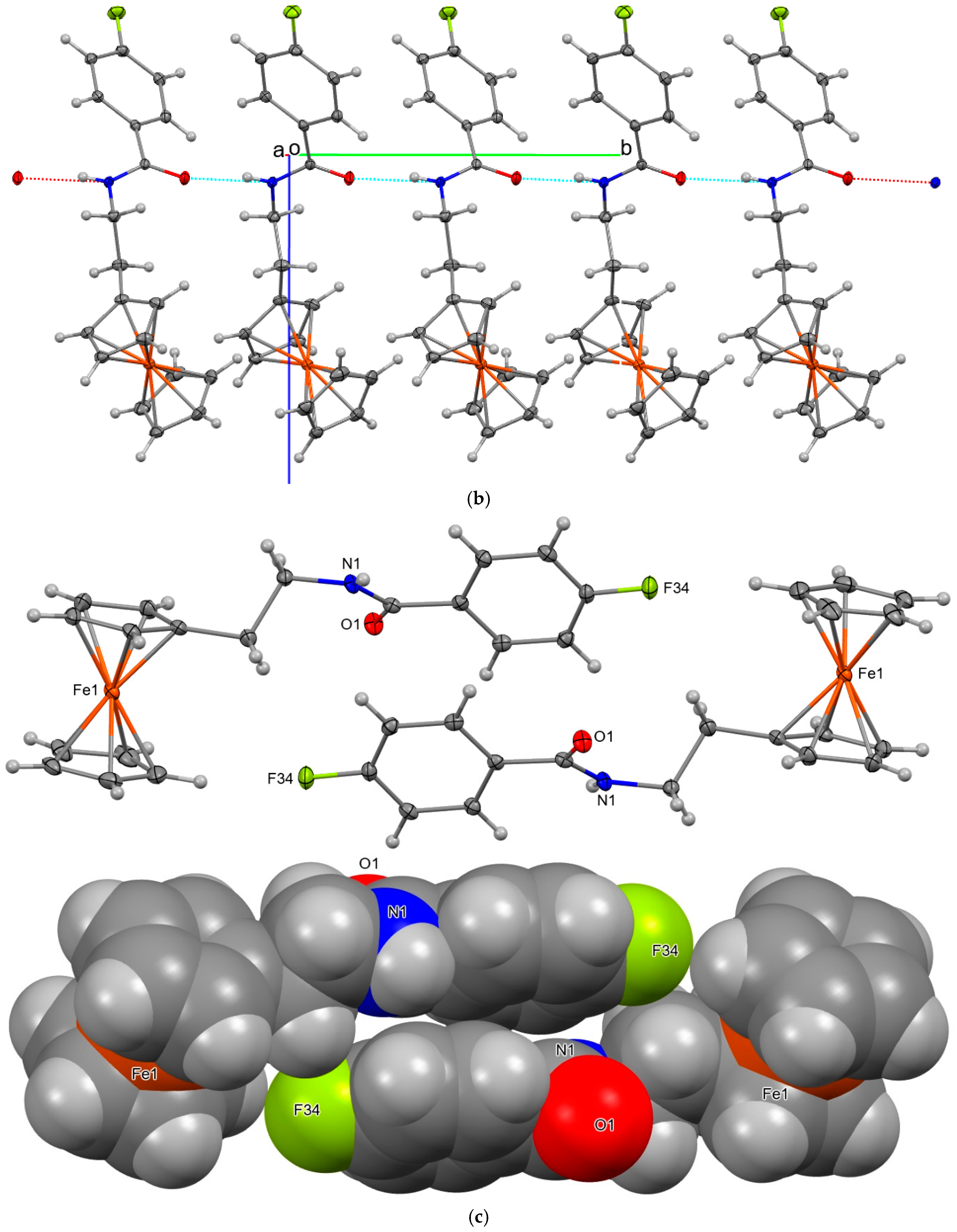
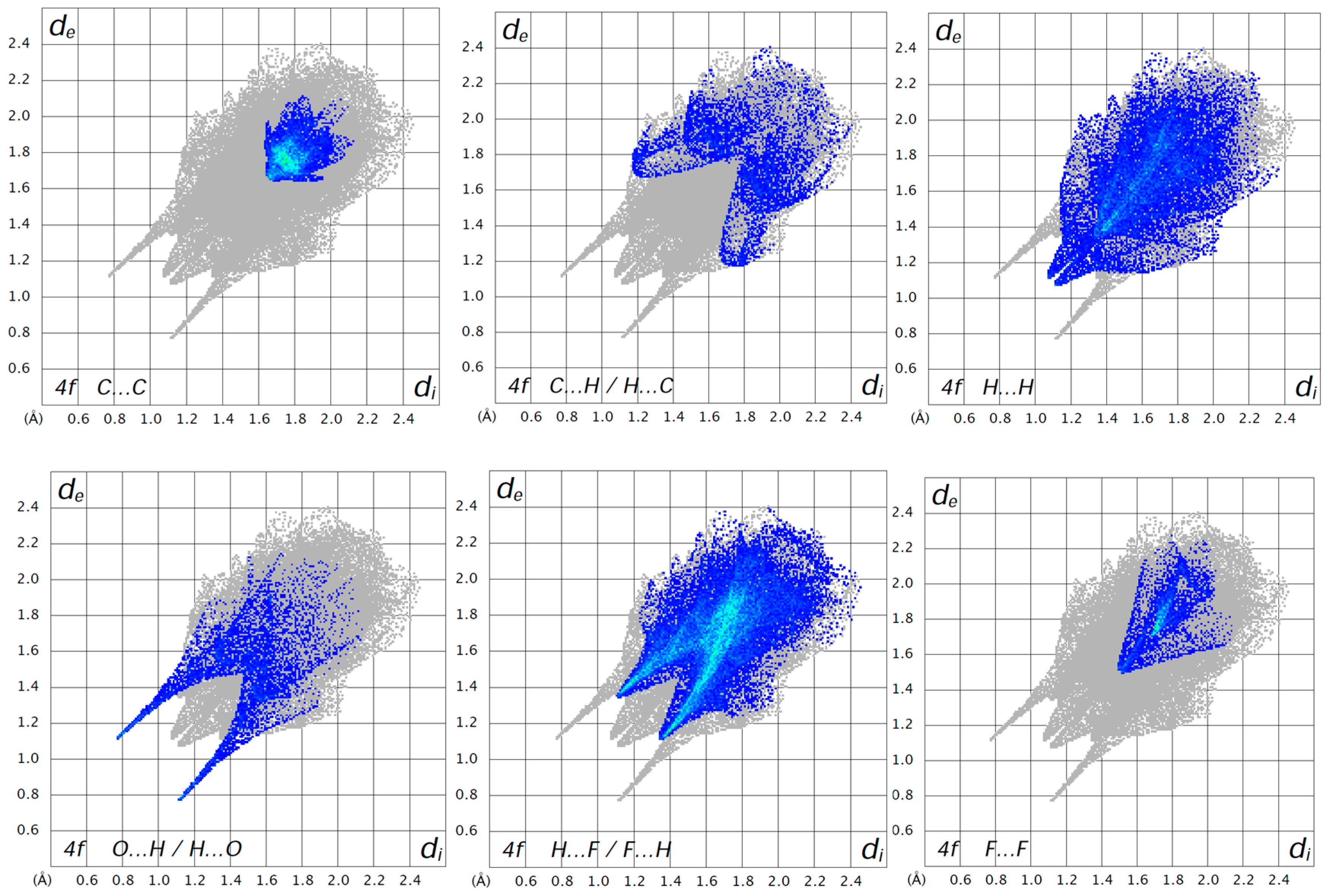
| Structures | Crystal System; Space Group | Z’ | Volume (Å3); KPI | R, wR2R-Factors, GoF |
|---|---|---|---|---|
| 4a | Monoclinic; P21/n | 2 | 2851.36 (18); 71.3 | 0.044, 0.126, 0.98 |
| 4d [14] | Orthorhombic; P212121 | 1 | 1435.97 (4); 71.8 | 0.031, 0.065, 1.05 |
| 4e | Monoclinic; P21/a | 1 | 1524.92 (8); 69.3 | 0.034, 0.085, 1.05 |
| 4f | Monoclinic; P21/c | 1 | 1577.44 (10); 71.2 | 0.034, 0.086, 1.06 |
| 5 | Orthorhombic; Pbca | 1 | 3145.27 (16); 69.8 | 0.037, 0.092, 1.03 |
| Structures | C5H4/C6 (°) | C5H4/Amide (°) | Amide…Amide Interaction (Å) | O=C-N-Ctorsion |
|---|---|---|---|---|
| 4a | 73.16 (11)/79.12 (11) | 64.77 (12)/68.16 (12) | 2.924 (3)/2.869 (3) | −1.6 (4)/4.1 (4) |
| 4d [14] | 77.80 (8) | 69.80 (10) | 2.972 (3) | 2.1 (3) |
| 4e | 81.24 (7) | 68.55 (10) | 2.819 (2) | −7.6 (3) |
| 4f | 11.34 (11) | 57.31 (9) | 2.892 (2) | 3.0 (3) |
| 5 | 61.66 (7) | 34.08 (12) | 2.853 (2) | −3.8 (3) |
| Geometric Data | SUFLIR (100 K) [5] (Å,°) | 4f (Å,°) |
|---|---|---|
| Fe⋯C5 (centroid); (Å,°) a | 1.6467 (8), 1.6479 (8); 179.53 (4) | 1.6482 (9), 1.6530 (10); 178.75 (6) |
| C5⋯C6F6 [5] or C5⋯C6F5 b | 3.5825 (10); 3.6052 (11) | 3.5399 (13); 3.5720 (12) |
| Dihedral angle (planes)° c | 7.67( 9); 8.86 (9) d | 2.76 (12); 2.88 (10) |
| C-F⋯C5 ring centroid (Å) | 3.5949 (13); 3.6210 (14) | 3.5179 (16); 3.6620 (15) |
| C-F⋯C5 ring centroid (Å) | 3.3342 (18); 3.3370 (18) | 3.257 (2); 3.377 (2) |
| C-F⋯C5 (°) | 68.05 (8); 67.13 (8) | 67.79 (10); 67.22 (9) |
| Shortest stack cpC⋯Carene | 3.310 (2), 3.337 (2), 3.340 (2) | 3.319 (3), 3.327 (3), 3.387 (3) |
| Shortest stack cpH⋯F-C | 3.089, 3.167, 3.176, 3.226 | 3.21, 3.29, 3.29, 3.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallagher, J.F.; Jelsch, C.; Kenny, P.T.M.; Lough, A.J. Impressive 1D (Ferrocenyl⋯C6F5R⋯)n Stacking Due to Cooperative Interactions in N-(Ferrocenylmethyl)Pentafluorobenzenecarboxamide: Four Crystal Structures and Contacts Analyses in N-(Ferrocenylalkyl)Benzenecarboxamides. Crystals 2025, 15, 299. https://doi.org/10.3390/cryst15040299
Gallagher JF, Jelsch C, Kenny PTM, Lough AJ. Impressive 1D (Ferrocenyl⋯C6F5R⋯)n Stacking Due to Cooperative Interactions in N-(Ferrocenylmethyl)Pentafluorobenzenecarboxamide: Four Crystal Structures and Contacts Analyses in N-(Ferrocenylalkyl)Benzenecarboxamides. Crystals. 2025; 15(4):299. https://doi.org/10.3390/cryst15040299
Chicago/Turabian StyleGallagher, John F., Christian Jelsch, Peter T. M. Kenny, and Alan J. Lough. 2025. "Impressive 1D (Ferrocenyl⋯C6F5R⋯)n Stacking Due to Cooperative Interactions in N-(Ferrocenylmethyl)Pentafluorobenzenecarboxamide: Four Crystal Structures and Contacts Analyses in N-(Ferrocenylalkyl)Benzenecarboxamides" Crystals 15, no. 4: 299. https://doi.org/10.3390/cryst15040299
APA StyleGallagher, J. F., Jelsch, C., Kenny, P. T. M., & Lough, A. J. (2025). Impressive 1D (Ferrocenyl⋯C6F5R⋯)n Stacking Due to Cooperative Interactions in N-(Ferrocenylmethyl)Pentafluorobenzenecarboxamide: Four Crystal Structures and Contacts Analyses in N-(Ferrocenylalkyl)Benzenecarboxamides. Crystals, 15(4), 299. https://doi.org/10.3390/cryst15040299






