Abstract
Ba1−xSrxFe12O19 (x = 0.0, 0.5 and 1.0) hard-magnetic nanohexaferrites prepared by autocombustion were primarily investigated using Mössbauer spectroscopy and optical studies. Morphological examination by electron scanning microscopy revealed that the particles agglomerated into grains with a hexagonal shape. The grain size increases with the amount of Sr content, from ca. 490 nm (x = 0.0) to ca. 700 nm (x = 1.0). Room-temperature Mössbauer spectroscopy showed that the mean hyperfine field increased with the substitution of Ba2+ by Sr2+, consistent with magnetization results. The preferential sites occupied by Fe ions in the hexaferrite structure were determined. Optical studies revealed that all compounds absorb up to ca. 1000 nm, and that the bandgap energy decreases with increasing Sr content.
1. Introduction
Hexaferrite materials (HFm) (including BaFe12O19, PbFe12O19, and SrFe12O19) have been used in numerous technological devices, especially in IT applications such as anti-electromagnetic interfaces, telecommunication, channel filters, microwave devices, magnetic recording, magneto-optics, gyromagnetic devices, and multilayer chip devices [1,2,3,4,5,6]. These materials are known to have high resistivity, high chemical stability, high coercive, and anisotropy fields [7].
The magnetic properties of HFm are linked to the intrinsic magnetic properties of M-type phase. The M-type hexaferrites crystallize in a hexagonal structure with 64 ions per unit cell distributed across 11 different symmetry sites, which contributes to their ferromagnetic behavior.
Recent research has confirmed the light absorption capabilities of HFm by examining its optical band gap energy [8]. It was confirmed that the synthesis technique, particle size, and concentration and nature of the doping metal ions induce significant modifications in band gap energy [9,10,11]. For example, Jayakumar et al. [12] reported that introducing Cd2+ and Ni2+ ions into Strontium hexaferrites (SrFe12O19) increases the optical band gap energy from 2.32 to 2.82 eV.
Generally, ion substitution in HFm leads to a change in site occupancy of Fe3+ ions in the five interstitial positions, with trivalent metal ions replacing them. This substitution strongly affects the structural, magnetic, and optical properties.
Mössbauer spectroscopy allows for detailed analysis of the iron sites in M-type hexaferrites. Previous studies have shown distinct hyperfine magnetic fields at different crystallographic sites (tetrahedral and octahedral), confirming the non-collinear magnetic structure of these materials. Variations in isomer shifts and quadrupole splitting provide insights into the electronic and magnetic environments surrounding the Fe ions. Mössbauer spectroscopy has been used to study the effects of substituting Fe3⁺ ions with other cations, examining how these substitutions impact the magnetic properties and stability of the hexaferrite structure [13,14].
In the earlier studies we examined, experimentally and theoretically, the structural, magnetic, electrical, and dielectric properties of Ba1−xSrxFe12O19 (x = 0; 0.5; 1) M-type hexaferrites synthesized through the autocombustion method [15,16].
In this study, we examine Ba1−xSrxFe12O19 hexaferrites prepared by autocombustion through scanning electron microscopy and energy dispersive X-ray spectroscopy. This allowed us to access particle morphology and compositional homogeneity. Additionally, Mössbauer spectroscopy was employed to determine the oxidation states of iron within the samples and to analyze the distribution of Fe across various sites in the hexaferrites. We also present optical studies conducted on the samples.
The combination of Mössbauer spectroscopy and optical studies provides crucial insight into the magnetic interactions and electronic structures of M-type hexaferrites. To continue the research in this area, the present results contribute to optimizing synthesis methods and exploring advanced doping strategies to further tailor the properties of M-type hexaferrites.
2. Experimental
The Ba1−xSrxFe12O19 (x = 0.0, 0.5 and 1.0) M-type hexaferrites were synthesized through an autocombustion method detailed in our earlier publications [15,16]. Stoichiometric quantities of Fe(NO3)3·9H2O, Ba(NO3)2, and Sr(NO3)2 were used and dissolved in aqueous solution with glycine as fuel agent. The resulting black powders were compacted into pellets and subjected to a sintering process at consecutive temperatures of 900 °C for 30 min, 1000 °C for 30 min, and 1100 °C for 2 h.
The morphology of the samples was examined using a TESCAN VEGA3 SBH scanning electron microscope (SEM).
Mössbauer spectra were obtained at room temperature in transmission geometry using triangular velocity waveforms. A 57Co source embedded in an Rh matrix, with an activity of approximately 20 mCi, was employed. Powder samples were placed in Perspex holders for analysis. The spectra were evaluated using the least squares method with the NORMOS program [17], and isomer shifts were referenced to α-Fe at room temperature.
Solid state absorption spectra were recorded by collecting the reflectance using an AvaSpec-ULS-TEC Avantes Senseline Fiber Optic Spectrometer System coupled to a Mikropack DH-2000-BAL UV-Vis-NIR light source. A 45-degree angle probe tip fiber optic bundle for measurement of reflection in powders and thick fluids was used (FCR-UV200/600-2-IND 1211040). The 45-degree angle of the probe prevents the measurements of direct back reflection from the window. Background correction was performed by collecting the baseline with 100% and 0% reflectance (using a Polytetrafluoroethylene, PTFE, reference sample and the blocked beam, respectively) prior to the determination of the spectra of the solid samples. The conversion to absorption was conducted based on the Kubelka–Munk function, F(R) [18].
3. Results and Discussion
3.1. Structural and Mössbauer Studies
The structural characterization of our M-type hexaferrites using powder X-ray diffraction and Transmission electron microscopy (TEM) was reported in our previous studies [15,16]. The diffraction patterns were indexed to the hexagonal structure with P63/mmc space group of pure BaFe12O19. A minor secondary phase of hematite (α-Fe2O3) was found. This oxide was also unavoidable during the synthesis of hexaferrites in similar works [19].
The average size of the crystallites of the Ba1−xSrxFe12O19, (x = 0.0, x = 0.5 and x = 1.0) compounds was determined by the Scherrer equation, ranging from 56 nm to 44 nm, and the mean particle size obtained by TEM is ca. 230 nm to ca. 180 nm [15].
Figure 1 shows the SEM images of the hexaferrites that confirmed the coarseness in the samples and the hexagonal shape of the grains. It can also be observed that the hexagonal platelets are distributed homogeneously. This shape of grains is very distinct from those observed in other surface morphologies studies on Sr doped Ba hexaferrites [20]. One can observe that the average grain size decreases with the amount of Sr content, with the platelets’ mean length being approximately 1.5 μm, 900 nm, and 750 nm for Ba1−xSrxFe12O19, x = 0.0, x = 0.5, and x = 1.0, respectively. The decrease observed in the surface grains is in accordance with the decrease in crystallite and particle sizes with the increase of Sr content. In Figure 2, the chemical elements are shown to be uniformly distributed throughout the hexaferrite powders.
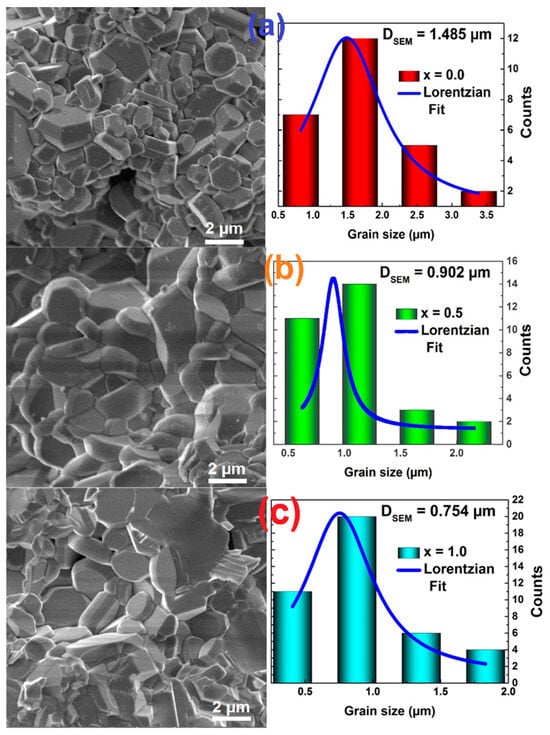
Figure 1.
SEM images of the Ba1−xSrxFe12O19 M-type hexaferrites: (a) x = 0.0, (b) 0.5, and (c) 1.0.
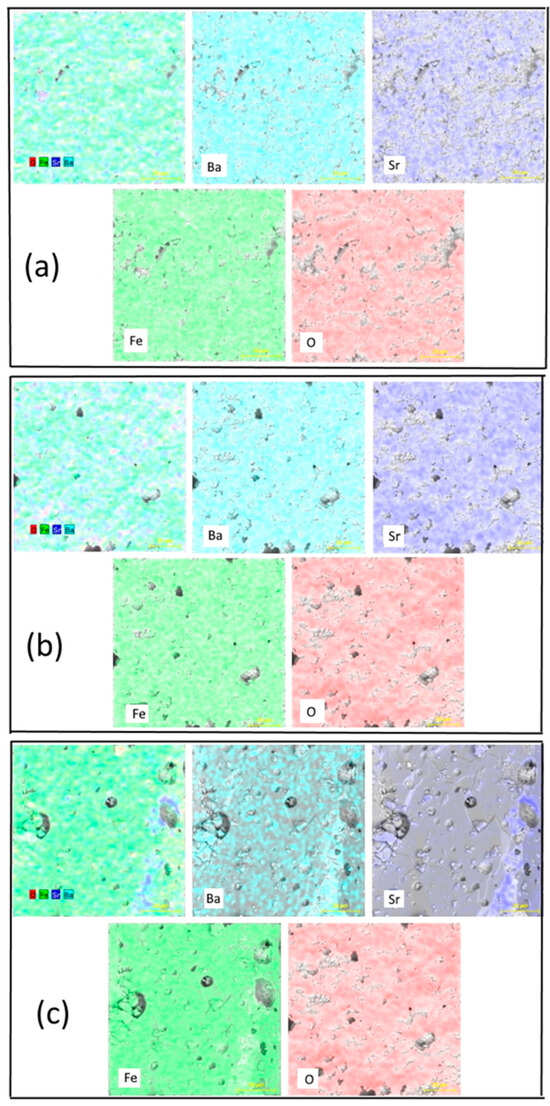
Figure 2.
Element mapping of the Ba1−xSrxFe12O19 samples: (a) x = 0.0, (b) x = 0.5, and (c) x = 1.0, determined by EDS.
Saturation magnetization (Ms), remanent magnetization (Mr), and coercivity (Hc) were extracted from the hysteresis loops shown in a previous work [16] and are presented in Table 1. In general, the hard magnetic materials’ properties increase concomitantly with the Sr doping content.

Table 1.
Magnetic parameters of Ba1−xSrxFe12O19 (x = 0.0, 0.5 and 1.0) M-type hexaferrites (ref. [14]).
The crystal structure of M-type hexaferrites consists of a unit cell composed of two molecular units, in a total of 64 ions (32 ions per unit). Each molecular unit features two distinct types of blocks—hexagonal (R) and cubic (S)—arranged in the sequence SRS*R*, where the S* and R* blocks are rotated 180° around the c-axis, overlapping with one another. Among the 64 ions, there are 38 O2⁻ ions and 24 Fe3⁺ ions occupying interstitial positions across five different crystallographic sites: three octahedral sites (12k, 4f2, and 2a), one tetrahedral site (4f1), and the bipyramidal 2b site, which is defined by a base formed from five oxygen atoms surrounding the Fe3⁺ ion [21]. The remaining two ions out of the total sixty-four can be either Ba2⁺ or Sr2⁺, depending on the specific composition [22]. In barium and strontium hexaferrites, Fe3⁺ serves as the ferromagnetic ion, and its distribution across the five crystallographic sites plays a critical role in determining the material’s overall magnetic properties. According to the Gortel model, within a given molecular unit, twelve Fe3⁺ ions are distributed as follows: Six occupy the 12k site, one occupies the 2a site (with spin up), one resides in the 2b site, and two are located in both the 4f1 and 4f2 sites (with spin down). This configuration results in eight Fe3⁺ ions with spin up and four with spin down. Consequently, the net magnetization of the hexagonal crystal is primarily due to the excess of spin-up Fe3⁺ ions [23,24].
The use of 57Fe Mössbauer spectroscopy is crucial for understanding the magnetic behavior of hexaferrites, particularly in relation to their structural characteristics and the preferential occupancy of various crystallographic sites. Fe3⁺ ions play a key role in this Mössbauer analysis.
Statistically, if Fe3+ ions are uniformly distributed by the five crystallographic sites, the occupation of sublattices 12k, 4f1, 4f2, 2a, and 2b is 50:17:17:8:8, respectively [22]. Any variation from this ratio can be ascribed to the substitution of other elements at this site, which may occur differently with different preparation methods, for instance.
The Mössbauer spectra of Ba1−xSrxFe12O19 (x = 0; 0.5 and 1) are shown in Figure 3. In all spectra, the Mössbauer contribution of the M-type phase was fitted using five magnetic components, each corresponding to the five distinct sites of the M-type crystal structure. The spectra were also adjusted with a small contribution of hematite. The fitted hyperfine parameters are shown in Table 2.
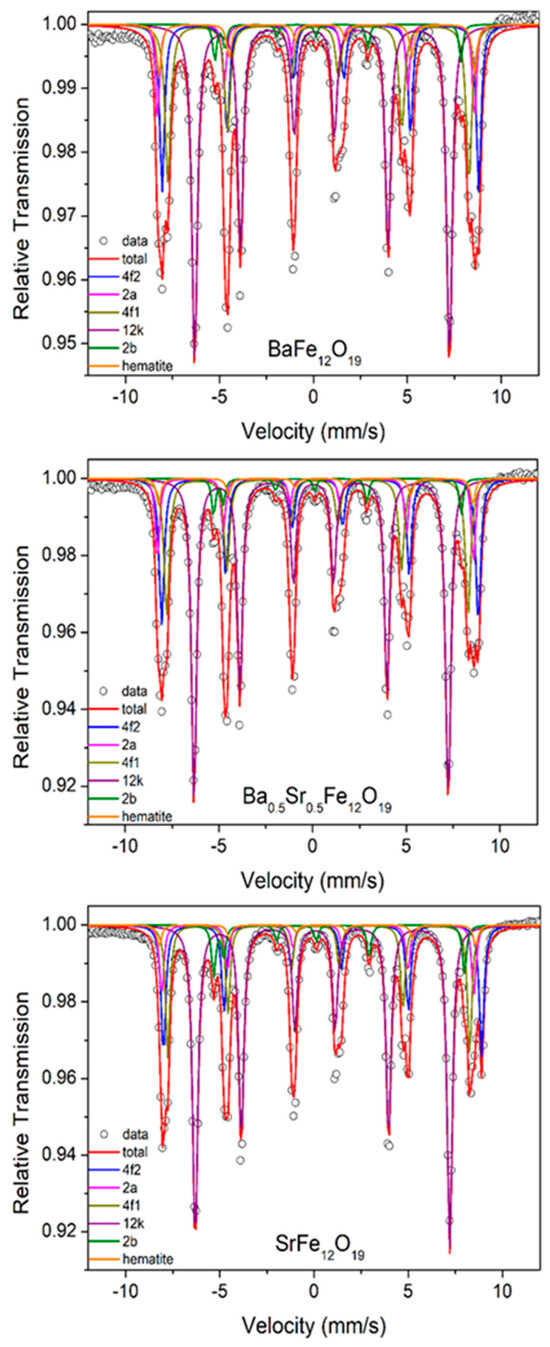
Figure 3.
Room-temperature Mössbauer spectra of the Ba1−xSrxFe12O19 M-type hexaferrites.

Table 2.
Hyperfine parameters obtained from the fitting of spectra shown in Figure 3: Isomer shift (δ), quadrupole splitting (Δ), hyperfine magnetic field (B), full width at half maximum (W), relative area (RA), and relative area only of the hexaferrite (RA*).
The contribution of Fe3⁺ ions at each site to the magnetic properties varies. Hyperfine Mössbauer parameters, including quadrupole splitting (Δ), isomer shift (δ), and hyperfine magnetic field (B), offer valuable insights into the characteristics of the different oxygen polyhedra present in the structure.
The fitting procedure was conducted according to the usual procedure following the literature, e.g., [25].
The spectrum for the 12k site displays the highest intensity (RA), while the 2b site, characterized by its trigonal bipyramidal symmetry, exhibits significant distortion, leading to larger quadrupole splitting values and reduced relative intensity. This pronounced quadrupole splitting in the 2b sublattice allows for a clear differentiation of the corresponding sextet from those of the other sublattices. The hyperfine magnetic field obtained from our fitting procedure is similar for 4f2 and 2a sites, but site 4f2 stands out due to its higher relative intensity.
The isomer shifts (δ) are of the order of 0.25 to 0.44 mm/s for the five sextets. It is widely recognized that in the magnetically ordered phase, the valence state of iron (Fe) can be primarily identified by the isomer shift values: 0.6–1.7 mm/s corresponds to Fe2⁺, 0.05–0.5 mm/s to Fe3⁺, and −0.15–0.05 mm/s to Fe4⁺ [8]. Therefore, all values of the isomer shift correspond to the Fe3+ state.
Substitution of Ba M-type hexaferrites by Dy and Cr [26], studied by Raman and Mössbauer spectroscopies, showed that the occupancy of those sites is very much modified, influencing the magnetic properties of the compounds.
The change of hyperfine magnetic field, isomer shift, and quadrupole splitting with composition x for Ba1−xSrxFe12O19 (x = 0; 0.5 and 1) is shown in Figure 4. B values change slightly with Sr substitution, except in the case of x = 1 for site 2a. In this case, one can observe that B decreases. The magnetization of the M-type phase can be assessed by calculating the average hyperfine field, denoted as <B>, which reflects the magnetic moment. This mean field is determined by assuming that the magnetic moments at the 12k, 2a, and 2b sites are aligned parallel to one another, while the moments at the 4f1 and 4f2 sites are oriented antiparallel, as outlined below:
where pi represents the relative Mössbauer percentage of the contribution from site “i” associated with the hyperfine field B(i) [27].
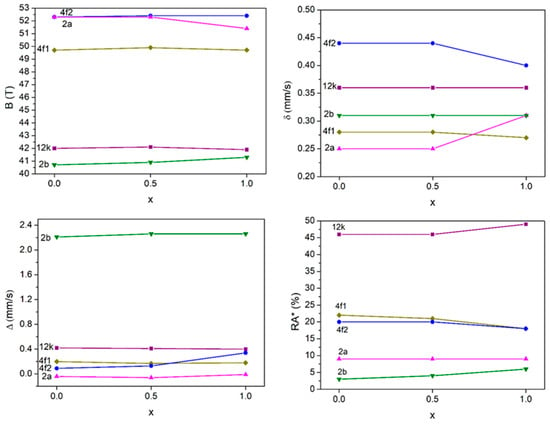
Figure 4.
Hyperfine magnetic field (B), isomer shift (δ), quadrupole splitting (Δ), and relative area (RA*) of Fe3+ ions in function of x for the Ba1−xSrxFe12O19 samples.
The mean hyperfine field <B> increases with the substitution of Ba2+ by Sr2+. The values obtained for <B> are 3.85; 4.75 and 8.77 T for Ba1−xSrxFe12O19, with x = 0; and 0.5 and 1, respectively. These results agree with the magnetization results.
The values of the isomer shift (δ) are almost constant for the 12k, 2b, and 4f1 sites. The values of isomer shift decrease due to increased s electron density at the Fe3+ nuclei. This behavior shows that the s electron density at the Fe3+ nuclei on the 4f1 site, and especially on the 4f2 site, increases with replacement by Sr. Moreover, the reduction in the isomer shift value is usually associated with a decrease in the interatomic distance between Fe and O. The increase in δ observed for Fe3+ on 2a sites in SrFe12O19 normally occurs when there is an increase in d electron density, which leads to a stronger shielding of the s electrons. This may be associated with the crystallographic and chemical environment of the 2a site [26], namely the nature of the nearby divalent cations.
With increasing Sr content, the values of the quadrupole splitting (Δ) are stable, except for the 4f2 site, where Δ increases for x = 1, showing a distortion of this site with the substitution. In general, the magnetic moments of Fe3+ ions remain aligned along the axial direction.
According to the relative areas (RAs*), there are some differences between the theoretical relative populations and those obtained experimentally. The discrepancy is attributed to variations in the Lamb–Mössbauer factors across the different sites [19] and also to stacking faults [27].
3.2. Optical Studies
To elucidate the optical application of the three studied hexaferrites, UV–visible–NIR absorption spectroscopy in the 200–2500 nm optical region was obtained through the collection of the total reflectance of the samples and conversion into absorbance using the Kubelka–Munk function, F(R) [18]; see Figure 5. It was observed that all compounds absorb up to ca. 1000 nm (UV–visible and NIR regions).
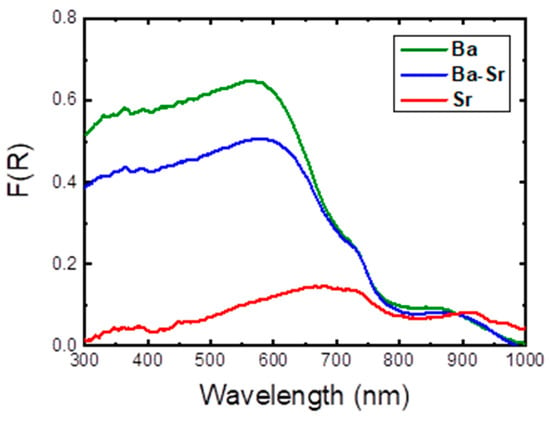
Figure 5.
Solid state diffuse reflectance spectra of the samples, as indicated: Ba1−xSrxFe12O19 (x = 0.0, 0.5, and 1.0), after Kubelka–Munk function, F(R), transformation.
We used the absorbance values to calculate the parameter α, using the following equation:
where, Abs is the absorbance, and t the thickness of the samples.
It is important to mention that, in order to create a graph, the absorption coefficient must be calculated as function of the photon energy, as described by the following equation [28]:
In this equation, is the photon frequency, Eg is the band gap energy, and A is a characteristic parameter of transition, which depends on the value of n. For direct transition, n is equal to 2; for indirect allowed transition, n is 1/2; and n is equal to 1/3 or 2/3 for indirect and direct forbidden transitions, respectively. Hexaferrite materials are known to exhibit indirect transitions (n = 1/2) [29,30]. The latter equation becomes:
The band gap energy, Eg, values could be deduced from extrapolation of a linear portion of the vs. Tauc plots, as presented by dark line in Figure 6. This procedure is frequently employed for characterizing semiconductors and is well-documented in the literature [31,32,33].
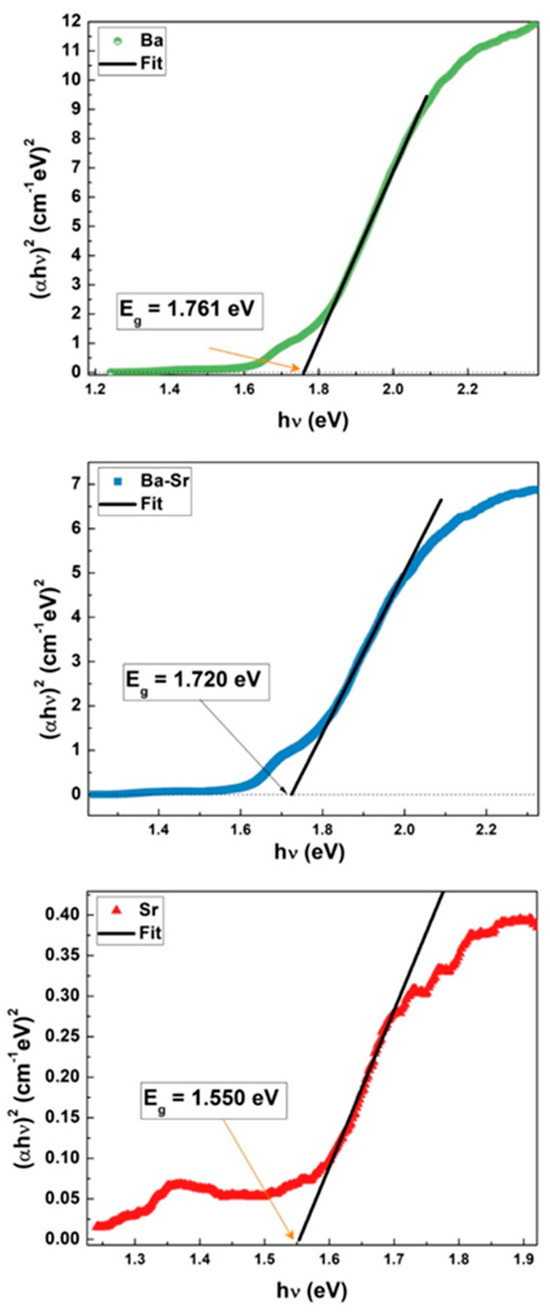
Figure 6.
Tauc plots for indirect band gap of Ba1−xSrxFe12O19 (x = 0.0, 0.5, and 1.0) samples.
The obtained values for Eg are within the ones reported for another Ba and for Sr hexaferrites [20,34,35].
Size, metal substitution ratio, geometry, and synthesis method differences might lead to different optical band gap energies [36,37]. The results obtained for Eg are plotted in Figure 7. From those values, the semiconductor nature of the produced compounds can be inferred [35], and one can see that Eg decreases with the increase of Sr content.
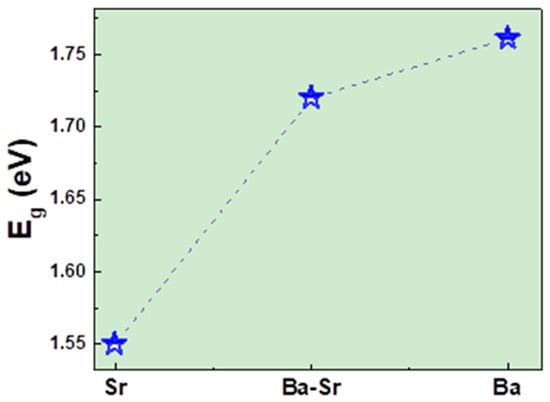
Figure 7.
Bandgap energy (Eg) vs. Sr content for the Ba1−xSrxFe12O19 (x = 0.0, 0.5, and 1.0) samples.
These values are inferior to those obtained by Raghuram et al. [20] for Sr doped Ba hexaferrites prepared by hydrothermal methods, where the particles were much smaller than those of our compounds, in accordance with the blue shift of Eg observed with the decrease in particle size. Our compounds, with higher grain sizes, can express better applications in optoelectronic devices and photocatalytic and sensor areas.
4. Conclusions
We have studied Ba1−xSrxFe12O19 hard-magnetic nanohexaferrites, synthesized by autocombustion, specifically focusing on the effects of Sr substitution on their properties. Morphologically, the hard-magnetic nanoferrites agglomerated in hexagonal-shaped grains, consistent with typical hexaferrite structures. Grain size increases with Sr content, which can be related to the changes in crystallinity as Sr substitutes for Ba.
Mössbauer spectroscopy was used to study the magnetic properties and site occupancy of Fe ions in the hexaferrite structure. It showed that as Sr2+ replaced Ba2+, the mean hyperfine field increased in agreement with the increase of saturation magnetization. The occupancy of the 4f1 and 4f2 sites by Fe3+ also increases with replacement of Ba by Sr. This suggests that Sr might be influencing the magnetic interactions or the distribution of Fe ions in the structure, aligning with the changes in magnetization. These results are valuable in understanding how tuning the composition of these materials could affect their performance in applications like permanent magnets and magnetic storage devices.
The optical investigations revealed that the title hexaferrites absorb light up to 1000 nm, and the bandgap energy decreases as Sr content increases. This indicates a shift in the electronic structure of the hexaferrites as the Sr substitution influences the bandgap. All compositions have low band gap energy, making their photocatalytic activity under visible light more practicable, especially for SrFe12O19 nanoparticles. This is important for applications where optical properties, such as visible light photocatalytic activities, are relevant.
Author Contributions
Conceptualization and methodology: B.F.O.C. Investigation, data curation, and formal analysis: B.F.O.C., A.B., B.J.C.V., J.C.W., J.P., Y.M. and E.D. Supervision: B.F.O.C. Funding acquisition: B.F.O.C., B.J.C.V., J.C.W. and J.P. Writing—original draft: B.F.O.C. and A.B. Writing—review and editing: B.F.O.C., B.J.C.V., J.C.W., J.P. and E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCT—Fundação para a Ciência e Tecnologia, I.P. through the projects UIDB/04564/2020 and UIDP/04564/2020, with DOI identifiers 10.54499/UIDB/04564/2020 and 10.54499/UIDP/04564/2020, respectively, and by FCT through project UID/Multi/04349/2020 and the National Infrastructure Roadmap, LTHMFL-NECL, LISBOA-01-0145-FEDER-022096. And also by FCT through projects UIDB/00313/2020 (DOI:10.54499/UIDB/00313/2020) and UIDP/00313/2020 (hDOI:10.54499/UIDP/00313/2020) and LA/P/0056/2020 (national funds).
Data Availability Statement
Data will be made available on request.
Acknowledgments
Access to TAIL-UC facility funded under QREN-Mais Centro Project No. ICT_2009_02_012_1890 is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Albanese, G.; Watts, B.E.; Leccabue, F.; Castanon, S.D. Mössbauer and magnetic studies of PbFe12−xCrxO19 hexagonal ferrites. J. Magn. Magn. Mater. 1998, 184, 337–343. [Google Scholar] [CrossRef]
- Ounnunkada, S.; Winotai, P. Properties of Cr-substituted M-type barium ferrites prepared by nitrate–citrate gel-autocombustion process. J. Magn. Magn. Mater. 2006, 301, 292–300. [Google Scholar] [CrossRef]
- Slimani, Y.; Baykal, A.; Manikandan, A. Effect of Cr3+ substitution on AC susceptibility of Ba hexaferrite nanoparticles. J. Magn. Magn. Mater. 2018, 458, 204–212. [Google Scholar] [CrossRef]
- Slimani, Y.; Almessiere, M.A.; Baykal, A. AC susceptibility study of Cu substituted BaFe12O19 nanohexaferrites. Ceram. Int. 2018, 44, 13097–13105. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Baykal, A. Exchange spring magnetic behavior of Sr0.3Ba0.4Pb0.3Fe12O19/(CuFe2O4)x nanocomposites fabricated by a one-pot citrate sol-gel combustion method. J. Alloys Compd. 2018, 762, 389–397. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; El Sayed, H.S.; Baykal, A. Ce-Y co-substituted strontium nanohexaferrites: AC susceptibility and Mössbauer studies. Ceram. Int. 2018, 44, 12520–12527. [Google Scholar] [CrossRef]
- Wohlfarth, E.P. (Ed.) Ferromagnetic Materials; North-Holland Publ. Co.: Amsterdam, The Netherlands, 1982. [Google Scholar] [CrossRef]
- Bañuelos-Frías, A.; Martínez-Guajardo, G.; Alvarado-Perea, L.; Canizalez-Dávalos, L.; Ruiz, F.; Valero-Luna, C. Light absorption properties of mesoporous barium hexaferrite, BaFe12O19. Mater. Lett. 2019, 252, 239–243. [Google Scholar] [CrossRef]
- Banihashemi, V.; Ghazi, M.E.; Izadifard, M. Dual Ca–Zn substituted strontium hexaferrite; investigation of structural, magnetic and optical properties. Phys. B Condens. Matter 2021, 605, 412670. [Google Scholar] [CrossRef]
- Mohammed, J.; Suleiman, A.B.; Carol, T.T.T.; Hafeez, H.Y.; Sharma, J.; Maji, P.K.; Kumar, S.G.; Srivastava, A.K. Enhanced dielectric and optical properties of nanoscale barium hexaferrites for optoelectronics and high frequency application. Chin. Phys. B 2018, 27, 128104. [Google Scholar] [CrossRef]
- Asiri, S.; Amir, M.; Güner, S.; Gungunes, H.; Batoo, K.M.; Sertkol, M.; Imran, A. Structural, Optical and Mössbauer Study of Ba1−xCuxFe12O19 (0.5 ≤ x) Nano Hexaferrites. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1446–1456. [Google Scholar] [CrossRef]
- Jayakumar, T.; Raja, C.R.; Arumugam, S. Elucidation of structural, optical, and magnetic properties of Cd/Ni-doped strontium hexaferrite. J. Mater. Sci. Mater. Electron. 2020, 31, 16308–16313. [Google Scholar] [CrossRef]
- Nishkala, K.R.; Rao, R.R.; Mutalik, S.; Daivajna, M.D. Effect of La substitution on the structural and chemical properties of Barium hexaferrite via Mossbauer spectroscopy. Hyperfine Interact. 2023, 244, 7. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Trukhanov, S.V.; Panina, L.V. Effect of In substitution on structural, magnetic, and Mössbauer properties of M-type hexaferrites. Ceram. Int. 2018, 44, 11676–11680. [Google Scholar] [CrossRef]
- Marouani, Y.; Massoudi, J.; Noumi, M.; Benali, A.; Dhahri, E.; Sanguino, P.; Graça, M.P.F.; Valente, M.A.; Costa, B.F.O. Electrical conductivity and dielectric properties of Sr doped M-type barium hexaferrite BaFe12O19. RSC Adv. 2021, 11, 1531. [Google Scholar] [CrossRef] [PubMed]
- Marouani, Y.; Mabrouki, A.; Dhahri, R.; Dhahri, E.; Costa, B.F.O. Experimental and theoretical studies of structural, magnetic and electronic properties of Ba1−xSrxFe12O19 (x = 0, 0.5, 1) hexaferrites. Inorg. Chem. Com. 2022, 136, 109163. [Google Scholar] [CrossRef]
- Brand, R.A. Normos Mössbauer Fitting Program v.90; Wissel GMbH: Stanberg, Germany, 1994. [Google Scholar]
- Law, D.P.; Blakeney, A.B.; Tkachuk, R. The Kubelka–Munk Equation: Some Practical Considerations. J. Near Infrared Spectrosc. 1996, 4, 189–193. [Google Scholar] [CrossRef]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191. [Google Scholar] [CrossRef]
- Raghuram, N.; Rao, T.S.; Naidu, C.B. Investigations on functional properties of hydrothermally synthesized Ba1−xSrxFe12O19 (x = 0.0–0.8) nanoparticles. Mater. Sci. Semicond. Process. 2019, 94, 136–150. [Google Scholar] [CrossRef]
- Silva, W.M.S.; Ferreira, N.S.; Soares, J.M. Investigation of structural and magnetic properties of nanocrystalline Mn-doped SrFe12O19 prepared by proteic sol–gel process. J. Magn. Magn. Mater. 2015, 395, 263–270. [Google Scholar] [CrossRef]
- Auwal, I.A.; Korkmaz, A.D.; Amir, M.D.; Asiri, S.M.; Baykal, A.; Gungunes, H.; Shistah, S.E. Mössbauer analysis and cation distribution of Zn substituted BaFe12O19 Hexaferrites. J. Sup. Nov. Magn. 2018, 31, 151–156. [Google Scholar] [CrossRef]
- Choudhary, H.K.; Kumar, R.; Anupama, A.V.; Sahoo, B. Effect of annealing temperature on the structural and magnetic properties of Ba-Pb-hexaferrite powders synthesized by sol-gel auto-combustion method. Ceram. Int. 2018, 44, 8877–8889. [Google Scholar] [CrossRef]
- Evans, B.J.; Grandjean, F.; Lilot, A.P.; Vogel, R.H.; Gerard, A. 57Fe hyperfine interactions parameters and selected magnetic properties of high purity MFe12O19 (M = Sr, Ba). J. Magn. Magn. Mater. 1987, 67, 123–129. [Google Scholar] [CrossRef]
- Baykal, A.; Yokuş, S.; Güner, S.; Güngüneş, H.; Sözeri, H.; Amir, M. Magneto-optical properties and Mössbauer Investigation of BaxSryPbzFe12O19 Hexaferrites. Ceram. Int. 2017, 43, 3475–3482. [Google Scholar] [CrossRef]
- Mohammed, J.; Sharma, J.; Yerima, K.U.; Carol, T.T.; Basandrai, D.; Kumar, A.; Maji, P.K.; Srivastava, A.K. Magnetic, Mössbauer and Raman spectroscopy of nanocrystalline Dy3+-Cr3+ substituted barium hexagonal ferrites. Phys. B 2020, 585, 412115. [Google Scholar] [CrossRef]
- Lechevalier, L.; Le Breton, J.M.; Wang, J.F.; Harris, I.R. Structural analysis of hydrothermally synthesized Sr1−xSmxFe12O19. J. Magn. Magn. Mater. 2004, 269, 192–196. [Google Scholar] [CrossRef]
- Karmakar, M.; Mondal, B.; Pal, M.; Mukherjee, K. Acetone and ethanol sensing of barium hexaferrite particles: A case study considering the possibilities of non-conventional hexaferrite sensor. Sens. Actuators B Chem. 2014, 190, 627–633. [Google Scholar] [CrossRef]
- Thirupathy, C.; Lims, S.C.; Sundaram, S.J.; Mahmoud, A.H.; Kaviyarasu, K. Equilibrium synthesis and magnetic properties of BaFe12O19/NiFe2O4 nanocomposite prepared by co precipitation method. J. King Saud Univ. Sci. 2020, 32, 1612–1618. [Google Scholar] [CrossRef]
- Subramanyam, G.; Rao, N.K.; Daivajna, M.D. La3+-Induced Band-Gap Modifications in Barium Hexaferrite: An Investigation of the Structural, Optical, and Dielectric Properties. Eng. Proc. 2023, 55, 94. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z—What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Kisch, H. (Ed.) Semiconductor photocatalysis. In Semiconductor Photocatalysis: Principles and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014. [Google Scholar] [CrossRef]
- Güner, S.; Baykal, A.; Amir, M.; Güngüneş, H.; Geleri, M.; Sözeri, H.; Shirsath, S.E.; Sertkol, M. Synthesis and characterization of oleylamine capped MnxFe1−xFe2O4 nanocomposite: Magneto-optical properties, cation distribution and hyperfine interactions. J. Alloys Comp. 2016, 688 Pt A, 675–686. [Google Scholar] [CrossRef]
- Basha, D.B.; Kumar, N.S.; Naidu, K.C.B.; Kumar, G.R. Structural, electrical, and magnetic properties of nano Sr1−xLaxFe12O19 (x = 0.2–0.8). Sci. Rep. 2022, 12, 12723. [Google Scholar] [CrossRef]
- Sekhar, D.C.; Rao, T.S.; Naidu, K.C.B. Hexagonal microstructure, magnetic and dielectric properties of iron deficient BaNixZnxFe12-xO19 (x = 0.0–0.5) hexaferrites. Appl. Phys. A 2021, 127, 841. [Google Scholar] [CrossRef]
- Saqib, M.; Ali, S.S.; Zulqarnain, M.; Qadri, M.U.; Riaz, M.; Hasan, M.S.; Khan, M.I.; Tahir, M.; Arshad, M.I.; Rani, H.S. Temperature-Dependent Variations in Structural, Magnetic, and Optical Behavior of Doped Ferrites Nanoparticles. J. Supercond. Nov. Magn. 2021, 34, 609–616. [Google Scholar] [CrossRef]
- Baykal, A.; Güner, S.; Demir, A. Synthesis and magneto-optical properties of triethylene glycol stabilized Mn1−xZnxFe2O4 nanoparticles. J. Alloys Comp. 2015, 619, 5–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).