Abstract
The growing plastic waste crisis calls for innovative and sustainable solutions that go beyond traditional recycling methods. Electrochemical upcycling has emerged as a promising approach for converting waste plastics into valuable chemicals, fuels, and functional materials. Recent advancements in electrochemical strategies for plastic valorization focus on key catalysts, reaction mechanisms, and process efficiencies. Recent studies place special emphasis on new techniques aimed at improving selectivity, energy efficiency, and scalability. Additionally, integrating renewable energy sources and optimizing electrode materials have significantly enhanced process sustainability. This review specifically focuses on recent research, which addresses the key challenges in the electrochemical upcycling of plastic waste.
1. Introduction
Growing global concerns about energy and environmental issues from the use of plastic products are becoming increasingly widespread, as disposable plastics remain some of the most used items in daily life [1,2,3,4]. From packaging materials to single-use utensils, these products contribute significantly to pollution and waste accumulation. Approximately 9.7 billion tons of plastic were manufactured between 1950 and 1980. Projections indicate that plastic production will double by 2025 and triple by 2050 [5]. Additionally, an estimated 343 million tons of plastic waste are generated annually [6].
Pure plastics need additives like plasticizers and stabilizers to improve their properties. Without these additives, pure plastics break down more easily and perform worse in terms of heat, strength, and physical characteristics. Plastics have made our lives easier, but they also create a lot of waste [7,8]. This waste can be very harmful to both living things and the environment. Polyethylene terephthalate (PET) is one of the commonly produced plastics that is extensively utilized for packaging, containers, films, and fibers because of its outstanding characteristics, including water resistance, chemical stability, high durability, and minimal permeability to CO2 [9,10,11]. However, the widespread use of PET and other plastics has led to growing concerns about their environmental impact [12,13,14].
Biological degradation, catalytic transformation, and electrochemical upcycling are distinct methods for addressing plastic waste. Biological degradation uses microorganisms or enzymes to break down plastics, offering a sustainable approach but with slow processing and limited applicability to certain plastics [15,16]. Catalytic transformation involves using catalysts to convert plastics into valuable products, offering faster and more efficient results but requiring high energy and specialized conditions [17,18]. Electrochemical upcycling utilizes electrical energy to degrade plastics into useful materials under milder conditions, potentially integrating with renewable energy. Each method has unique advantages and challenges, with their effectiveness dependent on plastic types, efficiency, and scalability. For instance, the electrocatalytic upcycling of ethylene glycol (EG), a byproduct of PET plastic waste, into formate has been investigated as a sustainable method for chemical production. According to the IMARC Group website, the price of methyl formate reached USD 1840 per metric ton in the United States in September 2024.
Improper disposal and few recycling options have caused serious plastic pollution, especially in oceans and waterways, putting marine ecosystems at risk [19,20]. To address this issue, researchers are looking into new solutions like electrochemical upcycling, which turns waste plastics into valuable products [21,22]. Electrochemical processes are chemical reactions that involve the transfer of electrons between materials, often using an electric current [23]. Unlike traditional recycling, which can reduce plastic quality, electrochemical upcycling uses advanced catalysts and chemical reactions to decompose plastic waste into useful chemicals, fuels, and other industrial materials [11,24].
Hydrogen (H2) is a high-energy, eco-friendly fuel, making it a promising green energy source. Water splitting is a common method of H2 production, where hydrogen at the cathode and oxygen (O2) forms at the anode [25,26,27]. However, large-scale adoption is hindered by the slow oxygen evolution reaction (OER). To enhance energy efficiency, anodic glucose oxidation, which occurs at a lower potential than OER, can be used as a substitute. This not only reduces energy consumption but also generates valuable byproducts, making the process more efficient and economically viable [28,29,30,31,32]. Electrochemical techniques, powered by renewable energy (solar, wind, hydro), efficiently upcycle waste plastics with low energy use. Though monomer recovery remains challenging, new electroconversion methods depolymerize plastics into hydrogen energy and useful monomers. As shown in Figure 1, PET can be broken down into potassium terephthalate and EG in an alkaline solution, and EG can be transformed into valuable chemicals via electro-oxidation (EGOR) [33,34,35]. Traditional recycling reclaims only 20% of PET bottles, leaving most in landfills. In contrast, electrocatalytic upcycling converts ethylene glycol (EG) into high-value formate, offering a promising and sustainable alternative to produce valuable chemicals from plastic waste, as highlighted in recent studies [36,37,38].
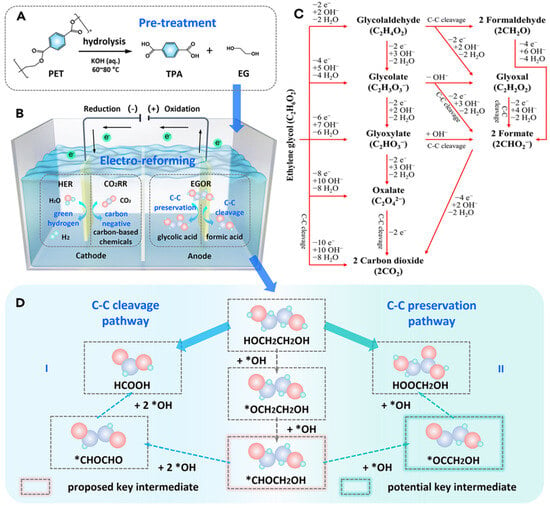
Figure 1.
Electrochemical conversion of PET. (A) Breakdown of PET into terephthalic acid (TPA) and EG through hydrolysis. (B) Development of an electro-reforming system, highlighting the reactions occurring at the cathode and anode [39]. (C) Representative EG oxidation reaction pathways under alkaline conditions [11]. (D) Two pathways for EGOR conversion: (I) C–C bond cleavage mechanism and (II) C–C bond retention mechanism [39].
This review explores the advancements in the electrochemical upcycling of PET waste into valuable chemicals, fuels, and functional materials. Traditional recycling methods, such as mechanical and chemical recycling, face limitations in efficiency, energy consumption, and product quality. Electrochemical upcycling offers a promising alternative by leveraging electrocatalysts and renewable energy sources to selectively break down PET into high-value compounds like formate and glycolic acid. The review discusses recent developments in catalyst design for enhancing reaction efficiency and selectivity. Challenges such as process scalability, feedstock variability, and energy efficiency remain significant barriers to the widespread adoption of electrochemical upcycling. Addressing these challenges through further research and technological advancements could enhance its feasibility, positioning electrochemical upcycling as a transformative approach to plastic waste management while contributing to a circular economy and reducing environmental pollution.
2. Principles of Electrochemical Plastic Upcycling
Electrochemical plastic upcycling represents a cutting-edge strategy that employs electrochemical reactions to convert plastic waste into valuable chemicals and fuels. This method utilizes electrocatalysts to facilitate selective oxidation and reduction reactions under controlled conditions, enabling the efficient depolymerization and transformation of plastic-derived intermediates. The process is governed by key mechanisms, including electron transfer reactions, surface catalysis, and the electrochemical cleavage of polymer bonds, all of which impact the selectivity and efficiency of the operation. Various factors, such as the choice of electrode material, the composition of the electrolyte, the applied potential, and the reaction environment, play a vital role in optimizing performance. Understanding these foundational principles provides valuable insights into the benefits of electrochemical upcycling compared to traditional recycling methods.
2.1. Advantages of Electrochemical Upcycling over Traditional Methods
Electrochemical upcycling has been identified as a promising method for PET plastic waste valorization, offering several distinct advantages over conventional treatment methods such as mechanical recycling, incineration, and pyrolysis. The key benefits of electrochemical approaches include lower energy requirements, enhanced selectivity, and the potential for value-added product generation under mild conditions.
Traditional methods for PET recycling, such as chemical and mechanical processes, often require significant amounts of energy. Mechanical recycling involves physical processes like grinding and melting, which can be energy-intensive and may lead to material degradation [40]. Chemical recycling, while more efficient for decomposing PET into its monomers (e.g., terephthalic acid and ethylene glycol), still requires substantial energy inputs for hydrolysis or other chemical reactions [21,41]. In contrast, electrochemical upcycling can utilize the inherent chemical energy stored in PET hydrolysates, such as ethylene glycol, to produce high-value chemicals like formate and hydrogen with lower energy inputs [42]. This approach leverages electrocatalysis to facilitate reactions to the anode and cathode, reducing the overall energy required for the process.
Electrochemical upcycling also offers the potential for selective degradation and recovery of materials. Unlike traditional methods, which may result in a mixture of products or require additional purification steps, electrochemical processes can be tailored to produce specific high-value chemicals. For instance, the use of catalysts like Pd-deposited hierarchical aligned NiTe nanoarrays on Ni foam (Pd-NiTe/NF) allows for the efficient oxidation of PET hydrolysates to formate with high Faradaic efficiency (95.6%) while concurrently producing hydrogen at the cathode with a Faradaic efficiency of 98.6% [42]. This selectivity is crucial for maximizing the economic and environmental benefits of PET upcycling by ensuring that the resulting products are of high purity and value.
Table 1 provides a comprehensive comparison of various plastic recycling methods, including mechanical, chemical, thermal, biological, and electrochemical upcycling. It highlights key aspects such as advantages, limitations, and the typical scale of treated plastics, offering a structured overview of each approach. By summarizing these methods, we can easily compare their efficiencies, challenges, and applicability in waste management.

Table 1.
A summary of the various plastic recycling methods, including mechanical, chemical, biological approaches, and electrochemical upcycling.
2.2. Electrochemical Processes for Plastic Upcycling
Electrochemical processes for upcycling plastic waste can operate under mild conditions, such as lower temperatures and pressures, reducing energy consumption and environmental impact. These methods use electricity to drive chemical reactions, which can often be powered by renewable energy sources, making the process more sustainable. Additionally, electrochemical techniques offer the potential for selective transformations, which can improve efficiency and reduce the production of unwanted byproducts.
Various plastics, including PET, HDPE, PVC, LDPE, PP, PS, and others, can be processed through thermochemical, photocatalytic, electrocatalytic, and photoelectrocatalytic methods. For plastic upcycling via electrochemical processes, the method typically involves three key steps (Figure 2). First, depolymerization breaks plastic down into its fundamental monomers, for example, PET breaks down into terephthalic acid (TPA) and EG, through electrochemically induced hydrolysis or reductive depolymerization [47]. Second, the oxidation of EG converts it into valuable products like formate, glycolic acid, etc. Lastly, there is the selective conversion into valuable chemicals, which plays a pivotal role in advancing sustainable industrial processes. By targeting specific pathways, this approach minimizes waste and enhances the efficiency of resource utilization, paving the way for innovative applications in pharmaceuticals, agriculture, and material science [48,49].
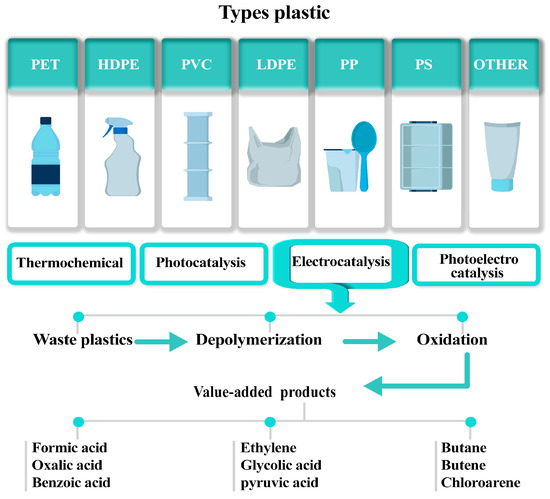
Figure 2.
An overview of plastic types and their electrochemical upcycling pathways.
The choice of electrolyte solutions and electrode materials is essential for the effectiveness and selectivity of electrochemical plastic upcycling processes. Aqueous electrolytes are widely used due to their cost-effectiveness and environmental benefits, while their pH can significantly impact reaction pathways. For instance, Tessa H. T. Myren et al. demonstrated that a water/MeOH-based electrolyte could create a basic local environment to facilitate PET depolymerization [50]. Additionally, organic electrolytes and ionic liquids have been explored to enhance plastic waste solubility and improve reaction kinetics [51,52,53,54].
The selection of electrode materials further dictates process efficiency. Among anode materials, boron-doped diamond (BDD) electrodes exhibit high efficiency in generating hydroxyl radicals for plastic degradation, while metal oxide electrodes, such as iridium oxide [55], have also been investigated. Carbon-based electrodes like graphite rods and carbon paper [56] have proven effective for the electrooxidation of organic acids derived from plastic decomposition. Cathode materials also play a crucial role, with titanium dioxide/graphite (TiO2/C) cathodes showing promise for polyvinyl chloride (PVC) dichlorination [57]. Recent advancements have focused on integrating electrochemical processes with other upcycling techniques to enhance efficiency. Pichler et al. developed a two-step process combining polyethylene oxidative depolymerization with diluted nitric acid, followed by photo- or electrocatalytic decarboxylation to generate gaseous hydrocarbons [58]. This review highlights the growing potential of electrochemical methodologies in sustainable plastic upcycling, offering promising pathways for efficient waste valorization.
3. Recent Developments in Electrochemical Plastic Upcycling
Electrocatalytic plastic waste upcycling is an emerging treatment technology that aims to convert waste plastics into higher-value chemicals or fuels through electrochemical methods [41]. Electrochemical recycling is highlighted as a versatile solution. The development of catalysts is essential for improving the efficiency of electrochemical reactions, particularly in controlling oxidation processes [11].
J. Wang et al. demonstrated an innovative approach to upcycling PET plastic waste by combining its electro-reforming with a reduction reaction of CO2, producing formic acid at the anode and cathode [59]. This process achieves high catalytic activity and Faradaic efficiencies (90% at the anode and 82% at the cathode) while requiring a low bias requirement of 1.55 V, 200 mV lower than systems without PET hydrolysate (Figure 3). The system also achieves a remarkable Faradaic efficiency of 155% for production of formate at 1.9 V due to simultaneous generation at both electrodes. Using low-cost, earth-abundant NiCo2O4 nanowire and SnO2 nanosheet electrodes, this method offers a scalable, energy-efficient solution for valorizing PET waste and producing formic acid concurrently.
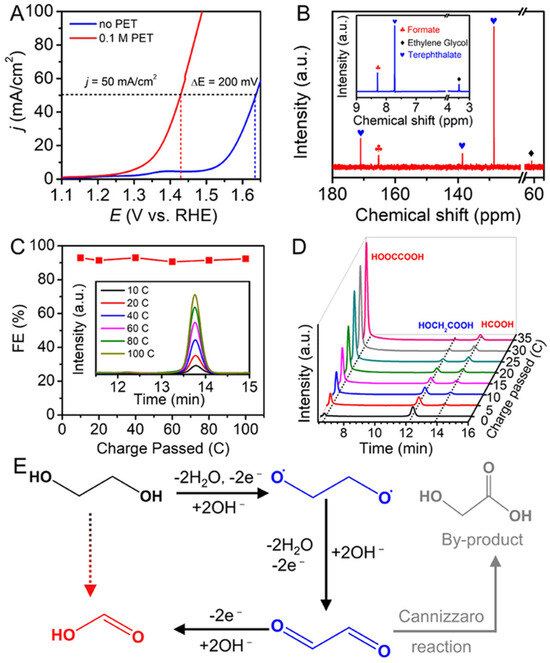
Figure 3.
The NiCo2O4/CFP electrode performance for oxidation of PET hydrolysate. (A) Linear sweep voltammetry (LSV) curves in 1 M NaOH compare NiCo2O4/CFP with and without 0.1 M PET hydrolysate. (B) The electrolyte’s composition after electrolysis is analyzed using 1H NMR and 13C NMR (inset) spectroscopy. (C) Faradaic efficiencies (FEs) for formate production are measured at 1.45 V with varying charge inputs (inside: the high-performance liquid chromatography (HPLC) chromatograms). (D) HPLC for glycolic acid oxidation products at 1.45 V under varying charge conditions. (E) A reaction mechanism for formate production [59].
In another study, Z. Tu et al. present self-supporting CuPd alloy nanosheets as bifunctional catalysts for the efficient upcycling of PET waste and nitrate-laden wastewater, achieving approximately 93% Faradaic efficiency for converting ethylene glycol (EG) from PET hydrolysate into high-value glycolic acid (GA) and about 92% efficiency for nitrate reduction to ammonium [60]. The catalysts utilize a synergistic interaction between Pd and Cu to enhance activity, preserve C–C bonds, and prevent overoxidation, as demonstrated by in situ FTIR spectroscopy and density functional theory (DFT) calculations (Figure 4). An innovative electroforming architecture combines EG oxidation with nitrate reduction or oxygen reduction reactions, allowing for flexible daytime production of GA and ammonium or nighttime electricity generation. This low-energy, multifunctional system provides a competitive and sustainable pathway for plastic upcycling and wastewater resource utilization, addressing environmental and energy challenges.
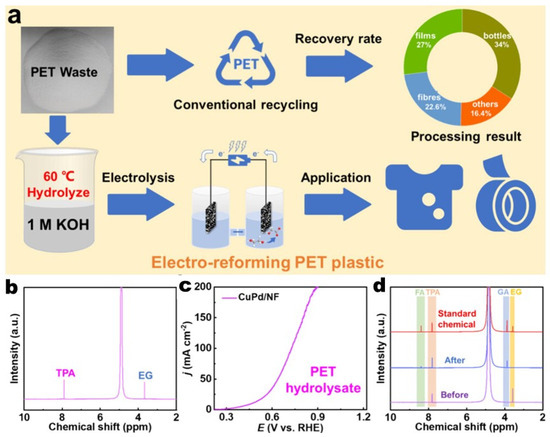
Figure 4.
An illustration of the conventional route of PET recycling and electrocatalytic upcycling of PET into commercial chemicals; (a) 1H NMR spectrum of PET hydrolysate; (b) LSV curves of CuPd/NF for PET hydrolysate oxidation; (c) 1H NMR spectra of PET hydrolysate before and after electrolysis; (d) a schematic illustration of the process of PET electro-reforming and product separation; inset: 1H NMR spectra and photographs of the collected TPA and GA [60].
M. Du et al. present a cascade strategy for upcycling PET waste into glycolate with a high total yield of 92.6% under standard conditions. The process begins with enzymatic depolymerization using an engineered hydrolase that achieves 95.6% PET conversion into ethylene glycol (EG) monomers within 12 h. As shown in Figure 5, it is followed by an electrochemical conversion of EG into glycolate using a Pd/Ni(OH)2 catalyst, achieving a Faradaic efficiency of 97.5%. The mild operating conditions of this hybrid process eliminate the need for high temperatures, pressures, and concentrated alkali. This integrated process demonstrates a highly profitable and low-carbon alternative for PET upcycling, complementing existing recycling methods such as mechanical recycling and pyrolysis. The strategy also holds potential for the upcycling of other post-consumer plastic waste [61].
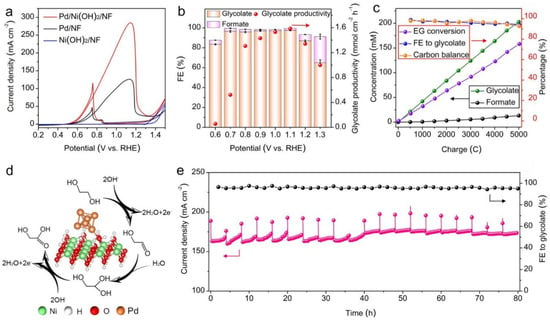
Figure 5.
This figure illustrates the electrocatalytic performance of Pd/Ni(OH)2/NF for ethylene glycol (EG) oxidation. (a) Cyclic voltammetry (CV) profiles compare Pd/Ni(OH)2/NF and Ni(OH)2/NF at a 5 mV s−1 scan rate in a 1.0 M KOH solution containing 0.3 M EG. (b) Faradaic efficiency (FE) and glycolate production rates over Pd/Ni(OH)2/NF are analyzed across a potential range of 0.6 to 1.3 V. (c) The relationships between consumed charge and various factors, including glycolate and formate concentrations, FE toward glycolate, carbon balance, and EG conversion, are presented. (d) The reaction pathway depicting EG oxidation to glycolate over Pd/Ni(OH)2 is outlined. (e) Chronoamperometric stability tests at 1.1 V evaluate long-term performance, with the anolyte being refreshed every 4 h [61].
D. Xiang et al. introduce a novel electrochemical upcycling method for transforming polylactic acid (PLA) hydrolysate into valuable products such as acetate and green hydrogen fuel. By using N- and P-doped CuOx nanowires (NW) on nickel foam (NF) as the electrocatalyst, the system effectively drives lactate oxidation with high selectivity and a lower onset potential compared to water oxidation, achieving a saving of 224 mV at 30 mA/cm2 (Figure 6). The catalyst, derived from a Cu(OH)2 NW/NF precursor and Saccharomycetes as a source of nitrogen and phosphorus, enables both lactate oxidation and hydrogen generation. In situ, Raman spectroscopy identifies divalent copper species as active sites. The NW structure enhances activity, robustness, and selectivity for key intermediates, pyruvate and acetate, through a tandem oxidation–decarboxylation pathway. This approach offers a cost-effective route for hybrid water electrolysis and sustainable upcycling of PLA waste into C2 chemicals [62].
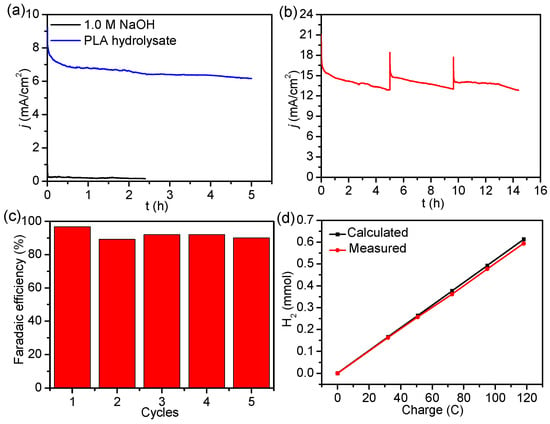
Figure 6.
(a) Electrolysis at a constant potential for the LOR at 1.42 V using N- and P-doped CuOx NW/NF in 1.0 M NaOH and PLA hydrolysate. (b) Current-time plot for N- and P-doped CuOx NW/NF at 1.48 V with periodic addition of PLA hydrolysate. (c) Calculated Faradaic efficiencies for LOR over a series of five consecutive cycles. (d) A comparison between the measured and theoretical quantities of H2 [62].
The nickel-modified cobalt phosphide (CoNi0.25P) electrocatalyst demonstrated high efficiency in the study by H. Zhou et al. [33]. This study presents an efficient electrocatalytic process for upcycling PET waste into valuable chemicals, including potassium diformate (KDF), terephthalic acid (PTA), and hydrogen (H2) fuel, using a bifunctional CoNi0.25P catalyst. As shown in Figure 7, the process selectively oxidizes ethylene glycol (EG) with >80% formate selectivity at high current densities (>100 mA cm−2) while achieving >80% Faradaic efficiency at 500 mA cm−2 (the catalyst undergoes surface reconstruction into metal oxy(hydroxide), enhancing its performance). A membrane-electrode assembly (MEA) reactor enables 500 mA cm−2 at <1.8 V, improving efficiency. The process yields 389.2 g formate, 818.5 g PTA, and 16.9 g H2 per kg PET and is compatible with impure PET. Techno-economic analysis (TEA) suggests a revenue of ~$350 per tonne of PET, making it a scalable and sustainable plastic waste upcycling solution.
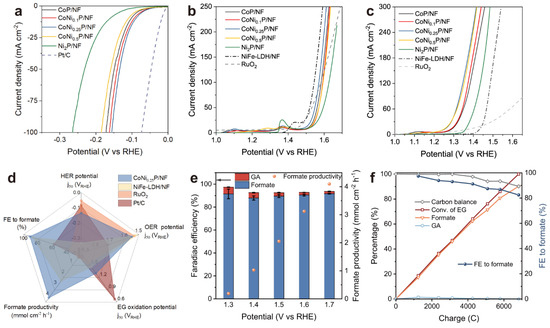
Figure 7.
(a) HER polarization curves with 100% iR correction. (b,c) LSV curves with 85% iR correction for OER (b) and EG oxidation (c). (d) A comparison of the catalytic performance of CoNi0.25P/NF with other reported catalysts. (e) The Faradaic efficiency (FE) and formate production rate on CoNi0.25P/NF at various potentials, with error bars representing the standard deviation from three measurements. (f) Kinetic curves for EG conversion at 1.5 V vs. RHE. Reaction conditions: All experiments were conducted in a 1 M KOH solution within an H-cell separated by an AEM, using a scan rate of 5 mV s−1 for polarization curves, with an EG concentration of 0.3 M [33].
T. Zhang et al. present a groundbreaking photovoltaic-driven electrocatalytic strategy for upcycling PET plastic waste into valuable formic acid and green hydrogen while significantly reducing energy consumption compared to conventional methods [63]. The process uses KOH to break down PET, which is then fed into an electrochemical flow reactor (EFR) powered by silicon photovoltaic (PV) panels. The CuO nanowire (NW) anode and 20% Pt/C cathode achieve an impressive solar-to-chemical (STC) efficiency of 32.6% under simulated sunlight. As shown in Figure 8, the system exhibits high Faradaic efficiencies of 67% for formic acid and 90% for green hydrogen, with exceptional long-term stability over 120 h. This method highlights the potential of utilizing renewable energy sources such as solar, wind, and hydropower to transform plastic waste into valuable chemicals and fuels, providing a sustainable and effective solution for plastic recycling and green energy generation.
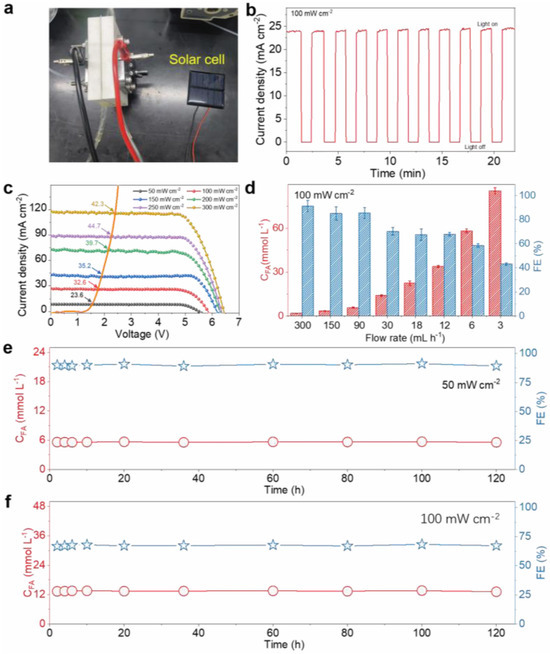
Figure 8.
The PV-EFR system performance using a 1.0 M KOH + 0.1 M PET hydrolysate. (a) A photograph of a commercial Si solar cell-powered electrocatalytic system. (b) Chopped Chronoamperometric Analysis of the PV–electrolysis system under 100 mW cm−2 illumination. (c) j-V curves of the commercial Si solar cell and the electrocatalytic system (geometric area: 6 × 6 cm, 50–300 mW cm−2, catalyst electrode area ~2 cm2) along with the corresponding solar-to-chemical efficiency. (d) The relationship between FA concentration and PET hydrolysate flow rate at an overall current density of 24 mA cm−2. A stability test for continuous FA production at concentrations of (e) 6.0 mmol L−1 and (f) 13.8 mmol L−1, with a PET hydrolysate flow rate of 30 mL h−1 [63].
Y. Li et al. introduce an Fe–Ni2P/NF bifunctional electrocatalyst for electrochemically upgrading PET plastic waste while simultaneously producing hydrogen and valuable chemicals like formic acid [64]. By doping iron into nickel phosphide porous nanosheets via a hydrothermal-phosphating process, the catalyst achieves enhanced HER and ethylene glycol oxidation reaction (EGOR) performance. Its ultrathin, porous structure increases active sites, while Fe doping optimizes the electronic structure, improving efficiency. The PET hydrolysate electrolyzer operates at a low electrolysis potential of 1.39 V for 10 mA cm−2, outperforming conventional water splitting. The system exhibits excellent durability, maintaining performance after 1000 cycles (Figure 9). This approach offers greater energy efficiency, profitability, and sustainability, highlighting the potential of plastic waste upcycling for green hydrogen production.
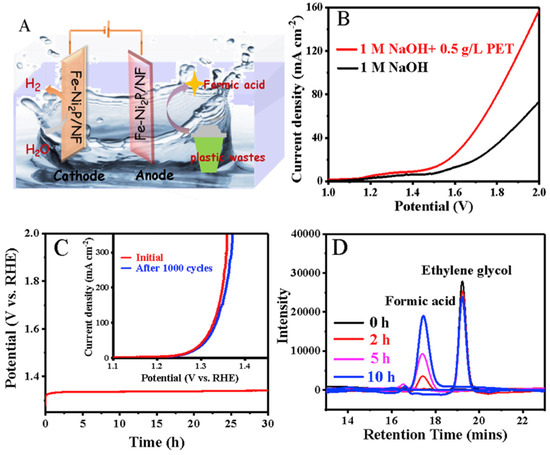
Figure 9.
(A) A picture representing the electro-reforming of PET for H2 generation using a Fe–Ni2P/NF electrode. (B) Polarization curves of the Fe–Ni2P/NF||Fe–Ni2P/NF electrolyzer in an alkaline electrolyte, with and without 0.5 g/L PET plastic waste. (C) Chronopotentiometric analysis of Fe1.5–Ni2P/NF in an alkaline solution containing 0.5 M EG at a current density of 50 mA cm−2 (inset: LSV curves of Fe1.5–Ni2P/NF before and after 1000 cycles). (D) Liquid chromatography analysis of electro-oxidation products [64].
A sustainable ammonia production method for integrating PET hydrolysate oxidation with nitrate reduction (NO3RR) in a coelectrolysis system was studied by T. Ren et al. [65]. Using Ru-incorporated Co-based MOFs as bifunctional precatalysts that transform into Ru-CoOOH at the anode and Ru-Co (OH)2 at the cathode, the system efficiently converts PET plastic waste and NO3 wastewater into valuable products like ammonia and formate. Operating at 50 mA cm−2 and 1.53 V, it achieves high ammonia yield (0.244 mmol h−1 cm−2) with 94.3% Faradaic efficiency and 98.57% selectivity. The oxidation of PET hydrolysate also generates terephthalic acid, with Ru-CoOOH/NF demonstrating superior efficiency (96.53%) for ethylene glycol oxidation. Ru incorporation enhances catalytic performance by optimizing hydrogen adsorption–desorption properties (Figure 10). The system offers a more energy-efficient alternative to OER-based processes, providing a scalable solution for NO3 wastewater treatment and plastic waste upcycling.
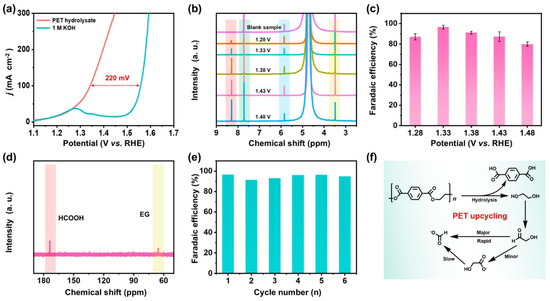
Figure 10.
(a) LSV curves (95% iR compensated) for PET hydrolysate at 1 M KOH for Ru-CoOOH/NF. (b) 1H NMR spectra of PET hydrolysate and its oxidation products over Ru-CoOOH/NF at varying potentials (ethylene glycol (yellow), maleic acid (blue), terephthalic acid (PTA) (green), and formate (red)). (c) Faradaic efficiency (FE) of formate production from PET hydrolysate oxidation across different potentials. (d) 13C NMR spectrum for oxidation products of the PET hydrolysate. (e) FE of Ru-CoOOH/NF for formate over multiple cycles at 1.33 VRHE. (f) A picture representing of the upcycling process of the PET [65].
Effective plastic upcycling depends on the Faradaic efficiency (FE) of electrocatalysts, which facilitate the conversion of plastics into valuable compounds, such as glycolic acid and formic acid, through the oxidation process. Table 2 summarizes the performances of various electrocatalysts in plastic upcycling and their roles in electrochemical systems designed for this purpose.

Table 2.
A summary of recent electrocatalysts used for plastic upcycling.
4. Conclusions
Electrochemical upcycling has emerged as a groundbreaking approach to tackling the global plastic waste issue, offering a sustainable and energy-efficient alternative to traditional recycling methods. This review highlights significant advancements in catalyst development, process efficiency, and integration with renewable energy sources, all of which contribute to the economic and environmental viability of plastic upcycling. Despite remarkable progress, challenges such as process scalability, catalyst stability, and waste feedstock variability remain. Future research should focus on optimizing reaction conditions, developing cost-effective catalysts, and exploring hybrid strategies that combine biological, thermal, and electrochemical processes. By leveraging interdisciplinary innovations, electrochemical upcycling has the potential to revolutionize plastic waste management, contributing to a circular economy and a more sustainable future.
Author Contributions
J.K., S.M., Y.P. and S.L. contributed to the conceptualization, literature review, and drafting of the manuscript. D.K. and Z.M. supervised this study, provided critical insights, and contributed to the revision and finalization of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was supported by the 2023 Research Fund of the University of Ulsan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dokl, M.; Copot, A.; Krajnc, D.; Van Fan, Y.; Vujanović, A.; Aviso, K.B.; Tan, R.R.; Kravanja, Z.; Čuček, L. Global projections of plastic use, end-of-life fate and potential changes in consumption, reduction, recycling and replacement with bioplastics to 2050. Sustain. Prod. Consum. 2024, 51, 498–518. [Google Scholar] [CrossRef]
- Williams, A.T.; Rangel-Buitrago, N. The past, present, and future of plastic pollution. Mar. Pollut. Bull. 2022, 176, 113429. [Google Scholar] [CrossRef]
- Singh, N.; Walker, T.R. Plastic recycling: A panacea or environmental pollution problem. npj Mater. Sustain. 2024, 2, 17. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, B.; Xiong, L.; Liu, W.; Wang, D.; Ma, W.; Jiang, L.; Yang, J.; Wang, P.; Xiao, T.; et al. Highly selective upcycling of plastic mixture waste by microwave-assisted catalysis over Zn/b-ZnO. Nat. Commun. 2025, 16, 1726. [Google Scholar] [CrossRef]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 2017, 9, 1346–1360. [Google Scholar] [CrossRef]
- Geyer, R. Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling. Environmental Impact, Societal Issues, Prevention, and Solutions; Letcher, T.M., Ed.; Academic Press: London, UK, 2020; pp. 13–32. [Google Scholar] [CrossRef]
- Dhaka, V.; Singh, S.; Anil, A.G.; Naik, T.S.S.K.; Garg, S.; Samuel, J.; Kumar, M.; Ramamurthy, P.C.; Singh, J. Occurrence, toxicity and remediation of polyethylene terephthalate plastics. A review. Environ. Chem. Lett. 2022, 20, 1777–1800. [Google Scholar] [CrossRef]
- Kamali, A.R.; Li, S. Molten salt-assisted valorization of waste PET plastics into nanostructured SnO2@terephthalic acid with excellent Li-ion storage performance. Appl. Energy 2023, 334, 120692. [Google Scholar] [CrossRef]
- Chan, K.; Zinchenko, A. Aminolysis-assisted hydrothermal conversion of waste PET plastic to N-doped carbon dots with markedly enhanced fluorescence. J. Environ. Chem. Eng. 2022, 10, 107749. [Google Scholar] [CrossRef]
- Soong, Y.-H.V.; Sobkowicz, M.J.; Xie, D. Recent Advances in Biological Recycling of Polyethylene Terephthalate (PET) Plastic Wastes. Bioengineering 2022, 9, 98. [Google Scholar] [CrossRef]
- Cho, J.; Kim, B.; Kwon, T.; Lee, K.; Choi, S.-I. Electrocatalytic upcycling of plastic waste. Green Chem. 2023, 25, 8444–8458. [Google Scholar] [CrossRef]
- Babaei, M.; Jalilian, M.; Shahbaz, K. Chemical recycling of Polyethylene terephthalate: A mini-review. J. Environ. Chem. Eng. 2024, 12, 112507. [Google Scholar] [CrossRef]
- Sarda, P.; Hanan, J.C.; Lawrence, J.G.; Allahkarami, M. Sustainability performance of polyethylene terephthalate, clarifying challenges and opportunities. J. Polym. Sci. 2022, 60, 7–31. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Guo, X.; Chen, Y.; Sun, W.; Peng, C. Electrocatalytic reforming of polyethylene terephthalate waste plastics into high-value-added chemicals with green hydrogen generation. J. Colloid Interface Sci. 2025, 685, 29–37. [Google Scholar] [CrossRef]
- Haq, F.; Kiran, M.; Khan, I.A.; Mehmood, S.; Aziz, T.; Haroon, M. Exploring the pathways to sustainability: A comprehensive review of biodegradable plastics in the circular economy. Mater. Today Sustain. 2025, 29, 101067. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Hernando-Morales, V.; Zeghal, E.; Niemann, H. Microbial Degradation of Marine Plastics: Current State and Future Prospects. In Biotechnology for Sustainable Environment; Springer: Singapore, 2021; pp. 111–154. [Google Scholar] [CrossRef]
- Oh, S.; Stache, E.E. Chemical Upcycling of Commercial Polystyrene via Catalyst-Controlled Photooxidation. J. Am. Chem. Soc. 2022, 144, 5745–5749. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, H.; Zhao, K.; Wang, S.; Zhang, D.; Li, Y.; Fan, R.; Li, J.; Chen, X.; Zhou, X.; et al. Catalytic oxidation upcycling of polyethylene terephthalate to commodity carboxylic acids. Nat. Commun. 2024, 15, 10732. [Google Scholar] [CrossRef]
- Oliveira, J.; Belchior, A.; da Silva, V.D.; Rotter, A.; Petrovski, Ž.; Almeida, P.L.; Lourenço, N.D.; Gaudêncio, S.P. Marine Environmental Plastic Pollution: Mitigation by Microorganism Degradation and Recycling Valorization. Front. Mar. Sci. 2020, 7, 567126. [Google Scholar] [CrossRef]
- Watt, E.; Picard, M.; Maldonado, B.; Abdelwahab, M.A.; Mielewski, D.F.; Drzal, L.T.; Misra, M.; Mohanty, A.K. Ocean plastics: Environmental implications and potential routes for mitigation—A perspective. RSC Adv. 2021, 11, 21447–21462. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Xi, W.; Zhou, H.; Yang, P.; Yao, J.; Jiang, X.; Wu, D. Upcycling plastic waste to carbon materials for electrochemical energy storage and conversion. Chem. Eng. J. 2023, 461, 141962. [Google Scholar] [CrossRef]
- Wang, X.-H.; Zhang, Z.-N.; Wang, Z.; Ding, Y.; Zhai, Q.-G.; Jiang, Y.-C.; Li, S.-N.; Chen, Y. Ultra-thin CoNi0.2P nanosheets for plastics and biomass participated hybrid water electrolysis. Chem. Eng. J. 2023, 465, 142938. [Google Scholar] [CrossRef]
- Masoumi, Z.; Tayebi, M.; Tayebi, M.; Lari, S.A.M.; Sewwandi, N.; Seo, B.; Lim, C.-S.; Kim, H.-G.; Kyung, D. Electrocatalytic Reactions for Converting CO2 to Value-Added Products: Recent Progress and Emerging Trends. Int. J. Mol. Sci. 2023, 24, 9952. [Google Scholar] [CrossRef] [PubMed]
- Plastic upcycling. Nat. Catal. 2019, 2, 945–946. [CrossRef]
- Adame-Solorio, J.; Kimmel, S.W.; Bailey, K.O.; Rhodes, C.P. Chromium Substitution Within Ruthenium Oxide Aerogels Enables High Activity Oxygen Evolution Electrocatalysts for Water Splitting. Crystals 2025, 15, 116. [Google Scholar] [CrossRef]
- Ahmad, K.; Oh, T.H. Progress in Boron Nitride-Based Materials as Catalysts for Energy Storage and Electrochemical Application. Crystals 2025, 15, 27. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Yewale, M.A.; Jeon, C.-W. Effect of Annealing Temperature on Morphology and Electrochromic Performance of Electrodeposited WO3 Thin Films. Crystals 2024, 14, 1038. [Google Scholar] [CrossRef]
- Tayebi, M.; Masoumi, Z.; Seo, B.; Lim, C.-S.; Hong, C.H.; Kim, H.J.; Kyung, D.; Kim, H.-G. Production of H2 and Glucaric Acid Using Electrocatalyst Glucose Oxidation by the Ta NiFe LDH Electrode. ACS Appl. Mater. Interfaces 2024, 16, 26107–26120. [Google Scholar] [CrossRef]
- Tayebi, M.; Masoumi, Z.; Lee, H.; Hong, D.; Seo, B.; Lim, C.; Kyung, D.; Kim, H. MOF-Derived FeCoO/N-Doped C Bifunctional Electrode for H2 Production Through Water and Glucose Electrolysis. Adv. Sustain. Syst. 2024, 8, 2400342. [Google Scholar] [CrossRef]
- Chang, J.; Song, F.; Hou, Y.; Wu, D.; Xu, F.; Jiang, K.; Gao, Z. Molybdenum, tungsten doped cobalt phosphides as efficient catalysts for coproduction of hydrogen and formate by glycerol electrolysis. J. Colloid Interface Sci. 2024, 665, 152–162. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Li, F.-M.; Dang, Z.; Gao, P. Ultrathin NiSe Nanosheets on Ni Foam for Efficient and Durable Hydrazine-Assisted Electrolytic Hydrogen Production. ACS Appl. Mater. Interfaces 2021, 13, 34457–34467. [Google Scholar] [CrossRef]
- Liu, S.; Shang, S.; Zhang, L.; Cao, B.; Meng, L.; Ding, Y.; Wu, H. Co2P@MoO3/NF composite as dual-functional electrocatalyst for energy-saving hydrogen production and urea oxidation. Fuel 2024, 373, 132362. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Y.; Li, Z.; Xu, M.; Wang, Y.; Ge, R.; Kong, X.; Zheng, L.; Duan, H. Electrocatalytic upcycling of polyethylene terephthalate to commodity chemicals and H2 fuel. Nat. Commun. 2021, 12, 4679. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kong, D.; Zheng, X.; Park, J.H. Upcycling plastic wastes into value-added products via electrocatalysis and photoelectrocatalysis. J. Energy Chem. 2024, 91, 522–541. [Google Scholar] [CrossRef]
- Ma, F.; Wang, S.; Gong, X.; Liu, X.; Wang, Z.; Wang, P.; Liu, Y.; Cheng, H.; Dai, Y.; Zheng, Z.; et al. Highly efficient electrocatalytic hydrogen evolution coupled with upcycling of microplastics in seawater enabled via Ni3N/W5N4 janus nanostructures. Appl. Catal. B Environ. Energy 2022, 307, 121198. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, J.; Baumann, D.; Peng, L.; Xu, Y.; Shakir, I.; Huang, Y.; Duan, X. Hierarchical 3D electrodes for electrochemical energy storage. Nat. Rev. Mater. 2018, 4, 45–60. [Google Scholar] [CrossRef]
- Ge, K.; Shao, H.; Lin, Z.; Taberna, P.-L.; Simon, P. Advanced characterization of confined electrochemical interfaces in electrochemical capacitors. Nat. Nanotechnol. 2024, 20, 196–208. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Tang, J.; Hu, Z.; Zhang, Y.; Ge, H.; Jian, N.; Zhao, J.; Cabot, A.; Li, J. Electrochemical PET recycling to formate through ethylene glycol oxidation on Ni–Co–S nanosheet arrays. J. Mater. Chem. A Mater. 2024, 12, 33917–33925. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Wang, L.; Kuang, M.; Wang, S.; Yang, J. Toward carbon neutrality: Selective conversion of waste plastics into value-added chemicals. Matter 2023, 6, 3322–3347. [Google Scholar] [CrossRef]
- Petersen, H.A.; Myren, T.H.T.; O’sullivan, S.J.; Luca, O.R. Electrochemical methods for materials recycling. Mater. Adv. 2021, 2, 1113–1138. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Zhang, L.; Matos, J.; Wang, L.; Yang, J. Electrocatalytic upcycling of plastic waste: Progress, challenges, and future. Electron 2024, 2, e63. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Li, X.; Deng, K.; Yu, H.; Xu, Y.; Wang, H.; Wang, Z.; Wang, L. Electrocatalytic upcycling of polyethylene terephthalate plastic to formic acid coupled with energy-saving hydrogen production over hierarchical Pd-doped NiTe nanoarrays. Appl. Catal. B Environ. 2024, 340, 123236. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, e2000415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.-H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 1979 2020, 370, 437–441. [Google Scholar] [CrossRef]
- Abdelfatah, A.M.; Hosny, M.; Elbay, A.S.; El-Maghrabi, N.; Fawzy, M. From Waste to Worth: Upcycling Plastic into High-Value Carbon-Based Nanomaterials. Polymers 2025, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Park, H.; Ackermann, Y.S.; Avérous, L.; Ballerstedt, H.; Besenmatter, W.; Blázquez, B.; Bornscheuer, U.T.; Branson, Y.; Casey, W.; et al. Exploring biotechnology for plastic recycling, degradation and upcycling for a sustainable future. Biotechnol. Adv. 2025, 81, 108544. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, H.; Xu, S.-M.; Yang, J.; Hao, P.; Cai, X.; Ren, Y.; Xu, M.; Kong, X.; Shao, M.; et al. Electrocatalytic Upcycling of Biomass and Plastic Wastes to Biodegradable Polymer Monomers and Hydrogen Fuel at High Current Densities. J. Am. Chem. Soc. 2023, 145, 6144–6155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Lin, J.; Lu, M.; Li, L.; Xu, P.; Liu, X.; Chen, L. Potential cycling boosts the electrochemical conversion of polyethylene terephthalate-derived alcohol into valuable chemicals. Nat. Commun. 2024, 15, 8463. [Google Scholar] [CrossRef]
- Siracusa, C.; Celestre, V.; Quartinello, F.; Damonte, G.; Madsen, J.; Guebitz, G.M.; Daugaard, A.E.; Pellis, A. There and Back Again: Recovery of Terephthalic Acid from Enzymatically Hydrolyzed Polyesters for Resynthesis. ACS Sustain. Resour. Manag. 2025, 2, 334–342. [Google Scholar] [CrossRef]
- Myren, T.H.T.; Stinson, T.A.; Mast, Z.J.; Huntzinger, C.G.; Luca, O.R. Chemical and Electrochemical Recycling of End-Use Poly(ethylene terephthalate) (PET) Plastics in Batch, Microwave and Electrochemical Reactors. Molecules 2020, 25, 2742. [Google Scholar] [CrossRef]
- Kim, Y.; Jeevika, A.; Suwa, T.; Kubo, K.; Iimura, K.-I. Asymmetric Imidazolium-Based Ionic Liquid Crystal with Enhanced Ionic Conductivity in Low-Temperature Smectic Phases. Crystals 2024, 14, 1053. [Google Scholar] [CrossRef]
- Murray, J.S.; Riley, K.E.; Brinck, T. A Revival of Molecular Surface Electrostatic Potential Statistical Quantities: Ionic Solids and Liquids. Crystals 2024, 14, 995. [Google Scholar] [CrossRef]
- Mao, Y.; Fan, S.; Li, X.; Shi, J.; Wang, M.; Niu, Z.; Chen, G. Trash to treasure: Electrocatalytic upcycling of polyethylene terephthalate (PET) microplastic to value-added products by Mn0.1Ni0.9Co2O4-δ RSFs spinel. J. Hazard. Mater. 2023, 457, 131743. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.H.; Barlow, S.; Marder, S.R.; Luca, O.R. Electricity-driven recycling of ester plastics using one-electron electro-organocatalysis. Chem Catal. 2023, 3, 100675. [Google Scholar] [CrossRef]
- Henke, A.H.; Saunders, T.P.; Pedersen, J.A.; Hamers, R.J. Enhancing Electrochemical Efficiency of Hydroxyl Radical Formation on Diamond Electrodes by Functionalization with Hydrophobic Monolayers. Langmuir 2019, 35, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Kothandam, G.; Singh, G.; Guan, X.; Lee, J.M.; Ramadass, K.; Joseph, S.; Benzigar, M.; Karakoti, A.; Yi, J.; Kumar, P.; et al. Recent Advances in Carbon-Based Electrodes for Energy Storage and Conversion. Adv. Sci. 2023, 10, 2301045. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef]
- Pichler, C.M.; Bhattacharjee, S.; Rahaman, M.; Uekert, T.; Reisner, E. Conversion of Polyethylene Waste into Gaseous Hydrocarbons via Integrated Tandem Chemical–Photo/Electrocatalytic Processes. ACS Catal. 2021, 11, 9159–9167. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wang, M.; Zhang, T.; Chai, X.; Lu, J.; Wang, T.; Zhao, Y.; Ma, D. Electrocatalytic Valorization of poly(ethylene terephthalate) Plastic and CO2 for Simultaneous Production of Formic Acid. ACS Catal. 2022, 12, 6722–6728. [Google Scholar] [CrossRef]
- Tu, Z.; He, X.; Liu, X.; Xiong, D.; Xue, S.; Wu, D.; Wang, J.; Chen, Z. Upcycling Polyethylene Terephthalate Plastic to C2 Chemicals in Parallel with Nitrate Reduction to Ammonia or Electric Energy Generation. Chem. Mater. 2025, 37, 1195–1204. [Google Scholar] [CrossRef]
- Du, M.; Xue, R.; Yuan, W.; Cheng, Y.; Cui, Z.; Dong, W.; Qiu, B. Tandem Integration of Biological and Electrochemical Catalysis for Efficient Polyester Upcycling under Ambient Conditions. Nano Lett. 2024, 24, 9768–9775. [Google Scholar] [CrossRef]
- Xiang, D.; Zhou, K.; Huang, J.; Kang, Q.; Li, H.; Duan, Y.; Du, J.; Liu, H. Electrochemical Upgrading of Waste Polylactic Acid Plastic for the Coproduction of C2 Chemicals and Green Hydrogen. Molecules 2024, 29, 5323. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Wang, J.; Miao, Y.; Wang, T.; Qian, X.; Zhao, Y. Photovoltaic-driven electrocatalytic upcycling poly(ethylene terephthalate) plastic waste coupled with hydrogen generation. J. Hazard. Mater. 2023, 450, 131054. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Wang, X.-H.; Chen, C.; Li, M.; Wang, J.-Y.; Li, S.-N. Electrochemical upgrading of PET plastic wastes for hydrogen production using porous Fe–Ni2P nanosheets. Int. J. Hydrogen Energy 2024, 96, 794–802. [Google Scholar] [CrossRef]
- Ren, T.; Yu, Z.; Yu, H.; Deng, K.; Wang, Z.; Li, X.; Wang, H.; Wang, L.; Xu, Y. Sustainable Ammonia Electrosynthesis from Nitrate Wastewater Coupled to Electrocatalytic Upcycling of Polyethylene Terephthalate Plastic Waste. ACS Nano 2023, 17, 12422–12432. [Google Scholar] [CrossRef]
- Shi, R.; Liu, K.-S.; Liu, F.; Yang, X.; Hou, C.-C.; Chen, Y. Electrocatalytic reforming of waste plastics into high value-added chemicals and hydrogen fuel. Chem. Commun. 2021, 57, 12595–12598. [Google Scholar] [CrossRef] [PubMed]
- Si, D.; Xiong, B.; Chen, L.; Shi, J. Highly selective and efficient electrocatalytic synthesis of glycolic acid in coupling with hydrogen evolution. Chem Catal. 2021, 1, 941–955. [Google Scholar] [CrossRef]
- Li, Y.; Lee, L.Q.; Yu, Z.G.; Zhao, H.; Zhang, Y.-W.; Gao, P.; Li, H. Coupling of PET waste electroreforming with green hydrogen generation using bifunctional catalyst. Sustain. Energy Fuels 2022, 6, 4916–4924. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Hu, M.-K.; Wei, W.; Zhou, S.-H.; Wu, X.-T.; Zhu, Q.-L. Ordered macroporous superstructure of bifunctional cobalt phosphide with heteroatomic modification for paired hydrogen production and polyethylene terephthalate plastic recycling. Appl. Catal. B Environ. 2022, 316, 121667. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Zhao, H.; Wang, Z.; Li, H.; Gao, P. A bifunctional catalyst of ultrathin cobalt selenide nanosheets for plastic-electroreforming-assisted green hydrogen generation. J. Mater. Chem. A Mater. 2022, 10, 20446–20452. [Google Scholar] [CrossRef]
- Liu, F.; Gao, X.; Shi, R.; Guo, Z.; Tse, E.C.M.; Chen, Y. Concerted and Selective Electrooxidation of Polyethylene-Terephthalate-Derived Alcohol to Glycolic Acid at an Industry-Level Current Density over a Pd–Ni(OH)2 Catalyst. Angew. Chem. 2023, 135, e202300094. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Kang, S.; Xu, C.; Ma, Y.; Cai, L.; Zhu, Y.; Chai, Y.; Qiu, B. Electrochemical Production of Glycolate Fuelled by Polyethylene Terephthalate Plastics with Improved Techno-Economics. Small 2023, 19, 2303693. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, F.; Kuang, M.; Wang, L.; Wang, H.; Li, W.; Yang, J. Unveiling synergy of strain and ligand effects in metallic aerogel for electrocatalytic polyethylene terephthalate upcycling. Proc. Natl. Acad. Sci. USA 2024, 121, e2318853121. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, L.; Wu, D.; Xu, F.; Jiang, K.; Guo, Y.; Gao, Z. Concurrent electrocatalytic hydrogen evolution and polyethylene terephthalate plastics reforming by self-supported amorphous cobalt iron phosphide electrode. J. Colloid Interface Sci. 2024, 655, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Lian, Z.; Wang, W.; Yu, J.; Yu, H.; Wang, Z.; Xu, Y.; Wang, L.; Wang, H. Lattice Strain and Charge Redistribution of Pt Cluster/Ir Metallene Heterostructure for Ethylene Glycol to Glycolic Acid Conversion Coupled with Hydrogen Production. Small 2024, 20, 2305000. [Google Scholar] [CrossRef]
- Liu, X.; He, X.; Xiong, D.; Wang, G.; Tu, Z.; Wu, D.; Wang, J.; Gu, J.; Chen, Z. Electro-Reforming of PET Plastic to C2 Chemicals with Concurrent Generation of Hydrogen and Electric Energy. ACS Catal. 2024, 14, 5366–5376. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).