Advancements in the Research on the Preparation and Growth Mechanisms of Various Polymorphs of Calcium Carbonate: A Comprehensive Review
Abstract
:1. Introduction
2. Properties and Preparation of Various Polymorphs of Nano-Calcium Carbonate
2.1. Structure of Nano-Calcium Carbonate
2.2. Methodologies for the Synthesis of Nano-Calcium Carbonate
3. Crystal Polymorphism Control
3.1. Influence of Polymorphism Controllers
3.1.1. Inorganic Ions
3.1.2. Carbohydrates
3.1.3. Alcohols
3.1.4. Acids
3.2. Influence of Process Conditions
- ▪
- Temperature
- ▪
- Stirring Rate
- ▪
- pH
- ▪
- Mixing Rate of the Solution
3.3. Influence of Reactor Structure
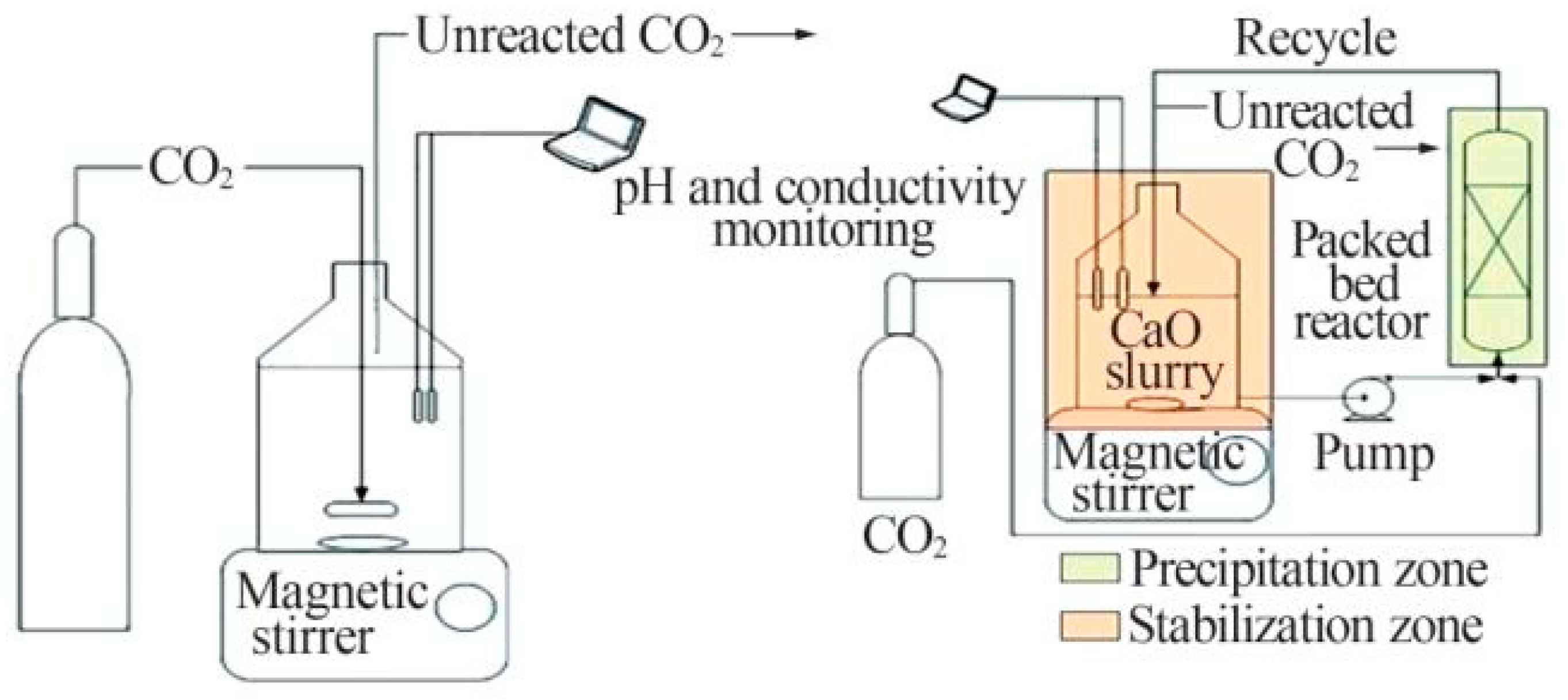
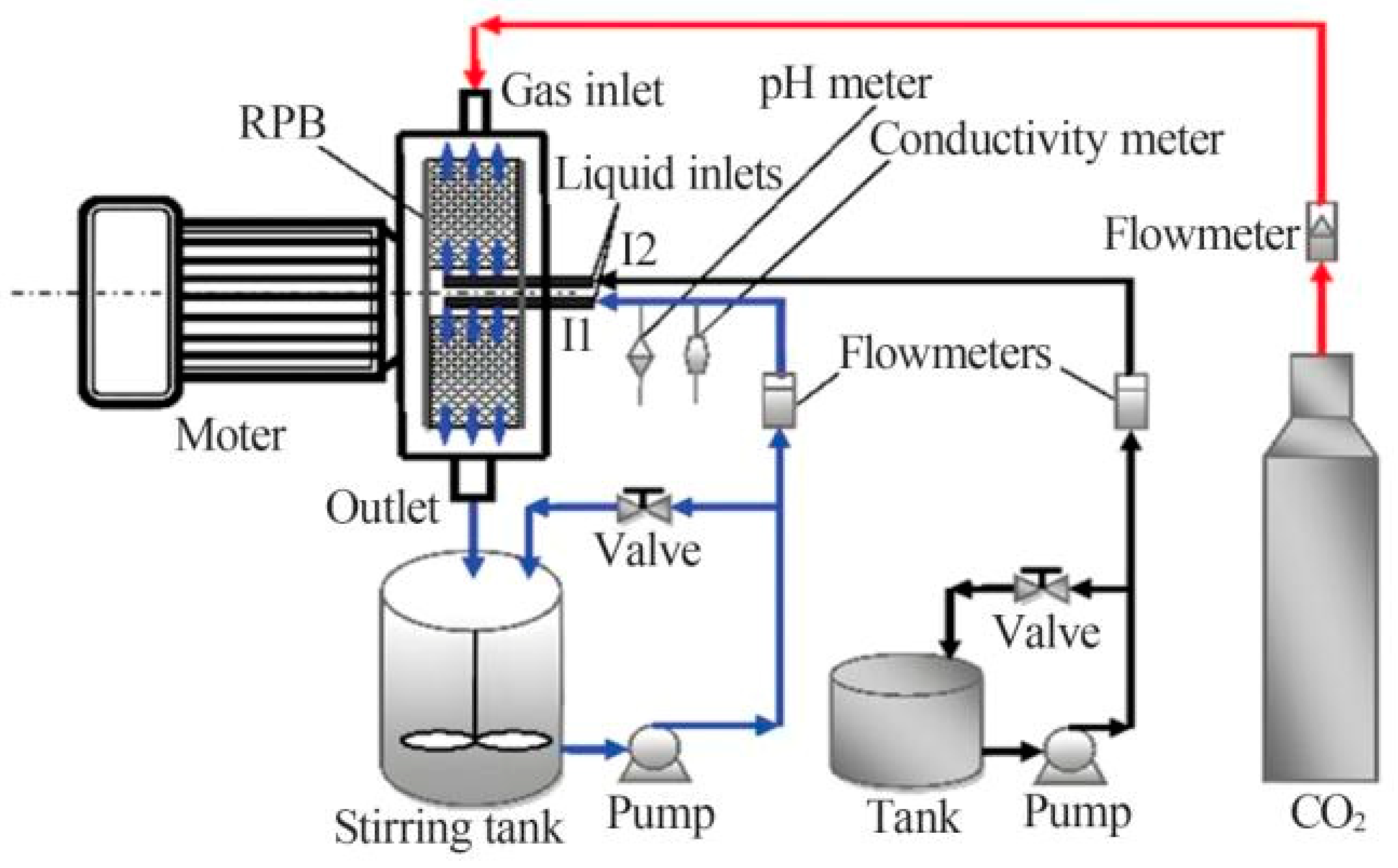
3.3.1. Rotating Packed Bed Reactor
3.3.2. High-Gravity Reactor
4. Fundamentals of Crystallization
4.1. Crystal Nucleation
4.2. Crystal Growth
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.G.; Wang, J.; Guo, Y.; Jia, Y.R. Exploring the Influence of Reaction Parameters on the Preparation of Calcium Carbonate by Spontaneous Precipitation. Cryst. Res. Technol. 2018, 53, 1700120. [Google Scholar] [CrossRef]
- Sovova, S.; Abalymov, A.; Pekar, M.; Skirtach, A.G.; Parakhonskiy, B. Calcium Carbonate Particles: Synthesis, Temperature and Time Influence on the Size, Shape, Phase, and Their Impact on Cell Hydroxyapatite Formation. J. Mater. Chem. B 2021, 9, 8308–8320. [Google Scholar] [CrossRef] [PubMed]
- Gentili, D.; Gazzano, M.; Melucci, M.; Jones, D.; Cavallini, M. Polymorphism as an Additional Functionality of Materials for Technological Applications at Surfaces and Interfaces. Chem. Soc. Rev. 2019, 48, 2502–2517. [Google Scholar] [CrossRef] [PubMed]
- Fadia, P.; Tyagi, S.; Bhagat, S.; Nair, A.; Panchal, P.; Dave, H.; Dang, S.; Singh, S. Calcium Carbonate Nano- and Microparticles: Synthesis Methods and Biological Applications. 3 Biotech 2021, 11, 457. [Google Scholar] [CrossRef]
- Adavi, K.; Molaei Dehkordi, A. Synthesis and Polymorph Controlling of Calcite and Aragonite Calcium Carbonate Nanoparticles in a Confined Impinging-Jets Reactor. Chem. Eng. Process.-Process Intensif. 2021, 159, 108239. [Google Scholar] [CrossRef]
- Escardino, A.; García-Ten, J.; Feliu, C.; Moreno, A. Calcium Carbonate Thermal Decomposition in White-Body Wall Tile during Firing. I. Kinetic Study. J. Eur. Ceram. Soc. 2010, 30, 1989–2001. [Google Scholar] [CrossRef]
- Clark, S.M.; Colas, B.; Jacob, D.E.; Neuefeind, J.C.; Wang, H.-W.; Page, K.L.; Soper, A.K.; Schodder, P.I.; Duchstein, P.; Zubiri, B.A.; et al. The Nano- and Meso-Scale Structure of Amorphous Calcium Carbonate. Sci. Rep. 2022, 12, 6870. [Google Scholar] [CrossRef]
- Qiu, J.; Lyu, J.-W.; Yang, J.-L.; Cui, K.-B.; Liu, H.-Z.; Wang, G.-F.; Liu, X. Review on Preparation, Modification and Application of Nano-Calcium Carbonate. Part. Part. Syst. Charact. 2024, 41, 2400097. [Google Scholar] [CrossRef]
- Ševčík, R.; Pérez-Estébanez, M.; Viani, A.; Šašek, P.; Mácová, P. Characterization of Vaterite Synthesized at Various Temperatures and Stirring Velocities without Use of Additives. Powder Technol. 2015, 284, 265–271. [Google Scholar] [CrossRef]
- Chilakala, R.; Thenepalli, T.; Huh, J.-H.; Ahn, J.-W. Precipitated Calcium Carbonate Synthesis by Simultaneous Injection to Produce Nano Whisker Aragonite. J. Korean Ceram. Soc. 2016, 53, 222–226. [Google Scholar] [CrossRef]
- Ševčík, R.; Šašek, P.; Viani, A. Physical and Nanomechanical Properties of the Synthetic Anhydrous Crystalline CaCO3 Polymorphs: Vaterite, Aragonite and Calcite. J. Mater. Sci. 2018, 53, 4022–4033. [Google Scholar] [CrossRef]
- Hussein, A.I.; Ab-Ghani, Z.; Che Mat, A.N.; Ab Ghani, N.A.; Husein, A.; Rahman, I.A. Synthesis and Characterization of Spherical Calcium Carbonate Nanoparticles Derived from Cockle Shells. Appl. Sci. 2020, 10, 7170. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, G.; Fang, Y.; Cao, L.; Guo, Z.; Wang, K.; Li, J. Calcium Carbonate Crystallization Process from the Mineralization of Calcium Chloride Waste. Sep. Purif. Technol. 2023, 319, 124066. [Google Scholar] [CrossRef]
- Kogo, M.; Suzuki, K.; Umegaki, T.; Kojima, Y. Control of Aragonite Formation and Its Crystal Shape in CaCl2-Na2CO3-H2O Reaction System. J. Cryst. Growth 2021, 559, 125964. [Google Scholar] [CrossRef]
- Tabassum, S.; Sahadat Hossain, M.; Bin Mobarak, M.; Nigar, F.; Ahmed, S. Synthesis of Nano Calcium Silicates from Waste Calcite and Aragonite Phases for Efficient Adsorptive Removal of Industrial Organic Pollutants. Arab. J. Chem. 2024, 17, 105901. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.S.; Choudhary, N.; Gnanamoorthy, G.; Tirth, V.; Prasad, S.; Khan, A.H.; Islam, S.; Khan, N.A. The Processing of Calcium Rich Agricultural and Industrial Waste for Recovery of Calcium Carbonate and Calcium Oxide and Their Application for Environmental Cleanup: A Review. Appl. Sci. 2021, 11, 4212. [Google Scholar] [CrossRef]
- Fu, H.-Y.; Qi, S.-X.; Shi, Z.-N.; Zeng, L.; Zhao, H.-B. Effect of Nano-CaCO3 on the Physical and Mechanical Properties of Analogue to Silty Mudstone Materials. Arab. J. Geosci. 2021, 14, 2502. [Google Scholar] [CrossRef]
- Li, W.; Huang, Y.; Wang, T.; Fang, M.; Li, Y. Preparation of Calcium Carbonate Nanoparticles from Waste Carbide Slag Based on CO2 Mineralization. J. Clean. Prod. 2022, 363, 132463. [Google Scholar] [CrossRef]
- Shang, D.; Zhou, N.; Dai, Z.; Song, N.; Wang, Z.; Du, P. Formation of Calcium Carbonate Nanoparticles through the Assembling Effect of Glucose and the Influence on the Properties of PDMS. RSC Adv. 2022, 12, 13600–13608. [Google Scholar] [CrossRef]
- Chen, X.-F.; Jiao, C.-J. Effect of Construction Wastes on the Rheo-Physical Behavior of Photocatalytic Mortar. Case Stud. Constr. Mater. 2022, 16, e01049. [Google Scholar] [CrossRef]
- Zhao, P.; Tian, Y.; You, J.; Hu, X.; Liu, Y. Recent Advances of Calcium Carbonate Nanoparticles for Biomedical Applications. Bioengineering 2022, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Huang, Z.; Zhu, Y.; Han, Y.; Tong, Z. Preparation of Nano-Sized Calcium Carbonate in Solution Mixing Process. J. Cryst. Growth 2021, 571, 126247. [Google Scholar] [CrossRef]
- Tian, B.; Ding, Z.; Zong, S.; Yang, J.; Wang, N.; Wang, T.; Huang, X.; Hao, H. Manipulation of Pharmaceutical Polymorphic Transformation Process Using Excipients. Curr. Pharm. Des. 2020, 26, 2553–2563. [Google Scholar] [CrossRef]
- Seepma, S.Y.M.H.; Ruiz-Hernandez, S.E.; Nehrke, G.; Soetaert, K.; Philipse, A.P.; Kuipers, B.W.M.; Wolthers, M. Controlling CaCO3 Particle Size with {Ca2+}:{CO32−} Ratios in Aqueous Environments. Cryst. Growth Des. 2021, 21, 1576–1590. [Google Scholar] [CrossRef]
- Nessi, E.; Dimoliani, M.; Papadopoulos, A.I.; Ntourou, G.; Voutetakis, S.; Intzes, K.; Dimitriadis, G.; Seferlis, P. Experimental Testing for Calcium Carbonate Nanoparticles Production in a Rotating Packed Bed. Chem. Eng. Trans. 2022, 94, 727–732. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, L.; Li, Y.; Zhang, J. Controllable Crystallization of Pure Vaterite Using CO2-Storage Material and Different Ca2+ Sources. J. CO2 Util. 2021, 48, 101520. [Google Scholar] [CrossRef]
- Milner, M.P.; Yang, M.; Compton, R.G. Vaterite Dissolution: Mechanism and Kinetics. J. Phys. Chem. C 2024, 128, 10388–10396. [Google Scholar] [CrossRef]
- Huang, Y.; Skirtach, A.G.; Parakhonskiy, B. V Systematic Study of Stability, Loading Efficiency and Release Mechanisms, and Cellular Interaction of Vaterite with Various Sizes. Ceram. Int. 2024, 50, 7469–7479. [Google Scholar] [CrossRef]
- Song, X.; Hua, X.; Yang, R.; Tuo, Y.; Wang, S.; Wang, J.; He, P.; Luo, X. Synergetic Effects of Initial NH4+ and Ca2+ Concentration on the Formation Vaterite Using Steamed Ammonia Liquid Waste as a Direct Carbonation. Powder Technol. 2023, 419, 118363. [Google Scholar] [CrossRef]
- Chen, W.-Z.; Jiao, C.-J.; Zhang, X.-C.; Yang, Y.; Chen, X.-F. Study on the Microstructure of Recycled Aggregate Concrete Strengthened by the Nano-SiO2 Soaking Method. Structures 2023, 58, 105388. [Google Scholar] [CrossRef]
- Wang, W.; Bao, Y.; Wang, K.; Qi, X.; Jia, Z.; Guo, S. A Novel Strategy to Enhance the Toughness of Foamed Concrete Utilizing the Synergistic Effect of Polyvinyl Alcohol Fibers and In-Situ Grown Calcium Carbonate. Constr. Build. Mater. 2025, 462, 139973. [Google Scholar] [CrossRef]
- Maslyk, M.; Mondeshki, M.; Tremel, W. Amorphous Calcium Carbonate Monohydrate Containing a Defect Hydrate Network by Mechanochemical Processing of Mono-Hydrocalcite Using Ethanol as Auxiliary Solvent. CrystEngComm 2022, 24, 4687–4697. [Google Scholar] [CrossRef]
- Remya, K.P.; Kim, S.; Kim, M.-J. Surfactant-Free Hydrothermal Fabrication of Vaterite CaCO3 with Hexagonal Bipyramidal Morphologies Using Seawater. Powder Technol. 2022, 410, 117865. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Antipina, M.N. Size-Controlled Synthesis of Vaterite Calcium Carbonate by the Mixing Method: Aiming for Nanosized Particles. Cryst. Growth Des. 2016, 16, 1311–1319. [Google Scholar] [CrossRef]
- Hood, M.A.; Landfester, K.; Muñoz-Espí, R. The Role of Residue Acidity on the Stabilization of Vaterite by Amino Acids and Oligopeptides. Cryst. Growth Des. 2014, 14, 1077–1085. [Google Scholar] [CrossRef]
- Lai, Y.; Chen, L.; Bao, W.; Ren, Y.; Gao, Y.; Yin, Y.; Zhao, Y. Glycine-Mediated, Selective Preparation of Monodisperse Spherical Vaterite Calcium Carbonate in Various Reaction Systems. Cryst. Growth Des. 2015, 15, 1194–1200. [Google Scholar] [CrossRef]
- Luo, J.; Kong, F.; Ma, X. Role of Aspartic Acid in the Synthesis of Spherical Vaterite by the Ca(OH)2–CO2 Reaction. Cryst. Growth Des. 2016, 16, 728–736. [Google Scholar] [CrossRef]
- Xu, A.-W.; Dong, W.-F.; Antonietti, M.; Cölfen, H. Polymorph Switching of Calcium Carbonate Crystals by Polymer-Controlled Crystallization. Adv. Funct. Mater. 2008, 18, 1307–1313. [Google Scholar] [CrossRef]
- Xiao, H.; Hu, C.; Chen, C.; Tao, C.; Wu, Y.; Jiang, J. The Advantage of Alcohol–Calcium Method on the Formation and the Stability of Vaterite against Ethanol–Water Binary Solvent Method. J. Mater. Res. 2020, 35, 289–298. [Google Scholar] [CrossRef]
- Oral, Ç.M.; Ercan, B. Influence of PH on Morphology, Size and Polymorph of Room Temperature Synthesized Calcium Carbonate Particles. Powder Technol. 2018, 339, 781–788. [Google Scholar] [CrossRef]
- Han, Y.S.; Hadiko, G.; Fuji, M.; Takahashi, M. Influence of Initial CaCl2 Concentration on the Phase and Morphology of CaCO3 Prepared by Carbonation. J. Mater. Sci. 2006, 41, 4663–4667. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Wang, L.; Li, G.; Li, Y. Facile Preparation of CaCO3 with Diversified Patterns Modulated by N-[(2-Hydroxyl)-Propyl-3-Trimethylammonium] Chitosan Chloride. Powder Technol. 2016, 299, 51–61. [Google Scholar] [CrossRef]
- Quan, C.-Q.; Jiao, C.-J.; Chen, W.-Z.; Xue, Z.-C.; Liang, R.; Chen, X.-F. The Impact of Fractal Gradation of Aggregate on the Mechanical and Durable Characteristics of Recycled Concrete. Fractal Fract. 2023, 7, 663. [Google Scholar] [CrossRef]
- Liendo, F.; Arduino, M.; Deorsola, F.A.; Bensaid, S. Optimization of CaCO3 Synthesis through the Carbonation Route in a Packed Bed Reactor. Powder Technol. 2021, 377, 868–881. [Google Scholar] [CrossRef]
- Sun, B.-C.; Wang, X.-M.; Chen, J.-M.; Chu, G.-W.; Chen, J.-F.; Shao, L. Synthesis of Nano-CaCO3 by Simultaneous Absorption of CO2 and NH3 into CaCl2 Solution in a Rotating Packed Bed. Chem. Eng. J. 2011, 168, 731–736. [Google Scholar] [CrossRef]
- Kang, F.; Wang, D.; Pu, Y.; Zeng, X.-F.; Wang, J.-X.; Chen, J.-F. Efficient Preparation of Monodisperse CaCO3 Nanoparticles as Overbased Nanodetergents in a High-Gravity Rotating Packed Bed Reactor. Powder Technol. 2018, 325, 405–411. [Google Scholar] [CrossRef]
- Nielsen, M.H.; Aloni, S.; Yoreo, J.J. De In Situ TEM Imaging of CaCO3 Nucleation Reveals Coexistence of Direct and Indirect Pathways. Science 2014, 345, 1158–1162. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The Kinetics and Mechanisms of Amorphous Calcium Carbonate (ACC) Crystallization to Calcite, via Vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Bots, P.; Benning, L.G.; Rodriguez-Blanco, J.-D.; Roncal-Herrero, T.; Shaw, S. Mechanistic Insights into the Crystallization of Amorphous Calcium Carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. [Google Scholar] [CrossRef]
- Giuffre, A.J.; Gagnon, A.C.; De Yoreo, J.J.; Dove, P.M. Isotopic Tracer Evidence for the Amorphous Calcium Carbonate to Calcite Transformation by Dissolution–Reprecipitation. Geochim. Cosmochim. Acta 2015, 165, 407–417. [Google Scholar] [CrossRef]
- Goodwin, A.L.; Michel, F.M.; Phillips, B.L.; Keen, D.A.; Dove, M.T.; Reeder, R.J. Nanoporous Structure and Medium-Range Order in Synthetic Amorphous Calcium Carbonate. Chem. Mater. 2010, 22, 3197–3205. [Google Scholar] [CrossRef]
- Liendo, F.; Arduino, M.; Deorsola, F.A.; Bensaid, S. Nucleation and Growth Kinetics of CaCO3 Crystals in the Presence of Foreign Monovalent Ions. J. Cryst. Growth 2022, 578, 126406. [Google Scholar] [CrossRef]
- Beck, R.; Seiersten, M.; Andreassen, J.-P. The Constant Composition Method for Crystallization of Calcium Carbonate at Constant Supersaturation. J. Cryst. Growth 2013, 380, 187–196. [Google Scholar] [CrossRef]
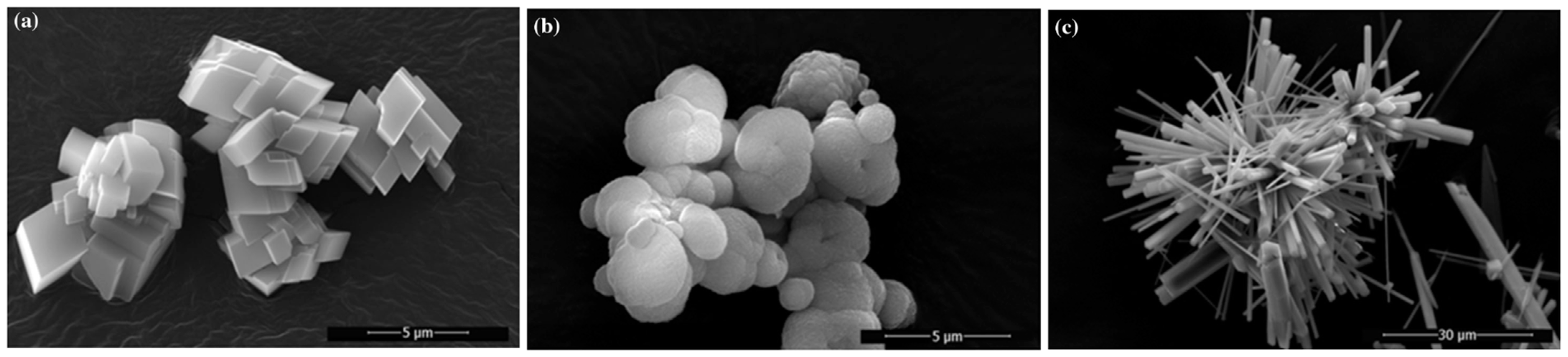



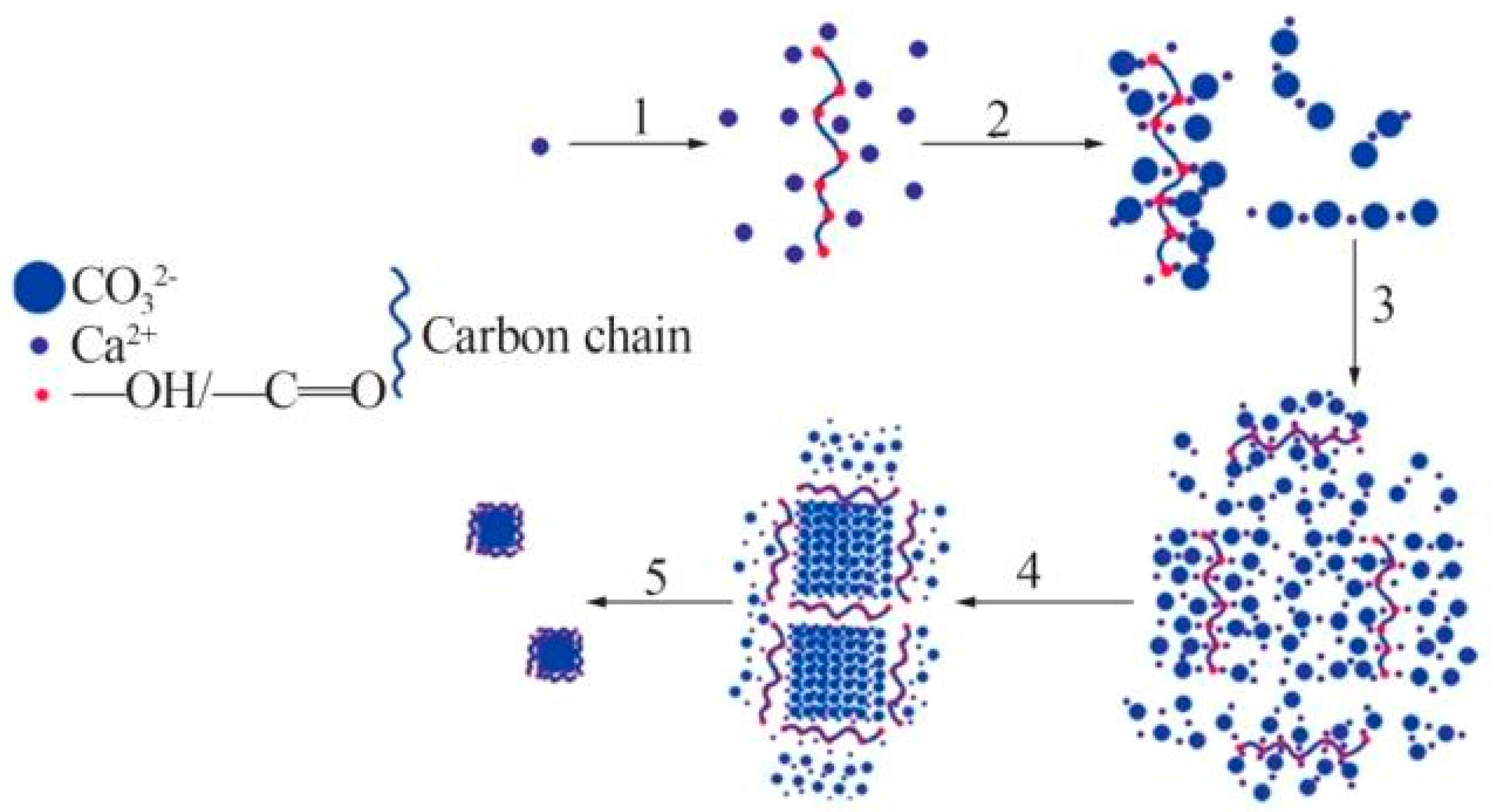

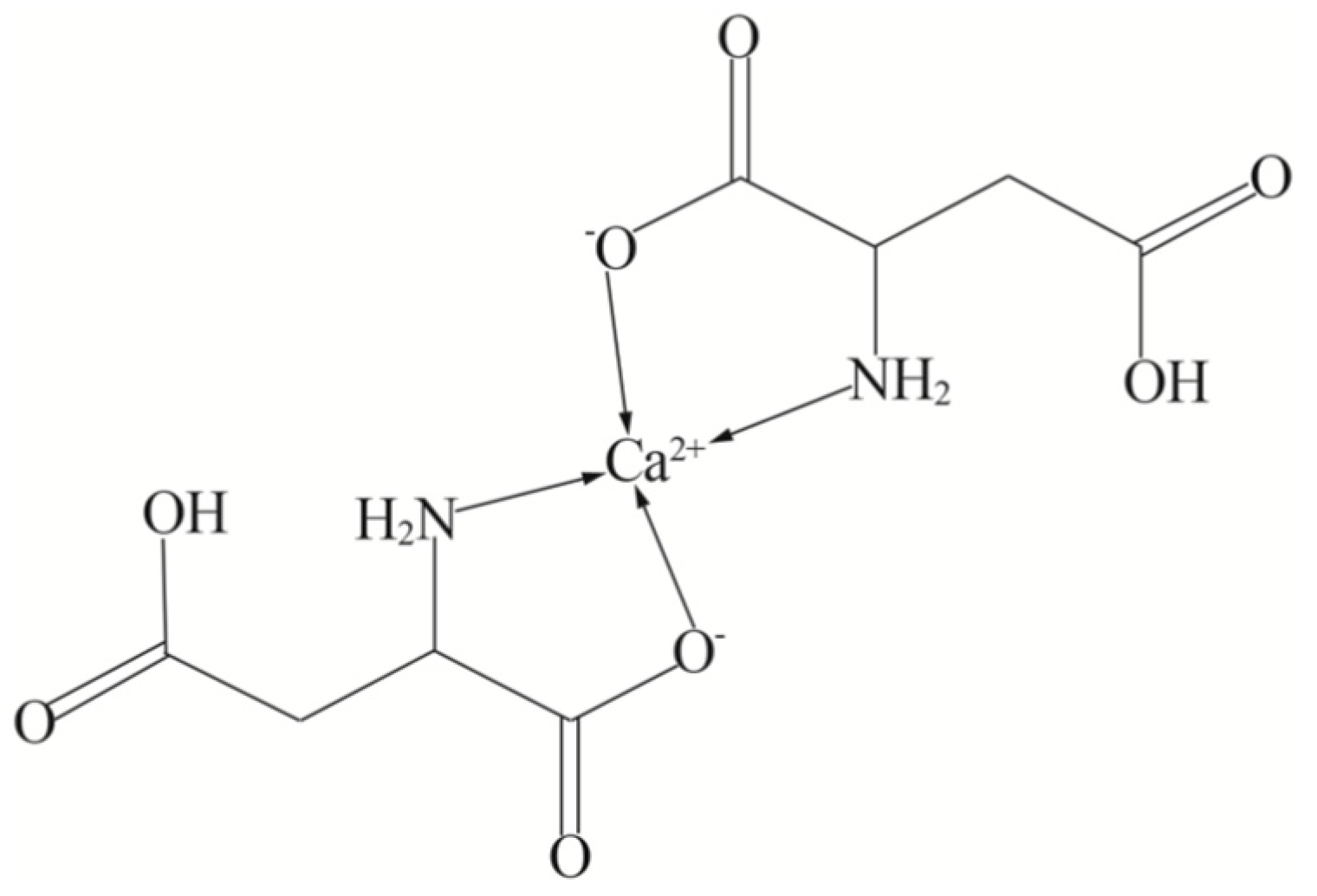
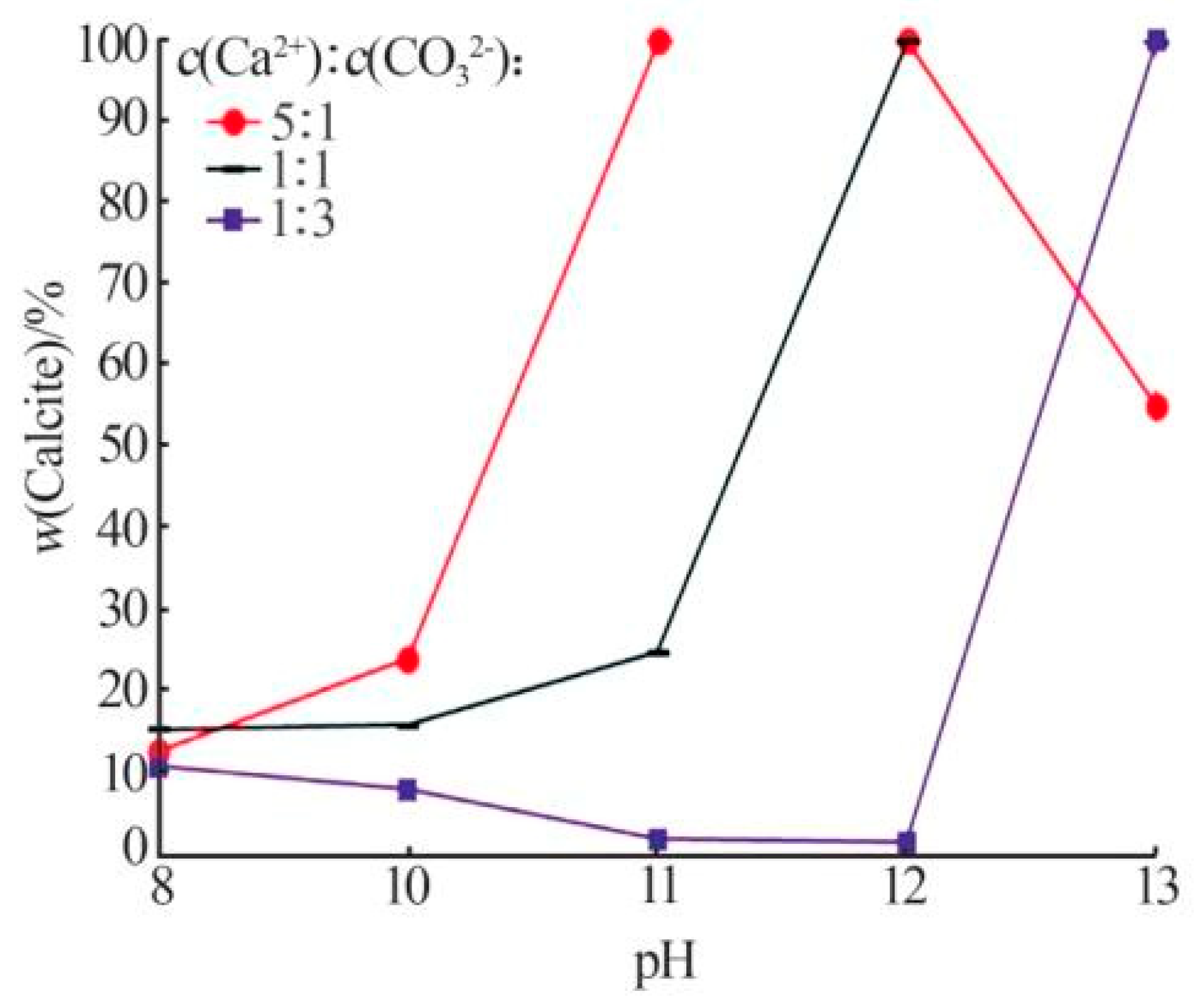




| Main Factors | Secondary Factors |
|---|---|
| Supersaturation | Reaction Solvent |
| Temperature | Polymorph Control Agent |
| Stirring Rate | Two-Phase Interface |
| Mixing Rate of Reactant Solution | pH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-G.; Jiao, C.-J.; Zhang, X.-C.; Zheng, J.-S.; Chen, X.-F. Advancements in the Research on the Preparation and Growth Mechanisms of Various Polymorphs of Calcium Carbonate: A Comprehensive Review. Crystals 2025, 15, 265. https://doi.org/10.3390/cryst15030265
Lu C-G, Jiao C-J, Zhang X-C, Zheng J-S, Chen X-F. Advancements in the Research on the Preparation and Growth Mechanisms of Various Polymorphs of Calcium Carbonate: A Comprehensive Review. Crystals. 2025; 15(3):265. https://doi.org/10.3390/cryst15030265
Chicago/Turabian StyleLu, Cheng-Gong, Chu-Jie Jiao, Xiu-Cheng Zhang, Jian-Sheng Zheng, and Xue-Fei Chen. 2025. "Advancements in the Research on the Preparation and Growth Mechanisms of Various Polymorphs of Calcium Carbonate: A Comprehensive Review" Crystals 15, no. 3: 265. https://doi.org/10.3390/cryst15030265
APA StyleLu, C.-G., Jiao, C.-J., Zhang, X.-C., Zheng, J.-S., & Chen, X.-F. (2025). Advancements in the Research on the Preparation and Growth Mechanisms of Various Polymorphs of Calcium Carbonate: A Comprehensive Review. Crystals, 15(3), 265. https://doi.org/10.3390/cryst15030265






