Abstract
Hollow flower-like multi-metallic nanocrystals have attracted significant research attention due to their exceptional catalytic properties, which stem from their high surface area-to-volume ratio and abundant active sites. Nevertheless, conventional synthesis methods for noble metal nanocrystals typically involve complex procedures or require harsh reaction conditions. In this work, we developed a facile and environmentally benign strategy for fabricating hollow flower-shaped trimetallic nanocrystals at ambient temperature. Our approach employs AgCl nanocubes, derived from AgNO3 and HAuCl4, as self-sacrificing templates. Through ascorbic acid-mediated reduction of metal precursors, we successfully synthesized three distinct types of hollow flower-like nanocrystals: AuAgCu, AuAgPt, and AuAgPd. Comprehensive characterization confirmed the well-defined morphology and precise composition control of the as-prepared nanocrystals. The catalytic performance was systematically evaluated through in situ UV–vis spectroscopy monitoring of 4-nitrophenylthiophenol reduction, revealing the following activity trend: AuAgCu > AuAgPt > AuAgPd. This study not only provides a versatile platform for constructing sophisticated multi-metallic nanostructures but also offers valuable insights into the structure–activity relationship of complex catalysts.
1. Introduction
Noble metal nanocrystals have garnered significant interest from scientists due to their unique electronic and optical properties. In particular, these nanocrystals with multifunctional capabilities demonstrate considerable potential for applications in sensing, detection, catalysis, and biomedicine [1,2,3,4,5,6,7]. In catalytic applications, noble metal nanocrystals exhibit a higher concentration of low-coordination surface atoms compared to bulk metals, providing distinct advantages in the catalytic degradation of environmental pollutants. Nitroaromatic compounds represent a notable category of second-generation water pollutants, commonly utilized as solvents in the production of fuels, pesticides, and explosives, thereby posing a potential threat to human safety [8]. Recent years have seen extensive investigations into the nitroaromatic reduction reactions involving various noble metal nanocrystals, including Au, Ag, Pt, and Pd [9,10,11,12,13,14,15,16,17]. Research indicates that the catalytic activity of these nanocrystals is influenced by their size, morphology, and composition. Furthermore, it has been observed that nanocrystals with different morphologies and compositions often exhibit varying catalytic activities. Extensive research has led to significant advancements in the synthesis of noble metal nanocrystals with precisely controlled architectures, including polyhedral [18,19,20,21,22], nanorod [23], nanoflower [24], hollow [25,26,27], and porous structures [28,29,30]. Among these, hollow and flower-like nanostructures have attracted particular attention due to their unique structural advantages. These configurations expose a higher density of low-coordination atoms, leading to enhanced atomic utilization efficiency and consequently superior catalytic performance. A notable example is the work by Kan et al., who developed a sophisticated approach for fabricating gold nanobipyramids with tunable dimensions encapsulated within AgPt hollow nanostructures. These hybrid nanostructures demonstrated exceptional capabilities in surface-enhanced Raman spectroscopy for real-time monitoring of redox processes [31]. Building upon these developments, our research group previously established a novel strategy for the controlled synthesis of hollow flower-like Au nanoparticles. This approach utilized in situ generated AgCl nanocubes as sacrificial templates, enabling the precise fabrication of hollow flower-shaped Au nanocrystals with tunable sizes ranging from 170 to 550 nm. The resulting nanostructures exhibited remarkable catalytic activity in the reduction of 4-nitrothiophenol, demonstrating the potential of such precisely engineered nanocrystals in catalytic applications [12].
In addition to the morphology of nanocrystals, the elemental composition of noble metal nanocrystals plays a decisive role in their catalytic activity. Compared to single component metal nanocrystals, noble metal nanocrystals composed of multiple elements often exhibit superior performance, which can be attributed to the synergistic effects of these multiple elements [32,33]. Piao and collaborators developed a simple and scalable method to construct CuAu bimetallic hollow particles from Cu2O nanoparticle aggregates for the catalytic reduction of 4-nitrophenol. The activity factor of the hollow CuAu bimetallic particles is 2.4 times that of the hollow Cu catalyst [13]. Oh et al. reported that Pd-based bimetallic catalysts, including Pd-Pt, Pd-Au, Pd-Ni, Pd-Cu, and Pd-Ag, synergistically enhance the reduction ability of 4-nitrophenol [34]. However, because the alloying of noble metals is constrained by thermodynamics, it often results in core-shell structures during the preparation process [35]. Therefore, preparing multi-element noble metal alloys with specific morphologies remains a challenge. To our knowledge, there are few reports on the preparation of multi-metal nanocrystals with a hollow flower-like structure.
In this work, we present a straightforward synthesis method for the preparation of Au-based hollow flower-like trimetallic nanocrystals. Specifically, we successfully synthesized three types of nanocrystals: AuAgCu, AuAgPd, and AuAgPt. These nanocrystals were subsequently employed to catalyze the reduction of 4-nitrothiophenol using NaBH4. The catalytic reaction process was monitored in situ via UV–vis spectroscopy, and the catalytic performance of the three noble metal nanocrystals was investigated.
2. Materials and Methods
2.1. Materials and Characterization
The reagents and consumables utilized in this experiment were obtained from commercial sources and employed directly without prior purification. Chloroauric acid (HAuCl4·3H2O, Mw = 393.83, 99.9% trace metals basis), silver nitrate (AgNO3, A.R., 99.8%), ascorbic acid (AA, AR, >99.0%), Potassium palladium (IV) chloride (K2PdCl6, Mw = 397.33, Pd ≥ 26.3%), Potassium hexachloroplatinate (IV) (K2PtCl6, Mw = 485.99, ≥99.9% metals basis), Copper(II) chloride dihydrate (CuCl2·2H2O, Mw = 170.48, ≥99.99% metals basis), polyvinylpyrrolidone (PVP), 4-nitrothiophenol (4-NTP, ≥80%), 4-aminothiophenol (4-ATP, 98%), nitric acid (HNO3, AR), and hydrochloric acid (HCl, AR) were procured from Aladdin. The water used in this experiment was ultrapure, purified by a Milli-Q Lab system (18.2 MΩ·cm). The morphologies and structures of the flower-like hollow nanocrystals were examined using scanning electron microscopy (SEM, 3.0 kV, SU70, Hitachi, Japan), transmission electron microscopy (TEM), and TEM-EDS mapping. The catalytic reaction was monitored using a UV–vis spectrophotometer (Cary 60).
2.2. Synthesis of Flower-like Trimetallic Nanocrystals
To prepare AuAgPt nanocrystals, we began by adding 40 mg of PVP to a 50 mL flask containing 20 mL of water while maintaining magnetic stirring at room temperature. The resulting solution was vigorously stirred and mixed for 5 min at room temperature. Subsequently, 0.2 mL of 0.5 M HAuCl4·3H2O and 40 μL of 0.5 M AgNO3 were introduced into the well-mixed PVP solution, continuing vigorous stirring for 15 min. Following this, 0.2 mL 0.1 M K2PtCl6 was added and the solution was stirred for an additional 10 min. Finally, 3.0 mL of 0.2 M ascorbic acid as the reducing agent was incorporated into the solution, and the reaction was completed by stirring magnetically at room temperature for 1 h.
To synthesize AuAgPd trimetallic nanocrystals, 40 mg of PVP was added to 20 mL of ultrapure water at room temperature and magnetically stirred for 5 min to ensure complete dissolution. Subsequently, 0.2 mL of 0.5 M HAuCl4·3H2O and 40 μL of 0.5 M AgNO3 were simultaneously introduced into the solution. Following magnetic stirring, a milky white turbidity was observed. Next, 0.2 mL of 0.1 M K2PdCl6 solution was added, and stirring continued for an additional 10 min before introducing 3.0 mL of 0.2 M ascorbic acid as a reducing agent. The reaction was allowed to proceed at room temperature for 60 min.
For the preparation of AuAgCu hollow flower-shaped trimetallic nanocrystals, 40 mg of PVP was similarly dissolved in 20 mL of ultrapure water under magnetic stirring at room temperature. To this PVP solution, 0.2 mL of 0.5 M HAuCl4·3H2O and 40 μL of 0.5 M AgNO3 were added while stirring. Following this, 0.2 mL of 0.1 M CuCl2 solution was added, and stirring was maintained for 10 min before the addition of 3.0 mL of 0.2 M ascorbic acid as a reducing agent. The reaction was conducted at room temperature for 1 h. The resulting nanocrystal was then centrifuged at 6000 rpm for 15 min, after which the supernatant was discarded. The precipitate was washed twice with water and ethanol and subsequently stored in pure water for future use. Ultimately, the prepared nanocrystals were immersed in ammonia aqueous solution for 1 h to remove AgCl from the nanocrystals, and finally a hollow structure was formed.
2.3. Catalytic Reduction of 4-Nitrothiophenol by Trimetallic Nanocrystals
To determine the UV–visible (UV–vis) absorption peak positions of the reactants and products, we initially tested the UV–vis absorption spectra of the reactant 4-NTP and the product 4-ATP standard solution. To ensure consistency in the tests, we mixed 0.1 mM 4-NTP with 1.9 mL of 0.1 mM 4-ATP and 1 mL of 0.1 M NaBH4. Following this, the reduction process of 4-NTP catalyzed by three metal nanocrystals was monitored in situ using UV–vis spectroscopy in a standard quartz cuvette with a path length of 1 cm. First, 1.9 mL of 0.1 mM 4-NTP and 1 mL of 0.1 M NaBH4 solution were added to a standard quartz cell and mixed thoroughly. Subsequently, the prepared hollow flower-shaped trimetallic nanocrystals were introduced to initiate monitoring of the reaction.
3. Results and Discussion
3.1. Characterizations of the Hollow Flower-like Nanocrystals
The crystal structure and phase composition were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and energy dispersive spectrometry (EDS). Figure 1A,B present the SEM and TEM images of the AuAgPt nanocrystals, respectively. The SEM image (Figure 1A) reveals that the surface of the AuAgPt nanocrystals is covered with a dense, flower-like structure, accompanied by small particles distributed across the surface of the flower sheet. The TEM image (Figure 1B) indicates that the prepared AuAgPt nanocrystals exhibit a uniform size of approximately 200 nm. Figure 1C displays the high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image, which shows that the central region of the AuAgPt nanocrystals appears as a gray area, while the periphery exhibits a bright edge, suggesting the presence of a hollow structure within. The corresponding EDS mapping (Figure 1D–F) clearly illustrates the distribution of Au, Ag, and Pt elements. The EDS elemental spectra (Figure S1) indicate that the contents of Au, Ag, and Pt are 80%, 17%, and 1%, respectively.
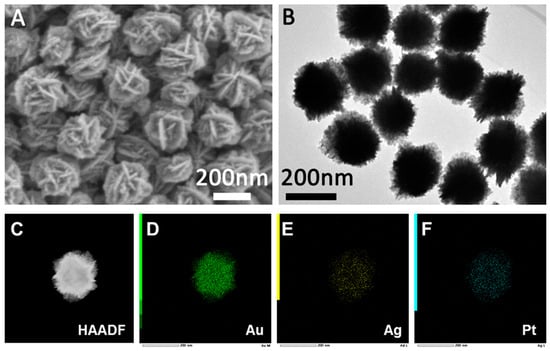
Figure 1.
SEM (A) and TEM (B) images of the prepared AuAgPt nanoparticles. (C) HAADF-STEM image and (D–F) corresponding elemental mappings of the AuAgPt nanoparticles.
The morphology, structure, and composition of AuAgPd and AuAgCu nanocrystals were characterized using SEM, TEM, HAADF-STEM, and EDS techniques. As illustrated in Figure 2A and Figure 3A, the SEM images reveal that both AuAgPd and AuAgCu nanocrystals exhibit flake-like structures. The corresponding TEM images (Figure 2B and Figure 3B) indicate that the prepared AuAgPd and AuAgCu nanocrystals have relatively uniform sizes, measuring approximately 200 nm and 180 nm, respectively. The structure of the nanocrystals can also be characterized by HAADF-STEM and EDS analyses. Figure 2C and Figure 3C present the HAADF-STEM images of the two nanocrystals. Notably, the bright areas on the edges of the nanoparticles surround a gray area in the center, suggesting that the interiors are hollow. The EDS analysis of AuAgPd nanocrystals (Figure 2D–F) demonstrates that Au, Ag, and Pd are distributed across the entire surface of the nanocrystals, with a corresponding atomic mole ratio of approximately 87:12:2 for Au, Ag, and Pd in the ternary AuAgPd nanoalloy (Figure S2). Additionally, the EDS element mapping results for AuAgCu nanocrystals reveal a uniform distribution of Au, Ag, and Cu (Figure 3C), with atomic ratios of approximately 51%, 5%, and 44%, respectively (Figure S3).
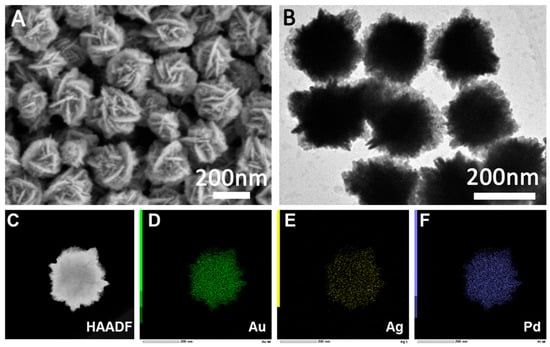
Figure 2.
SEM (A) and TEM (B) images of the prepared AuAgPd nanoalloys. (C) HAADF-STEM image and (D–F) corresponding elemental mappings of the AuAgPd nanoparticles.
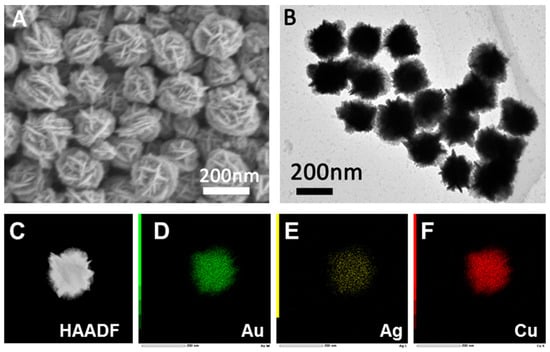
Figure 3.
SEM (A) and TEM (B) images of the prepared AuAgCu nanoparticles. (C) HAADF-STEM image and (D–F) corresponding elemental mappings of the AuAgCu nanoparticles.
X-ray diffraction (XRD) analysis is widely recognized as a powerful technique for determining the composition of synthesized materials. Figures S4–S7 show the XRD patterns of AuAg, AuAgCu, AuAgPd, and AuAgPt, respectively. As we can see, all synthesized samples exhibit similar characteristic peaks corresponding to the face-centered cubic (FCC) structure of Au. The XRD patterns of the three nanocrystals display distinct diffraction peaks associated with the (111), (200), (220), (311), and (222) crystallographic planes. The absence of detectable impurity peaks suggests that the hollow flower-like crystals are well-crystallized and of high purity. Notably, the intensity of the (111) peak is significantly higher than that of other peaks, indicating a preferential orientation of the (111) crystal planes in the synthesized structures. The detailed XRD peak positions for the three nanocrystals are summarized in Table S1.
3.2. Factors Affecting the Preparation of Nanocrystals
3.2.1. Effects of the Ascorbic Acid
The concentration of the reducing agent significantly influences the reduction rate and consequently dictates the reaction kinetics. In this study, we employed ascorbic acid (0.4 M) as the reducing agent to convert metal precursor ions into trimetallic nanocrystals. By varying the concentration of ascorbic acid in the reaction system, we investigated its impact on the resulting nanocrystals. Specifically, we added (A) 3.0 mL, (B) 2.5 mL, (C) 2.0 mL, (D) 1.0 mL, (E) 0.5 mL, and (F) 0.2 mL to the reaction system for the reduction process, with the corresponding SEM images of the prepared nanocrystals depicted in Figure 4. The addition of 3.0 mL resulted in irregular gold nanocrystals (Au NCs), as illustrated in Figure 4A. When the volume of ascorbic acid was reduced to 2.5 mL, 2.0 mL, 1.0 mL, 0.5 mL, and 0.2 mL, we obtained distinct AuAg NCs, as shown in Figure 4B–F, respectively. The corresponding HAADF-STEM and EDS mappings was shown in Figure S8. Notably, as the amount of reducing agent decreased, the petal structures of the flower-like AuAg NCs gradually diminished (Figure 4C,D). At a reducing agent volume of 0.5 mL, the quantity was insufficient to produce flower-like AuAg NCs, resulting instead in irregular particles (Figure 4E). Further reduction to 0.2 mL yielded irregular polyhedral particles (Figure 4F). In summary, an excessive amount of reducing agent accelerates the nucleation of Au NCs, leading to the formation of irregular small gold nanoparticles. Conversely, a decrease in reducing agent concentration slows the formation rate of gold atoms, resulting in preferential deposition of newly formed gold atoms onto the existing gold nano-petals, which makes the petals of the flower-like nano-Au less numerous and thicker. This result is consistent with previous reports [36]. When the concentration of the reducing agent is too low, it becomes inadequate to reduce the formed AgCl, preventing the production of hollow flower-like AuAg NCs.
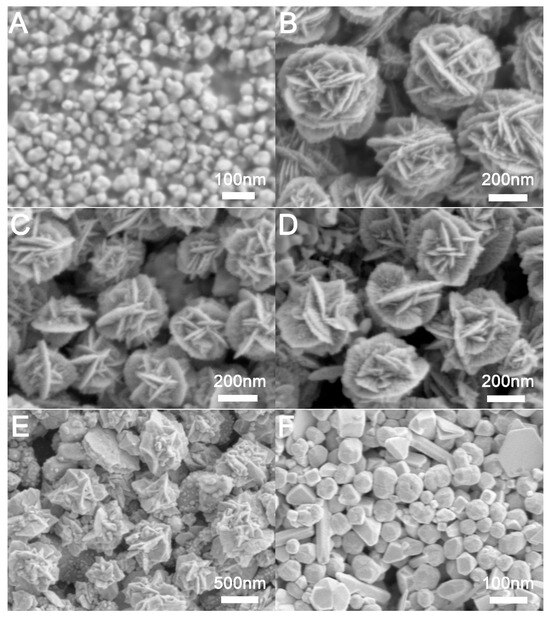
Figure 4.
SEM images of nanoparticles prepared under different amounts of AA: (A) 3.0 mL, (B) 2.5 mL, (C) 2.0 mL, (D) 1.0 mL, (E) 0.5 mL, (F) 0.2 mL.
3.2.2. Effects of the PVP
PVP not only controls the formation of precursor nanoparticles at the initial stage of the reaction, but also controls the morphological transformation of nanostructures grown on it [37]. To investigate the impact of ligands on the synthesis of Au NCs, we examined both the absence of ligands and the application of PVP, CTAC, and PDDA as ligands. As illustrated in Figure 5A, the addition of PVP results in the formation of flower-like nanocrystals. In contrast, the absence of a ligand yields spherical nanocrystals, as depicted in Figure 5B, which are characterized by a surface covered with island-like gold nanospheres. The use of PDDA leads to the production of dendritic Au NCs, as shown in Figure 5C. When CTAC is employed as a ligand, irregular gold nanoparticles are obtained, as seen in Figure 5D. These observations suggest that PVP is crucial for the formation of flower-like Au NCs. Research findings indicate that PVP exhibits stronger binding affinity to the {111} facets of gold nanoparticles compared to the {100} facets, resulting in the preferential formation of Au {111}-rich surfaces [38,39]. This is consistent with our XRD experimental results. Additionally, we investigated the effect of varying amounts of PVP on the hollow flower-like structure. When the PVP concentration was reduced to 10 mg, irregular nanocrystals were produced, as shown in Figure S9. The regular flower-like structure was no longer evident, indicating that an adequate amount of PVP is necessary for the formation of the flower-like structure.
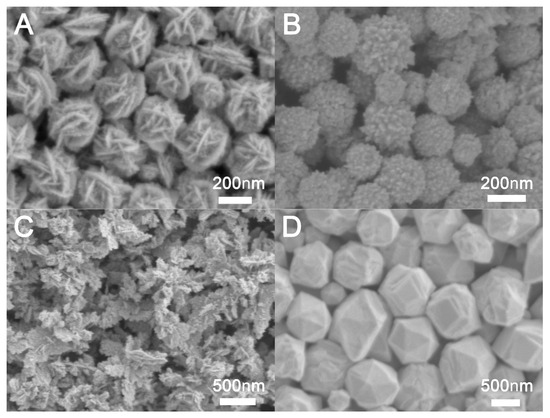
Figure 5.
SEM images of Au NCs prepared with different ligands: (A) PVP, (B) without ligand, (C) PDDA, and (D) CTAB.
3.3. Formation Mechanism
In a typical preparation process, HAuCl4 and AgNO3 are first added to the PVP solution, resulting in a bright yellow solution that turns milky white upon mixing. This phenomenon occurs due to the interaction between Cl− ions from the precursor HAuCl4 and Ag+ ions from AgNO3, leading to the formation of AgCl particles, which is consistent with previous reports [3]. The corresponding SEM characterization images reveal that the formed AgCl particles exhibit a cubic morphology (Figure S10). Subsequently, a third metal precursor ion (K2PdCl6, K2PtCl6, or CuCl2) is introduced into the solution, followed by the addition of the reducing agent ascorbic acid (AA). As the reaction progresses, the metal precursor ions are reduced to atoms and deposited onto the AgCl surface. When a smaller amount of reducing agent (0.2 M AA, 0.2 mL) is used, gold ions are preferentially reduced to gold atoms, which deposit on the pre-formed AgCl cubes (Figure S11). As the concentration of the reducing agent AA increases, a greater number of precursor ions are reduced to atoms and deposited on the AgCl particles, resulting in a flake-like structure guided by PVP. This result is consistent with previous reports [36]. Increasing the amount of reducing agent further enhances the nucleation sites, leading to the formation of more flower-like structures. Throughout the entire reduction process, the AgCl particles are inevitably partially reduced by AA, resulting in the presence of a small amount of Ag in the final nanocrystals. Concurrently, under the influence of the reducing agent, the precursor ions K2PdCl6, K2PtCl6, and CuCl2 are also reduced, yielding AuAgCu, AuAgPt, and AuAgPd nanocrystals with a flower-like architecture. Under the action of ammonia, the internal AgCl is dissolved and finally forms a hollow structure (Figure S12).
3.4. Catalytic Properties of the Hollow Flower-like Nanocrystals
The catalytic performance of AuAgPt, AuAgPd, and AuAgCu hollow flower-like nanocrystals was investigated by catalyzing the reduction of 4-NTP to 4-ATP in the presence of excess NaBH4. Previous studies have shown that 4-NTP is first converted to DMAB under the action of reducing agent and catalyst, and DMAB is further converted to 4-ATP as the reaction proceeds [40,41]. Initially, we mixed 1.9 mL of 0.1 mM 4-NTP and 1.9 mL of 0.1 mM 4-ATP with 1 mL of 0.1 M NaBH4, and subsequently conducted a UV–vis test. The results, depicted in Figures S13 and S14, show that the UV–vis spectrum peaks of the 4-NTP and 4-ATP standards mixed with NaBH4 are located at 410 nm and 270 nm, respectively. As illustrated in Figure S15, in the absence of a catalyst, the UV–vis spectrum of 4-NTP remains essentially unchanged after a reaction time of 60 min, indicating that NaBH4 alone is insufficient to reduce 4-NTP to 4-ATP. Under consistent conditions with a catalyst volume of 0.1 mL (10 mg/mL) and a reaction time of 14 min, we employed UV–vis spectroscopy to monitor the progress of the reduction reaction of three metal nanocrystals catalyzed by 4-NTP, aiming to evaluate their catalytic activity. During the reaction, UV–vis measurements were conducted every 2 min.
As illustrated in Figure 6A, the addition of AuAgCu nanocrystals resulted in a gradual decrease in the UV–vis absorption peak of the reactant 4-NTP at 410 nm, indicating the onset of the reaction. Furthermore, as the reaction progressed, new peaks emerged at 340 nm. Previous studies have identified this peak at 340 nm as the reaction intermediate DMAB [12]. As the reaction proceeded, the peak corresponding to the reactant 4-NTP (410 nm) continued to weaken, while the peak of the intermediate DMAB (340 nm) gradually increased. When the reaction proceeded to 8 min, the peak corresponding to 4-NTP (410 nm) completely disappeared, indicating that the reactants were completely converted. At the same time, the product 4-ATP peak was detected at 270 nm. As the reaction proceeded, the intermediate DMAB peak gradually weakened, and the product 4-ATP peak continued to increase. The reaction proceeded to 14 min, and the reactant 4-NTP was completely converted into product 4-ATP.
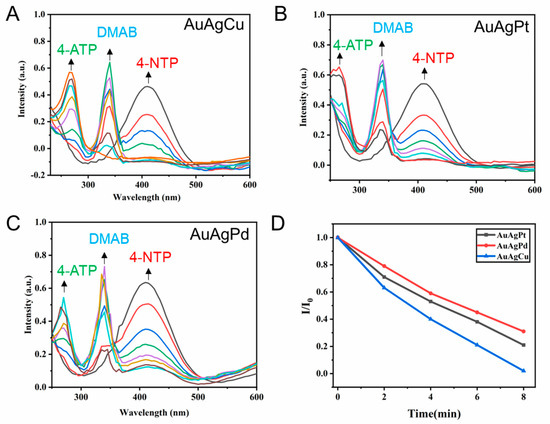
Figure 6.
UV–vis absorption spectra during the reduction of 4-nitrophenolate with different kinds of catalysts: (A) AuAgCu NPs, (B) AuAgPt NCs, (C) AuAgPd NCs. (D) The ratio of UV–vis absorption peak intensity to initial intensity of 4-NTP varies with reaction time under different catalyst conditions.
When AuAgPt nanoalloy was used as catalyst (Figure 6B), the complete conversion of 4-NTP required 12 min. However, when AuAgPd nanoalloy was used as catalyst (Figure 6C), the peak of 4-NTP at 410 nm still existed when the reaction was carried out for 14 min, indicating that the reactant was still not completely reacted. Furthermore, we compared the time required for the conversion of 4-NTP under different catalyst conditions to investigate the catalyst activity. As shown in Figure 6D, we recorded the ratio of the UV–vis spectral absorption peak intensity of the reactant 4-NTP to the initial intensity of the reaction 8 min before the reaction. It can be seen from the Figure 6A that the reactants are basically completely converted under the action of AuAgCu catalyst, and the reactants are completely converted within 8 min under the conditions of AuAgPt and AuAgPd catalysts, and the catalytic activity of AuAgPt is stronger than that of AuAgPd.
4. Conclusions
In conclusion, this study presents a straightforward method for the preparation of hollow flower-shaped trimetallic nanocrystals. Three types of nanocrystals were successfully synthesized, including AuAgCu, AuAgPd, and AuAgPt. The structure, morphology, composition, and catalytic activity of the synthesized metal nanocrystals were characterized using SEM, TEM, HAADF-STEM, and EDS analyses. The results indicate that the AuAgM nanocrystals (M = Cu/Pt/Pd) with a hollow flower structure. And the formation mechanism of hollow flower-like nanocrystals was explored. The effects of ligand type, concentration, and reducing agent concentration on crystal preparation were investigated, leading to further inference regarding the formation mechanism of the hollow flower-like crystals. Furthermore, we compared the catalytic activity of AuAgCu, AuAgPd, and AuAgPt nanocrystals in the reduction of 4-NTP using NaBH4. The catalytic test results demonstrate that the catalytic activity of the prepared AuAgCu nanocrystals surpasses that of both AuAgPd and AuAgPt. Our method holds promise for broader application in the synthesis of hollow flower-shaped metal nanocrystals with diverse elemental compositions to achieve enhanced catalytic activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst15030246/s1.
Author Contributions
Conceptualization, Y.Q. and Y.H.; methodology, Y.Q. and Y.H.; software, J.T., H.Z. and F.L.; Data curation, visualization, investigation, Y.Q., H.Z., J.T. and F.L.; writing—original draft preparation, Y.Q. and Y.H.; writing—review and editing, Y.Q. and Y.H.; supervision, funding acquisition, Y.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LTGC24B050008), Key Laboratory of Drug Prevention and Control Technology of Zhejiang Province, National Narcotics Laboratory Zhejiang Regional Center (No. NNLZRC-2024-013), and National College Students Innovation and Entrepreneurship Training Program (202411483018).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Choi, J.H.; Wang, H.; Oh, S.J.; Paik, T.; Sung, P.; Sung, J.; Ye, X.; Zhao, T.; Diroll, B.T.; Murray, C.B.; et al. Exploiting the colloidal nanocrystal library to construct electronic devices. Science 2016, 352, 205–208. [Google Scholar] [CrossRef]
- Kuznetsova, V.; Coogan, Á.; Botov, D.; Gromova, Y.; Ushakova, E.V.; Gun’Ko, Y.K. Expanding the Horizons of Machine Learning in Nanomaterials to Chiral Nanostructures. Adv. Mater. 2024, 36, 2308912. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.; Xu, J.J.; Xia, X.H.; Chen, H.Y. Emerging advances in plasmonic nanoassemblies for biosensing and cell imaging. Chin. Chem. Lett. 2023, 34, 108165. [Google Scholar] [CrossRef]
- Hwang, E.Y.; Lee, J.H.; Kang, M.J.; Lim, D.W. Stimuli-responsive plasmonic core–satellite hybrid nanostructures with tunable nanogaps. J. Mater. Chem. B 2023, 11, 1692–1704. [Google Scholar] [CrossRef]
- Qin, Y.Z.; Wu, Y.Z.; Wang, B.J.; Wang, J.Y.; Yao, W.X. Facile synthesis of Ag@Au core-satellite nanowires for highly sensitive SERS detection for tropane alkaloids. J. Alloy. Compd. 2021, 884, 161053. [Google Scholar] [CrossRef]
- Piotrowski, M.; Ge, Z.S.; Han, X.; Wang, Y.X.; Bandela, A.K.; Thumu, U. A facile post-assembly approach for the fabrication of non-close-packed gold nanocrystal arrays from binary nanocrystal superlattices. Nanoscale 2023, 15, 5188–5192. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Z.; Wu, Y.Z.; Wang, B.J.; Wang, J.Y.; Zong, X.S.; Yao, W.X. Controllable preparation of sea urchin-like Au NPs as a SERS substrate for highly sensitive detection of the toxic atropine. RSC Adv. 2021, 11, 19813. [Google Scholar] [CrossRef]
- McCormick, N.G.; Feeherry, F.E.; Levinson, H.S. Microbial transformation of 2,4,6-trinitrotoluene. Appl. Environ. Microbiol. 1976, 31, 949–958. [Google Scholar] [CrossRef]
- Huang, Y.F.; Wu, D.Y.; Zhu, H.P.; Zhao, L.B.; Liu, G.K.; Ren, B.; Tian, Z.Q. Surface-enhanced Raman spectroscopic study of p-aminothiophenol. Phys. Chem. Chem. Phys. 2012, 14, 8485–8497. [Google Scholar] [CrossRef]
- Kang, L.L.; Xu, P.; Zhang, B.; Tsai, H.H.; Han, X.J.; Wang, H. Laser wavelength- and power-dependent plasmon-driven chemical reactions monitored using single particle surface enhanced Raman spectroscopy. Chem. Commun. 2013, 49, 3389–3391. [Google Scholar] [CrossRef]
- Cui, Q.L.; Shen, G.Z.; Yan, X.H.; Li, L.D.; Möhwald, H.M.; Bargheer, M. Fabrication of Au@Pt Multibranched Nanoparticles and Their Application to In Situ SERS Monitoring. ACS Appl. Mater. Interfaces 2014, 6, 17075–17081. [Google Scholar] [CrossRef]
- Qin, Y.Z.; Lu, Y.X.; Pan, W.F.; Yu, D.D.; Zhou, J.G. One-pot synthesis of hollow hydrangea Au nanocrystals as a dual catalyst with SERS activity for in situ monitoring of a reduction reaction. RSC Adv. 2019, 9, 10314. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.H.; Jiang, J.W.; Yoon, S.H. Hollow porous Cu–Au particles with high catalytic activity for the reduction of 4-nitrophenol. CrystEngComm 2020, 22, 4386–4392. [Google Scholar]
- Zhou, X.; Huang, H.; Yang, Y.; Zhou, H.; Liang, R.; Zhao, Y.; Cui, Q.; Tang, Y.; Chen, S.; Li, P.; et al. Dendritic alloy shell Au@AgPt yolk sensor with excellent dual SERS enhancement and catalytic performance for in-situ SERS monitoring reaction. Sens. Actuators B Chem. 2023, 394, 134385. [Google Scholar] [CrossRef]
- Ten, A.; Lomonosov, V.; Boukouvala, C.; Ringe, E. Magnesium Nanoparticles for Surface-Enhanced Raman Scattering and Plasmon-Driven Catalysis. ACS Nano 2024, 18, 18785–18799. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, Y.W.; Wang, Y.; Cao, J.; Wang, J.J.; Zheng, Y.Y.; Pan, J.Q.; Zhong, W.W.; Li, C.R. A defect-enriched PdMo bimetallene for ethanol oxidation reaction and 4-nitrophenol reduction. Chem. Commun. 2024, 60, 3323–3326. [Google Scholar] [CrossRef]
- Zheng, X.; Ye, Z.; Akmal, Z.; He, C.; Zhang, J.; Wang, L. Recent progress in SERS monitoring of photocatalytic reactions. Chem. Soc. Rev. 2024, 53, 9954. [Google Scholar] [CrossRef]
- Hong, J.W.; Lee, S.U.; Lee, Y.W.; Han, S.W. Hexoctahedral Au Nanocrystals with High-Index Facets and Their Optical and Surface-Enhanced Raman Scattering Properties. J. Am. Chem. Soc. 2012, 134, 4565–4568. [Google Scholar] [CrossRef]
- Qin, Y.Z.; Pan, W.F.; Yu, D.D.; Lu, Y.X.; Wu, W.H.; Zhou, J.G. Stepwise evolution of Au micro/nanocrystals from an octahedron into a truncated ditetragonal prism. Chem. Commun. 2018, 54, 3411. [Google Scholar] [CrossRef]
- Qin, Y.Z.; Lu, Y.X.; Yu, D.D.; Zhou, J.G. Controllable synthesis of Au nanocrystals with systematic shape evolution from an octahedron to a truncated ditetragonal prism and rhombic dodecahedron. CrystEngComm 2019, 21, 5602–5609. [Google Scholar] [CrossRef]
- Zhao, G.; Lochon, F.; Dembélé, K.; Florea, I.; Baron, A.; Ossikovski, R.; Güell, A.G. Rapid and Facile Synthesis of Gold Trisoctahedrons for Surface-Enhanced Raman Spectroscopy and Refractive Index Sensing. ACS Appl. Nano Mater. 2024, 7, 5598–5609. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.J.; Kwon, S.; Lee, S.; Jung, I.; Park, S. Octahedron in a Cubic Nanoframe: Strong Near-Field Focusing and Surface-Enhanced Raman Scattering. ACS Nano 2024, 18, 7656–7665. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, J.F.; Guan, B.Y.; Lu, Y.; Lou, X.W. Hierarchical hollow nanoprisms based on ultrathin Ni-Fe layered double hydroxide nanosheets with enhanced electrocatalytic activity towards oxygen evolution. Angew. Chem. Int. Ed. 2018, 57, 172–176. [Google Scholar] [CrossRef]
- Meng, X.; Dyer, J.; Huo, Y.; Jiang, C. Greater SERS Activity of Ligand-Stabilized Gold Nanostars with Sharp Branches. Langmuir 2020, 36, 3558–3564. [Google Scholar] [CrossRef]
- Kwon, T.; Hwang, H.; Sa, Y.J.; Park, J.; Baik, H.; Joo, S.H.; Lee, K. Cobalt assisted synthesis of IrCu hollow octahedral nanocages as highly active electrocatalysts toward oxygen evolution reaction. Adv. Funct. Mater. 2017, 27, 1604688. [Google Scholar] [CrossRef]
- Ge, J.J.; Wei, P.; Wu, G.; Liu, Y.; Yuan, T.; Li, Z.; Qu, Y.; Wu, Y.; Li, H.; Zhuang, Z.; et al. Ultrathin palladium nanomesh for electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 3435–3438. [Google Scholar] [CrossRef]
- Li, C.; Dag, Ö.; Dao, T.D.; Nagao, T.; Sakamoto, Y.; Kimura, T.; Terasaki, O.; Yamauchi, Y. Electrochemical Synthesis of Mesoporous Gold Films toward Mesospace-Stimulated Optical Properties. Nat. Commun. 2015, 6, 6608. [Google Scholar] [CrossRef]
- Jiang, B.; Li, C.; Dag, Ö.; Abe, H.; Takei, T.; Imai, T.; Hossain, M.S.A.; Islam, M.T.; Wood, K.; Henzie, J. Mesoporous Metallic Rhodium Nanocrystals. Nat. Commun. 2017, 8, 15581. [Google Scholar] [CrossRef]
- Xu, J.; Yun, Q.R.; Wang, C.S.; Li, M.M.; Cheng, S.; Ruan, Q.F.; Zhu, X.Z.; Kan, C.X. Gold nanobipyramid-embedded silver–platinum hollow nanostructures for monitoring stepwise reduction and oxidation reactions. Nanoscale 2020, 12, 23663–23672. [Google Scholar] [CrossRef]
- Lee, S.; Zhao, Q.; Lee, S.; Lee, Y.; Jung, I.; Park, S. Plasmonic Nanotrenches with 1 nm Nanogaps for Surface-Enhanced Raman Scattering-Based Screening of His-Tagged Proteins. Nano Lett. 2024, 24, 12315–12322. [Google Scholar] [CrossRef]
- Ilayaraja, N.; Prabu, N.; Lakshminarasimhan, N.; Murugan, P.; Jeyakumar, D. Au-Pt graded nanoalloy formation and its manifestation in small organics oxidation reaction. J. Mater. Chem. A. 2013, 1, 4048–4056. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Shang, H.Y.; Jin, L.J.; Chen, C.Y.; Wang, Y.; Yuan, M.Y.; Du, Y.K. Ir-doped Pd nanosheet assemblies as bifunctional electrocatalysts for advanced hydrogen evolution reaction and liquid fuel electrocatalysis. Inorg. Chem. 2020, 59, 3321–3329. [Google Scholar] [CrossRef]
- Oh, S.D.; Kim, M.R.; Choi, S.H.; Chun, J.H.; Lee, K.P.; Gopalan, A.; Hwang, C.G.; Kim, S.H.; Hoon, O.J. Radiolytic synthesis of Pd-M (M = Ag, Au, Cu, Ni and Pt) alloy nanoparticles and their use in reduction of 4-nitrophenol. J. Ind. Eng. Chem. 2008, 14, 687–692. [Google Scholar] [CrossRef]
- Alexander, D.T.L.; Forrer, D.; Rossi, E.; Lidorikis, E.; Agnoli, S.; Bernasconi, G.D.; Butet, J.; Martin, O.J.F.; Amendola, V. Electronic structure-dependent surface plasmon resonance in single Au–Fe nanocrystals. Nano Lett. 2019, 19, 5754–5761. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.F.; Zhang, W.; Lin, A.J.; Cheng, D.J. Low Pt-content ternary PtNiCu nanocrystals with hollow interiors and accessible surfaces as enhanced multifunctional electrocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 9600–9608. [Google Scholar] [CrossRef]
- Pedireddy, S.; Lee, H.K.; Tjiu, W.W.; Phang, I.Y.; Tan, H.R.; Chua, S.Q.; Troadec, C.; Ling, X.Y. One-step synthesis of zero-dimensional hollow nanoporous gold nanoparticles with enhanced methanol electrooxidation performance. Nat. Commun. 2014, 5, 4947–5947. [Google Scholar] [CrossRef]
- Keisuke, K.; Akito, I. Synthesis and Optical Properties of Flower and Spiky-Ball-Like Silver Gold Nanoparticles. Bull. Chem. Soc. Jpn. 2014, 7, 780–791. [Google Scholar]
- Pastoriza-Santos, I.; Sánchez-Iglesias, A.; García de Abajo, F.J.; Liz-Marzán, L.M. Environmental optical sensitivity of gold nanodecahedra. Adv. Funct. Mater. 2007, 17, 1443–1450. [Google Scholar] [CrossRef]
- Kemal, L.; Jiang, X.C.; Wong, K. Experiment and Theoretical Study of PoIy(vinyl pyrrolidone)-controlled Gold Nanoparticles. J. Phys. Chem. C 2008, 40, 112. [Google Scholar]
- Xie, W.; Herrmann, C.; Kömpe, K.; Haase, M.; Schlücker, S. Synthesis of Bifunctional Au/Pt/Au Core/Shell Nanoraspberries for in Situ SERS Monitoring of Platinum-Catalyzed Reactions. J. Am. Chem. Soc. 2011, 133, 19302–19305. [Google Scholar] [CrossRef]
- Zhang, J.W.; Winget, S.A.; Wu, Y.R.; Su, D.; Sun, X.J.; Xie, Z.X.; Qin, D. Ag@Au Concave Cuboctahedra: A Unique Probe for Monitoring Au-Catalyzed Reduction and Oxidation Reactions by Surface-Enhanced Raman Spectroscopy. ACS Nano 2016, 10, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).