Unraveling the Crystallization, Mechanical, and Heat Resistance Properties of Poly(butylene adipate-co-terephthalate) Through the Introduction of Stereocomplex Crystallites
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PBAT/sc-PLA Composites
2.3. Characterization
2.3.1. Differential Scanning Calorimetry (DSC)
2.3.2. Rheology
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Heat Resistance Property
2.3.5. Tensile Property
3. Results and Discussion
3.1. Crystallinity
3.1.1. Stereocomplex-PLA Formation
3.1.2. Effect of PLA Content on the Crystallinity of PBAT/sc-PLA Composites
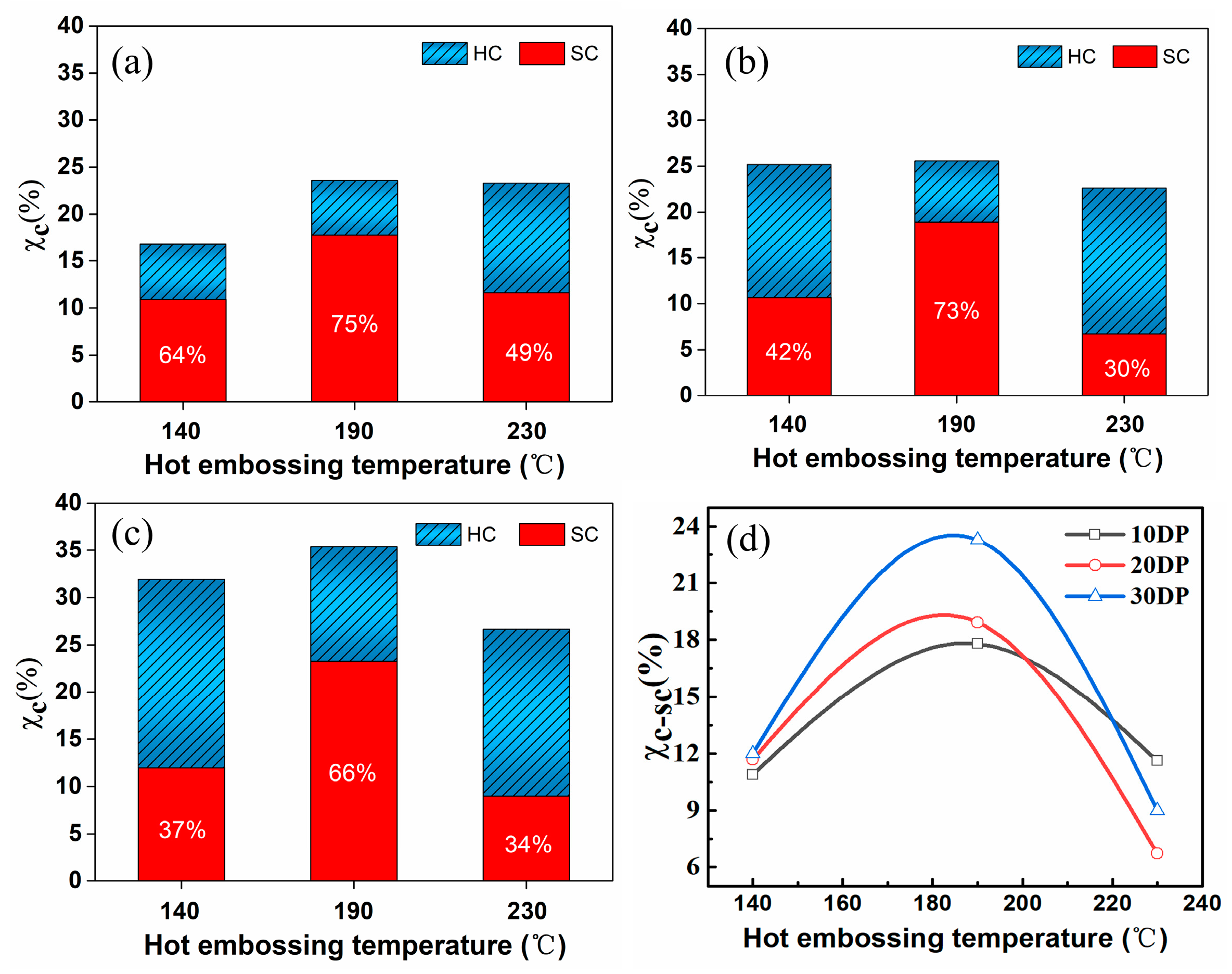
3.1.3. Effect of Hot Embossing Temperature on the Crystallinity of PBAT/sc-PLA Composites
3.2. Rheological Behavior
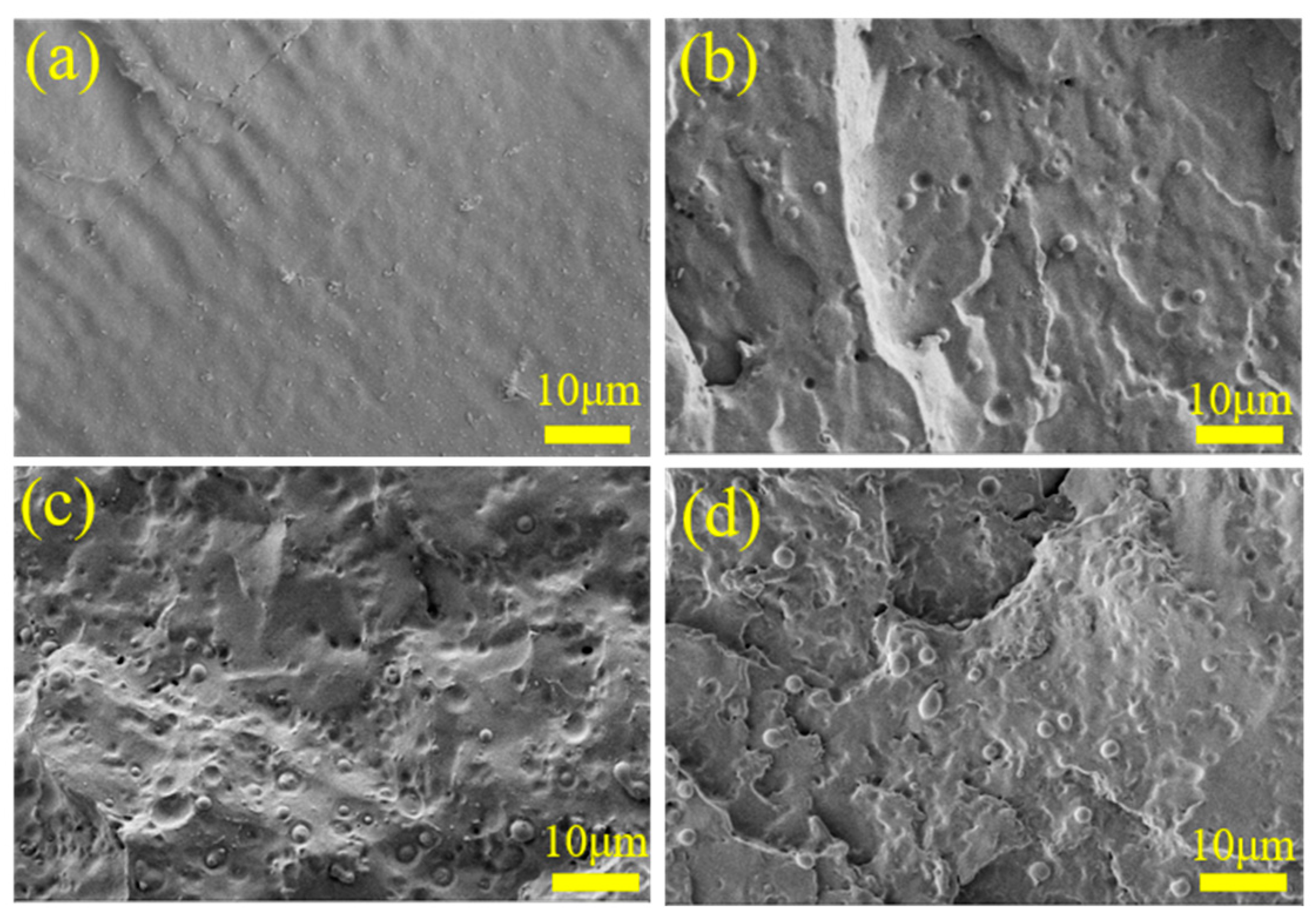
3.3. Phase Morphology
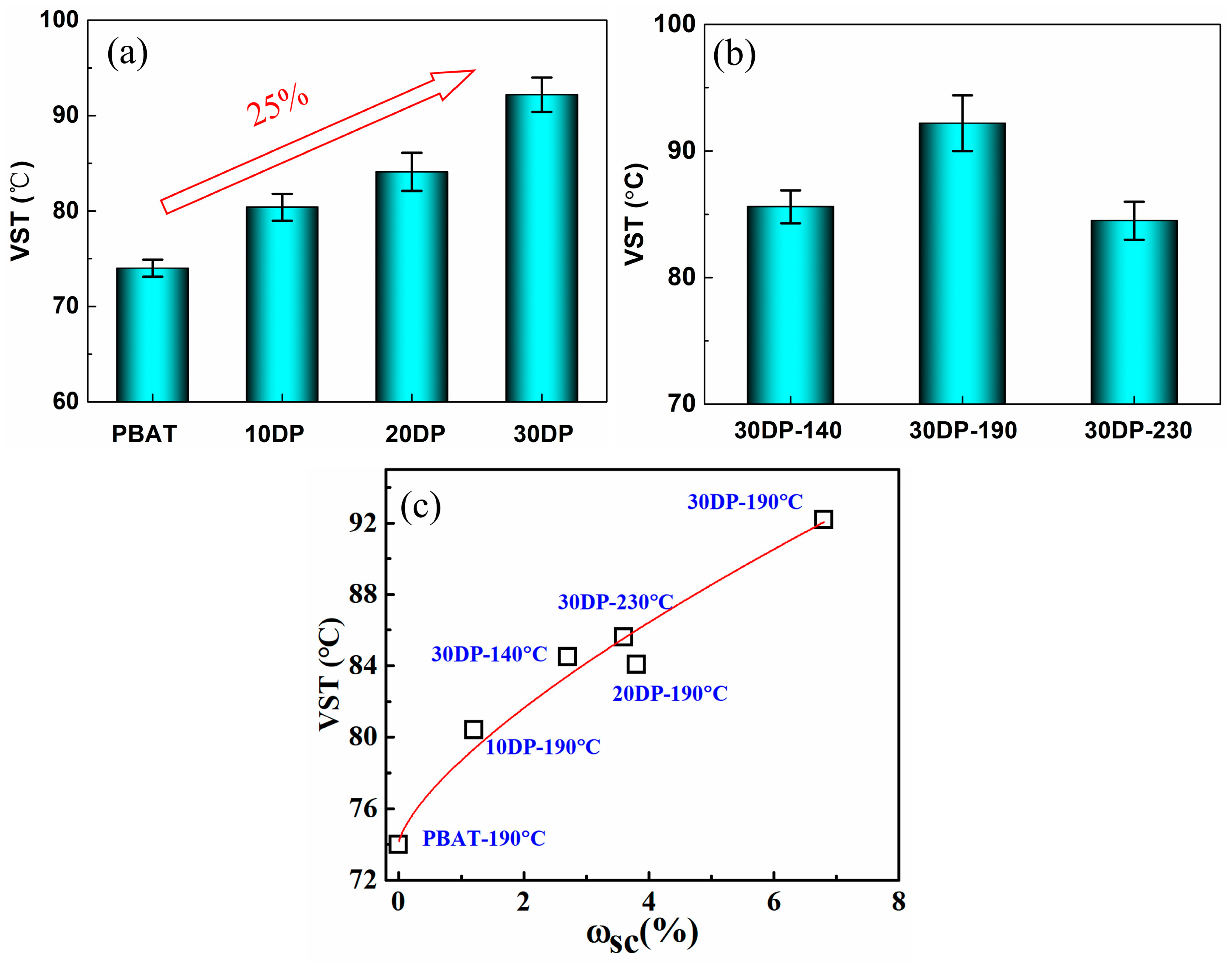
3.4. Heat Resistance
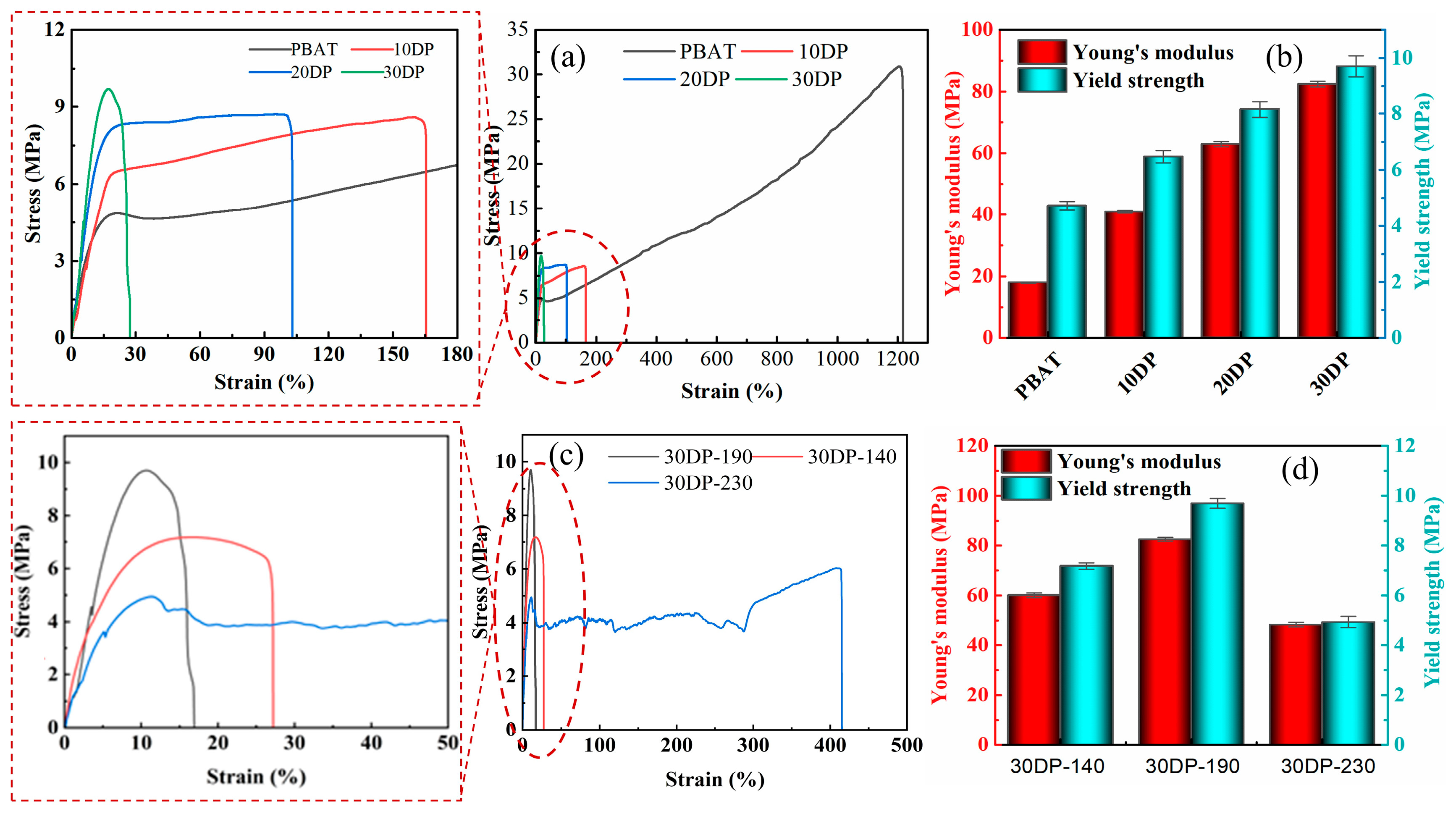
3.5. Mechanical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Liu, H.; Yang, Y.L.A.Y. Blends of poly(butylene adipate-co-terephthalate) (PBAT) and stereocomplex polylactide with improved rheological and mechanical properties. RSC Adv. 2020, 10, 10482–10490. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.T.H.; Tang, Z.T.Z.; Zhuang, X.Z.X.; Chen, X.C.X.; Jing, X.J.X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Mukherjee, C.; Varghese, D.; Krishna, J.S.; Boominathan, T.; Rakeshkumar, R.; Dineshkumar, S.; Rao, C.V.S.B.; Sivaramakrishna, A. Recent advances in biodegradable polymers—Properties, applications and future prospects. Eur. Polym. J. 2023, 192, 112068. [Google Scholar] [CrossRef]
- Wu, L.; Shi, X.; Wu, Z.S. Recent advancements and perspectives of biodegradable polymers for supercapacitors. Adv. Funct. Mater. 2023, 33, 1. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Amanda Grizzo, D.M.D.S. Multifunctional bilayer membranes composed of poly(lactic acid), beta-chitin whiskers and silver nanoparticles for wound dressing applications. Int. J. Biol. Macromol. 2023, 251, 126314. [Google Scholar]
- Ye Fu, G.W.X.B. Biodegradation Behavior of Poly(Butylene Adipate-Co-Terephthalate) (PBAT), Poly(Lactic Acid) (PLA), and Their Blend in Freshwater with Sediment. Molecules 2020, 25, 3946. [Google Scholar] [CrossRef]
- Torres, S.; Navia, R.; Murdy, R.C.; Cooke, P.; Misra, M.; Mohanty, A.K. Green composites from residual microalgae biomass and poly(butylene adipate-co-terephthalate): Processing and plasticization. ACS Sustain. Chem. Eng. 2015, 3, 614–624. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A.; Billon, N.; Combeaud, C. Effect of the simultaneous biaxial stretching on the structural and mechanical properties of PLA, PBAT and their blends at rubbery state. Eur. Polym. J. 2015, 68, 288–301. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmuş, A. Ductility improvements of PLA-based binary and ternary blends with controlled morphology using PBAT, PBSA, and nanoclay. Compos. Part B Eng. 2020, 182, 107661. [Google Scholar] [CrossRef]
- Chen, P.; Bai, D.; Tang, H.; Liu, H.; Wang, J.; Gao, G.; Li, L. Polylactide aerogel with excellent comprehensive performances imparted by stereocomplex crystallization for efficient oil-water separation. Polymer 2022, 255, 125128. [Google Scholar] [CrossRef]
- Özogul, F.; Hamed, I.; Gokdogan, S. The impact of natural clinoptilolite on ammonia, cadaverine and other polyamine formation by food-borne pathogen in lysine decarboxylase broth. LWT Food Sci. Technol. 2016, 65, 703–710. [Google Scholar] [CrossRef]
- Iguchi, Y.; Asai, S.A.S. Formation of PLA stereocomplex crystals during melt-blending of asymmetric PLLA/PDLA/PMMA blends of varying miscibility. Polym. J. 2019, 29, 225–235. [Google Scholar] [CrossRef]
- Li, C.L.C.; Dou, Q.D.Q.; Bai, Z.B.Z.; Lu, Q.L.Q. Non-isothermal crystallization behaviors and spherulitic morphology of poly(lactic acid) nucleated by a novel nucleating agent. J. Therm. Anal. Calorim. 2015, 122, 407–417. [Google Scholar] [CrossRef]
- Jongpanya-Ngam, P.; Khankrua, R.; Seadan, M.; Suttiruengwong, S. Effect of synthesized sulfonate derivatives as nucleating agents on crystallization behavior of poly(lactic acid). Des. Monomers Polym. 2022, 25, 115–127. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polym. Int. J. Sci. Technol. Polym. 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
- Ramos, F.J.H.T.; de Fátima Vieira Marques, M.; Rodrigues, J.G.P.; de Oliveira Aguiar, V.; Luz, F.S.D.; de Azevedo, A.R.G.; Monteiro, S.N. Development of novel geopolymeric foam composites coated with polylactic acid to remove heavy metals from contaminated water. Case Stud. Constr. Mater. 2022, 16, e795. [Google Scholar] [CrossRef]
- Ma, B.; Wang, X.; He, Y.; Dong, Z.; Zhang, X.; Chen, X.; Liu, T. Effect of poly(lactic acid) crystallization on its mechanical and heat resistance performances. Polymer 2021, 212, 123280. [Google Scholar] [CrossRef]
- Pan, G.P.G.; Xu, H.X.H.; Mu, B.M.B.; Ma, B.M.B.; Yang, J.Y.J.; Yang, Y.Y.Y. Complete stereo-complexation of enantiomeric polylactides for scalable continuous production. Chem. Eng. J. 2017, 328, 759–767. [Google Scholar] [CrossRef]
- Xie, Y.X.Y.; Lan, X.L.X.; Bao, R.B.R.; Lei, Y.L.Y.; Cao, Z.C.Z.; Yang, M.Y.M.; Yang, W.Y.W.; Wang, Y.W.Y. High-performance porous polylactide stereocomplex crystallite scaffolds prepared by solution blending and salt leaching. Mater. Sci. Eng. C (Mater. Biol. Appl.) 2018, 90, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Jing Yang, W.L.B.M. Simultaneous toughness and stiffness of 3D printed nano-reinforced polylactide matrix with complete stereo-complexation via hierarchical crystallinity and reactivity. Int. J. Biol. Macromol. 2022, 202, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Shapira-Furman, T.; Nyska, A.; Domb, A.J. Biocompatibility of insulin-PLA stereocomplex. J. Bioact. Compat. Pol. 2024, 39, 429–439. [Google Scholar] [CrossRef]
- Quero, E.; Müller, A.J.; Signori, F.; Coltelli, M.B.; Bronco, S. Isothermal cold-crystallization of PLA/PBAT blends with and without the addition of acetyl tributyl citrate. Macromol. Chem. Phys. 2012, 213, 36–48. [Google Scholar] [CrossRef]
- Bao, R.B.R.; Yang, W.Y.W.; Jiang, W.J.W.; Liu, Z.L.Z.; Xie, B.X.B.; Yang, M.Y.M. Polymorphism of racemic poly(l -lactide)/Poly(d -lactide) blend: Effect of melt and cold crystallization. J. Phys. Chem. B 2013, 117, 3667–3674. [Google Scholar] [CrossRef]
- Michell, R.M.; Ladelta, V.; Silva, E.D.; Müller, A.J.; Hadjichristidis, N. Poly(lactic acid) Stereocomplexes based Molecular Architectures: Synthesis and Crystallization. Prog. Polym. Sci. 2023, 146, 101742. [Google Scholar] [CrossRef]
- Li, Y.L.Y.; Han, C.H.C.; Zhang, X.Z.X.; Dong, Q.D.Q.; Dong, L.D.L. Effects of molten poly(D,L-lactide) on nonisothermal crystallization in stereocomplex of poly(L-lactide) with poly(D-lactide). Thermochim. Acta 2013, 573, 193–199. [Google Scholar] [CrossRef]
- Zhao, H.Z.H.; Bian, Y.B.Y.; Li, Y.L.Y.; Dong, Q.D.Q.; Han, C.H.C.; Dong, L.D.L. Bioresource-based blends of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and stereocomplex polylactide with improved rheological and mechanical properties and enzymatic hydrolysis. J. Mater. Chem. A 2014, 5, 8881–8892. [Google Scholar] [CrossRef]
- Woo, E.M.; Chang, L. Crystallization and morphology of stereocomplexes in nonequimolar mixtures of poly(l-lactic acid) with excess poly(d-lactic acid). Polymer 2011, 52, 6080–6089. [Google Scholar] [CrossRef]
- Li, Y.L.Y.; Han, C.H.C.; Bian, Y.B.Y.; Dong, Q.D.Q.; Zhao, H.Z.H.; Zhang, X.Z.X.; Xu, M.X.M.; Dong, L.D.L. Miscibility, thermal properties and polymorphism of stereocomplexation of high-molecular-weight polylactide/poly(D,L-lactide) blends. Thermochim. Acta 2014, 508, 53–62. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Cao, W.; Guo, Z.; Fang, Z.; Li, J.; Chen, P. Ultra-high flame-retardant efficiency of phosphorous-silicon hybrid microsphere in poly (butylene adipate-co-terephthalate). Compos. Part B Eng. 2023, 263, 110861. [Google Scholar] [CrossRef]
- López-Rodríguez, N.; de Arenaza, I.M.; Meaurio, E.; Sarasua, J.R. Improvement of toughness by stereocomplex crystal formation in optically pure polylactides of high molecular weight. J. Mech. Behav. Biomed. 2014, 37, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, Y.; Pang, X.; Chen, X. Poly(Lactic Acid): Recent Stereochemical Advances and New Materials Engineering. Adv. Mater. 2024, e2412185. [Google Scholar] [CrossRef] [PubMed]
- Raef, M.; Sarasua, J.; Etxeberria, A.; Ugartemendia, J.M. Stereocomplexation: From molecular structure to functionality of advanced polylactide systems. Polymer 2023, 280, 126066. [Google Scholar] [CrossRef]
- Hou, Y.; Jia, H.; Pan, Y.; Liu, C.; Shen, C.; Liu, X. Porous Poly(l-lactide)/Poly(d-lactide) Blend Film with Enhanced Flexibility and Heat Resistance via Constructing a Regularly Oriented Pore Structure. Macromolecules 2023, 56, 7606–7616. [Google Scholar] [CrossRef]
- Tan, B.H.; Muiruri, J.K.; Li, Z.; He, C. Recent Progress in Using Stereocomplexation for Enhancement of Thermal and Mechanical Property of Polylactide(Article). ACS Sustain. Chem. Eng. 2016, 4, 5370–5391. [Google Scholar] [CrossRef]
- Gu, Z.; Zhang, J.; Cao, W.; Liu, X.; Wang, J.; Zhang, X.; Chen, W.; Bao, J. Extraordinary toughness and heat resistance enhancement of biodegradable PLA/PBS blends through the formation of a small amount of interface-localized stereocomplex crystallites during melt blending. Polymer 2022, 262, 125454. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, L.; Han, C. Enhanced rheological, crystallization, mechanical, and heat resistance performance of poly(L-lactide)/basalt fibers composites via in situ formation of stereocomplex polylactide crystals. J. Appl. Polym. Sci. 2024, 141, e56280. [Google Scholar] [CrossRef]
- Shi, X.S.X.; Qin, J.Q.J.; Wang, L.W.L.; Ren, L.R.L.; Rong, F.R.F.; Li, D.L.D.; Wang, R.W.R.; Zhang, G.Z.G. Introduction of stereocomplex crystallites of PLA for the solid and microcellular poly(lactide)/poly(butylene adipate-co-terephthalate) blends. RSC Adv. 2018, 8, 11850–11861. [Google Scholar] [CrossRef]
- Sarasua, J.R.; Arraiza, A.L.; Balerdi, P.; Maiza, I. Crystallinity and mechanical properties of optically pure polylactides and their blends. Polym. Eng. Sci. 2005, 45, 745–753. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. The effect of morphology and chemical characteristics of cellulose reinforcements on the crystallinity of polylactic acid. J. Appl. Polym. Sci. 2006, 101, 300–310. [Google Scholar] [CrossRef]
- Tsuji, H.; Horii, F.; Nakagawa, M.; Ikada, Y.; Odani, H.; Kitamaru, R. Stereocomplex formation between enantiomeric poly(lactic acid)s. 7. Phase structure of the stereocomplex crystallized from a dilute acetonitrile solution as studied by high-resolution solid-state carbon-13 NMR spectroscopy. Macromolecules 1992, 25, 4114–4118. [Google Scholar] [CrossRef]
- Hye-Seon Park, C.H. Relationship between the Stereocomplex Crystallization Behavior and Mechanical Properties of PLLA/PDLA Blends. Polymers 2021, 13, 1851. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Karst, D.; Yang, Y. Molecular modeling study of the resistance of PLA to hydrolysis based on the blending of PLLA and PDLA. Polymer 2006, 47, 4845–4850. [Google Scholar] [CrossRef]
- Tuccitto, A.V.; Anstey, A.; Sansone, N.D.; Park, C.B.; Lee, P.C. Controlling stereocomplex crystal morphology in poly(lactide) through chain alignment. Int. J. Biol. Macromol. 2022, 218, 22–32. [Google Scholar] [CrossRef]
- Mohammadi, M.; Heuzey, M.; Carreau, P.J.; Taguet, A. Interfacial localization of CNCs in PLA/PBAT blends and its effect on rheological, thermal, and mechanical properties. Polymer 2021, 233, 124229. [Google Scholar] [CrossRef]
- Wang, X.A.; Peng, S.A.; Chen, H.A.; Yu, X.A.; Zhao, X.A.X.C. Mechanical properties, rheological behaviors, and phase morphologies of high-toughness PLA/PBAT blends by in-situ reactive compatibilization. Compos. Part B Eng. 2019, 173, 107028. [Google Scholar] [CrossRef]
- Tong, M.; Ma, B.; Wang, X.; He, Y.; Yu, J. A feasible strategy to balance the performance of stereo-complexed polylactide by incorporating poly(butylene adipate-co-terephthalate). Int. J. Biol. Macromol. 2023, 228, 366–373. [Google Scholar] [CrossRef]
- Li, J.; Xu, M.; He, H.; Gao, Q.; Zhang, Y.; Zhang, H.; Gu, Q.; Xue, B.; Zhu, Z. Enhancing Tensile Strength and Toughness via Moderate Orientation of Amorphous Molecular Chains in Biobased Biodegradable Poly(lactic acid). ACS Appl. Polym. Mater. 2022, 4, 6969–6977. [Google Scholar] [CrossRef]
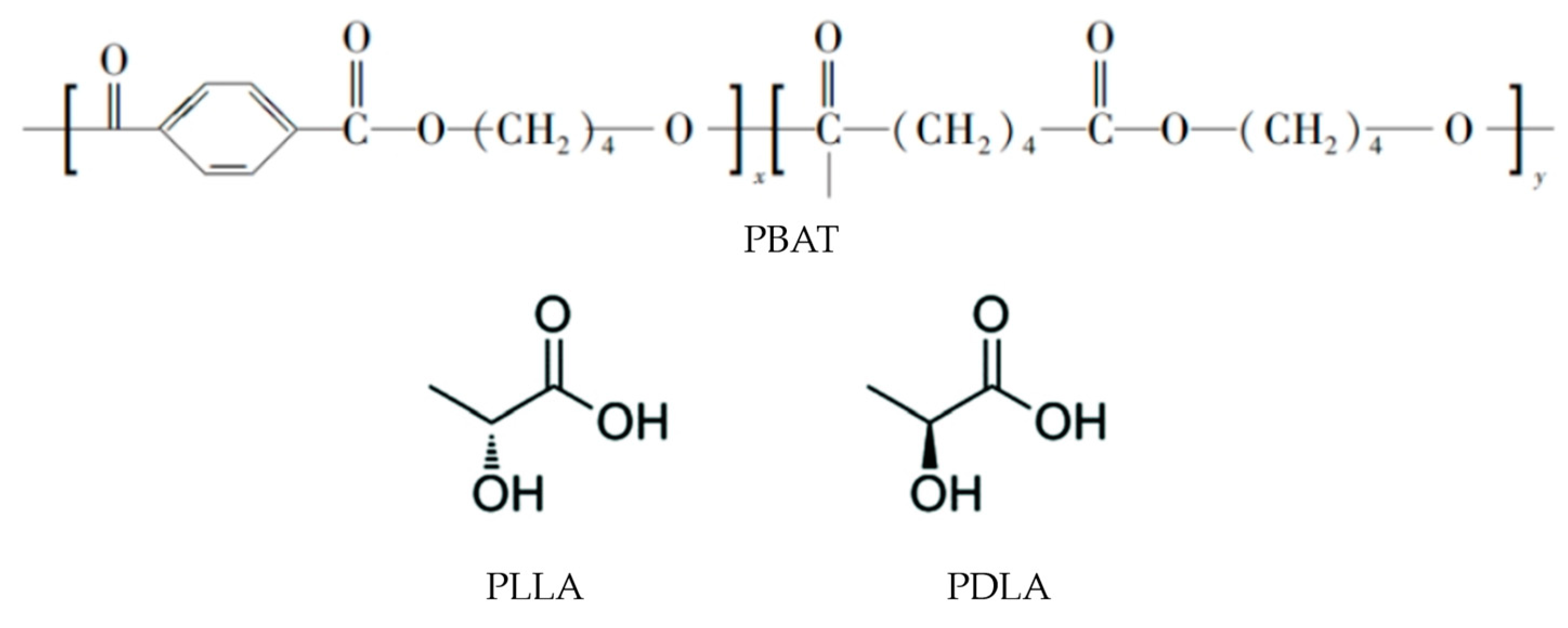




| Samples | PBAT (wt.%) | PLLA (wt.%) | PDLA (wt.%) | Hot Embossing Temperature |
|---|---|---|---|---|
| PBAT | 100 | 0 | 0 | 190 °C |
| 10DP | 90 | 5 | 5 | 190 °C |
| 20DP | 80 | 10 | 10 | 190 °C |
| 30DP | 70 | 15 | 15 | 190 °C |
| L20/D10 | 70 | 20 | 10 | - |
| L15/D15 | 70 | 15 | 15 | - |
| L10/D20 | 70 | 10 | 20 | - |
| 30DP-140 | 70 | 15 | 15 | 140 °C |
| 30DP-230 | 70 | 15 | 15 | 230 °C |
| Sample | ΔHc (J/g) | Tg (°C) | ΔHm-sc (J/g) | ΔHm-hc (J/g) | Xc-sc (%) | Xc (%) | fsc (%) |
|---|---|---|---|---|---|---|---|
| L20/D10 | 3.66 ± 0.23 | 9.87 ± 0.53 | 3.47 ± 0.18 | 22.56 ± 1.05 | 7.94 | 30.49 | 26 |
| L15/D15 | 4.27 ± 0.16 | 9.22 ± 0.65 | 5.29 ± 0.18 | 19.56 ± 1.21 | 11.22 | 30.78 | 37 |
| L10/D20 | 4.23 ± 0.20 | 9.68 ± 0.63 | 3.81 ± 0.22 | 20.63 ± 0.88 | 8.15 | 28.80 | 28 |
| 30PLLA | 2.26 ± 0.13 | 10.12 ± 0.59 | - | 28.14 ± 1.31 | - | 28.14 | - |
| Sample | ΔHm-sc (J/g) | ΔHm-hc (J/g) | Xc-sc (%) | Ωsc (%) |
|---|---|---|---|---|
| 10DP | 1.26 ± 0.13 | 2.46 ± 0.17 | 11.51 | 1.15 |
| 20DP | 4.75 ± 0.21 | 2.48 ± 0.09 | 18.98 | 3.79 |
| 30DP | 8.69 ± 0.23 | 3.81 ± 0.20 | 22.79 | 6.83 |
| Sample | Tc (°C) | ΔHm-sc (J/g) | ΔHm-hc (J/g) | Xc-sc (%) | Xc-hc (%) | Xc (%) | Ƒsc (%) |
|---|---|---|---|---|---|---|---|
| 140 | 1.36 ± 0.23 | 0.74 ± 0.13 | 10.89 | 5.92 | 16.80 | 64 | |
| 10DP | 190 | 2.27 ± 0.15 | 1.02 ± 0.16 | 17.79 | 5.79 | 23.57 | 75 |
| 230 | 1.89 ± 0.14 | 1.89 ± 0.21 | 11.62 | 11.68 | 23.30 | 49 | |
| 140 | 2.43 ± 0.19 | 3.30 ± 0.25 | 10.67 | 14.50 | 25.18 | 42 | |
| 20DP | 190 | 4.89 ± 0.15 | 1.72 ± 0.14 | 18.92 | 6.65 | 25.58 | 73 |
| 230 | 2.37 ± 0.24 | 5.40 ± 0.29 | 6.71 | 15.90 | 22.61 | 30 | |
| 140 | 4.00 ± 0.26 | 6.66 ± 0.28 | 11.98 | 19.93 | 31.90 | 37 | |
| 30DP | 190 | 9.06 ± 0.31 | 4.70 ± 0.24 | 23.27 | 12.08 | 35.35 | 66 |
| 230 | 4.09 ± 0.29 | 8.04 ± 0.36 | 8.99 | 17.66 | 26.65 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, M.; Zhang, T.; Jiang, J.; Jia, C.; Li, Y.; Wang, X.; Li, Q. Unraveling the Crystallization, Mechanical, and Heat Resistance Properties of Poly(butylene adipate-co-terephthalate) Through the Introduction of Stereocomplex Crystallites. Crystals 2025, 15, 247. https://doi.org/10.3390/cryst15030247
Qiao M, Zhang T, Jiang J, Jia C, Li Y, Wang X, Li Q. Unraveling the Crystallization, Mechanical, and Heat Resistance Properties of Poly(butylene adipate-co-terephthalate) Through the Introduction of Stereocomplex Crystallites. Crystals. 2025; 15(3):247. https://doi.org/10.3390/cryst15030247
Chicago/Turabian StyleQiao, Min, Tao Zhang, Jing Jiang, Caiyi Jia, Yangyang Li, Xiaofeng Wang, and Qian Li. 2025. "Unraveling the Crystallization, Mechanical, and Heat Resistance Properties of Poly(butylene adipate-co-terephthalate) Through the Introduction of Stereocomplex Crystallites" Crystals 15, no. 3: 247. https://doi.org/10.3390/cryst15030247
APA StyleQiao, M., Zhang, T., Jiang, J., Jia, C., Li, Y., Wang, X., & Li, Q. (2025). Unraveling the Crystallization, Mechanical, and Heat Resistance Properties of Poly(butylene adipate-co-terephthalate) Through the Introduction of Stereocomplex Crystallites. Crystals, 15(3), 247. https://doi.org/10.3390/cryst15030247







