Abstract
Environmental legislation has focused its attention on improving air quality. In this context, the presence of sulfur compounds in fuels, such as diesel and gasoline, is undesirable. When sulfur is combusted, compounds are emitted as SOx (SO2 and SO3) into the atmosphere, causing acid rain and respiratory diseases. For this reason, environmental norms have been established to reduce the sulfur content of fuels. Sulfur (mainly as alkylbenzothiophenes, dibenzothiophenes and alkyldibenzothiophenes) is removed in refineries through a process called hydrodesulfurization (HDS). HDS is performed at an industrial level with the use of NiMo, CoMo or NiW catalysts supported on alumina. Unsupported MoS2 (bulk) catalysts have recently attracted attention due to their high activity and selectivity in HDS. In this study, bulk NiMo catalyst precursors were synthesized using solvothermal methods with varying pH and solvothermal synthesis time. The precursors and catalysts were characterized using scanning electron microscopy with energy dispersive X-ray spectroscopy (EDS) microanalysis, X-ray diffraction (XRD), textural properties using liquid nitrogen physisorption at 77 K, Raman spectroscopy and high-resolution transmission electron microscopy (HTREM). The results indicate that the morphology of the NiMoO4 precursors synthesized in an ethanol/water mixture varies, forming “grains,” “flakes” or “rods,” depending on the dwell time and synthesis conditions. The catalytic activity results show that the bulk NiMo catalyst synthesized at 2 h presented higher selectivity and catalytic activity in the HDS of 4,6-DMDBT when compared to a supported reference catalyst (NiMo/γ-Al2O3).
1. Introduction
Oil has been the energy source for transportation for a long time and is expected to still be used for some time to come despite the incursion of new green technologies [1]. Sulfur compounds present in diesel are considered a source of pollution as, when they are burned in internal combustion engines, sulfur compounds, including sulfur dioxides (SO2 and SO3), are emitted to the atmosphere; such compounds can have undesirable consequences, such as generating acid rain. In humans, sulfur dioxides can cause difficulty in breathing—especially in asthmatic people—among other issues [2]. The main organosulfur compounds present in oil are mercaptans, thiophene, benzthiophene, di-benzthiophene, 4,6-dimethyl dibenzothiophene and their respective alkylated compounds; the more the molecular weight increases, as for 4,6-dimethyl dibenzothiophene, the more difficult the removal of sulfur becomes due to steric hindrance. There are many forms of technology for sulfur removal, such as oxidative desulfurization, adsorption desulfurization and bio-desulfurization processes. Compared with these technologies, hydrodesulfurization (HDS) has been widely used in refineries to produce ultra-low sulfur fuels [3].
The HDS process is performed using catalysts such as NiMo, CoMo, NiW, CoW and Ni-Mo-W sulfides supported on alumina [4]. The operating conditions depend on the feed to be processed (naphtha, diesel, vacuum gas oil or atmospheric residue). For diesel, the following conditions are utilized: LHSV = 2–4 h−1, hydrogen/hydrocarbon ratio = 240, T = 613–633 K and P = 30–40 atm [5].
To obtain sulfur content as low as 10 ppm and comply with national and international standards, more active catalysts are sought. Research [6] has shown that bulk catalysts can achieve high activity, although with the disadvantage of a low specific surface area; to counteract this, nanoparticles with various morphological arrangements have been synthesized, such as nanoflowers, nanosheets, nanorods, nanoblocks, and so on [7,8,9]. According to Chianelli and Yan [6,8,10], supported catalysts such as NiMoS on alumina enable a strong interaction between MoS2 and its support, thus diminishing the catalysts’ activity. On the other hand, Ni (or Co) promoters are prone to form a spinel phase with the alumina support, which also affects the catalytic activity. The above issues do not affect unsupported catalysts; thus, more active sites are generated (CUSs: coordinatively unsaturated sites).
For the synthesis of bulk catalysts, oxides or sulfides, NiMoO4, decomposition of thiosalts, template-assisted synthesis, template-free synthesis and hydrothermal or solvothermal methods are used [11]. Hydrothermal or solvothermal methods—also known as soft chemistry methods—offer energy advantages when compared to solid-state methods as they require mild reaction conditions (e.g., with temperatures around 686 K) in a medium such as water and ethanol. Additionally, it is possible to change the morphology of the material, depending on the solvothermal reaction time used in the synthesis.
In this work, bulk NiMoO4 catalytic precursors were prepared using solvothermal methods, applying the postulates of green chemistry [12] by using water and ethanol under mild temperature conditions. The materials obtained were tested for their catalytic activity in the hydrodesulfurization of one of the more refractory organosulfur compounds; namely, 4,6-dimethyldibenzothiophene (4,6-DMDBT). To the best of our knowledge, no attempts have been made to relate synthesis time to the size of the NiMoS crystallites in turn to the distribution of products.
2. Materials and Methods
2.1. Synthesis of Bulk NiMo Catalyst Precursors by Solvothermal Methods
The synthesis of NiMoO4 was performed under an ethanol-assisted solvothermal process on an isothermal batch reactor at 423 K, taking into consideration that the pH, time and temperature of the reaction have a crucial influence on the synthesis and evolution of the growth and morphology of the final product. Amounts of 10 mL of 0.1 M solutions of Ni(NO3)2·6H2O (Aldrich) and Na2MoO4·2H2O (J.T. Baker) were prepared with a nominal molar ratio of Ni/Mo = 1 in a solution with a volumetric ratio of ethanol (J.T. Baker)/water (distilled) of 2:1. Initially, a 1 M Na2MoO4 solution was added very slowly to the 0.1 M Ni(NO3)2 aqueous solution under vigorous magnetic stirring. The mixture was maintained for 30 min after the addition step. During preparation, the pH of the mixture was adjusted to 7 with 0.01 M HNO3 and NH4OH solutions (with each drop, instant clouding of the mixture was observed). Two NiMoO4 precursors were synthesized using different times (2 h and 6 h). Once they had finished, the batch reactor was quickly cooled with ice. The resulting solution was centrifuged to separate a yellow precipitate. The supernatant liquid (ethanol/water mixture) was removed. The precipitate was washed with anhydrous ethanol under stirring and then centrifuged again to remove the supernatant liquid (ethanol). After that, the precipitate was stored in a vacuum desiccator overnight. Finally, it was dried in an oven at 363 K for one hour.
2.2. Characterization Techniques of Bulk Catalyst Precursors
2.2.1. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy (EDS)
The determination of the morphology, textural properties and composition of the NiMoO4 bulk catalyst precursors was carried out on a JEOL JSM-5900LV (Jeol Ltd., Tokyo, Japan) instrument with an EDS Oxford model ISIS detector for elemental identification and quantification.
2.2.2. X-Ray Diffraction (XRD)
The diffraction patterns of the NiMoO4 samples were determined on an X-ray diffraction machine, the Siemens D-5000 (Siemens AG, Munich, Germany), using CuKα radiation (λ = 1.5406 Å). The data acquisition was performed in the 2θ angle range from 10° to 70°.
2.2.3. Textural Properties Using Liquid Nitrogen Physisorption at 77 K
Before carrying out the measurements, the samples were degassed at 543 K for 3 h under medium vacuum (200 mTorr). The specific area of the NiMo bulk catalyst precursors was determined on a Micromeritics Tristar SAPA (Micromeritics Instrument Corporation, Norcross, GA, USA) instrument using the BET method and the pore size distribution using the BJH method.
2.2.4. Raman Spectroscopy
The Raman spectra were obtained using a Nicolet Almega XR Dispersive Raman spectrometer (Thermo Fisher Scientific, Massachusetts, USA). A BX51 microscope (Olympus Corporation, Tokyo, Japan) with a 50X objective was used to focus the laser beam onto the sample and to collect the scattered light in a 180° (backscattering) configuration. The spectra were obtained with 100 s and with a resolution of ~4 cm−1. The wavelength of 532 nm was used as the excitation source (Nd: YVO4 laser).
2.2.5. High-Resolution Transmission Electron Microscopy (HR-TEM)
The identification of sulfides in the bulk catalysts was carried out on a JEM-2010 TEM/STEM (Jeol Ltd., Tokyo, Japan) with an acceleration voltage of 200 kV with a point-to-point resolution of 1.9 Å. Previously, the samples were dispersed in heptane for 20 min.
2.3. Catalyst Activation
Prior to the reaction, it was necessary to change the bulk NiMo precursors (NiMoO4) to the NiMoS sulfided phase. To this end, the samples were sulfided under the following conditions: 4 h at 673 K with a flow of 30 mL/min of an H2S/H2 mixture (15% H2S) with a heating rate of 3 K/min.
2.4. Catalytic Test
The reaction was carried out in a batch reactor using 50 mg of sulfided bulk catalyst, except for the reference catalyst (NiMo/γ-Al2O3, mass = 200 mg). The reaction mixture consisted of 40 mL of n-decane with 1000 ppm of sulfur (as 4,6-DMDBT). The operating temperature was 598 K, and there was an initial H2 pressure of 1100 psia. Reaction samples were taken every hour for six hours, and the products were analyzed using an HP 6890 gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a 105 m × 0.2 mm × 0.5 µm PONA capillary column equipped with a flame ionization detector (FID).
3. Results and Discussion
3.1. Physicochemical Characterization of Bulk Catalyst Precursors
Elemental Composition Determined by Energy Dispersive X-Ray Spectroscopy (EDS)
The determination of the elemental composition of the catalyst precursors in their oxide form was carried out using EDS. The results are summarized in Table 1, where the composition of the precursors synthesized through 2 h (PrecEtOH(2 h)) and 6 h (PrecEtOH(6 h)) of solvothermal synthesis is shown. They are also compared with the theoretical values. The technique determined the presence of only three kinds of atoms: nickel, molybdenum and oxygen. The measured Ni/Mo molar ratios for the PrecEtOH(2 h) and PrecEtOH(6 h) precursors were 1.1806 and 1.0102, respectively, as reported in Table 1.

Table 1.
Theoretical stoichiometric values for NiMoO4 and experimental values obtained by EDS for the synthesized material.
3.2. X-Ray Diffraction (XRD)
The X-ray results for the synthesized catalyst precursors are shown in Figure 1.
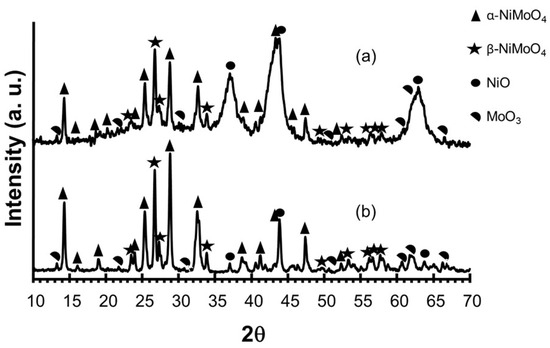
Figure 1.
X-ray diffraction patterns for PrecEtOH(2 h) (a); PrecEtOH(6 h) (b).
For the PrecEtOH(2 h) sample (Figure 1a), well-defined peaks are observed at 2θ = 14.14°, 25.08°, 26.48°, 28.75° and 32.5°, and broad peaks at 2θ = 37.26°, 43.21° and 62.8°. The well-defined peaks located at 2θ = 14.14°, 25.08°, 28.75° and 32.58° are characteristic reflections of the α-NiMoO4 compound (JCPDS 33-0948 card). A signal is observed at 2θ = 26.48°, which is characteristic of the crystallographic structure of β-NiMoO4 (JCPDS 45-0142), also reported by Liu et al. [13]. The broad signals at 2θ = 37.26°, 43.21° and 62.8° are assigned to characteristic reflections of NiO (JCPDS 71-1179). With less intensity, the characteristic reflections of MoO3 (JCPDS 5-0508) are observed at 2θ = 13.13, 20.70, 22.88, 25.24, 30.06, 51.23, 60.76, 62.33 and 66.47. In contrast, the diffraction pattern of PrecEtOH(6 h) (Figure 1b) shows well-defined reflections at 2θ = 14.11°, 25.19°, 26.38°, 28.70°, 32.54°, 43.72° and 47.34 which correspond to the reflections of the α-NiMoO4 (JCPDS 33-0948 card). Reflections at 2θ = 23.23°, 26.38°, 27.06° and 33.59° are associated with the β-NiMoO4 (JCPDS 45-0142 card) [13]. The main difference between the diffraction patterns in Figure 1a is that on the PrecEtOH(2 h) sample, NiO has not yet been integrated into the structure of NiMoO4; the behavior can be attributed to the shorter solvothermal synthesis time (crystallization time) of only 2 h. For the PrecEtOH(6 h) sample, the characteristic signals of NiO are observed with less intensity; it is assumed that it has already been incorporated into the crystalline structure of NiMoO4 (phases α and β), as observed in Figure 1b. The two samples present mixtures of both phases α and β of NiMoO4. Furthermore, with a greater synthesis time, an increase in intensities, a smaller peak width and a more defined baseline were observed, all signs that the material has a better crystalline arrangement; these results are similar to those reported in [13,14].
3.3. Scanning Electron Microscopy (SEM)
The results of scanning electron microscopy gave information on the morphology and size of the particles of the catalyst precursors PrecEtOH(2 h) and PrecEtOH(6 h) in their oxide forms, as shown in Figure 2a,b.
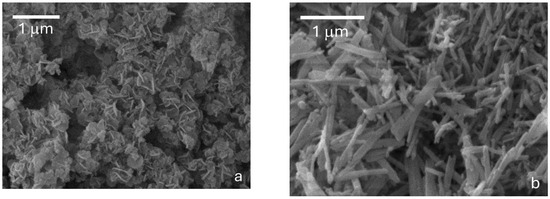
Figure 2.
Micrographs corresponding to the SEM analyses of the material synthesized with 2 h PrecEtOH(2 h) (a) and 6 h PrecEtOH(6 h) (b) using the solvothermal method EtOH/H2O.
The PrecEtOH(2 h) (Figure 2a) shows the presence of two types of grain in the form of irregular “flakes” that are integrated as nanoflowers and in the form of “rods”. The “flakes” are 310 nm long on average. After six hours of treatment (Figure 2b), it is observed that the “rods” measure, on average, 227 nm × 22 nm. Large bars, length approx. 1000 nm × 154 nm, but also thin and short “bars” with a length between 400 and 800 nm × 70 nm on average were observed. In general, the particles in Figure 2b are more geometric, homogeneous and larger than those in Figure 2a. This is a consequence of the synthesis time of each material, corroborating the increase in crystallinity reported by XRD. Similar results have been obtained by Théodet [15], using ethanol and calcining the samples at 673 K for 3 h.
3.4. Textural Properties by Nitrogen Physisorption at 77 K
The characteristic isotherms of the catalytic precursors synthesized in their oxide phase are shown in Figure 3a,b. The isotherms of the catalytic precursors PrecEtOH(2h) and PrecEtOH(6h) correspond to type III isotherms of the IUPAC classification and do not have hysteresis loops. This is characteristic of non-porous materials [16]; therefore, as expected, the synthesized crystals are not porous.
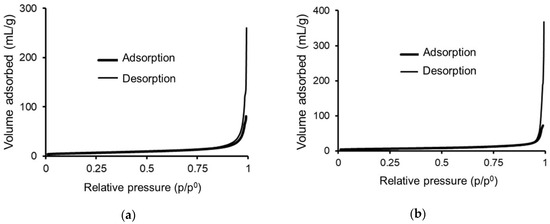
Figure 3.
Adsorption–desorption isotherm of PrecEtOH(2 h) (a) and adsorption–desorption isotherm of PrecEtOH(6 h) (b).
The isotherms of the PrecEtOH(2 h) catalytic precursors, Figure 3a, were 188 m2/g, and for PrecEtOH(6 h), they were 25 m2/g. According to Olivas [17], for bulk catalysts, low surface areas (approximately 30 m2/g) are generally reported; this area is a function of the grain size, which, in turn, depends on the synthesis method applied and the crystallization time. According to SEM determinations at low crystallization time (2 h), the grain has a heterogeneous distribution of sizes and shapes, flakes integrated into the “nanoflower” clusters, thus contributing to the high specific area. The increase in synthesis time to 6 h, PrecEtOH(6 h), leads to particles of a single shape, needles, with a larger size and homogeneous distribution, associated with a more crystalline arrangement [15] and, in turn, with a decrease in the specific area, due to sintering.
The pore size distribution of the catalyst precursors in the oxidic phase is shown in Figure 4. The catalyst precursor, PrecEtOH(2 h), Figure 4a, presents a multimodal pore size distribution with a local maximum at 60 Å in the mesopore region, reflecting the low crystallinity of the material synthesized. The rest of the distribution is in the macropore region (>500 Å), related to the macropore result of the agglomeration of the particles. Figure 4b, the PrecEtOH(6 h) catalyst precursor, does not show any porous distribution since this material is crystalline, as seen in XRD and SEM, and the surface of the particles lacks pores.
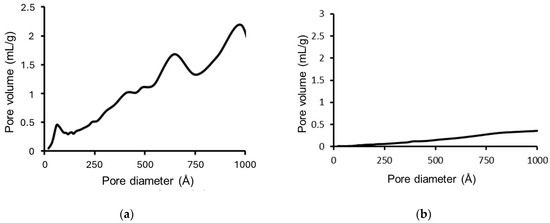
Figure 4.
Pore size distributions for PrecEtOH(2 h) (a) and PrecEtOH(6 h) (b).
3.5. Characterization of Sulfurized Bulk Catalysts
3.5.1. Raman Spectroscopy Analysis
As the sulfides of NiMo catalysts oxidize upon contact with oxygen in the air to MoO3 in a short time, the identification of the sulfide phases from being carried out by XRD was prevented; thus, it was necessary to perform Raman spectroscopy on the sulfided catalysts. The Raman spectra of the catalysts (CatEtOH(2 h) and CatEtOH(6 h)) are presented in Figure 5a,b.
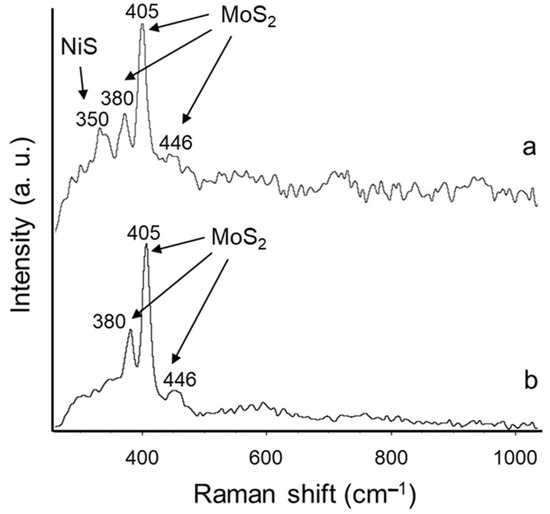
Figure 5.
Raman spectra of sulfurized CatEtOH(2 h) (a) and sulfurized CatEtOH(6 h) catalyst (b).
The Raman spectrum of the sulfurized bulk catalyst, CatEtOH(2 h), in Figure 5a, shows bands at 350, 380, 405 and 446 cm−1, the most intense being located at 380 and 405 cm−1. The band at 350 cm−1 is assigned to Ni-S bond vibrations [18]. The strong bands at 405 and 380 cm−1 and the weak one at 446 cm−1 correspond to MoS2 [19]. In Figure 5b, the Raman spectrum of CatEtOH(6 h) is observed, with bands at 380, 405 and 446 cm−1. This corresponds to MoS2; unlike the Raman spectrum of CatEtOH(2 h), it does not show the signal at 350 cm−1, which is assigned to the Ni-S bond.
3.5.2. High-Resolution Transmission Electron Microscopy (HR-TEM)
The sulfided bulk catalysts were characterized by HR-TEM. The high-resolution transmission micrographs of the sulfided bulk catalysts CatEtOH(2 h) and CatEtOH(6 h) are presented in Figure 6a and Figure 6b, respectively.
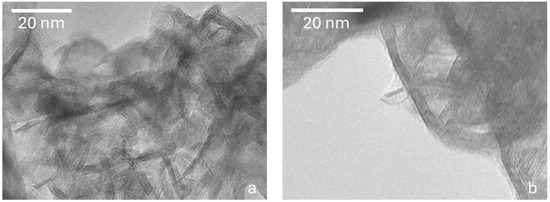
Figure 6.
High-resolution transmission electron microscopy micrographs of the sulfided bulk catalysts: (a) CatEtOH(2 h) and (b) CatEtOH(6 h).
Particles of the active phase (MoS2), their morphology, stacking and lengths were observed.
The micrograph in Figure 6a shows the geometric arrangement of alternating dark and light bands, typical of Ni-promoted MoS2 sulfides. Micrographs show many of these structures. Crystallites of 2–5 layers with lengths between 6 and 11 nm are observed. Some of these crystallites are apparently curved; along with the fact that the precursor had no pores, this suggests that only the outer part of the flakes was sulfided; i.e., most of the molybdenum sulfides are located around the catalyst particles.
In micrograph 6b, molybdenum sulfide stacks with five layers on average and lengths ranging from 15 to 30 nm are observed. Comparing these with Figure 2b, it leads us to think that, in this case also, most of the molybdenum sulfides are located around the catalyst particles. In this catalyst, particles containing only two or three layers of molybdenum sulfide are observed at the grain boundaries (see micrograph in Figure 6b). It was observed that the length of the crystals and the number of layers in the crystal of the MoS2 particles change depending on the solvothermal synthesis time for the different materials and are a direct function of the specific area of the oxidized precursor. The amount of sulfurized Mo species was improved by decreasing the solvothermal synthesis time.
3.6. Catalytic Activity in HDS of 4,6-Dimethyldibenzothiophene (4,6-DMDBT)
The conversion of hydrodesulfurization of 4,6-DMDBT versus the time of reaction of our samples is shown in Figure 7. The most active catalyst was CatEtOH(2 h), which reached a total conversion of 4,6-DMDBT at the reaction time of 5 h; the CatEtOH(6 h) catalyst only reached a conversion of 0.6 of 4,6-DMDBT after 6 h. When comparing these bulk catalysts against a reference catalyst, NiMo/γ-Al2O3, the CatEtOH(2 h) catalyst performed better than the reference.
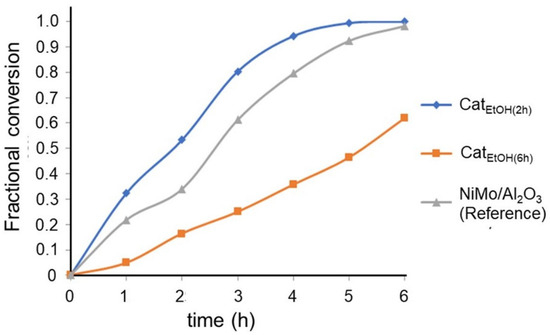
Figure 7.
Global conversion of 4,6 DMDBT as a function of time for the catalysts CatEtOH(2 h) and CatEtOH(6 h) (bulk), and NiMo/γ-Al2O3 (reference catalyst), batch reactor, P = 1100 psia T = 598 K.
The conversion curve of 4,6-DMDBT versus the time of the catalysts used was adjusted to a pseudo-first order kinetic model [20,21]. Table 2 shows the rate constants for the disappearance of 4,6-DMDBT obtained with the equation k’ = k/Mo atoms, where k (rate constant) was obtained from ln(CA/CA0) = −k(t), where CA is the concentration of 4,6-DMDBT at time t and CA0 is the initial concentration of 4,6-DMDBT at time zero (t0).

Table 2.
Rate constants of the catalysts evaluated in the HDS of 4,6-DMDBT.
Catalytic activity on the HDS reaction for 4,6-DMDBT of the bulk catalysts presents the following order: CatEtOH(2 h) > CatEtOH(6 h). As expected, the reaction rate constants per molybdenum atom indicated that the best catalyst for HDS of 4,6-DMDBT is CatEtOH(2 h).
Product Distribution
The products identified for the 4,6-DMDBT reaction are shown in Table 3.

Table 3.
Obtained products by HDS of 4,6-DMDBT.
The hydrodesulfurization (HDS) reaction of 4,6-dimethyldibenzothiophene (4,6-DMDBT) proceeds via two pathways [22]: (a) direct desulfurization (DSD), which yields 4,6-dimethylbiphenyl (4,6-DMDFL) as the primary product, and (b) hydrogenation (HYD), where desulfurization occurs following the hydrogenation of one of the aromatic rings in the 4,6-DMDBT molecule. This process produces 4,6-dimethylcyclohexyltoluene (4,6-DMCHT), which subsequently forms 4,6-dimethyldicyclohexyl (4,6-DMDCH). Therefore, the hydrogenation route includes both products. The HYD route leads to the formation of 4,6--DMDBT reaction intermediates: we grouped all the hydrogenated 4,6-DMDBT products (TH-4,6-DMDBT and HH-4,6-DMDBT) as hydrogenation compounds—Hyd-4,6-DMDBT; other detected products were 4,6-dimethylcyclohexyltoluene (4,6-DMCHT) and 4,6-dimethyldicyclohexyl (4,6-DMDCH). Figure 8a,b shows the product distribution for the 4,6-DMDBT reaction as a function of time for both catalysts, CatEtOH(2 h) and CatEtOH(6 h).
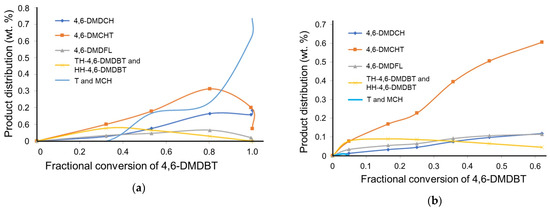
Figure 8.
Behavior of the product distribution in the HDS reaction of 4,6-DMDBT as a function of time for CatEtOH(2 h) (a) and CatEtOH(6 h) (b), batch reactor, P = 1100 psia, T = 598 K.
For the CatEtOH(2 h) sample, as the conversion of 4,6-DMDBT increases, the Hyd-4,6-DMDBT, 4,6-DMCHT and 4,6-DMDFL present a maximum as the conversion of 4,6-DMDBT progresses. As observed, hydrogenation occurs first to give TH-4,6-DMDBT and HH-4,6-DMDBT; the maximum proves that Hyd-4,6DMDBT is an intermediate in the reaction and produces 4,6-DMDCH. At a conversion of approximately XA = 0.8, it is observed that the concentration of 4,6-DMCHT and 4,6-DMDFL falls, and a rapid increase in other products such as light ones (toluene (T) and methylcyclohexane (MCH)) is observed; this indicates that toluene and MCH come from the breakdown of 4,6-DMCHT and 4,6-DMDFL. The concentration of 4,6-DMCHT is always higher than that of 4,6-DMDFL. This indicates that the main reaction route observed is hydrogenation, where the hydrogenation compounds of 4,6-DMDBT are converted into 4,6-DMCHT. On the other hand, direct desulfurization gives 4,6-DMDFL as a product. The concentration of 4,6-DMDFL reaches a maximum at 0.8 conversion of 4,6-DMDBT.
For CatEtOH(6 h), it can be observed from Figure 8b that as the conversion of 4,6-DMDBT increases, the concentration of 4,6-DMCHT also increases. The hyd-4,6-DMDBT compounds are observed at a maximum at low conversion of 4,6-DMDBT (0.2). As the conversion of 4,6-DMDBT increases, the concentration of hyd-4,6-DMDBT decreases. The concentration of 4,6-DMDFL and 4,6-DMDCH increases. Only traces of other products, such as light hydrocarbons (T and MCH), are observed.
With the above information, the pathway of reaction of 4,6-DMDBT is shown in Scheme 1.
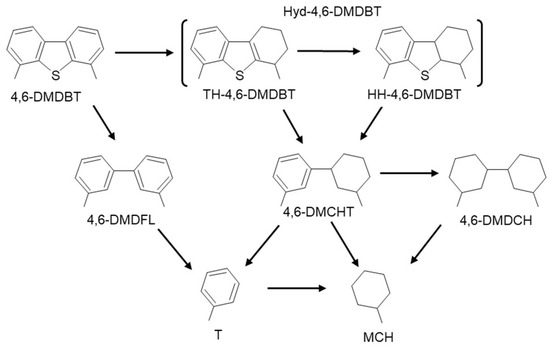
Scheme 1.
Pathway of 4,6-DMDBT reaction.
At the end of the catalytic evaluation, it was found that in both cases, the preferential reaction route of 4,6-DMDBT was as a first step for the hydrogenation to obtain partial hyd-4,6-DMDBT and that, later, being highly reactive, they were hydrodesulfurized to produce 4,6-MCHT. In general, it has been found that in the HDS of 4,6-DMDBT on NiMo sulfide catalysts, the methyl groups of 4,6-DMDBT prevent sulfur from adsorbing to the active NiMoS site [23]; however, in this experiment, a fraction of direct hydrodesulfurization is observed, producing 4,6-DMDFL. Bulk catalysts always contain more highly stacked NiMoS(II) phase sulfides, as observed in HR-TEM; these results coincide with those obtained by Lai et al. [7].
An unexpected result is that the reaction extended to the cracking of 4,6-DMCHT and 4,6-DMDCH to obtain light products (T and MCHT), probably caused by the acidity of the -SH groups generated from NiMoS, as described in [24,25].
The characterization results indicated that the 2 h solvothermal synthesis of the NiMoO4 catalyst precursor yields small particles with irregular “flake”-shaped structures. Meanwhile, the 6 h solvothermal synthesis resulted in the particles crystallizing with a long “rod” morphology with greater crystallinity and a consequent loss of specific surface area. This resulted in a decrease in catalytic activity for the HDS of 4,6-DMDBT. Regarding molybdenum sulfides, a greater quantity of MoS2 stacks with shorter lengths was obtained in CatEtOH(2 h) than in the case of CatEtOH(6 h), where only the edges of the “bars” of the catalyst were partially sulfided, which resulted in a smaller number of active sites for the HDS of 4,6-DMDBT. The comparison provided in Table 2 and Figure 7 demonstrates that it is possible to obtain a better bulk NiMo catalyst compared to the reference NiMo catalyst supported on alumina.
4. Conclusions
The solvothermal synthesis of bulk NiMo HDS precursors using EtOH/H2O mixtures and variable synthesis time resulted in materials with mixed-phase composition of α-NiMoO4 and β-NiMoO4 with attractive textural characteristics. Solvothermal methods offer an alternative for the preparation of bulk catalyst precursors through implementing green chemistry principles; in this way, mild reaction conditions and more environmentally friendly solvents can be used to obtain bulk catalyst precursors with high HDS activity for 4,6-DMDBT. It was shown that the bulk catalyst with a shorter solvothermal synthesis time, an ethanol/water ratio of 2, pH = 7, a larger surface area and a greater amount of β-NiMoO4 phase than α-NiMoO4 presented the best activity in the HDS reaction of 4,6-DMDBT. The described method enables the synthesis of materials with small grain sizes, which have crystallographic defects that are useful for the formation of a greater amount of the active phase on the amorphous surface of the grains. This results in high-performance HDS catalysts for refractory sulfur compounds such as 4,6-DMDBT. The CatEtOH(2 h) catalyst outperformed a commercial catalyst, NiMo/γ-Al2O3, in terms of HDS activity for 4,6-DMDBT via the hydrogenation route.
Author Contributions
Conceptualization, J.R.C.B., R.C.G., I.P.L., D.A.F.B. and M.H.C.; Methodology, J.R.C.B., R.C.G., I.P.L., D.A.F.B. and M.H.C.; Validation, J.R.C.B., R.C.G. and M.H.C.; Formal Analysis, J.R.C.B., R.C.G., D.A.F.B. and M.H.C.; Investigation, J.R.C.B., R.C.G., D.A.F.B. and M.H.C.; Resources, J.R.C.B., R.C.G., I.P.L., D.A.F.B. and M.H.C.; Data Curation, J.R.C.B., R.C.G., I.P.L., D.A.F.B. and M.H.C.; Writing—Original Draft Preparation, J.R.C.B. and M.H.C.; Writing—Review and Editing, J.R.C.B., R.C.G., I.P.L., D.A.F.B. and M.H.C.; Visualization, J.R.C.B., R.C.G. and M.H.C.; Supervision, J.R.C.B., R.C.G. and M.H.C.; Project Administration, J.R.C.B., R.C.G. and M.H.C.; Funding Acquisition, R.C.G. and M.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are thankful to the Secretaría de Investigación y Posgrado (SIP) of the Instituto Politécnico Nacional (IPN) for the financial support from the SIP-20241136 and SIP-20240987 projects to Macaria Hernández Chávez.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Unidad de Investigación en Catálisis (UNICAT) de la Facultad de Química de la UNAM, Cecilia Salcedo for the XRD work and Aida Gutiérrez Alejandre for her assistance in the Raman spectroscopy analysis. The comments and suggestions by the reviewers are deeply appreciated.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nadeina, K.A.; Budukva, S.V.; Vatutina, Y.V.; Mukhacheva, P.P.; Gerasimov, E.Y.; Pakharukova, V.P.; Klimov, O.V.; Noskov, A.S. Unsupported Ni—Mo—W hydrotreating catalyst: Influence of the atomic ratio of active metals on the HDS and HDN activity. Catalysts 2022, 12, 1671. [Google Scholar] [CrossRef]
- Singh, J.; Kaushik, R.; Chawla, M. Hazardous Gases: Risk Assessment on the Environment and Human Health, 1st ed.; Academic Press: London, UK, 2021; pp. 375–389. [Google Scholar]
- Wang, E.; Yang, F.; Song, M.; Chen, G.; Zhang, Q.; Wang, F.; Bing, L.; Wang, G.; Han, D. Recent advances in the unsupported catalysts for the hydrodesulfurization of fuel. Fuel Process. Technol. 2022, 235, 107386. [Google Scholar] [CrossRef]
- Üner, D. Advances in Refining Catalysis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 124. [Google Scholar]
- Weng, X.; Cao, L.; Zhang, G.; Chen, F.; Zhao, L.; Zhang, Y.; Gao, J.; Xu, C. Ultradeep Hydrodesulfurization of Diesel: Mechanisms, Catalyst Design Strategies, and Challenges. Ind. Eng. Chem. Res. 2020, 59, 21261–21274. [Google Scholar] [CrossRef]
- Chianelli, R.R.; Berhault, G.; Torres, B. Unsupported Transition Metal Sulfide Catalysts: 100 years of Science and Application. Catal. Today 2009, 147, 275–286. [Google Scholar] [CrossRef]
- Lai, W.; Chen, Z.; Zhu, J.; Yang, L.; Zheng, J.; Yi, X.; Fang, W. A NiMoS flower-like structure with self-assembled nanosheets as high-performance hydrodesulfurization catalysts. Nanoscale 2016, 8, 3823–3833. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hu, A.; Dai, Q.; Yang, Q.; Hou, R.; Liu, Z. Study on the Performance of Ni−MoS2 Catalysts with Different MoS2 Structures for Dibenzothiophene Hydrodesulfurization. ACS Omega 2023, 8, 41182–41193. [Google Scholar] [CrossRef]
- Chowdari, R.K.; De León, J.N.; Fuentes-Moyado, S. Effect of sulfidation conditions on the unsupported flower-like bimetallic oxide microspheres for the hydrodesulfurization of dibenzothiophene. Catal. Today 2022, 394–396, 13–34. [Google Scholar] [CrossRef]
- Yang, C.; Dai, Q.; Hu, A.; Yuan, H.; Yang, Q. The Influence of Metal–Support Interactions on the Performance of Ni-MoS2/Al2O3 Catalysts for Dibenzothiophene Hydrodesulfurization. Processes 2023, 11, 3181. [Google Scholar] [CrossRef]
- De León, J.D.; Chowdari, R.K.; Antúnez-García, J.; Fuentes-Moyado, S. Recent Insights in Transition Metal Sulfide Hydrodesulfurization Catalysts for the production of ultra low sulfur diesel: A short review. Catalysts 2019, 9, 87. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Liu, H.; Yin, C.; Li, X.; Chai, Y.; Li, Y.; Liu, C. Effect of NiMo phases on the hydrodesulfurization activities of dibenzothiophene. Catal. Today 2017, 282, 222–229. [Google Scholar] [CrossRef]
- Rammal, M.B.; Omanovic, S. Synthesis and characterization of NiO, MoO3, and NiMoO4 nanostructures through a green, facile method and their potential use as electrocatalysts for water splitting. Mater. Chem. Phys. 2020, 255, 123570. [Google Scholar] [CrossRef]
- Théodet, M.; Quilfen, C.; Martínez, C.; Aymonier, C. Continuous supercritical synthesis of unsupported and high specific surface area catalyst precursors for deep-hydrodesulfurization. J. Supercrit. Fluids 2016, 117, 252–259. [Google Scholar] [CrossRef]
- Condon, J.B. Surface Area and Porosity Determinations by Physisorption, 1st ed.; Elsevier: Amsterdam, The Netherland, 2006; pp. 6–14. [Google Scholar]
- Olivas, A.; Galván, D.H.; Alonso, G.; Fuentes, S. Trimetallic NiMoW unsupported catalysts for HDS. Appl. Catal. A 2009, 352, 10–16. [Google Scholar] [CrossRef]
- Bishop, D.; Thomas, P.; Ray, A. Raman spectra of nickel (II) sulfide. Mater. Res. Bull. 1998, 33, 1303–1306. [Google Scholar] [CrossRef]
- Jiménez Sandoval, S.; Yang, D.Y.; Frindt, R.F.; Irwin, J. Raman study and lattice dynamics of single molecular layers of MoS2. Phys. Rev. B Condens. Matter 1991, 44, 3955–3962. [Google Scholar] [CrossRef]
- Kabe, T.; Akamatsu, K.; Ishihara, A.; Otsuki, S.; Godo, M.; Zhang, Q.; Qian, W. Deep hydrodesulfurization of light gas oil. 1. Kinetics and mechanisms of dibenzothiophene hydrodesulfurization. Ind. Eng. Chem. Res. 1997, 36, 5146–5152. [Google Scholar] [CrossRef]
- Ma, X.; Sakanishi, K.; Mochida, I. Hydrodesulfurization reactivities of various sulfur compounds in diesel fuel. Ind. Eng. Chem. Res. 1994, 33, 218–222. [Google Scholar] [CrossRef]
- Whitehurst, D.; Isoda, T.; Mochida, I. Present State of the Art and Future Challenges in the Hydrodesulfurization of Polyaromatic Sulfur Compounds. Advan. Catal. 1998, 42, 345–471. [Google Scholar]
- Yoosuk, B.; Kim, J.H.; Song, C.; Ngamcharussrivichai, C.; Prasassarakich, P. Highly active MoS2, CoMoS2 and NiMoS2 unsupported catalysts prepared by hydrothermal synthesis for hydrodesulfurization of 4,6-dimethyldibenzothiophene. Catal. Today 2008, 130, 14–23. [Google Scholar] [CrossRef]
- Zhao, Y.; Prins, R. Mechanisms of hydrodenitrogenation of alkylamines and hydrodesulfurization of alkanethiols on NiMo/Al2O3, CoMo/Al2O3, and Mo/Al2O3. J. Catal. 2005, 229, 213–226. [Google Scholar] [CrossRef]
- Topsoe, N.Y.; Topsoe, H. FTIR studies of Mo/Al2O3-based catalysts: II. Evidence for the presence of SH groups and their role in acidity and activity. J. Catal. 1993, 139, 641–651. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).