Abstract
This study is focused on the sorption properties and the changes in the structure and state of Ti-25Al-25Nb (at.%) system alloys under thermal cyclic loading. These samples were produced by combining high-energy processing methods through mechanization and spark plasma sintering in the temperature range of 1100–1300 °C, followed by two-stage heat treatment at temperatures of 800 °C and 1250 °C. Thermal cyclic experiments on hydrogen sorption/desorption with samples of the Ti-25Al-25Nb (at.%) system were conducted at the VIKA experimental installation at a saturation temperature of about 500 °C and a degassing temperature of 610 °C. It took about 41 min to reach pressure equilibrium at 500 °C. The hydrogen diffusion coefficient was calculated based on the Barrer formula and was 9.1 × 10−5 cm2/s at 500 °C. The maximum hydrogen content was recorded after the first sorption/desorption cycle and was 1.91 wt%. Due to the multiple thermal cyclic effects in the hydrogen medium, the predominantly two-phase (O + B2) alloy structure underwent transformation to form a new structure (O-AlNbTi2). In the phase composition of the Ti-25Al-25Nb (at.%) alloy, the formation of hydrides in the form of independent phases as a result of thermal cycling was not detected. Hydrogen absorption is most likely to be associated with the formation of an interstitial solution based on existing crystalline phases.
1. Introduction
Hydrogen energy is a promising alternative to the use of non-renewable energy sources. One of the main obstacles to the widespread use of hydrogen in energy is the problem of its mobile storage and transportation [1,2]. As a result, there is increasing interest in multi-component alloys, as the way hydrogen is stored and transported in a chemically bonded state is safer than the way it is transported in liquid or gaseous states.
To date, solid-state metal hydride storage systems have demonstrated great potential for hydrogen storage in required quantities. Metal hydride [1,2,3] systems are the most attractive option for solid-state hydrogen storage and are distinguished by reliable, compact and repeatedly reversible sorbing properties. The current metal hydrides do not satisfy the most fundamental requirements for practical usage, so the hydrogen storage systems that were previously considered have not been put into action. The reasons for this include poor capacity, a slow chemical reaction rate, and intolerable temperatures for hydrogen sorption and desorption [3,4].
Nevertheless, powder metallurgy shows promise in the production of materials for safe hydrogen storage through the synthesis of intermetallic alloys (IMAs) based on titanium [3,4,5,6]. Due to the growing importance of the IMA of the Ti-25Al-25Nb (at.%) system as a building material, there have been substantial attempts to explore intermetallides based on titanium aluminum with a high niobium concentration [7,8]. Also, the Ti-Al-Nb system’s IMA data show that the material is highly hydrogen-absorption-friendly, making it a promising contender for use in resolving the issues surrounding solid-state hydrogen storage. The low density of the alloys used in this system gives them a significant edge when it comes to the possibility of generating a big hydrogen capacity.
Of all titanium aluminum, alloys based on B2, Ti2AlNb, and Ti3Al phases can contain and sorb a sufficient amount of hydrogen up to 5 wt%. In particular, Ti3Al phase-based intermetallids can extract a large amount of hydrogen (4 wt%) [8]. However, the practical use of such materials in hydrogen storage is hampered by the problem of their high temperature stability at desorption [9,10]. Bcc-based phases (B2, Ti2AlNb), which occur when adding Nb to Ti3Al, may have an even greater ability to absorb hydrogen, as poorly packaged bcc-based structures exceed closely packed fcc- and hcp-based structures in hydrogen absorption [11,12,13,14,15]. This is facilitated by the formed nanoscale phases, which have a large number of voids filled with hydrogen atoms.
The practical application of Ti-25Al-25Nb (at.%) alloys in hydrogen storage seems to necessitate extensive research into alloys with various controlled characteristics. The properties of metal hydrides must be stable under long-term cycling and thermal stability under the impact of elevated temperatures for their technical application. Hybrid systems with the desired phase state can be synthesized using a variety of production techniques, temperature regimes and alloyed element contents. Laboratory testing is the only way to find out how long they last under conditions close to those of an operational setting.
In connection with the above, the aim of this research is to determine the effect of the thermal cyclic processes of the sorption/desorption of hydrogen on the phase state of IMAs of the Ti-25Al-25Nb (at.%) system.
2. Materials and Methods
The research objects were samples of the Ti-25Al-25Nb alloy system (at.%) [16]. The alloys were formed by combining mechanical activation and spark plasma sintering from three-component powder composition of the Ti-Al-Nb system (the parameters are shown in Table 1). The following powders were used as the starting materials for producing hydrogen-intensive materials: titanium powder (main impurities: N < 0.1; O < 0.01; H < 0.001; C < 0.01; Si < 0.005; Ta < 0.1; Ti < 0.005; Fe < 0.005) with a particle size of 20–30 microns; niobium powder (main impurities: Ni < 0.001; Al < 0.002; Mg < 0.001; Mn < 0.001; Co < 0.001; Cu < 0.003; Zr < 0.001) with a particle size of 10–63 microns; aluminum powder with a particle size of 5 microns with an impurity content of no more than 0.001 (Hongwu International Group Ltd., Guangzhou, China).

Table 1.
Detailed parameters for obtaining a three-component alloy.
The analysis of the crystalline characteristics and phases of the alloy samples was performed using an Empyrean X-ray diffractometer manufactured by PanAlytical (Almelo, The Netherlands). A distinctive feature of this device is the detector manufactured using Pixcell technology, which has a high count rate, a wide dynamic range, and linearity. The PIXcel1D detector uses a scanning line operating mode to record diffraction patterns. Radiation: Cu Kα; voltage and current: 45 kV, 40 mA. A fixed divergence slit of 1° (the distance from the divergence slit to the focus of the tube is 87 mm), an antiscattering slit of 2°, and an incident beam mask 44 marked 10, providing an incident beam width of 9.9 mm, were used. The air temperature during the recording was 23 °C. Shooting mode: the scanning step size was 0.026° 2θ, the exposure time was 240.8 s, and the studied area of 2θ angles was from 10° to 135°. The method of processing and analyzing of diffraction patterns: The diffraction patterns were processed using the HighScore processing and search program. The profile of diffraction pattern peaks was described using the symmetric pseudo-Voigt function. The software achieves fitting of the calculated profile to the experimental one with the maximum match. Fitting of the calculated profile leads to the formation of numerical values of the peak parameters required to determine the phase composition. The main parameters for the qualitative phase analysis are the angular positions and relative intensities of the peaks. The COD database and the PDF-2 ICDD Release 2004 database were used to identify the phase composition. A special feature of the COD database is that the angular range of data in the standard cards is limited to 90° 2θ. The quantitative content of phases was assessed based on a visual assessment of the intensity of the peaks of the corresponding phases’ diffraction patterns.
A thermogravimetric analysis and a differential scanning calorimetry unit (TGA/DSC 2, Mettler-Toledo International Inc., Greifensee, Switzerland) were used to find the main temperatures and pressures of hydrogen sorption and desorption. The experiment used samples weighing 10–50 mg. Before conducting the study, the installation was calibrated for weight and temperature using standard samples. After that, the samples were placed in a reaction chamber with a controlled atmosphere containing high-purity hydrogen (99.999%). Pre-annealed quartz crucibles were used to prevent contamination of the samples. A precision pressure gauge monitored the hydrogen pressure inside the chamber and maintained it at 15 bar throughout the experiment.
Hydrogen sorption: The experiment was carried out with a stepwise increase in temperature from an initial temperature of 25 °C to 500 °C with a heating rate of 10 °C/min. After reaching the target temperature, the samples were kept in a hydrogen atmosphere at constant pressure until equilibrium was reached (about 30–60 min). The mass of the samples was recorded in real time with a step of 1 s, which made it possible to estimate the amount of absorbed hydrogen.
Hydrogen desorption: To remove hydrogen, the temperature was raised to 600 °C at a heating rate of 10 °C/min in an inert gas atmosphere (argon) or under vacuum (0.01 mbar). The thermal effects (endothermic peaks) were recorded using DSC, which made it possible to determine the desorption onset temperature and the maximum process rate.
Thermal cyclic experiments on the sorption/desorption of hydrogen with samples of the Ti-25Al-25Nb (at.%) system were conducted in the VIKA experimental installation [17,18]. This installation is designed for sorption/desorption processes of various materials in a temperature range from 20 °C to 1500 °C.
A previously manufactured and experimentally tested device was used in the experiments, which made it possible to place several test samples [17,18] inside its volume at once. Figure 1 presents the physical configuration of the VIKA installation and the experimental device (ED).
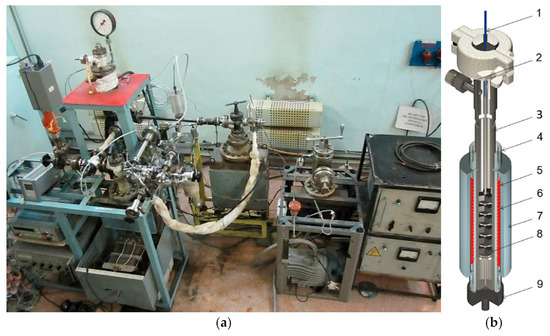
Figure 1.
VIKA experimental installation: (a) photos of VIKA installation; (b) experimental device (ED) (1—thermocouple; 2—pump and feed fittings; 3—ampoule vessel; 4—quartz pipe; 5—heater; 6—heater casing; 7—heat insulation; 8—test samples; 9—nitrogen feed for cooling).
Table 2 provides the original dimensions and weights of the samples.

Table 2.
Initial parameters of test samples.
Test conditions:
- − Sorption/desorption experiments—cyclic;
- − Saturation medium of samples—hydrogen;
- − Hydrogen saturation pressure difference—from 760 Torr to 300 Torr;
- − Saturation temperature—500–510 °C;
- − Degassing temperature of samples—600–610 °C;
- − Number of cycles—10;
- − Number of samples with reference values—1.
3. Results and Discussion
It is known that one method to determine the hydrogen content absorbed during sorption/desorption is the gravimetric method. This method is based on determining the mass change in the IMA of Ti-25Al-25Nb (at.%) system, which is caused by hydrogen absorption. The calculations and results of determination of hydrogen content of IMSA of the Ti-25Al-25Nb (at.%) system after the thermocyclic processes of sorption/desorption by hydrogen at a temperature of 500 °C are presented in Table 3 and Table 4.

Table 3.
Amount of hydrogen absorbed by Ti-Al-Nb system after 1st cycle.

Table 4.
Hydrogen weight content in IMA of Ti-25Al-25Nb (at.%) system after thermocyclic sorption/desorption.
As the calculations evidence, in the first cycle, the IMA of the Ti-25Al-25Nb (at.%) system shows a rather significant hydrogen absorption level, 3 wt%. This is primarily due to the saturation temperature of 500 °C. Increasing the number of sorption/desorption cycles results in an increase in the hydrogen absorbed to 3.2 wt%. However, at the 10th sorption/desorption cycle, there is a decrease in the hydrogen content to 2.98 wt%.
The results of the gravimetry show that increasing sorption/desorption cycles affect hydrogen uptake as follows: at the initial stage, with an increasing number of sorption/desorption cycles, the hydrogen absorbed is increased; however, the hydrogen concentration decreases at 10 cycles. The decrease in hydrogen concentration with increasing sorption/desorption cycles can be attributed to the following reasons: (1) a decrease in hydrogen solubility with increased sorption/desorption cycles; (2) the formation of an oxide layer that can act as a protective barrier against absorption; (3) the extraction of hydrogen from the mass caused by the high temperature of the process; (4) the evolution of the structural phase state of alloys of the Ti-25Al-25Nb (at.%) system during the action of multiple thermohydrogen exposure.However, no mechanical surface damage, such as cracking, splitting or chipping, was found in any of the test samples.
Studies of sorption properties and thermal cyclic stability of titanium alumina with an orthorhombic structure were conducted at a hydrogen pressure of 750 Torr and a temperature of 500 °C. During the entire test period, constant recording of pressure changes in the ampule device was conducted with test samples at the set saturation temperature. Figure 2 presents the kinetic curves of hydrogen uptake and pressure changes during desorption at thermal cyclic loads in the hydrogen medium.
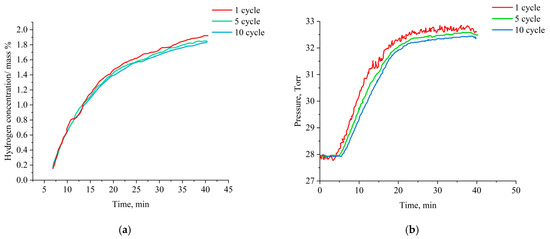
Figure 2.
Hydrogen absorption curves and pressure changes in ED during desorption by samples at thermal cyclic sorption/desorption: (a) hydrogen absorption curve; (b) change in pressure during desorption.
The results of this study show that hydrogen sorption in Ti-25Al-25Nb (at.%) alloys is characterized by logarithmic dependence, where after a short incubation period, the sorption rate increases, reaching equilibrium in 41 min at a temperature of 500 °C and a pressure of 750 Torr.
An increase in the number of cyclic loads on samples of titanium aluminides of the Ti-25Al-25Nb (at.%) system does not lead to significant changes in the kinetic curves of hydrogen absorption, which indicates a high degree of stability of the sorption properties of the obtained materials. The fluctuation after the first desorption experiment (Figure 2b) is associated with the purity of the data recording; subsequently, due to too many data, the recording time during the test was increased.
According to the authors of [19] (see Figure 3), when individual hydrides are formed, an equilibrium pressure shelf is formed on the curves (P-C-T) of hydrogen storage alloys, which indicates the formation of a hydride. However, in our experiments, up to the 10th cycle, no shelf formation was detected. That is, it can be argued that in our case, the absorbed hydrogen dissolves in the lattices of already existing phases, without the formation of individual hydride phases.
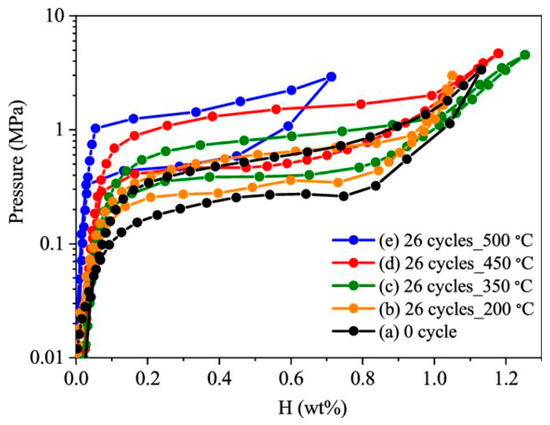
Figure 3.
Room Temperature Pressure composition isotherm curves of cycled TiFe alloy after hydrogen compression up to different temperatures. Reprinted with permission from Ref. [19]. Copyright 2023, Elsevier.
The time dependence of the rate of hydrogen absorption by the test samples at a temperature of 500 °C and a pressure of 750 Torr is shown in Figure 4. As the Figure shows, the rate of hydrogen absorption increases with increasing saturation time, since hydrogen diffusion is a thermal activation process. It can be seen that from 3 min into the tests, the hydrogen absorption rate already begins to increase, and in 5 min, it reaches its maximum. It should be borne in mind that this dependence is typical for two-phase (B2 + O) alloys of the Ti-25Al-25Nb (at.%) system, which were saturated with hydrogen at a temperature of 500 °C and a pressure of 750 Torr.

Figure 4.
The dependence of the hydrogen content in the ED on the saturation time at a temperature of 500 °C and a pressure of 750 Torr.
Previous studies [20,21] observed similar behavior. Dai et al. [20] demonstrated that the addition of niobium to Ti-Al-based alloys significantly enhanced their hydrogen storage capacity by increasing the number of defects such as interfaces and pores, as well as improving their thermal stability. This is because niobium atoms in the alloys promote the formation of body-centered cubic (bcc) phases, which are known to have higher hydrogen storage capacity compared to hexagonal closely packed (hcp) and face-centered cubic (fcc) phases.
Rui et al. [21] confirmed these findings by demonstrating that Ti-Al-Nb-based alloys exhibited accelerated hydrogen diffusion kinetics due to the high defect density generated by the addition of niobium. A special role in this process is played by grain boundaries and interphase regions between the bcc and orthorhombic (O) phases, which function as effective hydrogen diffusion pathways. Comparing the data from these works with the results of the present study, it can be argued that the addition of niobium not only improves the hydrogen absorption properties of Ti-25Al-25Nb alloys, but also ensures their thermal stability during multiple sorption/desorption cycles. In addition, the time to achieve equilibrium (~41 min) confirms the stability of the kinetic characteristics of this alloy, which makes it promising for use in hydrogen storage systems.
The effective diffusion coefficient of hydrogen DH in titanium aluminides of the Ti-25Al-25Nb (at.%) system was calculated using the Barrer formula [22]. Table 5 shows the sorption parameters determined by the calculation method for samples of the Ti-25Al-25Nb (at.%) system after one cycle.

Table 5.
The sorption parameters of the sample of the Ti-25Al-25Nb system (at.%).
The value of the effective diffusion coefficient for a two-phase alloy of the Ti-Al-Nb system is higher compared to other titanium-based alloys, which are given in the reference materials. However, the calculation of the diffusion coefficient for these alloys was conducted under atmospheric pressure and at room temperature. Thermodynamic theory states that when the temperature rises, so does the activation energy of atomic diffusion, which, in turn, causes the diffusion coefficient to rise. The high temperature promotes the absorption of hydrogen by the titanium aluminides. The higher diffusion coefficient is justified by the higher temperature.
In their efforts, the authors of [23,24] present data on the calculation of the diffusion coefficient of hydrogen in a polycrystalline titanium alloy. The results show that D is 2.6 × 10−10 cm2/s for lattice diffusion and 9.1 × 10−5 cm2/s for grain boundary diffusion. D_H, calculated as part of this work, is similar to the diffusion coefficient along the grain boundaries, which are given by the above authors. This suggests [24,25] that hydrogen diffusion is first concentrated along the grain boundaries of the B2 phase and between the sections of the O phase lamellae.
This value significantly exceeds the diffusion coefficient for γ-TiAl alloys obtained by Takasaki et al. [26], where DH was 1.0 × 10−5 cm2/s under similar conditions. This difference can be explained by the features of the crystal structure and composition of the alloys. In γ-TiAl alloys, hexagonal closely packed (hcp) and face-centered cubic (fcc) structures dominate, which are characterized by fewer defects and closer packing of atoms, which limits the diffusion of hydrogen.
At the same time, for the Ti-25Al-25Nb (at.%) system, the presence of the body-centered cubic (bcc) B2 phase contributes to the increase in the hydrogen diffusion coefficient. This is due to the fact that the structure of B2 phases has a lower packing density and a high density of defects, such as grain boundaries and interphase regions, which facilitates the movement of hydrogen atoms. The studies of Goyal et al. [27] emphasize that it is the grain boundaries of the B2 phase that are the main pathways for hydrogen diffusion in Ti-Al-Nb alloys, which is confirmed by the results of the present study.
Furthermore, Sundaram et al. [28] show that hydrogen diffusion in two-phase Ti-Al-Nb alloys occurs predominantly along the interphase boundaries between the bcc (B2) and orthorhombic (O) phases. In the present study, a similar mechanism is observed, confirming that diffusion along the grain and interphase boundaries dominates in Ti-25Al-25Nb alloys, which explains the high hydrogen diffusion rate.
The hydrogen absorption by titanium aluminides of the Ti-25Al-25Nb (at.%) system, according to the authors of [29], can be divided into several stages: the hydrogen molecule in the EI first concentrates around cracks, pores, and grain boundaries on the sample surface. The subsequent absorption of hydrogen molecules by the solid surface of the sample leads to its dissociation into hydrogen atoms (physical adsorption); by overcoming the solid surface, hydrogen atoms begin to penetrate into the volume of the test material (chemical adsorption) and diffuse through the crystal lattice of the existing phases, dissolving in it. After some time, the distribution of hydrogen atoms in the sample is uniform. Figure 5 shows the scheme of hydrogen absorption by samples of the Ti-25Al-25Nb (at.%) system.
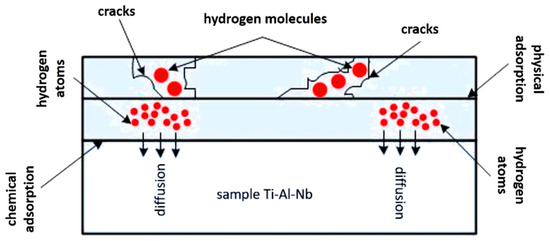
Figure 5.
A scheme of hydrogen absorption by a two-phase alloy of the Ti-25Al-25Nb system (at.%).
According to the obtained results, the hydrogen content increases with increasing temperature and/or pressure of hydrogen. The highest absorption intensity is observed at a hydrogen pressure of 750 Torr. However, it must be kept in mind that the hydrogen pressure of 750 Torr is limited by the specifications of the VIKA installation; accordingly, a subsequent increase in hydrogen pressure can lead to an improvement in the sorption properties of the obtained materials. The maximum hydrogen content in the Ti-25A-25Nb alloy (at.%) of about 1.91 (wt%) was recorded after the first cycle of sorption/desorption by hydrogen. The subsequent increase in high-temperature loads leads to a gradual decrease in sorbed hydrogen—1.85 (wt%) after 5 cycles and 1.83 (wt%) after 10 cycles.
The similar nature of the hydrogen kinetics upon saturation can be associated with the oxidation of the surface of the test materials. This is indicated by the difference in the values of the hydrogen content measured using two different methods, so when measured by the gravimetric method, the maximum hydrogen content reached 3.05 (wt%). The oxidation of the surface of titanium aluminides with an orthorhombic structure is facilitated by high temperatures (500–600 °C) and an increased duration (50–60 min) of sorption/desorption processes. With prolonged high-temperature exposure, the probability of oxidation of the test samples increases.
Details of the results of the microstructural and phase changes in samples after thermal cycling are given in [29,30].
Figure 6 illustrates segments of the diffraction patterns of the samples magnified in intensity following thermal cyclic loading in a hydrogen environment. Given that the conditions for recording the diffraction patterns of the samples before and after hydrogen saturation were identical, the alterations in the details of the peaks identified by the lines of the O-phase most likely accurately reflect the actual changes in the structural state of this phase.
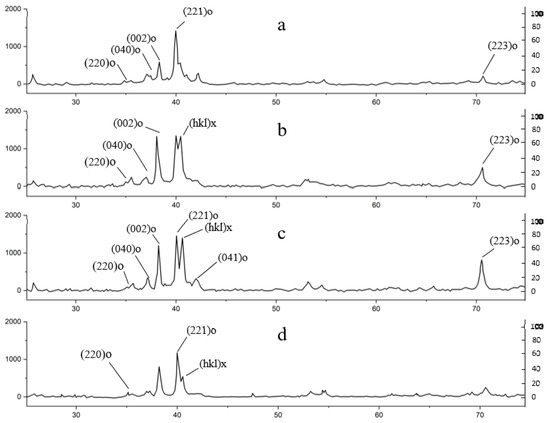
Figure 6.
The XRD results of the composition material Ti-25Al-25Nb (at.%): (a) reference sample, (b) after 1st cycle, (c) after 5 cycles, (d) after 10 cycles.
The angular positions of the lines in the bar diagrams were computed for potential phase composition options, selecting the appropriate values of the lattice parameters. The line intensities in the bar graphs correspond to the line intensities of the tabulated phases. The alterations in phase composition are shown by the emergence of a diffraction peak at an angle of approximately 40.6° 2θ. This peak is situated adjacent to the peak associated with the (221) line of the O-phase, characterized by an orthorhombic lattice, whose lines are reliably correlated with the peaks in the diffraction pattern of the original sample. The emergence and amplification of the intensity of this peak represent the most evident and distinctive alteration in the diffraction pattern from the surfaces of the samples in the series: ref.—1 cycle—5 cycles—10 cycles.
The appearance of peaks at 40.6° 2θ in the X-ray diffraction patterns indicates significant structural changes caused by repeated hydrogen saturation and desorption. These changes are consistent with the results of Chen et al. [24], where it was shown that hydrogen saturation leads to expansion of the O-phase crystal lattice in Ti-Al-Nb alloys due to the inclusion of hydrogen atoms in interatomic spaces (interstitial positions). Such behavior is typical of phases with an orthorhombic structure, which have a high capacity for reversible hydrogen absorption.
The selection of a candidate phase, characterized by a reasonable correlation with the additional peak, results in a favorable alignment of the diffraction peaks in the diffraction patterns of the B2 phase, which was previously detected in the phase composition of the initial sample. Many of the prominent lines in this phase exhibit near-angular-position coincidences with the lines of O*-AlNbTi2 (CmCm; a = 0.59 nm; b = 0.960 nm; c = 0.467 nm) of the orthorhombic phase, despite their lattice parameters differing from the original ones. The emergence of an orthorhombic phase with altered lattice properties is likely more advantageous for elucidating the structural alterations observed following hydrogen saturation tests. The alloy had the most significant structural alteration following the 10th cycle of thermal cycling.
The diffraction patterns of the sample after 10 cycles exhibited several peaks that align well with the positions of the Nb2Al-type phase (P42/mnm; a = 0.994 nm, c = 0.518 nm), indirectly corroborating the identification of previously observed precipitates. Illustrations of changes in the diffractograms of alloys as a result of thermal exposure in a hydrogen medium are shown in Figure 7.
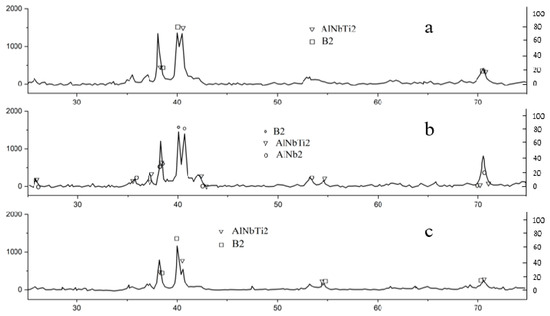
Figure 7.
The XRD results of the composition material Ti-25Al-25Nb (at.%): (a) after 1 cycle, (b) after 10 cycles, (c) after 5 cycles.
The alterations in the diffraction pattern of the samples due to consecutive saturation and desorption processes are most prominently reflected in the variations in the relative and absolute intensities of the peaks within the angular range of 39–41° 2θ. The intensity of the peak at an angular point of 40.6° 2θ increases to match the intensity of the peak at 40.0° after saturation and subsequently surpasses it following desorption.
It is interesting that this study did not show the formation of hydrides as separate phases. This is in line with what Liang et al. [31] found, which is that hydrogen in Ti-Al-Nb alloys prefers to form an interstitial solution that mixes with existing crystalline phases. This is a common behavior for bcc (B2) and orthorhombic (O) structures, which have a lot of free interatomic spaces that can hold hydrogen atoms. However, Zhang et al. [32] and Goyal et al. [27] show that the changes in phase in Ti-Al-Nb systems when they interact with hydrogen depend on the temperature and the number of thermal cycles. These studies back up the idea that an interstitial solution is a key way for Ti-Al-Nb alloys to store hydrogen, which is in line with what we found in this work.
The diffraction patterns of the samples after desorption showed changes similar to those after saturation. The peaks of the diffraction patterns after desorption that do not belong to the O-AlNbTi2 and B2 phases can be compared with the lines of such phases as, for example, B2 (0.331 nm) and O-AlNbTi2 (CmCm; a = 0.609 nm, b = 0.985 nm, c = 0.468 nm), whose lattice parameters differ from the tabular ones. Moreover, after 10 cycles, a number of peaks are in good agreement with the positions of the Nb2Al-type phase lines (P42/mnm; a = 0.994 nm, c = 0.5186 nm). Illustrations of changes in the diffractograms of alloys as a result of hydrogen desorption are shown in Figure 7. For each feasible phase composition, the angular positions of the lines in the bar diagrams are determined by selecting the relevant values of the lattice parameters.
The changes that are seen (see Figure 7, Table 6) can be thought of as structural transformations. The alloy’s mostly two-phase structure changes into a new structure, which stays the same in its initial state.

Table 6.
Results of X-ray diffraction of samples after 10 sorption/desorption cycles.
According to the results of the analysis of the alloy samples, the mechanism of transformation of the phase composition upon saturation, apparently, should be considered a shear one due to the simultaneous presence of phases of the initial and changed compositions in the diffraction patterns. In the case of defining the transformed phase as an interstitial solution in the O-AlNbTi2 phase, the presence of separating peaks corresponding to two compositions of solutions without a smooth transition speaks in favor of such an assumption.
At the same time, the alloy samples were also analyzed, and the results showed that the change in phase composition during saturation happened in a diffuse way, with the lattice parameters changing slowly as hydrogen was absorbed. This is because the peaks of the initial and transformed phases were clearly separated.
Thus, the structure of the Ti-25Al-25Nb (at.%) alloy system demonstrates high stability under thermal cycling effects, despite the decrease in the hydrogen content from 3.05 to 2.98 wt.% after 10 sorption/desorption cycles. This is due to the presence of a two-phase structure (O + B2), which retains its mechanical and crystalline properties even after multiple cycles of thermal hydride action. In addition, the addition of niobium helps to stabilize the phase composition and prevent the formation of brittle hydride phases, which is confirmed by the absence of changes in the X-ray diffraction patterns characteristic of hydrides.
It is also worth noting that the diffusion paths in Ti-25Al-25Nb alloys (grain boundaries and interphase regions) remain active throughout the testing, which ensures the reversibility of the process of hydrogen absorption and release.
4. Conclusions
The studies conducted demonstrated the high stability of the sorption/desorption characteristics of Ti-25Al-25Nb alloys under repeated thermal cycling loads in a hydrogen atmosphere. It was discovered that the main way hydrogen is absorbed is by creating a solid solution between the crystallized phases (O and B2). The observed phase changes show that the structure of the material is stable, that hydrides do not form, and that the alloy’s mechanical properties stay the same even after many cycles.
The kinetic curves of hydrogen absorption show logarithmic dependence, which means that there was a high rate of sorption at the beginning and that equilibrium was reached in the experimental conditions. The ability of the material to adapt to thermal hydride effects is shown by phase changes and the rearrangement of the O-phase structure. This alloy shows promise for use in hydrogen storage systems.
The results of this work confirm the importance of adding niobium, which helps stabilize the phase composition, increase the density of defects, and improve the sorption characteristics of the alloy. Ti-25Al-25Nb materials could be used in hydrogen energy because they are very stable structurally and the sorption/desorption processes can be undone.
Author Contributions
Conceptualization, Y.K., Y.T., N.M., A.U., M.A., A.K. and E.S.; Formal Analysis, Y.T., N.M., M.A. and A.K.; Methodology, Y.K., N.M., A.U., M.A. and E.S.; Supervision, Y.K.; Visualization, Y.T.; Writing—Original Draft, Y.K., Y.T., N.M., M.A. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. BR24992854).
Data Availability Statement
The data are contained within the article.
Acknowledgments
We express our sincere gratitude to the Intrachannel Testing Laboratory of the IAE Branch RSE NNC RK for their assistance in conducting the thermal cycling tests, and the Material Testing Department for their assistance in conducting the research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cheng, J.; Yang, J.; Zhang, X.; Zhong, H.; Ma, J.; Li, F.; Fu, L.; Bi, Q.; Li, J.; Liu, W. High temperature tribological behavior of a Ti-46Al-2Cr-2Nb intermetallics. Intermetallics 2012, 31, 120–126. [Google Scholar] [CrossRef]
- Cinca, N.; Roberto, C.; Lima, C.; Guilemany, J. An overview of intermetallics research and application: Status of thermal spray coatings. J. Mater. Res. Technol. 2013, 2, 75–86. [Google Scholar] [CrossRef]
- Bochenek, K.; Basista, M. Advances in processing of NiAl intermetallic alloys and composites for high temperature aerospace applications. Prog. Aerosp. Sci. 2015, 79, 136–146. [Google Scholar] [CrossRef]
- Xu, S.; Xu, Y.; Liang, Y.; Xu, X.; Gao, S.; Wang, Y.; He, J.; Lin, J. Phase equilibria of the Ti-Al-Nb system at 1300 °C. J. Alloys Compd. 2017, 724, 339–347. [Google Scholar] [CrossRef]
- Shuleshova, O.; Holland-Moritz, D.; Löser, W.; Voss, A.; Hartmann, H.; Hecht, U.; Witusiewicz, V.T.; Herlach, D.M.; Büchner, B. In situ observations of solidification processes in γ-TiAl alloys by synchrotron radiation. Acta Mater. 2010, 58, 2408–2418. [Google Scholar] [CrossRef]
- Kenel, C.; Leinenbach, C. Influence of cooling rate on microstructure formation during rapid solidification of binary TiAl alloys. J. Alloys Compd. 2015, 637, 242–247. [Google Scholar] [CrossRef]
- Wu, Y.; Zhen, L.; Li, X.; Yang, D.; Umakoshi, Y. Mechanical properties and oxidation behaviour of the Ti–24Al–14Nb–3V–0.5Mo alloy sheet. Mater. Sci. Eng. A 2006, 427, 42–50. [Google Scholar] [CrossRef]
- Jafari, R.; Eghbali, B.; Adhami, M. Influence of annealing on the microstructure and mechanical properties of Ti/Al and Ti/Al/Nb laminated composites. Mater. Chem. Phys. 2018, 213, 313–323. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, J.-H.; Kim, Y.-H.; Kim, J.-W.; Lee, K.-J.; Park, S.-J. Recent Progress Using Solid-State Materials for Hydrogen Storage: A Short Review. Processes 2022, 10, 304. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, N.; Wang, A.; Zhang, H.; Chen, C. Microstructure and high temperature oxidation behavior of Ti-Al-Nb-Si coatings on Ti-6Al-4V alloy. J. Alloys Compd. 2018, 765, 46–57. [Google Scholar] [CrossRef]
- Zhang, M.; Xiang, H.; Xu, L.; Feng, A.; Qu, S.; Chen, D. First-Principles Investigation on the Adsorption and Diffusion of Oxygen at the B2(110)–O(001) Interface in Ti2AlNb Alloys. Metals 2024, 14, 316. [Google Scholar] [CrossRef]
- Goyal, K.; Bera, C.; Sardana, N. Temperature-dependent structural, mechanical, and thermodynamic properties of B2-phase Ti2AlNb for aerospace applications. J. Mater. Sci. 2022, 57, 19553–19570. [Google Scholar] [CrossRef]
- Dadé, M.; Esin, V.; Nazé, L.; Sallot, P. Short- and long-term oxidation behaviour of an advanced Ti2AlNb alloy. Corros. Sci. 2019, 148, 379–387. [Google Scholar] [CrossRef]
- Kozhahmetov, Y.; Mukhamedova, N.; Urkunbay, A.; Tabieva, Y.; Yermolenko, M. Structural and mechanical properties of heat resistant titanium allows of the Ti-24.5Al-24.5Nb (at. %) system. Mater. Today Proc. 2023, 81, 1216–1222. [Google Scholar] [CrossRef]
- Senkevich, K.; Pozhoga, O. Experimental investigation of hydrogen absorption by commercial high alloyed Ti2AlNb-based alloy in cast and rapidly solidified state. Vacuum 2021, 191, 110379. [Google Scholar] [CrossRef]
- Kozhakhmetov, Y.; Batyrbekov, E.; Skakov, M.; Kurbanbekov, S.; Mukhamedova, N.; Mukhamedzhanova, R. Method for Obtaining Hydrogen Storage Rechargeable ICs. Utility Model Patent No. 5809, 29 January 2021. [Google Scholar]
- Gordienko, Y.; Ponkratov, Y.; Kulsartov, T.; Tazhibayeva, I.; Zaurbekova, Z.; Koyanbayev, Y.; Chikhray, Y.; Kenzhina, I. Research Facilities of IAE NNC RK (Kurchatov) for Investigations of Tritium Interaction with Structural Materials of Fusion Reactors. Fusion Sci. Technol. 2020, 76, 703–709. [Google Scholar] [CrossRef]
- Sadvakassova, A.; Tazhibayeva, I.; Kenzhin, E.; Zaurbekova, Z.; Kulsartov, T.; Gordiyenko, Y.; Chikhray, Y. Research of Reactor Radiation Influence upon Processes of Hydrogen Isotopes Interaction with Materials of the Fusion Facility. Fusion Sci. Technol. 2011, 60, 9–15. [Google Scholar] [CrossRef]
- Fangqin, G.; Toshiaki, K.; Ankur, J.; Hiroki, M.; Kouji, S.; Takayuki, I. Degradation and recovery properties in thermochemical hydrogen compression by using TiFe alloy. Int. J. Hydrog. Energy 2023, 48, 35164–35169. [Google Scholar] [CrossRef]
- Dai, J.; Li, S.; Zhang, H.; Yu, H.; Chen, C.; Li, Y. Microstructure and high-temperature oxidation resistance of Ti-Al-Nb coatings on a Ti-6Al-4V alloy fabricated by laser surface alloying. Surf. Coat. Technol. 2018, 344, 479–488. [Google Scholar] [CrossRef]
- Rui, X.; Yuyou, C.; Dong, L.; Zhuangqi, H.; Daming, X.; Qingchun, L. Microstructure Evolution of Rapidly Solidified Ti-3Al-2Nb Alloy. J. Mater. Sci. Technol. 1996, 12, 100–104. [Google Scholar]
- Baranov, V. Determination of effective hydrogen diffusion coefficients in deformed high-strength steels. Mod. Probl. Sci. Educ. 2007, 1, 38–41. [Google Scholar]
- Takasaki, A.; Tetsuya, H. Hydrogen evolution from cathodically charged titanium aluminide alloy Ti-24Al-11Nb. J. Alloys Compd. 2002, 340, 127–131. [Google Scholar] [CrossRef]
- Chen, R.; Ma, T.; Sun, Z.; Guo, J.; Ding, H.; Su, Y.; Fu, H. The hydrogen absorption behavior of high Nb contained titanium aluminides under high pressure and temperature. Int. J. Hydrogen Energy 2016, 41, 13254–13260. [Google Scholar] [CrossRef]
- Sundaram, P.; Wessel, E.; Clemens, H.; Kestler, H.; Ennis, P. Determination of the diffusion coefficient of hydrogen in gamma titanium aluminides during electrolytic charging. Acta Mater. 2000, 48, 1005–1019. [Google Scholar] [CrossRef]
- Takasaki, A.; Furuya, Y. Hydride formation and thermal desorption spectra of hydrogen of cathodically charged single-phase gamma titanium aluminide. Scr. Mater. 1999, 6, 595–599. [Google Scholar] [CrossRef]
- Goyal, K.; Sardana, N. Phase stability and microstructural evolution of Ti2AlNb alloys—A review. Mater. Today Proc. 2021, 41, 951–968. [Google Scholar] [CrossRef]
- Sundaram, P.; Wessel, E.; Ennis, P.; Quadakkers, W.; Singheiser, L. Diffusion coefficient of hydrogen in a cast gamma titanium aluminide. Scr. Mater. 1999, 41, 75–80. [Google Scholar] [CrossRef]
- Skakov, M.; Kozhakhmetov, Y.; Mukhamedova, N.; Miniyazov, A.; Sokolov, I.; Urkunbay, A.; Zhanbolatova, G.; Tulenbergenov, T. Effect of a High-Temperature Treatment on Structural-Phase State and Mechanical Properties of IMC of the Ti-25Al-25Nb at.% System. Materials 2022, 15, 5560. [Google Scholar] [CrossRef]
- Mukhamedova, N.; Kozhakhmetov, Y.; Skakov, M.; Kurbanbekov, S.; Mukhamedov, N. Microstructural stability of a two-phase (O + B2) alloy of the Ti-25Al-25Nb system (at.%) during thermal cycling in a hydrogen atmosphere. AIMS Mater. Sci. 2022, 9, 270–282. [Google Scholar] [CrossRef]
- Liang, C.; Zhao, J.F.; Chang, J.; Wang, H.P. Microstructure evolution and nano-hardness modulation of rapidly solidified Ti–Al–Nb alloy. J. Alloys Compd. 2020, 836, 155538. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, N.; Liang, H.; Liu, Y. Phase transformation and microstructure control of Ti2AlNb-based alloys: A review. J. Mater. Sci. Technol. 2021, 80, 203–216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).