Research on the Microstructure and Properties of Arc-Sprayed Austenitic Stainless Steel and Nickel-Based Alloy Composite Coatings with Different Spraying Distances
Abstract
1. Introduction
2. Experimental Materials and Methods
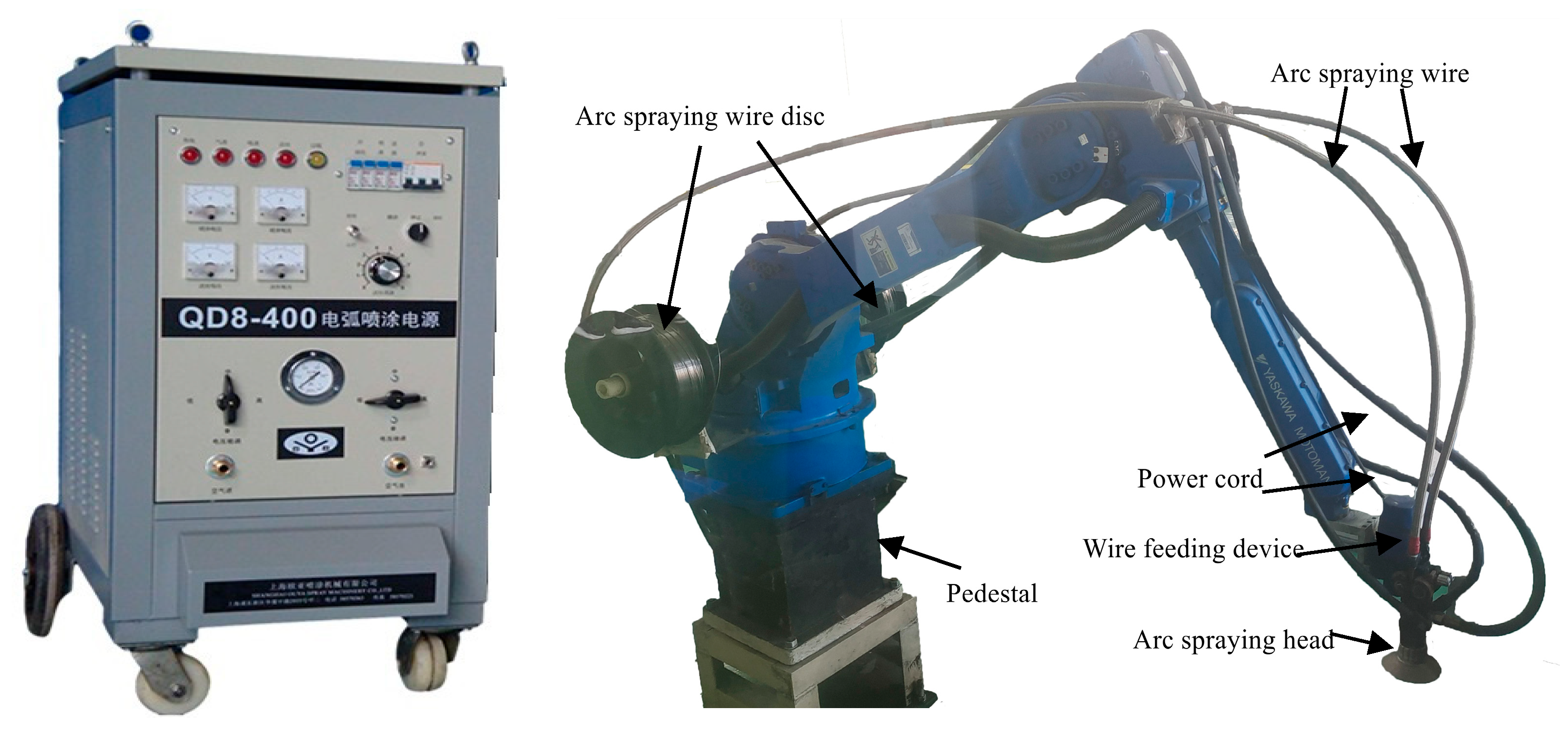
2.1. Coating Preparation
2.2. Coating Characterization Methods and Performance Testing
3. Results and Discussion
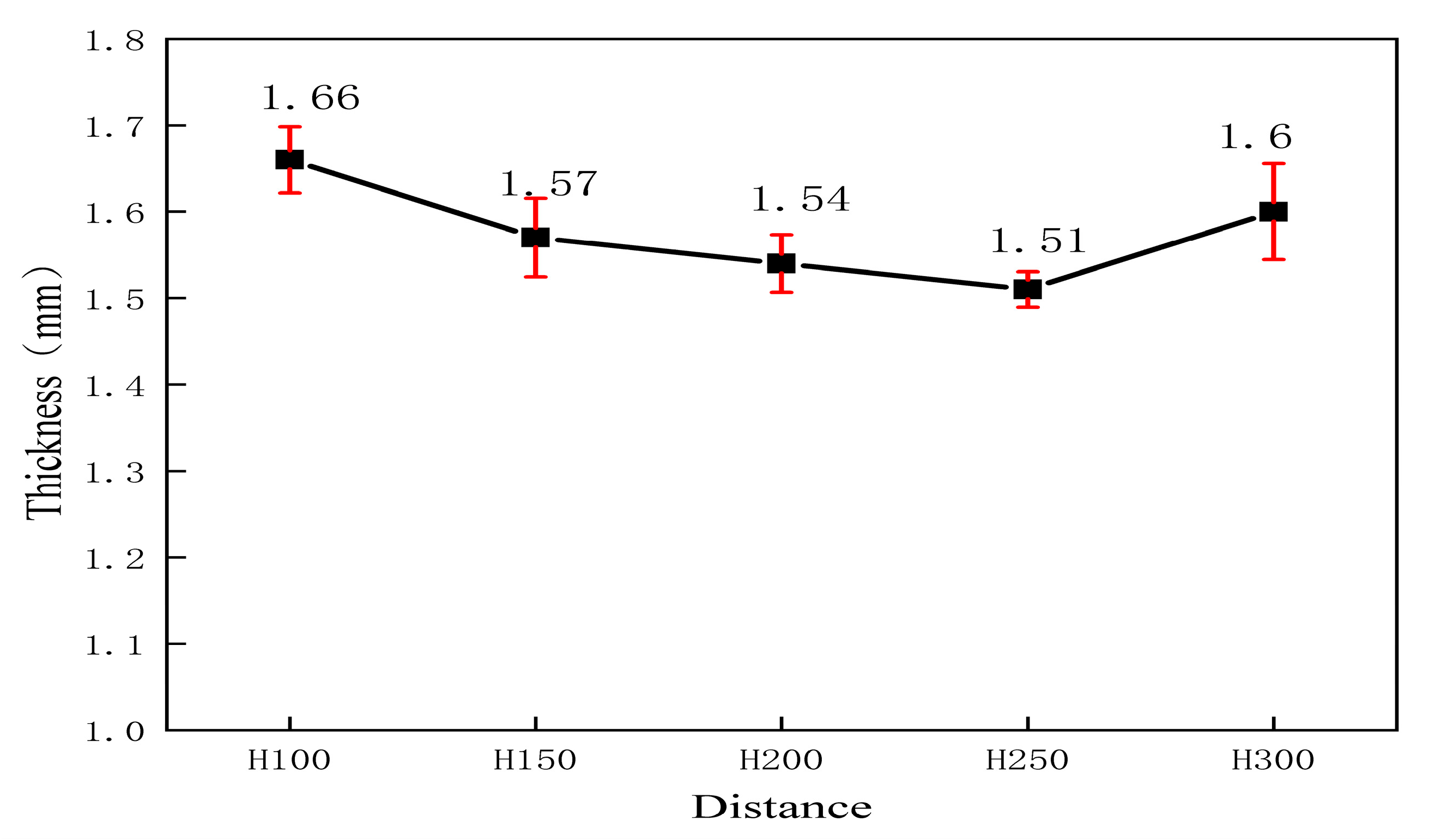
3.1. Sample Density
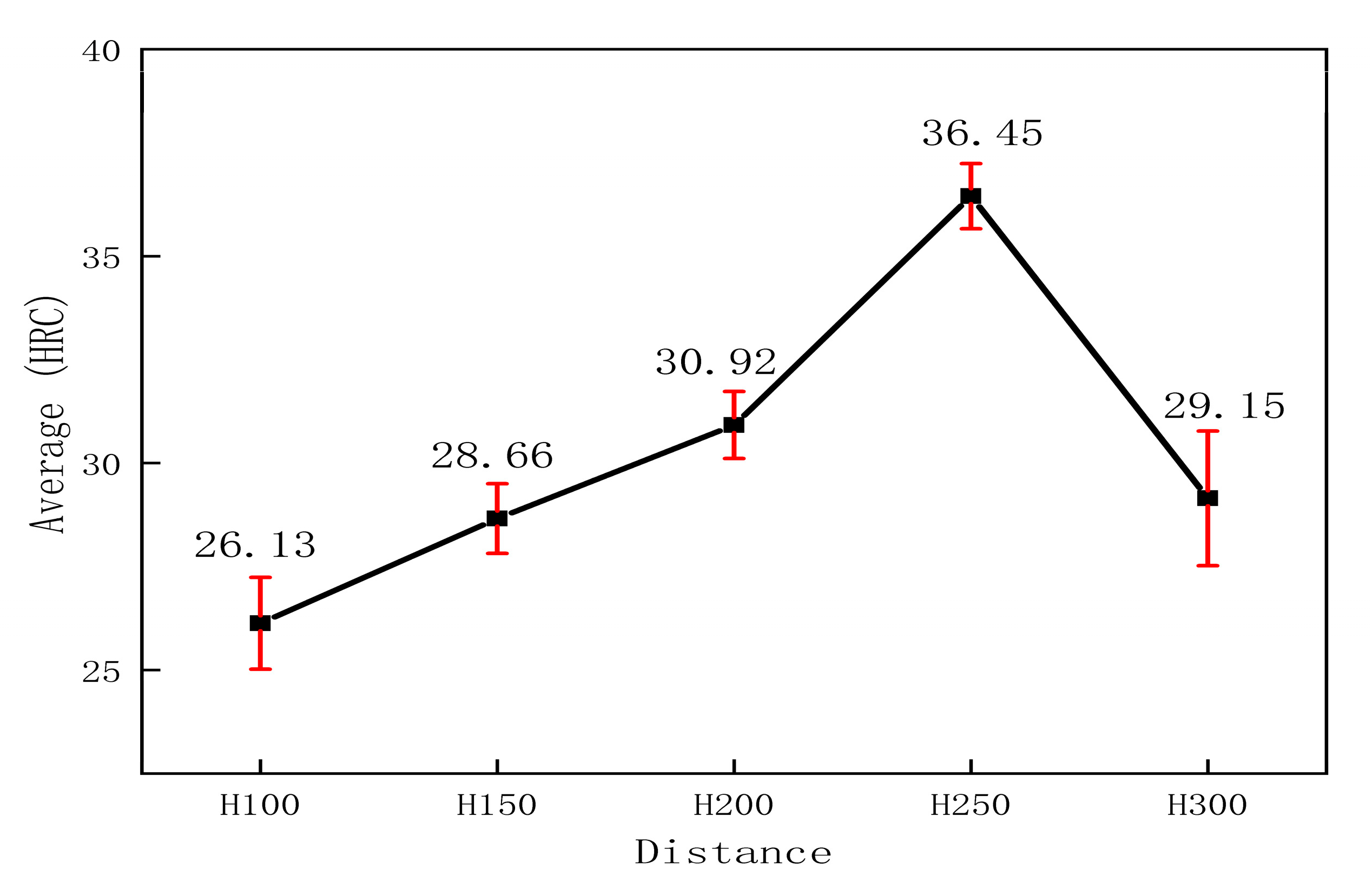
3.2. Coating Hardness
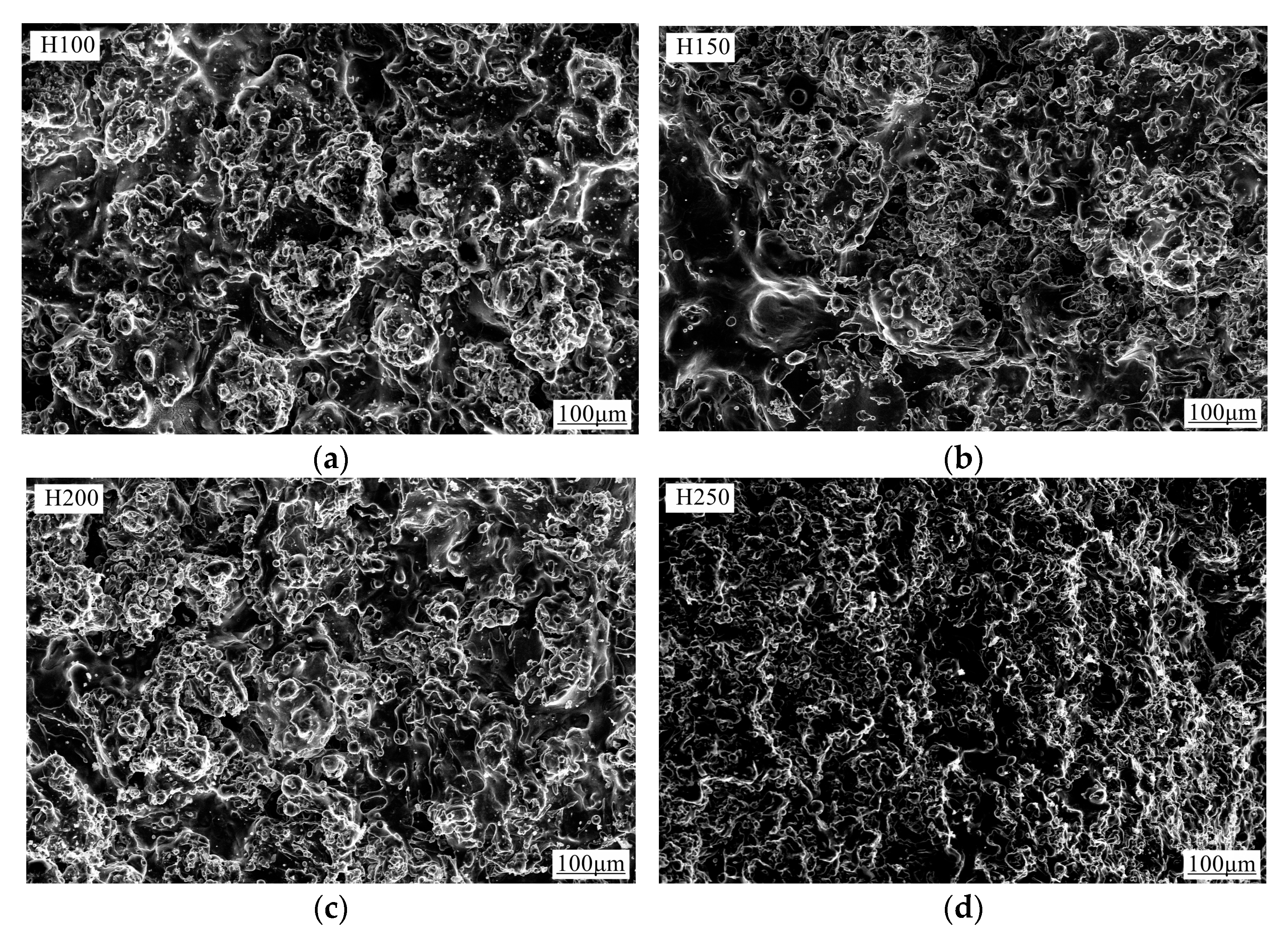
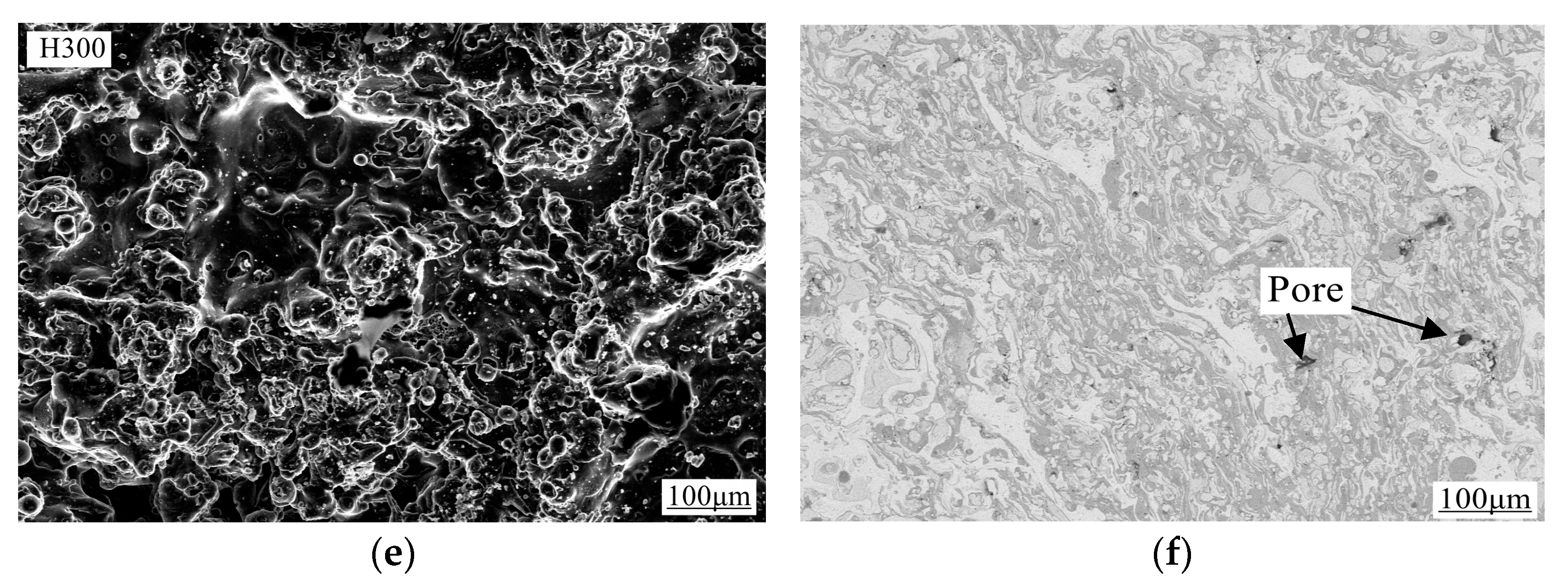
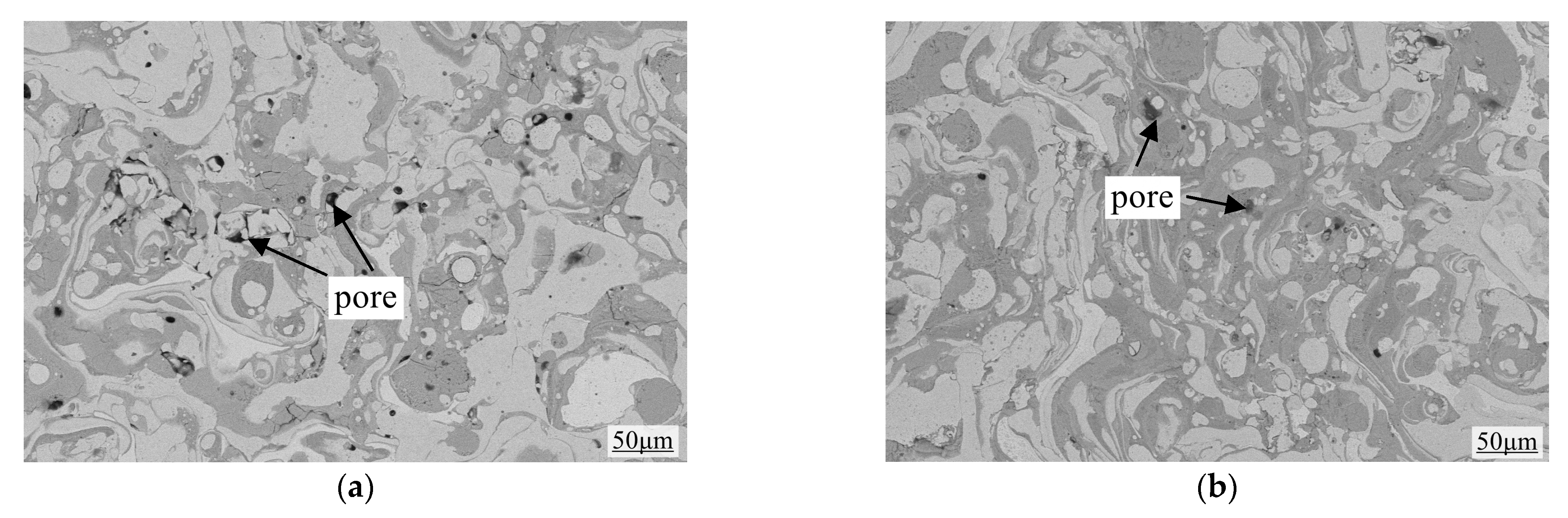
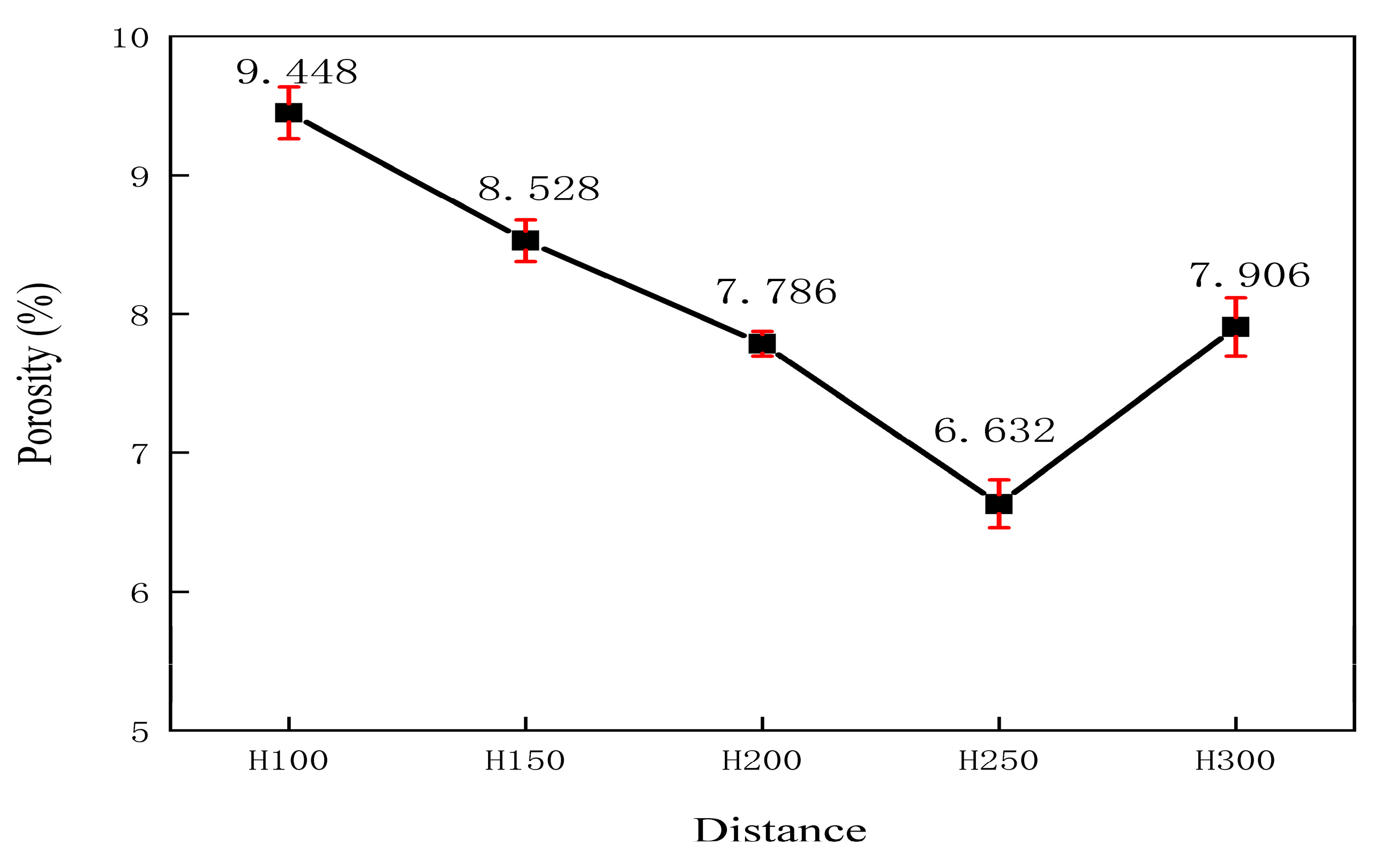
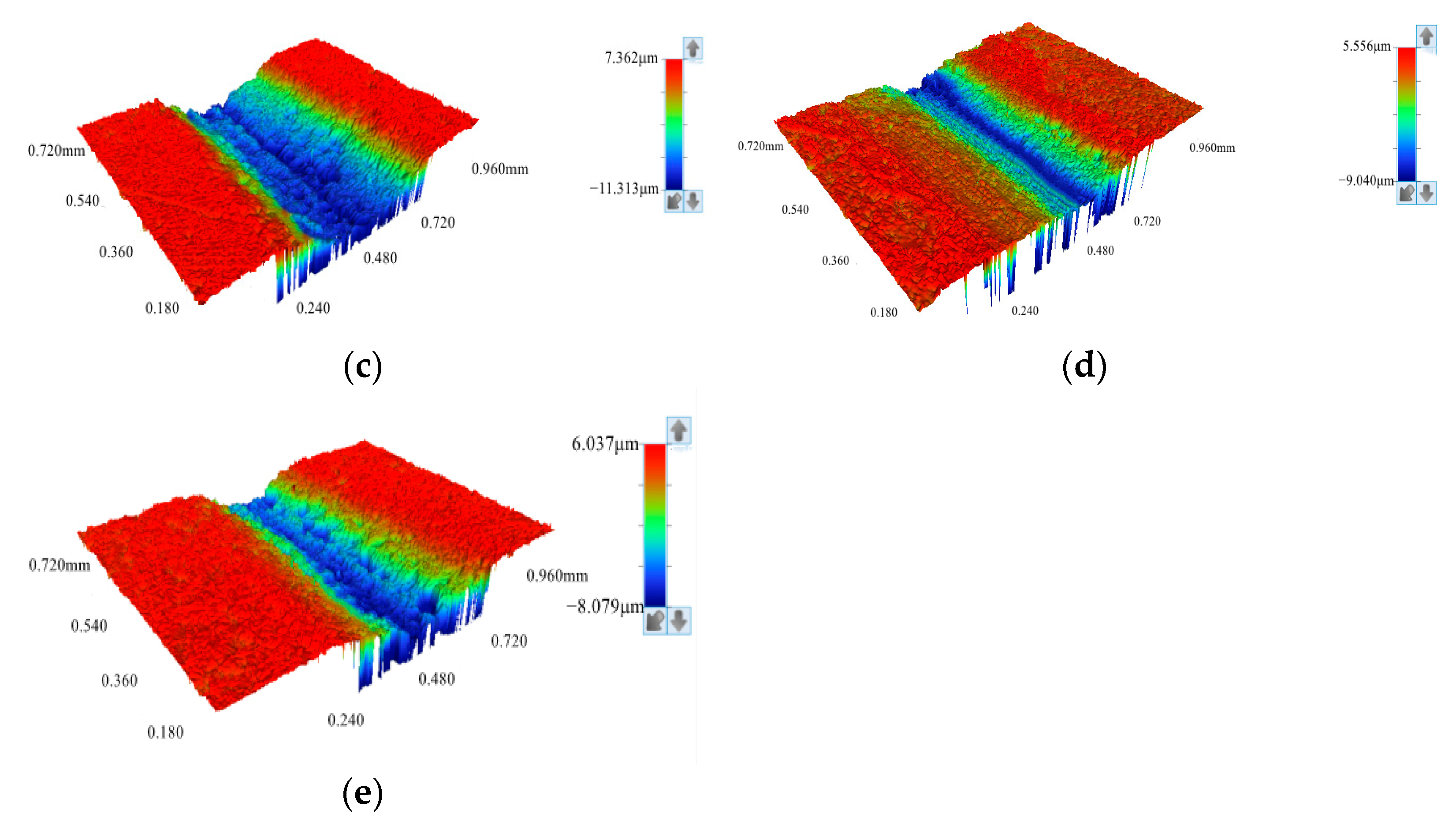
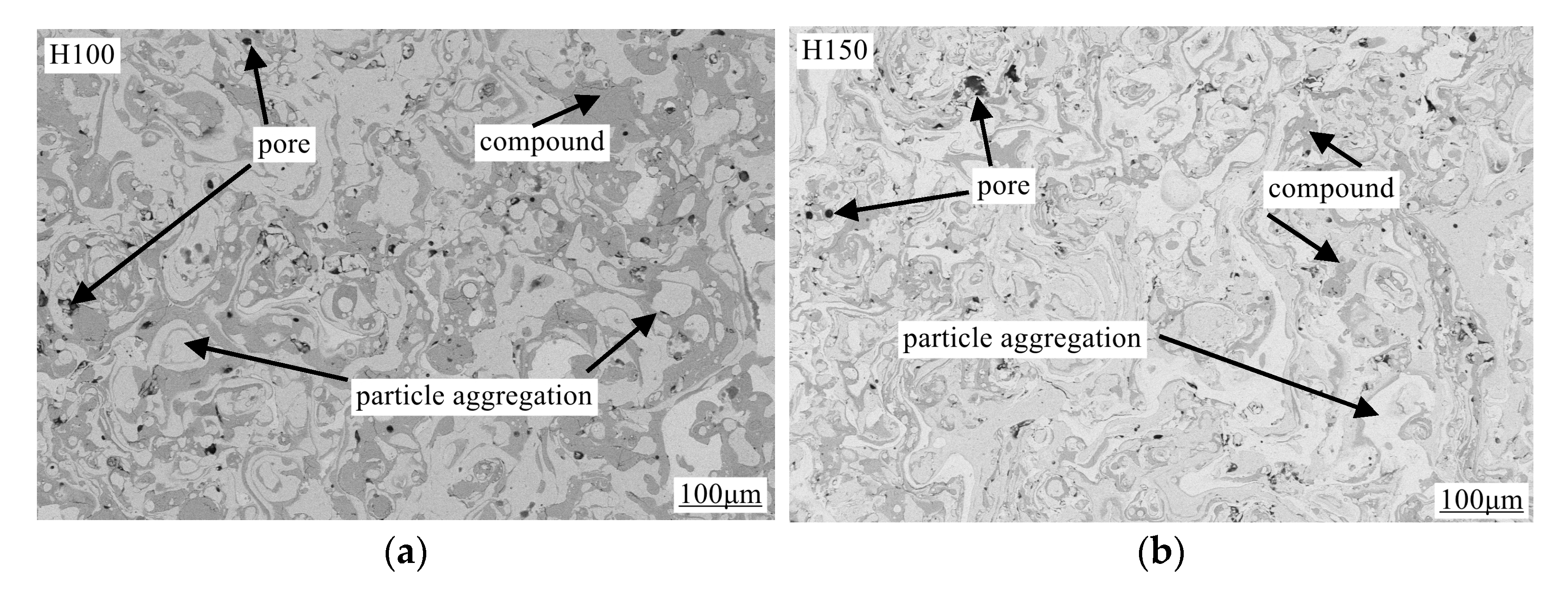
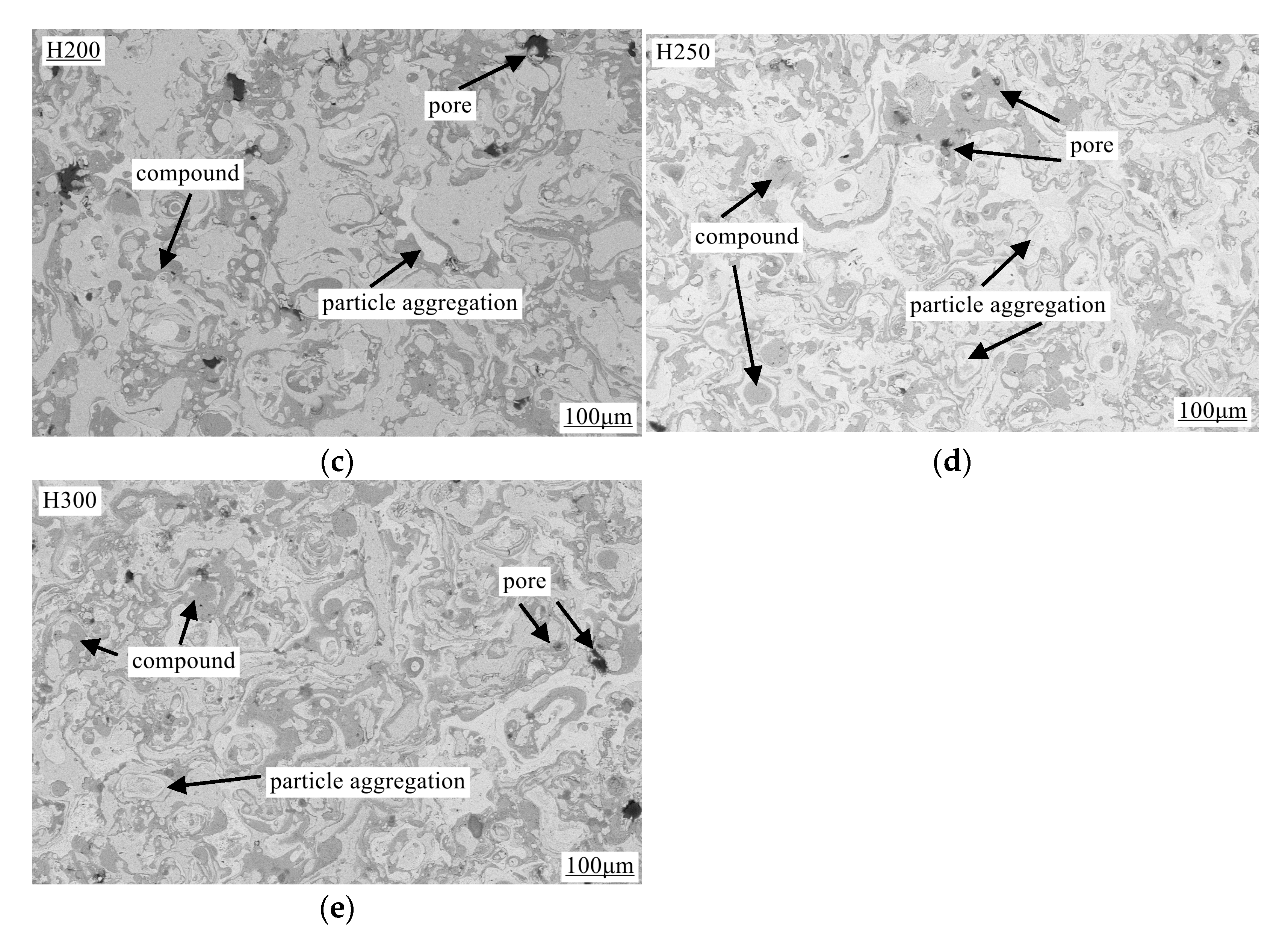
3.3. Characterization of Coating Surface Morphology and Calculation of Porosity
3.4. Analysis of Coating Friction and Wear Behavior
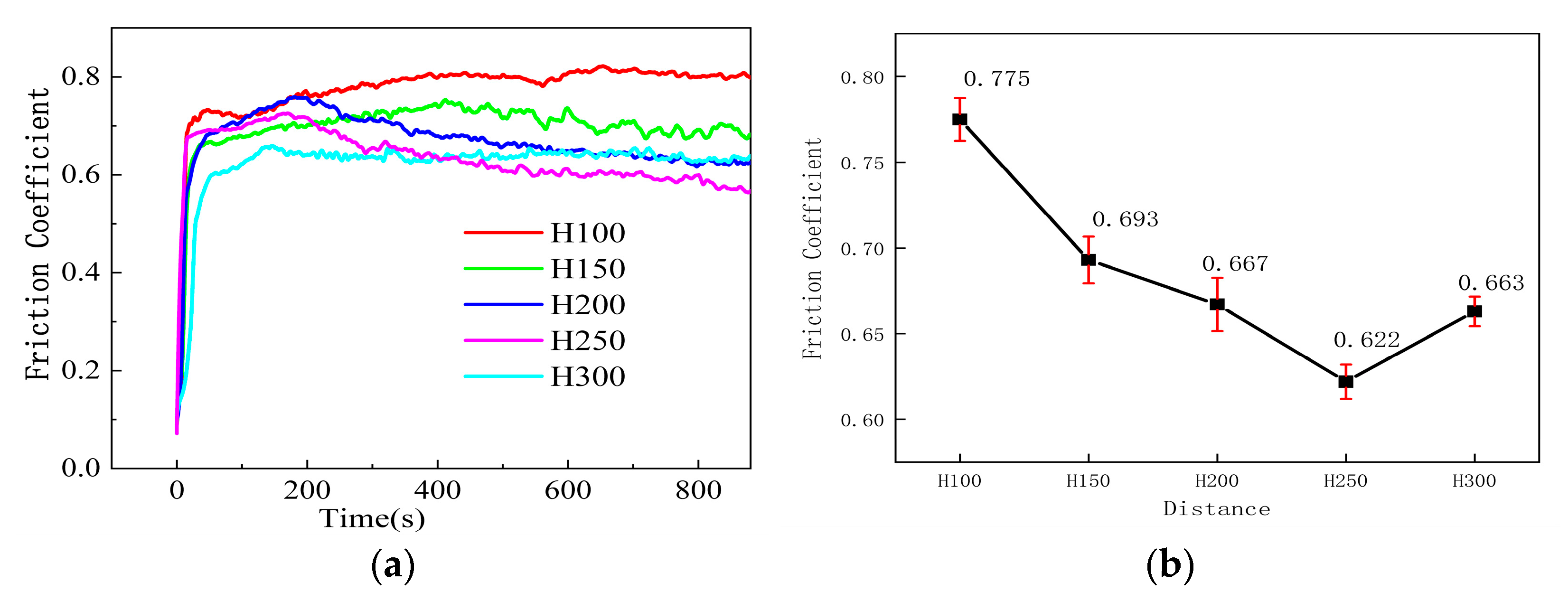
3.4.1. Coating Friction Factor Analysis
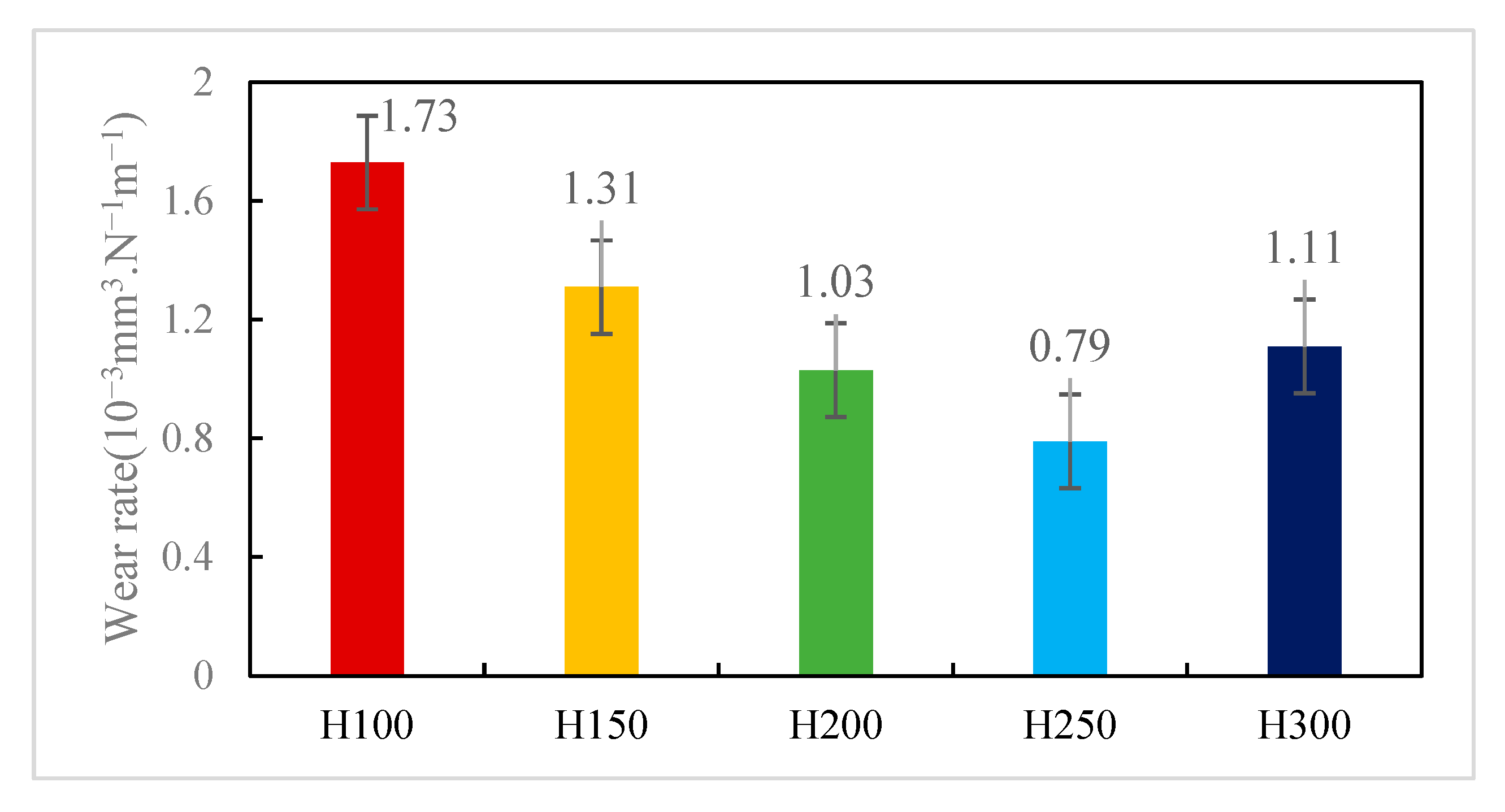
3.4.2. Analysis of Coating Wear Performance
3.5. Coating Phase Analysis
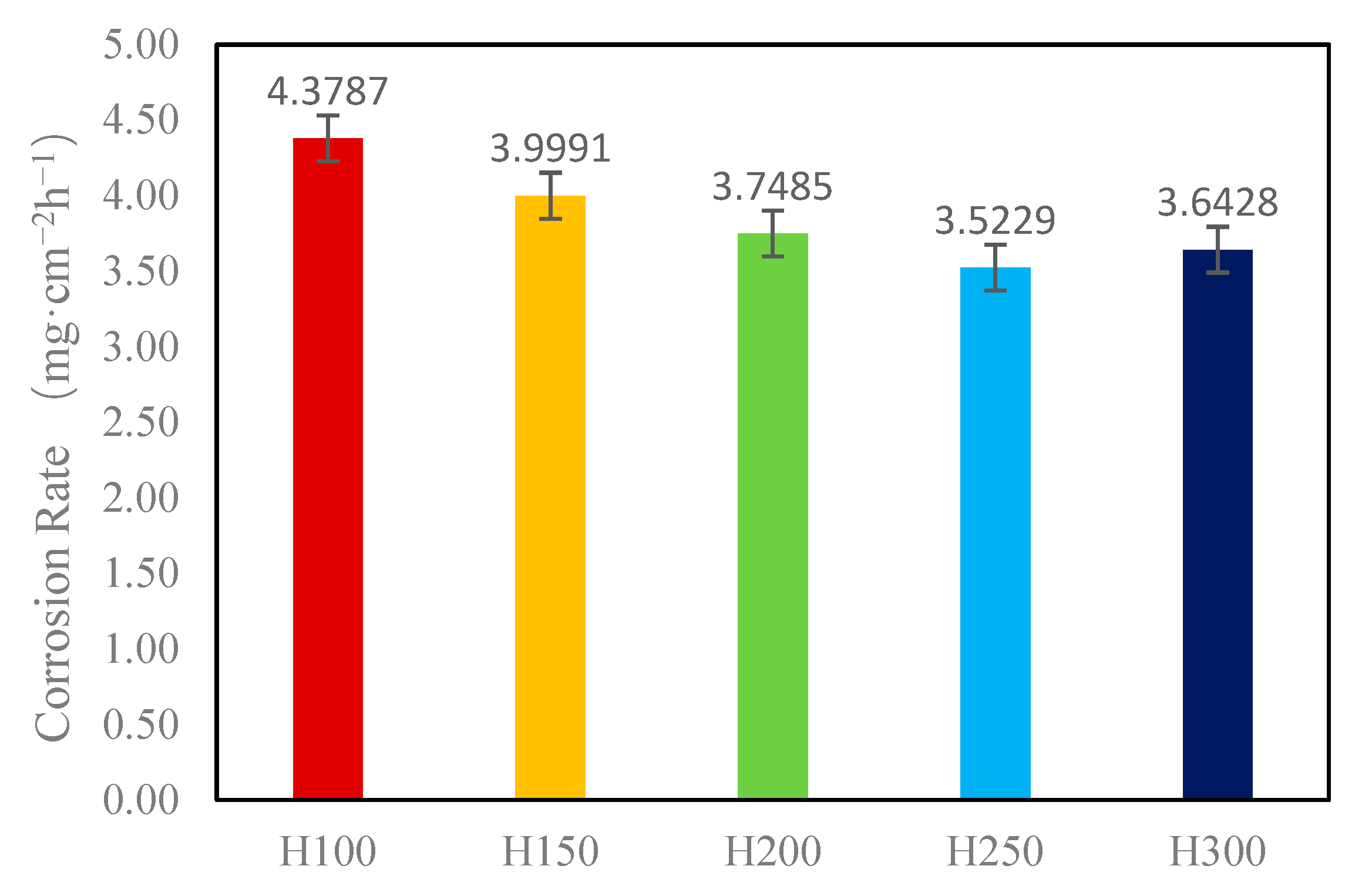
3.6. Coating Acid Corrosion Resistance Test
4. Conclusions
- The surface morphology of the sprayed coatings is relatively rough, with many pores between the particles. In the phase analysis of the sprayed coatings, at a spraying distance of 250 mm and a diffraction angle of 73°, a single Cr0.19Fe0.7Ni0.11 phase alloy precipitated, which contributed to the increase in the microhardness of the coating.
- From the friction and wear behavior analysis, the friction coefficient of the sprayed coatings is closely related to the density and hardness of the coatings. When the spraying distance was 100 mm, the friction coefficient reached its maximum value of 0.7752. As the spraying distance increased to 250 mm, the friction coefficient decreased to a minimum of 0.6214. The variation curve of the friction coefficient is positively correlated with density and hardness. At a diffraction angle of 43°, Cu and Cu3.8Ni phases were observed, indicating that the composition of the coating plays an active role in reducing the friction coefficient and inhibiting wear.
- In the acidic corrosion test, at a spraying distance of 250 mm, the coating exhibited the highest density of 6.4227 g/cm3, the highest Rockwell hardness of 31.3 HRC, a porosity of 6.63%, a wear rate of 0.79 × 10−3 mm3·N−1·m−1, the lowest friction coefficient of 0.6214, and the lowest acidic corrosion rate of 3.5229 mg·cm−2·h−1. Coatings sprayed at other distances had more pores and lower compactness. This reduced compactness increased the contact area between the acid and the coating, accelerating the corrosion process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qiang, W.; Kang, M.; Liu, J.; Ndumia, J.N. Microstructure and Wear Performance of High-Velocity Arc Sprayed FeMnCrNiBNbAl Coating. Coatings 2024, 14, 428. [Google Scholar] [CrossRef]
- Li, B.; Yang, D.; Liu, Z.; He, J.; Bai, J.; Jiang, H.; Tian, Y.; Zhang, Z.; Liu, S. Fabrication, Microstructure and Corrosion Resistance of Zn/Al Composite Coating by Arc Spraying. Coatings 2023, 13, 1406. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Magazov, N.; Kakimzhanov, D.; Apsezhanova, A.; Molbossynov, Y.; Kengesbekov, A. Influence of Spraying Process Parameters on the Characteristics of Steel Coatings Produced by Arc Spraying Method. Coatings 2024, 14, 1145. [Google Scholar] [CrossRef]
- Li, S.J.; Mao, D.Y.; He, Z.H.; Lu, B.; Liu, J.; Wang, Y.L.; Liu, H. Forming Performance of 1Cr18Ni9Ti Stainless Steel Laser Oscillating Welding Joints. Hot Work. Technol. 2025, 51–55, 64. [Google Scholar] [CrossRef]
- Xu, X.J.; Lin, W.Q.; Lu, H. Defect Reason on Outer Surface of 1Cr18Ni9Ti Stainless Steel Oil Return Tube. Corros. Prot. 2020, 41, 70–74. [Google Scholar]
- Wang, Y.F.; Zhao, Y.T.; Ma, H.H.; Ren, H.P. Effect of heat treatment on corrosion resistance of 1Cr18Ni9Ti and 2Cr13 steel plate welded joint. Heat Treat. Met. 2021, 46, 7–11. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, L.H.; Luo, S.H.; Hu, H.B.; Tang, T.G.; Zhang, Q.M. Effect of strain on microstructure evolution of 1Cr18Ni9Ti stainless steel during adiabatic shearing. J. Mater. Eng. Perform. 2016, 25, 29–37. [Google Scholar] [CrossRef]
- Gangloff, R.P.; Ha, H.M.; Burns, J.T.; Scully, J.R. Measurement and Modeling of Hydrogen Environment-Assisted Cracking in Monel K-500. Metall. Mater. Trans. A 2014, 45, 3814–3834. [Google Scholar] [CrossRef]
- Yang, M.H.; Li, X.W.; Sun, C.H.; Ruan, Y. Microstructure and Properties of Monel K-500 Alloy in Synergetic Modulation of Directional Solidification and Thermal Processing. Acta Metall. Sin. 2024, 61, 1–13. [Google Scholar]
- Jia, X.M.; Wang, J.; Liu, T.Y.; Zheng, H.B.; Wang, Q.Y.; Xi, Y.C.; Dong, L.J. Effect of aging temperature on microstructure and properties of Monel K500 alloy. Iron Steel Vanadium Titan. 2023, 44, 160–166. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Zheng, W.J.; Song, Z.G.; Feng, H. Development and Application of Nickel-copper Alloy in China. Hot Work. Technol. 2019, 48, 23–26. [Google Scholar] [CrossRef]
- Luo, L.X.; Liu, R.Z.; Xiao, Q.; Sun, H.; Ma, Y.L.; Shi, Y.J.; Zhang, C.; Ma, X.L.; Zhang, Z. Microstructure and properties of Zn–Al–Ni–Cu composite coating prepared by arc spraying. Electroplat. Finish. 2023, 42, 27–36. [Google Scholar] [CrossRef]
- Ge, Y.; Tan, P.; Li, Z.F.; Wang, J.Y.; Wang, Q.B.; Yang, B.J. Study on mechanical properties of Monel porous plate. Powder Metall. Ind. 2017, 27, 34–38. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Dubey, A.K. Recent trends in laser cladding and surface alloying. Opt. Laser Technol. 2021, 134, 106619. [Google Scholar] [CrossRef]
- Cheng, E.C.; Liu, J.; Zhang, J.; Wang, T.X.; Liu, L.H.; Li, Y.F. Microstructure and friction and wear properties of laser cladding Fe-Cr-Ni alloy layer. Trans. Mater. Heat Treat. 2022, 43, 143–152. [Google Scholar] [CrossRef]
- Yang, X.T.; Zhou, J.; Wang, X.H.; Wei, H.L.; Zeng, R.; Yang, Q.B.; Li, W.S. Effect of Cu Content on Microstructure and Tribological Properties of Ni-Based Directional Structure Alloy Coatings. Rare Met. Mater. Eng. 2022, 51, 1420–1426. [Google Scholar]
- Zhang, H.Y.; Wang, F.R.; Du, S.M.; Zeng, Z.X.; Cai, H.; Liu, E.Y. Effect of Ni Element on Microstructure and Tribological Properties of Plasma Sprayed Iron-Based Coatings. Min. Metall. Eng. 2021, 41, 150–155. [Google Scholar]
- Elttayef, A.K.; Abass, L.N.; Abd Al-Latif, L.G. Improvement mechanical properties of Inconel and Monel alloys synthesis by laser coating. Opt. Laser Technol. 2019, 109, 49–54. [Google Scholar] [CrossRef]
- Krelling, A.P.; Melo, F.S.; Almeida, E.A.S.; da Costa, C.E.; Milan, J.C.G. Microstructure and properties of borided Monel 400 alloy. Mater. Res. Express 2019, 6, 106410. [Google Scholar] [CrossRef]
- Kostryzhev, A.G.; Marenych, O.O.; Pan, Z.; Li, H.; van Duin, S. Strengthening mechanisms in Monel K500 alloyed with Al and Ti. J. Mater. Sci. 2023, 58, 4150–4164. [Google Scholar] [CrossRef]
- Wang, K.; Qi, H.; Ma, S.; Wang, L.; He, N.; Li, F. Effect of Nickel Addition on Solidification Microstructure and Tensile Properties of Cast 7075 Aluminum Alloy. Crystals 2023, 13, 1589. [Google Scholar] [CrossRef]
- Karlina, A.I.; Karlina, Y.I.; Kondratiev, V.V.; Kononenko, R.V.; Breki, A.D. Study of Wear of an Alloyed Layer with Chromium Carbide Particles After Plasma Melting. Crystals 2023, 13, 1696. [Google Scholar] [CrossRef]
- Wu, S.; Ning, Y.; Xie, H.; Tian, H.; Lv, J.; Liu, B. Study on the galvanic corrosion behavior of copper-nickel/titanium alloys under simulated seawater environment. J. Solid. State Electrochem. 2024, 28, 2315–2329. [Google Scholar] [CrossRef]
- Wielage, B.; Pokhmurska, H.; Student, M.; Gvozdeckii, V.; Stupnyckyj, T.; Pokhmurskii, V. Iron-Based Coatings Arc-Sprayed with Cored Wires for Applications at Elevated Temperatures. Surf. Coat. Technol. 2013, 220, 27–35. [Google Scholar] [CrossRef]
- Zeng, J.; Li, K.J.; LÜ, J.J.; Zhao, J.; Liu, Q.; He, Q.B. Effect of solution and two-stage aging on microstructure and hardness of Cu-containing nickel-based alloys. Trans. Mater. Heat Treat. 2024, 45, 113–122. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Yang, H.L.; Ruan, J.M. Microstructure of titanium-nickel alloy by mechanical alloying. J. Cent. South Univ. (Sci. Technol.) 2015, 46, 1202–1207. [Google Scholar]
- Liu, X.Z.; Shen, Q.W.; Liu, X.Z.; Chen, J.; Zhu, L.W.; Qi, J. Effect of Heat Treatment Temperature on the Spectral Properties of Cu-Ni Coating. Spectrosc. Spectr. Anal. 2015, 35, 1094–1098. [Google Scholar]
- Yang, X.; Wang, X.; Zhou, J.; Wei, H.; Zeng, R.; Li, W. Effect of Cu addition on the microstructure and tribological performance of Ni60 directional structure coating. Int. J. Miner. Metall. Mater. 2023, 30, 715–723. [Google Scholar] [CrossRef]
- Yao, K.; Liu, Z.D.; Liu, Q.B. Effect of Laser Power on Microstructure and Properties of Ni-Based Alloy Coatings on 30CrMnSiA Steel. J. Therm. Spray Technol. 2022, 31, 2136–2146. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Zhang, T.; Yu, T. Laser fabricated nickel-based coating with different overlap modes. Mater. Manuf. Process. 2021, 36, 1618–1630. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, C.; Chen, L.; Sun, J.; Yu, T. Effect of process parameters on the cladding track geometry fabricated by laser cladding. Optik 2020, 223, 165447. [Google Scholar] [CrossRef]
- Lu, S.Y. Super Stainless Steel and High Nickel Corrosion Resistant Alloys; Chemical Industry Press: Beijing, China, 2012; p. 355. [Google Scholar]
- Wang, H.Z.; Zhou, X.Y.; Liu, M.; Jia, L.; Huang, Y.F.; Wang, H.D. Research Progress of the Sealant Decreased Thermal Spray Coating Porosity. Mater. Rep. 2023, 37, 210–228. [Google Scholar]



| 45# | Fe | C | Si | Mn | P | S | Cr | Ni | Others |
| ≥97 | 0.42–0.5 | ≤0.35 | 0.5–0.8 | ≤0.04 | ≤0.05 | ≤0.25 | ≤0.25 | <0.5 |
| 1Cr18Ni9Ti | Cr | Ni | Mn | Si | P | C | S | Ti | Fe | Others |
| 18 | 9 | 2 | 1 | 0.035 | 0.12 | 0.03 | 0.6 | 68.415 | 0.8 | |
| Monel | Ni | Cu | Fe | Mn | C | Al | Si | S | Others | |
| 65 | 28 | 2.5 | 2 | 0.3 | 0.5 | 0.5 | 0.024 | 1.176 |
| Types of Silk Materials | Wire Feeding Voltage/V | Wire Feeding Current/A | Spray Current/A | Spray Voltage/V |
|---|---|---|---|---|
| 1Cr18Ni9Ti, Monel | 16 | 25 | 200 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Yang, Z.; Zhang, L.; Wang, J. Research on the Microstructure and Properties of Arc-Sprayed Austenitic Stainless Steel and Nickel-Based Alloy Composite Coatings with Different Spraying Distances. Crystals 2025, 15, 142. https://doi.org/10.3390/cryst15020142
Yan J, Yang Z, Zhang L, Wang J. Research on the Microstructure and Properties of Arc-Sprayed Austenitic Stainless Steel and Nickel-Based Alloy Composite Coatings with Different Spraying Distances. Crystals. 2025; 15(2):142. https://doi.org/10.3390/cryst15020142
Chicago/Turabian StyleYan, Jingang, Zhenming Yang, Limin Zhang, and Jianxin Wang. 2025. "Research on the Microstructure and Properties of Arc-Sprayed Austenitic Stainless Steel and Nickel-Based Alloy Composite Coatings with Different Spraying Distances" Crystals 15, no. 2: 142. https://doi.org/10.3390/cryst15020142
APA StyleYan, J., Yang, Z., Zhang, L., & Wang, J. (2025). Research on the Microstructure and Properties of Arc-Sprayed Austenitic Stainless Steel and Nickel-Based Alloy Composite Coatings with Different Spraying Distances. Crystals, 15(2), 142. https://doi.org/10.3390/cryst15020142





