Effect of Fungal Metabolism on Zn Minerals Formation: The Case of Aspergillus niger and Penicillium chrysogenum
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Experimental Conditions
2.2. Research Methods
2.2.1. Gas Chromatography–Mass Spectrometry (GC-MS)
2.2.2. Light Microscopy
2.2.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDX)
2.2.4. Powder X-Ray Diffraction (PXRD)
2.2.5. X-Ray Fluorescent (XRF) Analysis
3. Results
3.1. Morphological–Cultural and Physiological–Biochemical Properties of Fungi
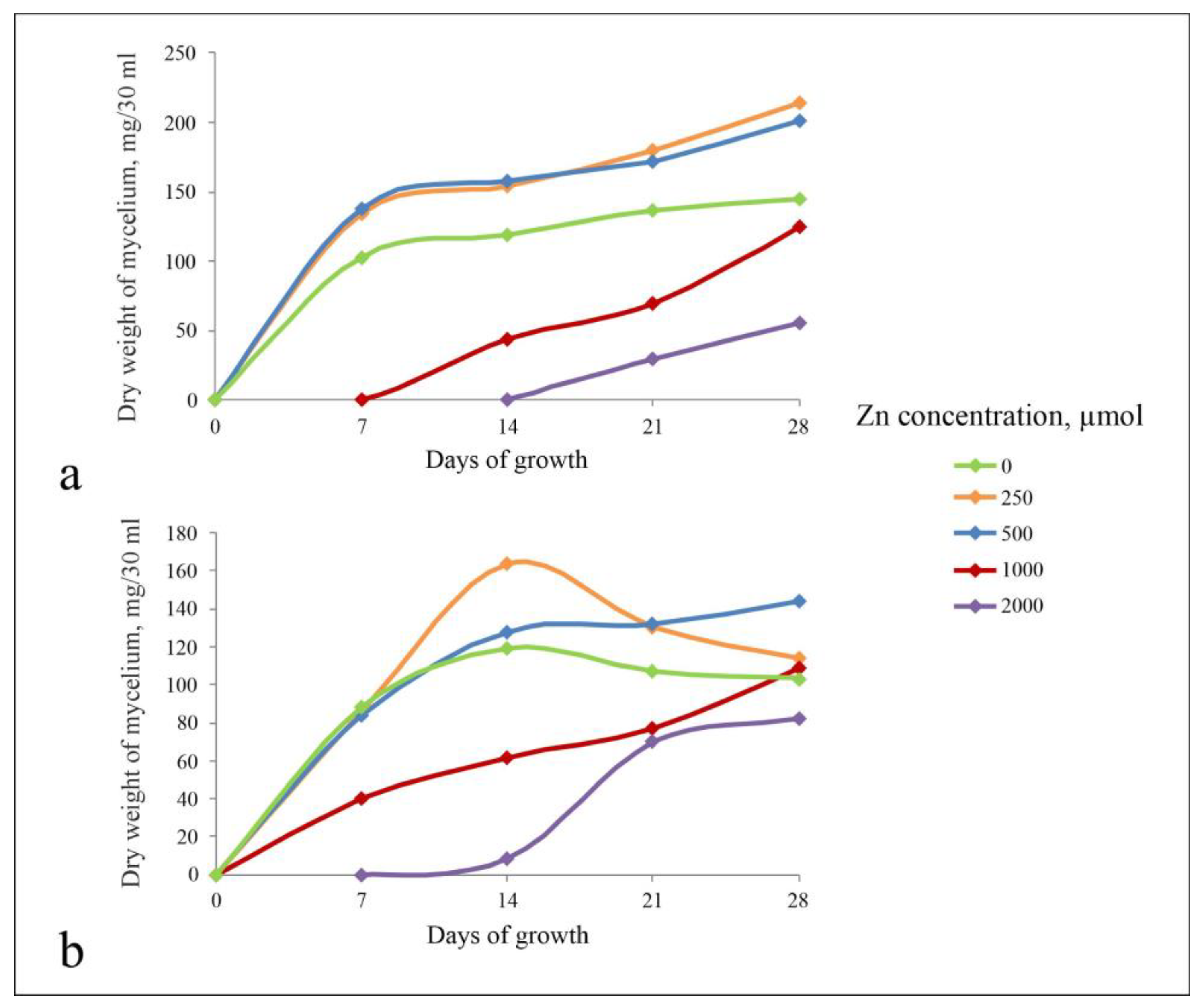
3.1.1. The Influence of Zn on the Morphological and Cultural Characteristics of Fungi
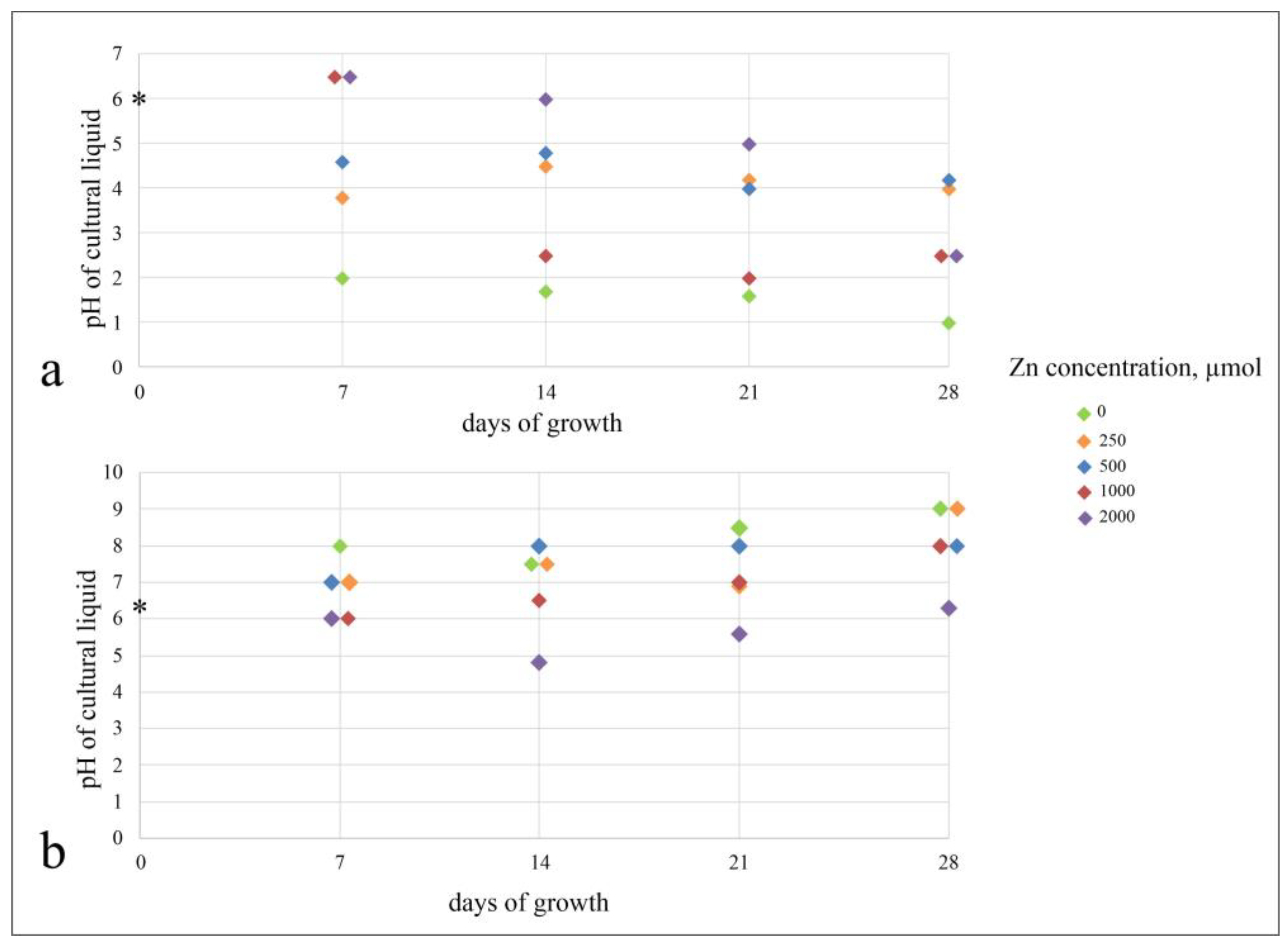
3.1.2. Metabolism of Fungi at Different Zn Concentrations in the Medium
3.2. Phase Formation in the Czapek–Dox Medium at Different Zn Concentrations
3.2.1. Phase Formation in the Absence of Fungi
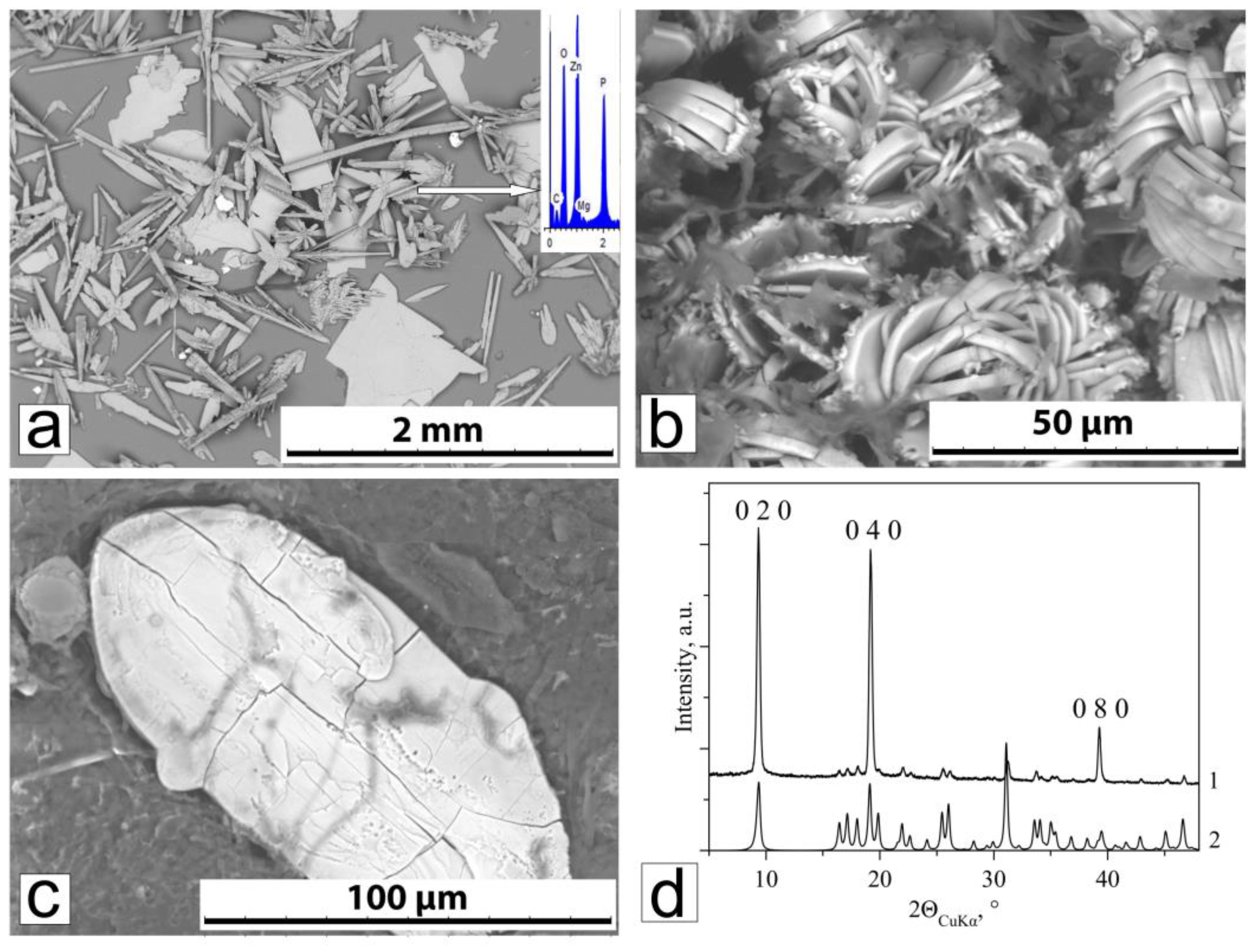
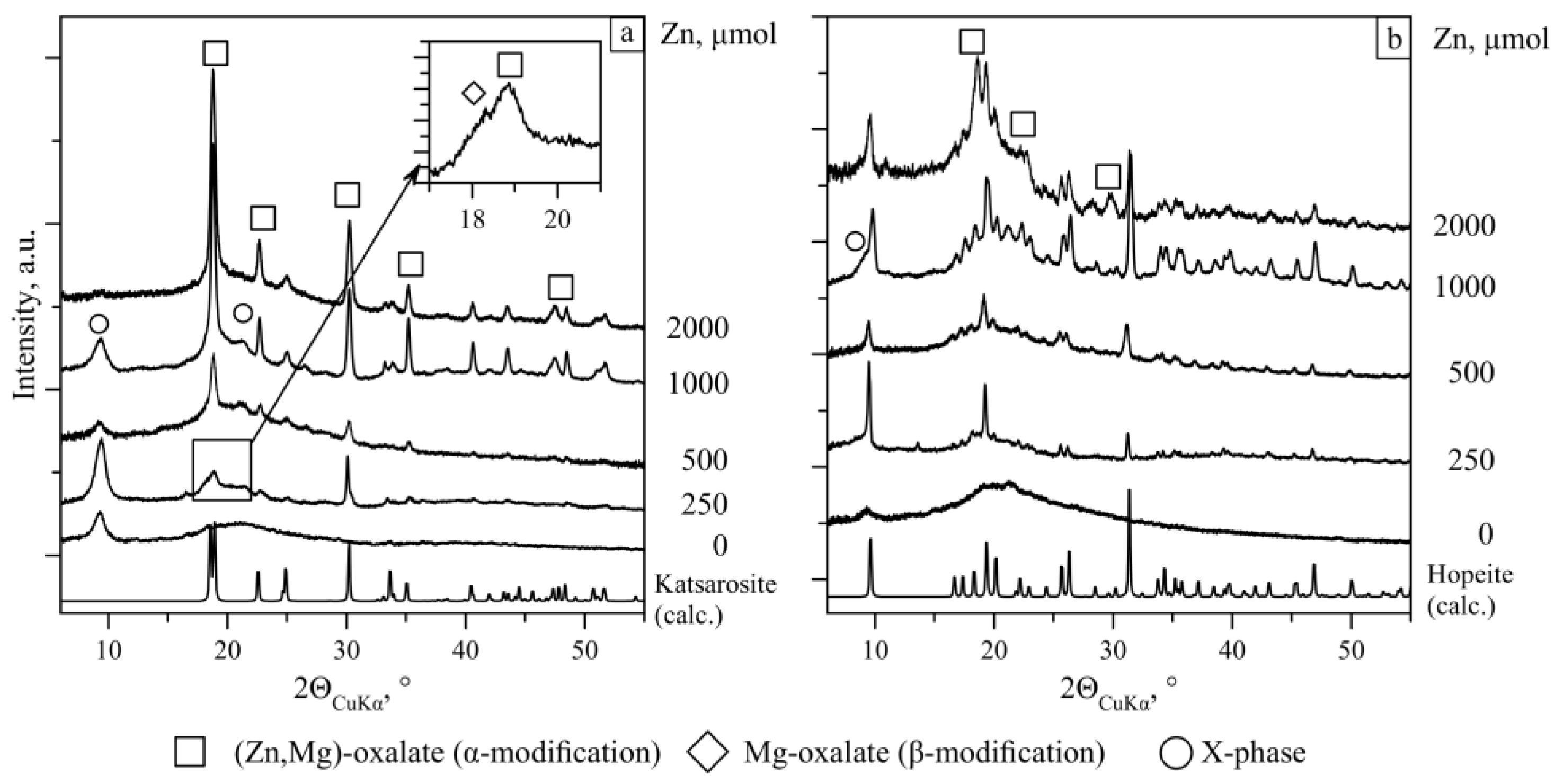
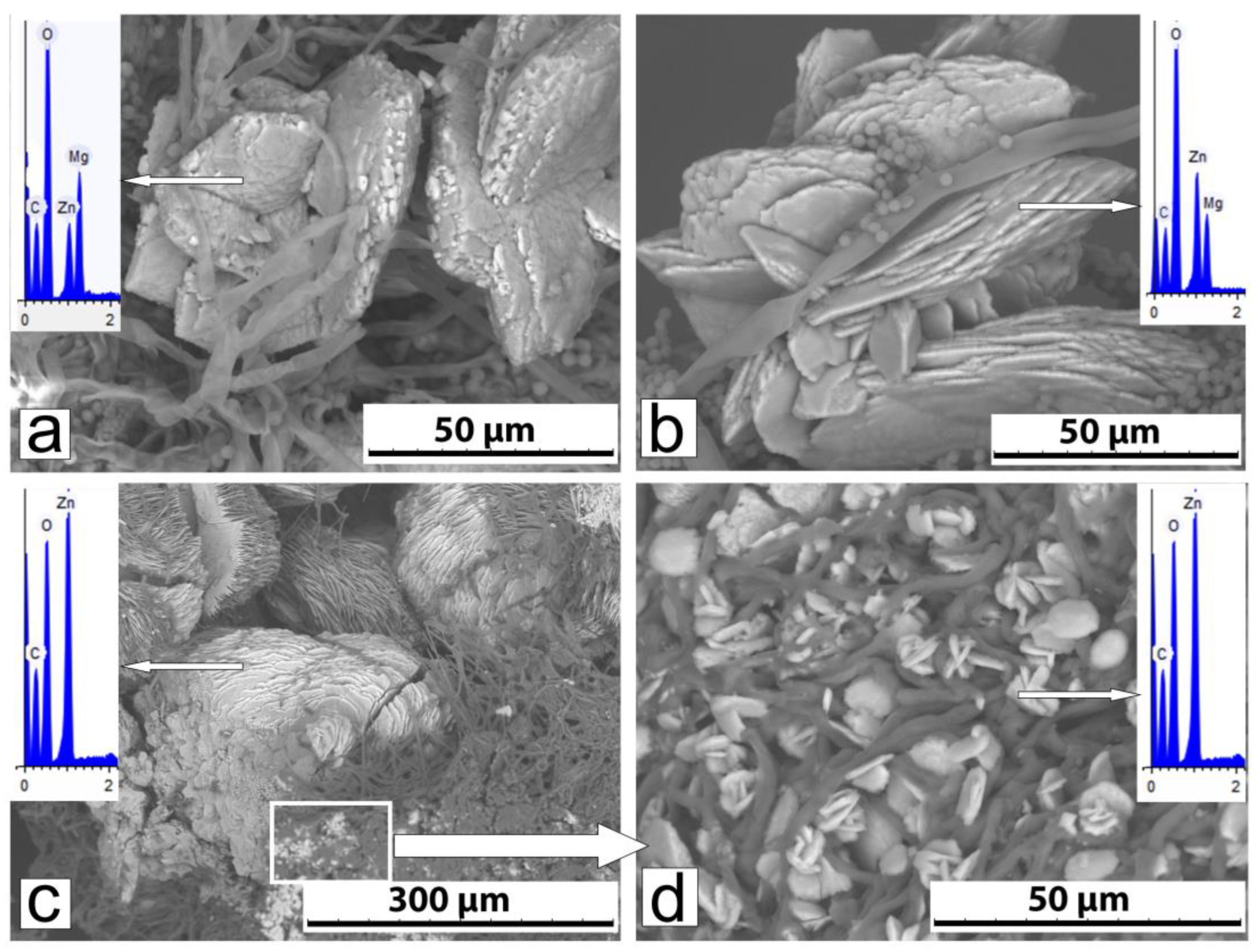
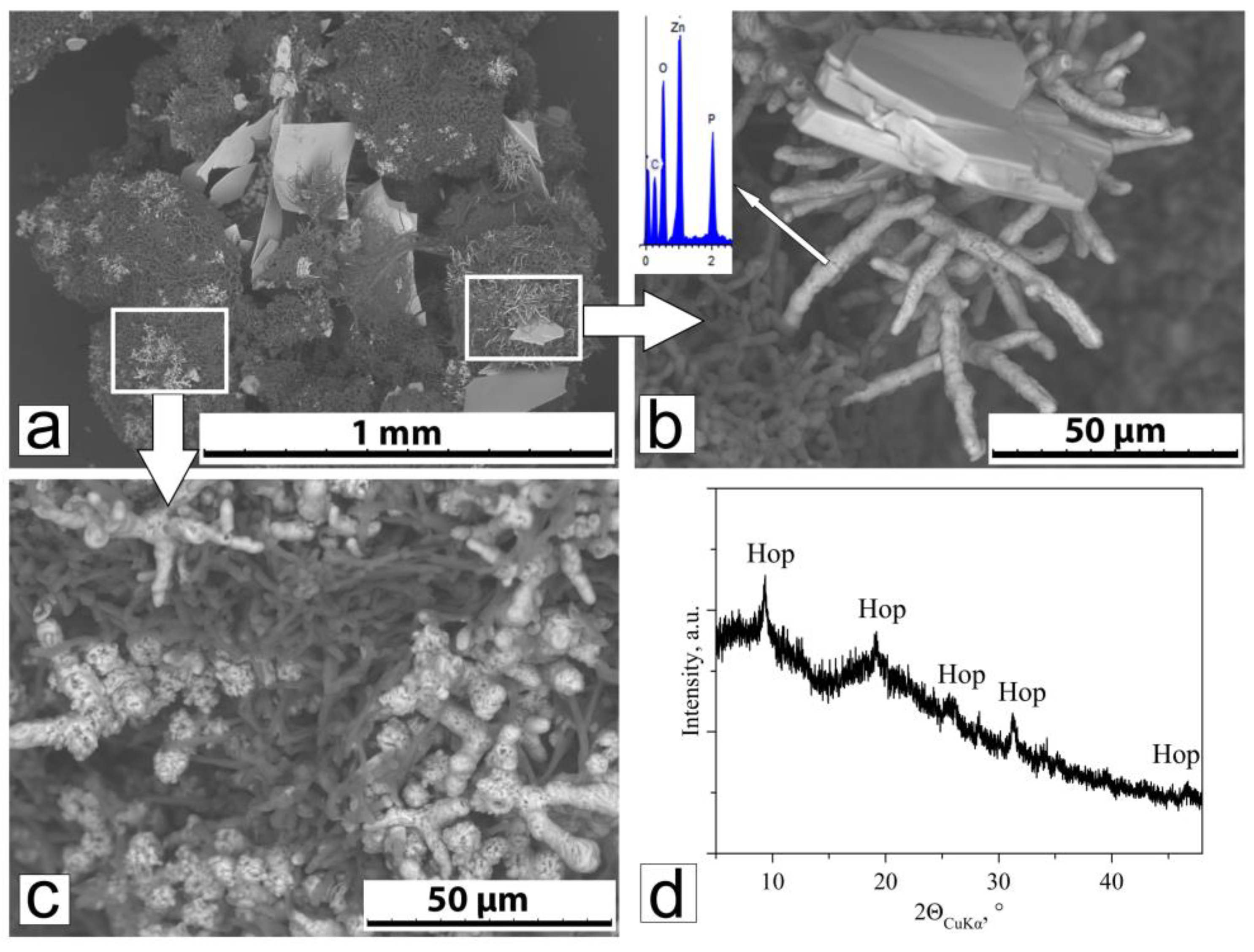
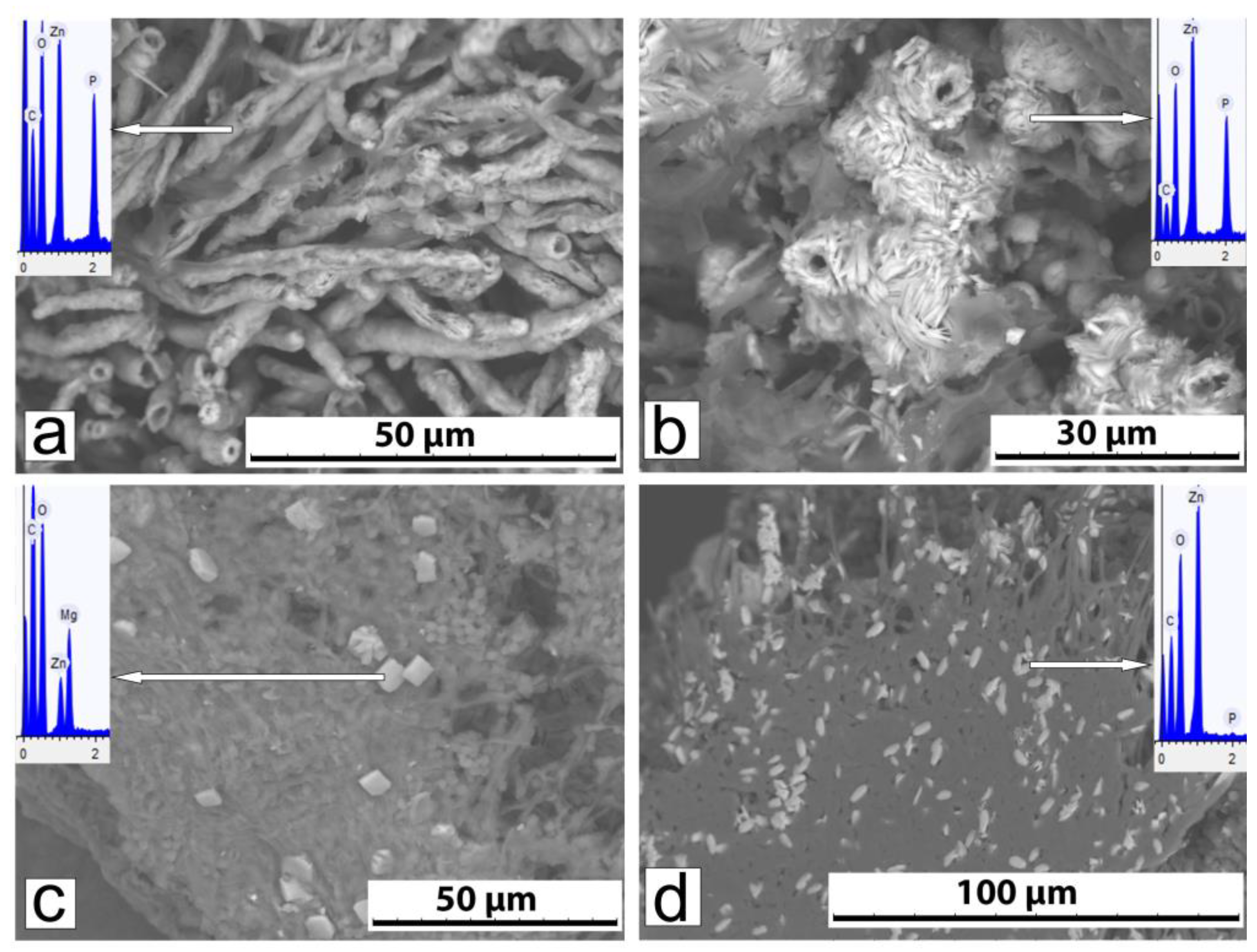
3.2.2. Phase Composition and Morphology of Crystals Formed Under Action of A. niger
3.2.3. Phase Composition and Morphology of Crystals Formed Under Action of P. chrysogenum
4. Discussion
4.1. Zinc Effect on the Growth and Metabolism of A. niger and P. chrysogenum
4.2. Zinc Concentration Effect of on Fungal Biomineralization
4.3. The Prospects for Using the A. niger and P. chrysogenum in Biotechnologies for Zn Detoxifying
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mukhopadhyay, M.; Noronha, S.B.; Suraishkumar, G.K. A review on experimental studies of biosorption of heavy metals by Aspergillus niger. Can. J. Chem. Eng. 2011, 89, 889–900. [Google Scholar] [CrossRef]
- Tian, D.; Jiang, Z.; Jiang, L.; Su, M.; Feng, Z.; Zhang, L.; Wang, S.; Li, Z.; Hu, S. A new insight into lead (II) tolerance of environmental fungi based on a study of Aspergillus niger and Penicillium oxalicum. Environ. Microbiol. 2019, 21, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.C.; Shijith, K.V.; Vipin, K.V.; Augusthy, A.R. Study on heavy metals toxicity biomarkers in Aspergillus niger. Int. J. Adv. Pharm. Biol. Chem. 2014, 3, 458–464. [Google Scholar]
- Ashruta, G.A.; Nanoty, V.; Bhalekar, U. Biosorption of heavy metals from aqueous solution using bacterial EPS. Int. J. Life Sci. 2014, 2, 373–377. [Google Scholar]
- Bhattacharya, A.; Gupta, A.; Kaur, A.; Malik, D. Simultaneous bioremediation of phenol and Cr (VI) from tannery wastewater using bacterial consortium. Int. J. Appl. Sci. Biotechnol. 2015, 3, 50–55. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, B.; Cai, Q.T. Bioremediation of hexavalent chromium pollution by Sporosarcinasaromensis m52 isolated from offshore sediments in Xiamen, China. Biomed. Environ. Sci. 2016, 29, 127–136. [Google Scholar] [CrossRef]
- Goher, M.E.; El-Monem, A.M.A.; Abdel-Satar, A.M.; Ali, M.H.; Hussian, A.-E.M.; Napiórkowska-Krzebietke, A. Biosorption of some toxic metals from aqueous solution using non-living algal cells of Chlorella vulgaris. J. Elem. 2016, 21, 703–714. [Google Scholar] [CrossRef]
- Marzan, L.W.; Hossain, M.; Mina, S.A.; Akter, Y.; Chowdhury, A.M.M.A. Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong city, Bangladesh: Bioremediation viewpoint. Egypt. J. Aquat. Res. 2017, 43, 65–74. [Google Scholar] [CrossRef]
- Saranya, K.; Sundaramanickam, A.; Shekhar, S.; Swaminathan, S.; Balasubramanian, T. Bioremediation of mercury by vibrio fluvialis screened from industrial effluents. BioMed Res. Int. 2017, 2017, 6509648. [Google Scholar] [CrossRef]
- Nayan, A.K.; Panda, S.S.; Basu, A.; Dhal, N.K. Enhancement of toxic Cr(VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and veltiveria of phytoremediation. J. Phytoremediation 2018, 20, 682–691. [Google Scholar] [CrossRef]
- Abioye, O.P.; Oyewole, O.A.; Oyeleke, S.B.; Adeyemi, M.O.; Orukotan, A.A. Biosorption of lead, chromium and cadmium in tannery effluent using indigenous microorganisms. Braz. J. Biol. Sci. 2018, 5, 25–32. [Google Scholar] [CrossRef]
- Rehan, M.; Alsohim, A.S. Bioremediation of Heavy Metals. In Environmental Chemistry and Recent Pollution Control Approaches; Saldarriaga-Noreña, H., Murillo-Tovar, M.A., Farooq, R., Dongre, R., Riaz, S., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Dhankhar, R.; Hooda, A. Fungal biosorption–an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ. Technol. 2011, 32, 467–491. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D. Remediation of copper-contaminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol. Eng. 2011, 37, 1601–1605. [Google Scholar] [CrossRef]
- Girbal, J.; Prada, J.L.; Rocabayera, R.; Argemi, M. Dating of Biodeposits of Oxalates at the Arc De Berà in Tarragona, Spain. Radiocarbon 2001, 43, 637–645. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef]
- Purvis, O.W.; Pawlik-Skowrońska, B.; Cressey, G.; Jones, G.C.; Kearsley, A.; Spratt, J. Mineral phases and element composition of the copper hyperaccumulator lichen Lecanora polytropa. Mineral. Mag. 2008, 72, 607–616. [Google Scholar] [CrossRef]
- Sazanova, K.; Osmolovskaya, N.; Schiparev, S.; Yakkonen, K.; Kuchaeva, L.; Vlasov, D. Organic Acids Induce Tolerance to Zinc- and Copper-Exposed Fungi Under Various Growth Conditions. Curr. Microbiol. 2015, 70, 520–527. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Zelenskaya, M.S.; Vlasov, A.D.; Rusakov, A.V.; Petrova, M.A. Carbonate and Oxalate Crystallization by Interaction of Calcite Marble with Bacillus subtilis and Bacillus subtilis–Aspergillus niger Association. Crystals 2020, 10, 756. [Google Scholar] [CrossRef]
- Zhao, J.; Csetenyi, L.; Gadd, G.M. Fungal-induced CaCO3 and SrCO3 precipitation: A potential strategy for bioprotection of concrete. Sci. Total Environ. 2022, 816, 151501. [Google Scholar] [CrossRef]
- Cotter-Howells, J.; Caporn, S. Remediation of contaminated land by formation of heavy metal phosphates. Appl. Geochem. 1996, 11, 335–342. [Google Scholar] [CrossRef]
- Basta, N.; McGowen, S.L. Evaluation of Chemical Immobilization Treatments for Reducing Heavy Metal Transport in a Smelter-Contaminated Soil. Environ. Pollut. 2004, 127, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Yiwen, Z.; Maxwell, S.L.; Runting, R.K.; Carrasco, L.R. Environmental destruction not avoided with the Sustainable Development Goals. Nat. Sustain. 2020, 3, 795–798. [Google Scholar] [CrossRef]
- Bajda, T.; Rogowska, M.; Pawłowska, R.A. Synthesis and solubility of hopeite Zn3(PO4)2 ·4H2O. Mineralogia 2023, 54, 78–81. [Google Scholar] [CrossRef]
- Fomina, M.; Alexander, I.J.; Hillier, S.; Gadd, G.M. Zinc Phosphate and Pyromorphite Solubilization by Soil Plant-Symbiotic Fungi. Geomicrobiol. J. 2004, 21, 351–366. [Google Scholar] [CrossRef]
- Doilom, M.; Guo, J.-W.; Phookamsak, R.; Mortimer, P.E.; Karunarathna, S.C.; Dong, W.; Liao, C.-F.; Yan, K.; Dhandevi, P.; Suwannarach, N.; et al. Screening of Phosphate-Solubilizing Fungi From Air and Soil in Yunnan, China: Four Novel Species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol. 2020, 11, 585215. [Google Scholar] [CrossRef]
- Suyamud, B.; Ferrier, J.; Csetenyi, L.; Inthorn, D.; Gadd, G.M. Biotransformation of struvite by Aspergillus niger: Phosphate release and magnesium biomineralization as glushinskite. Environ. Microbiol. 2020, 22, 1588–1602. [Google Scholar] [CrossRef]
- Kang, X.; Csetenyi, L.; Gao, X.; Gadd, G.M. Solubilization of struvite and biorecovery of cerium by Aspergillus niger. Appl. Microbiol. Biotechnol. 2022, 106, 821–833. [Google Scholar] [CrossRef]
- Bhalla, K.; Qu, X.; Kretschmer, M.; Kronstad, J.W. The phosphate language of fungi. Trends Microbiol. 2022, 30, 338–349. [Google Scholar] [CrossRef]
- Leng, Y.; Colston, R.; Soares, A. Understanding the biochemical characteristics of struvite bio-mineralising microorganisms and their future in nutrient recovery. Chemosphere 2020, 247, 125799. [Google Scholar] [CrossRef]
- Leng, Y.; Soares, A. The mechanisms of struvite biomineralization in municipal wastewater. Sci. Total Environ. 2021, 799, 149261. [Google Scholar] [CrossRef]
- Soares, А.; Veesam, M.; Simoes, F.; Wood, E.; Parsons, S.A.; Stephenson, T. Bio-Struvite: A New Route to Recover Phosphorus from Wastewater. Clean—Soil Air Water 2014, 42, 994–997. [Google Scholar] [CrossRef]
- Ennever, J.; Summers, F.E. Calcification by Candida albicans. J. Bacteriol. 1975, 122, 1391–1393. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.J.; Hillier, S.; Gadd, G.M. Lead Transformation to Pyromorphite by Fungi. Curr. Biol. 2012, 22, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hao, R.; Xu, H.; Lu, A. Removal mechanism of Pb(II) by Penicillium polonicum: Immobilization, adsorption, and bioaccumulation. Sci. Rep. 2020, 10, 9079. [Google Scholar] [CrossRef]
- Liang, X.; Hillier, S.; Pendlowski, H. Uranium phosphate biomineralization by fungi. Environ. Microbiol. 2015, 17, 2064–2075. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Zelenskaya, M.S.; Izatulina, A.R.; Korneev, A.V.; Vlasov, D.Y.; Frank-Kamenetskaya, O.V. Carbonate and Oxalate Crystallization Effected by the Metabolism of Fungi and Bacteria in Various Trophic Conditions: The Case of Penicillium chrysogenum and Penicillium chrysogenum with Bacillus subtilis. Crystals 2023, 13, 94. [Google Scholar] [CrossRef]
- Grassi, С.; Cecchi, S.; Baldi, A.; Zanchi, C.A.; Orlandini, S.; Pardini, A.; Napoli, M. Crop suitability assessment in remediation of Zn contaminated soil. Chemosphere 2020, 246, 125706. [Google Scholar] [CrossRef]
- Van, H.-T.; Hoang, V.H.; Nga, L.T.; Nguyen, Q.V.Q. Effects of Zn pollution on soil: Pollution sources, impacts and solutions. Results Surf. Interfaces 2024, 17, 100360. [Google Scholar] [CrossRef]
- Barreto, M.S.C.; Elzinga, E.J.; Rouff, A.A.; Siebecker, M.G.; Sparks, D.L.; Alleoni, L.R.F. Zinc speciation in highly weathered tropical soils affected by large scale vegetable production. Sci. Total Environ. 2024, 916, 170223. [Google Scholar] [CrossRef]
- Rahman, S.H.; Khanam, D.; Adyel, T.M.; Islam, M.S.; Ahsan, M.A.; Akbor, M.A. Assessment of Heavy Metal Contamination of Agricultural Soil around Dhaka Export Processing Zone (DEPZ), Bangladesh: Implication of Seasonal Variation and Indices. Appl. Sci. 2012, 2, 584–601. [Google Scholar] [CrossRef]
- Salnikova, E.V.; Burtseva, T.I.; Skalnaya, M.G.; Skalny, A.V.; Tinkov, A.A. Copper and zinc levels in soil, water, wheat, and hair of inhabitants of three areas of the Orenburg region, Russia. Environ. Res. 2018, 166, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Khan, M.; Sheikh, T.M.M.; Mumtaz, M.Z.; Chohan, T.A.; Shamim, S.; Liu, Y. Zinc Essentiality, Toxicity, and Its Bacterial Bioremediation: A Comprehensive Insight. Front. Microbiol. 2023, 17, 1133733. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, S.; Rovina, K.; Al Azad, S. Heavy Metal Contaminants Removal from Wastewater Using the Potential Filamentous Fungi Biomass: A Review. J. Microb. Biochem. Technol. 2015, 7, 384–393. [Google Scholar] [CrossRef]
- Sutjaritvorakul, T.; Gadd, G.M.; Whalley, A.; Sihanonth, P. Zinc Oxalate Crystal Formation by Aspergillus nomius. Geomicrobiology 2015, 33, 289–293. [Google Scholar] [CrossRef]
- Sayer, J.A.; Gadd, G.M. Binding of cobalt and zinc by organic acids and culture filtrates of Aspergillus niger grown in the absence or presence of insoluble cobalt or zinc phosphate. Mycol. Res. 2001, 105, 1261–1267. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.; Vlasov, D. Crystallization induced by fungi and bacteria. Acta Cryst. 2025, B81 Pt 1, 1–10. [Google Scholar] [CrossRef]
- Giester, G.; Rieck, B.; Lengauer, C.L.; Kolitsch, U.; Nasdala, L. Katsarosite Zn(C2O4)·2H2O, a new Humboldtine-Group Mineral from the Lavrion Mining District, Greece. Mineral. Pet. 2023, 117, 259–267. [Google Scholar] [CrossRef]
- Zelenskaya, M.S.; Izatulina, A.R.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y. Iron Oxalate Humboldtine Crystallization by Fungus Aspergillus Niger. Cryst. 2021, 11, 1591. [Google Scholar] [CrossRef]
- Atencio, D.; Coutinho, J.; Graeser, S.; Matioli, P.; Lindbergite, F.L. A New Mn Oxalate Dihydrate from Boca Rica Mine, Galiléia, Minas Gerais, Brazil, and Other Occurences. Am. Mineral. 2004, 89, 1087–1091. [Google Scholar] [CrossRef]
- Baran, E. Review: Natural oxalates and their analogous synthetic complexes. J. Coord. Chem. 2014, 67, 23–24. [Google Scholar] [CrossRef]
- Korneev, A.V.; Izatulina, A.R.; Kuz’mina, M.A.; Frank-Kamenetskaya, O.V. Solid Solutions of Lindbergite–Glushinskite Series: Synthesis, Ionic Substitutions, Phase Transformation and Crystal Morphology. Int. J. Mol. Sci. 2022, 23, 14734. [Google Scholar] [CrossRef] [PubMed]
- Sazanova, K.V.; Zelenskaya, M.S.; Korneev, A.V.; Vlasov, D.Y. Extracellular Zn Detoxication by Penicillium chrysogenum and Aspergillus niger. Mycol. Phytopathol. 2023, 57, 425–434b. [Google Scholar] [CrossRef]
- Herschke, L.; Enkelmann, V.; Lieberwirth, I.; Wegner, G. The role of hydrogen bonding in the crystal structures of zinc phosphate hydrates. Chem.—Eur. J. 2004, 10, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate-Solubilizing Bacteria, and Silicon to P Uptake by Plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Vlasov, D.Y.; Osmolovskay, N.G.; Schiparev, S.M.; Rusakov, A.V. Significance and regulation of acids production by rock-inhabited fungi. In Biogenic—Abiogenic Interactions in Natural and Anthropogenic Systems. Lecture Notes in Earth System Sciences; Frank-Kamenetskaya, O., Panova, E., Vlasov, D., Eds.; Springer: Cham, Switzerland, 2016; pp. 379–392. [Google Scholar]
- Sturm, E.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Zelenskaya, M.S.; Sazanova, K.V.; Rusakov, A.V.; Kniep, R. Crystallization of calcium oxalate hydrates by interaction of calcite marble with fungus Aspergillus niger. Am. Mineral. 2015, 100, 2559–2565. [Google Scholar] [CrossRef]
- Krake, S.; Conzelmann, C.; Heuer, S. Production of chitosan from Aspergillus niger and quantitative evaluation of the process using adapted analytical tools. Biotechnol. Bioprocess 2024, E29, 942–954. [Google Scholar] [CrossRef]
- Rusakov, A.; Kuz’mina, M.; Frank-Kamenetskaya, O. Biofilm Medium Chemistry and Calcium Oxalate Morphogenesis. Molecules 2021, 26, 5030. [Google Scholar] [CrossRef]
- Fomina, M.; Hillier, S.; Charnock, J.M.; Melville, K.; Alexander, I.J.; Gadd, G.M. Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveriacaledonica. Appl. Environ. Microbiol. 2005, 71, 371–381. [Google Scholar] [CrossRef]
- Hess, C.D.; Lu, W.; Rabinowitz, D.J.; Botstein, D. Ammonium toxicity and potassium limitation in yeast. PLoS Biol. 2006, 4, 2012–2023. [Google Scholar] [CrossRef]
- Berenjian, A.; Seifan, M. Mineral Formation by Microorganisms. Concepts and Applications; Berenjian, A., Seifan, M., Eds.; Springer: Cham, Switzerland, 2022; 387p. [Google Scholar]
- Colfen, H.; Mesocrystals, A.M. Nonclassical Crystallization; John Wiley & Sons: Hoboken, NJ, USA, 2008; 288p. [Google Scholar]
- L’vov, P.E.; Umantsev, A.R. Two-Step Mechanism of Macromolecular Nucleation and Crystallization: Field Theory and Simulations. Cryst. Growth Des. 2021, 21, 366–382. [Google Scholar] [CrossRef]
- Askhabov, A.M. Problem of building units in crystal growth and genesis of non-classical concepts of crystal formation. Vestn. Geosci. 2022, 11, 20–24. [Google Scholar] [CrossRef]
| Zn Concentration, μmol | Age of Culture, Days | Organic Acids, μg/mL | H2PO4− + HPO42− + PO43−, μg/mL | pH | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Soluble | Oxalic | Citric | Soluble | Insoluble | |||
| Soluble | Insoluble | Soluble | ||||||
| 0 | 0 | 14 | 197 ± 20 | 180 ± 35 | 280 ± 39 | 412 ± 93 | - | 2.8 |
| 28 | 222 ± 36 | 257 ± 29 | 449 ± 39 | 134 ± 18 | - | 2.0 | ||
| 250 | 192 | 14 | 127 ± 22 | 344 ± 43 | 103 ± 24 | 375 ± 41 | - | 4.2 |
| 28 | 204 ± 25 | 388 ± 56 | 57 ± 18 | 176 ± 22 | - | 3.9 | ||
| 500 | 432 | 14 | 105 ± 28 | 480 ± 47 | 68 ± 16 | 486 ± 64 | - | 5.0 |
| 28 | 110 ± 18 | 522 ± 28 | 52 ± 21 | 212 ± 37 | - | 5.2 | ||
| 1000 | 639 | 14 | 187 ± 34 | 640 ± 56 | 72 ± 11 | 375 ± 19 | - | 2.5 |
| 28 | 255 ± 37 | 720 ± 66 | 38 ± 7 | 176 ± 11 | - | 2.4 | ||
| 2000 | 1626 | 14 | - | - | - | 540 ± 52 | 22 ± 8 | 6.0 |
| 28 | 302 ± 8 | 988 ± 88 | - | 202 ± 64 | - | 2.8 | ||
| Zn Concentration, μmol | Age of Culture, Days | Organic Acids, μg/mL | H2PO4− + HPO42− + PO43−, μg/mL | pH | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Soluble | Oxalic | Citric | Soluble | Insoluble | |||
| Soluble | Insoluble | Soluble | ||||||
| 0 | 0 | 14 | - | 38 ± 6 | 3.1 ± 0.8 | 304 ± 18 | - | 8.0 |
| 28 | - | 64 ± 8 | trace | 194 ± 16 | - | 9.0 | ||
| 250 | 192 | 14 | - | 72 ± 11 | 4.8 ± 0.6 | 218 ± 18 | 35 ± 14 | 7.5 |
| 28 | - | 86 ± 22 | trace | 184 ± 12 | 29 ± 8 | 9.0 | ||
| 500 | 432 | 14 | - | 104 ± 9 | 6.4 ± 0.8 | 222 ± 9 | 48 ± 14 | 8.0 |
| 28 | - | 122 ± 86 | 5.5 ± 0.8 | 129 ± 8 | 37 ± 9 | 8.0 | ||
| 1000 | 639 | 14 | - | 52 ± 11 | 4.4 ± 0.7 | 228 ± 6 | 56 ± 22 | 6.5 |
| 28 | - | 108 ± 22 | 6.8 ± 0.6 | 117 ± 4 | 75 ± 13 | 8.0 | ||
| 2000 | 1626 | 14 | - | 34 ± 8 | 4.1 ± 0.4 | 234 ± 6 | 74 ± 28 | 4.8 |
| 28 | - | 42 ± 13 | 5.1 ± 0.7 | 111 ± 4 | 108 ± 31 | 6.3 | ||
| Days | Zn Concentration in Solution, μmol | |||||||
|---|---|---|---|---|---|---|---|---|
| 250 | 500 | 1000 | 2000 | |||||
| Phase Composition | pH | Phase Composition | pH | Phase Composition | pH | Phase Composition | pH | |
| 7 | Hop (ab), Zn-MgOx (α) | 3.8 | Hop (ab), Zn-MgOx (α) >> Mg-Kat | 4.6 | Hop (ab) | 6.5 | No fungal growth | 6.5 |
| 14 | Zn-MgOx | 4.5 | Zn-MgOx (α) >> Mg-Kat | 4.8 | Zn-MgOx (α)~ Mg-Kat | 2.5 | Hop (ab), Hop (bio) | 6.0 |
| 21 | 4.2 | 4.0 | 2.0 | Kat, Hop (ab), Hop (bio) | 5.0 | |||
| 28 | Zn-MgOx (α) >> Gl (β) | 4.0 | Zn-MgOx (α) >> Mg-Kat | 4.2 | 2.5 | Kat | 2.5 | |
| Days | Zn Concentration in Solution, μmol | |||||||
|---|---|---|---|---|---|---|---|---|
| 250 | 500 | 1000 | 2000 | |||||
| Phase Composition | pH | Phase Composition | pH | Phase Composition | pH | Phase Composition | pH | |
| 7 | Hop (ab) | 7.0 | Hop (ab) | 7.0 | Hop (ab) | 6.0 | No fungal growth | 6.0 |
| 14 | 7.5 | 8.0 | Hop (bio), Hop (ab) | 6.5 | Hop (ab) | 4.8 | ||
| 21 | 6.9 | 8.0 | Hop (bio), Hop (ab), (Zn0.5Mg0.5)Ox (α) > (Zn0.5Mg0.5)Ox (α) > Zn-MgOx (α) | 7.0 | Hop (bio), Hop(ab) >> Kat | 5.6 | ||
| 28 | Hop(ab), Zn-MgOx (α) > (Zn0.5Mg0.5)Ox (α) | 9.0 | Hop (ab), (Zn0.5Mg0.5)Ox (α) > Zn-MgOx (α) | 8.0 | 8.0 | Hop (bio), Hop(ab), Kat | 6.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sazanova, K.V.; Zelenskaya, M.S.; Korneev, A.V.; Bakhvalova, E.V.; Vlasov, D.Y.; Frank-Kamenetskaya, O.V. Effect of Fungal Metabolism on Zn Minerals Formation: The Case of Aspergillus niger and Penicillium chrysogenum. Crystals 2025, 15, 118. https://doi.org/10.3390/cryst15020118
Sazanova KV, Zelenskaya MS, Korneev AV, Bakhvalova EV, Vlasov DY, Frank-Kamenetskaya OV. Effect of Fungal Metabolism on Zn Minerals Formation: The Case of Aspergillus niger and Penicillium chrysogenum. Crystals. 2025; 15(2):118. https://doi.org/10.3390/cryst15020118
Chicago/Turabian StyleSazanova, Katerina V., Marina S. Zelenskaya, Anatoliy V. Korneev, Elena V. Bakhvalova, Dmitry Yu. Vlasov, and Olga V. Frank-Kamenetskaya. 2025. "Effect of Fungal Metabolism on Zn Minerals Formation: The Case of Aspergillus niger and Penicillium chrysogenum" Crystals 15, no. 2: 118. https://doi.org/10.3390/cryst15020118
APA StyleSazanova, K. V., Zelenskaya, M. S., Korneev, A. V., Bakhvalova, E. V., Vlasov, D. Y., & Frank-Kamenetskaya, O. V. (2025). Effect of Fungal Metabolism on Zn Minerals Formation: The Case of Aspergillus niger and Penicillium chrysogenum. Crystals, 15(2), 118. https://doi.org/10.3390/cryst15020118









