Comparative Study of Gemological and Spectroscopic Features and Coloration Mechanism of Three Types of Spodumene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Conventional Gemological Features
3.2. Crystal Structure and Chemical Composition Analysis
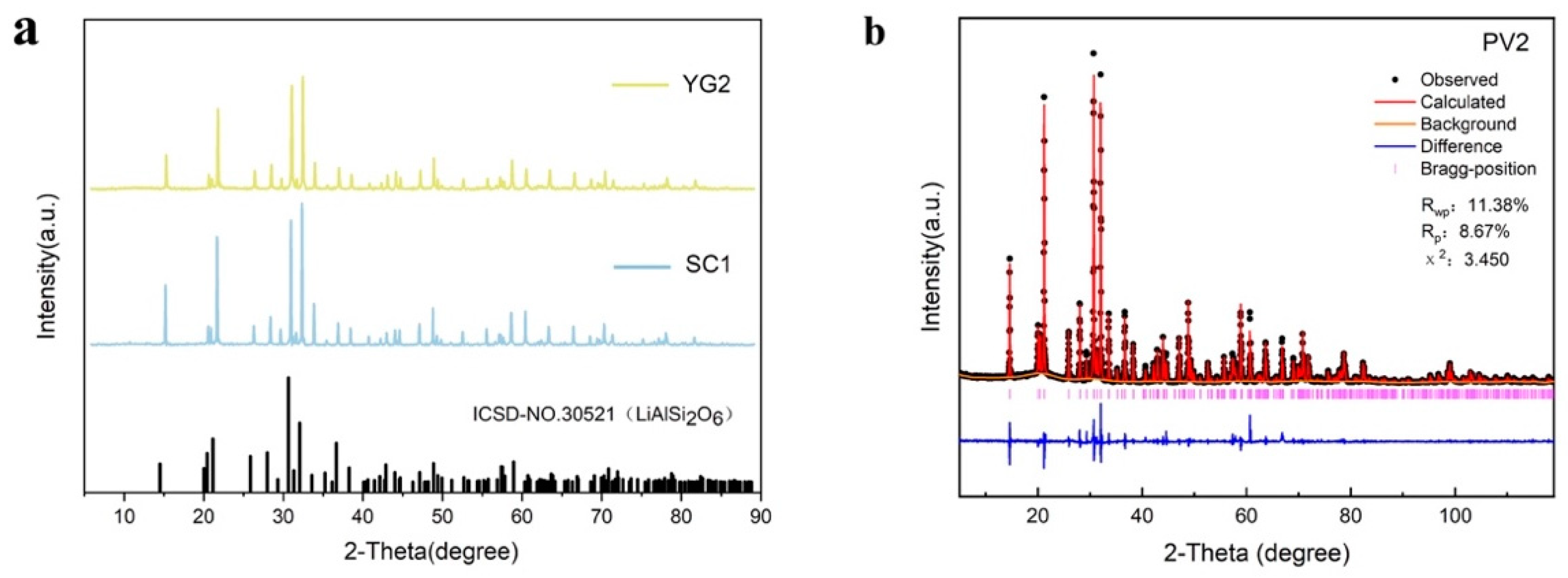
3.2.1. XRD Testing of Spodumene
3.2.2. Electron Microprobe Analysis
3.3. Spectral Characteristics and Chromogenic Mechanism Analysis
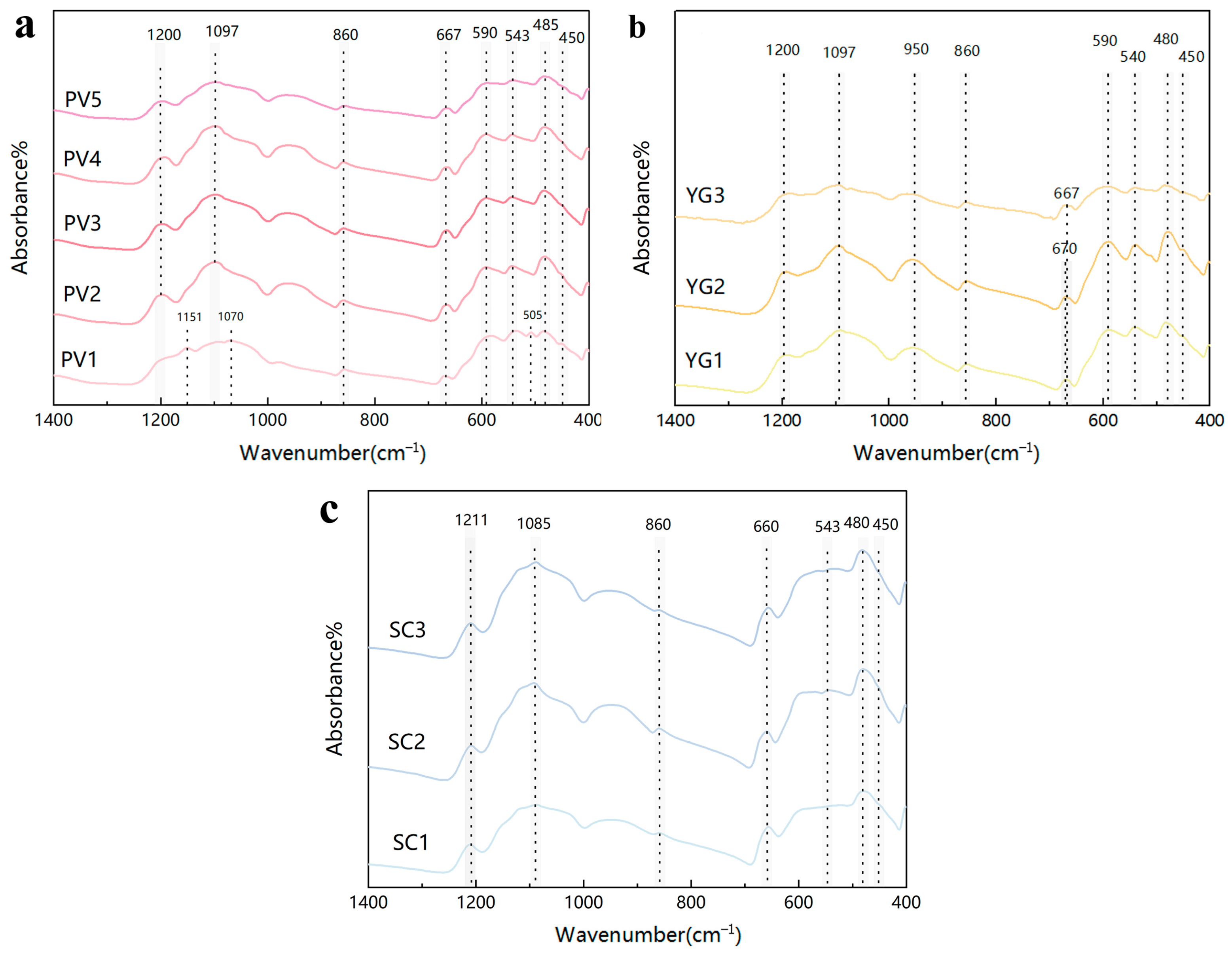
3.3.1. Infrared Spectra Analysis
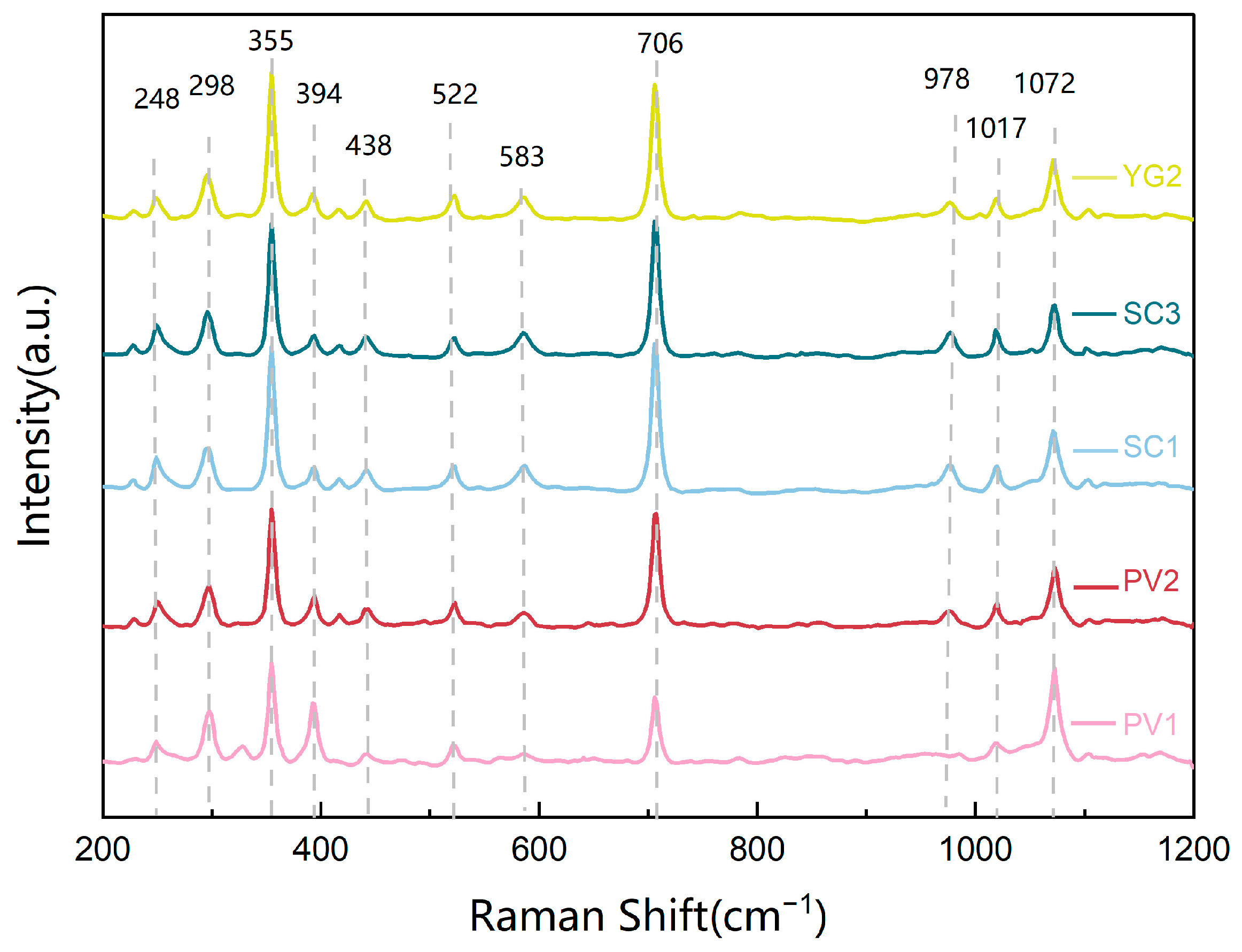
3.3.2. Raman Spectra Analysis
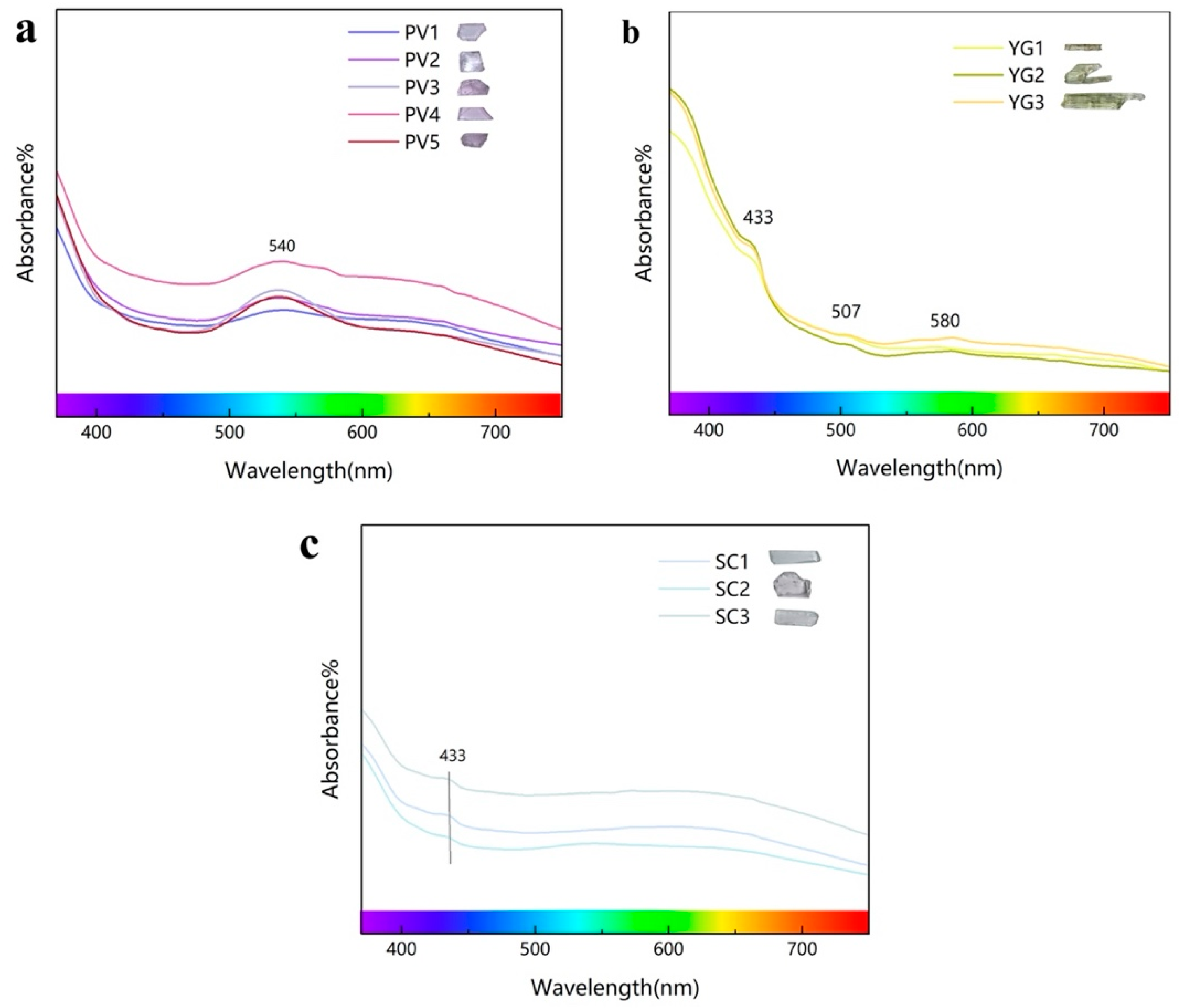
3.4. Ultraviolet–Visible Spectrum Analysis
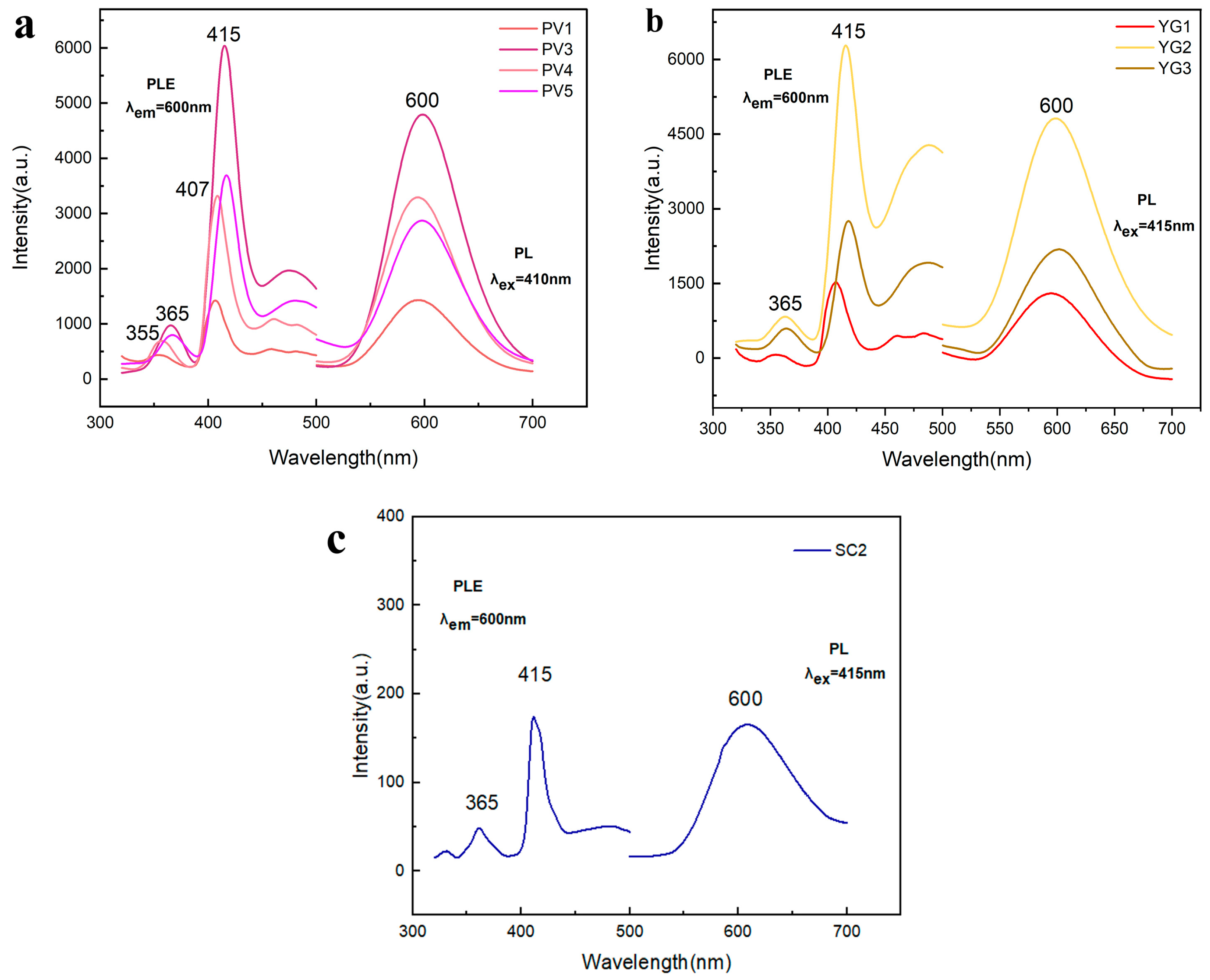
3.5. Luminescence Properties of Spodumene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Ma, B.; Lü, Y.; Wang, C.; Chen, Y. A review of lithium extraction from natural resources. Int. J. Miner. Metall. Mater. 2023, 30, 209–224. [Google Scholar] [CrossRef]
- Mohr, S.H.; Mudd, G.M.; Giurco, D. Lithium Resources and Production: Critical Assessment and Global Projections. Minerals 2012, 2, 65–84. [Google Scholar] [CrossRef]
- Sheikhi Gheshlaghi, R.; Ghorbani, M.; Sepahi, A.A.; Deevsalar, R.; Nakashima, K.; Shinjo, R. The origin of gem spodumene in the Hamadan Pegmatite, Alvand Plutonic Complex, western Iran. Can. Mineral. 2022, 60, 249–266. [Google Scholar] [CrossRef]
- Dessemond, C.; Soucy, G.; Harvey, J.-P.; Ouzilleau, P. Phase Transitions in the α–γ–β Spodumene Thermodynamic System and Impact of γ-Spodumene on the Efficiency of Lithium Extraction by Acid Leaching. Minerals 2020, 10, 519. [Google Scholar] [CrossRef]
- Mattson, S.M.; Rossman, G.R. Identifying characteristics of charge transfer transitions in minerals. Phys. Chem. Miner. 1987, 14, 94–99. [Google Scholar] [CrossRef]
- Walker, G.; El Jaer, A.; Sherlock, R.; Glynn, T.; Czaja, M.; Mazurak, Z. Luminescence spectroscopy of Cr3+ and Mn2+ in spodumene (LiAlSi2O6). J. Lumin. 1997, 72, 278–280. [Google Scholar] [CrossRef]
- Claffy, E.W. Composition, tenebrescence and luminescence of spodumene minerals. Am. Mineral. J. Earth Planet. Mater. 1953, 38, 919–931. [Google Scholar]
- Schmitz, B.; Lehmann, G. Color centers of manganese in natural spodumene LiAlSi2O6. Berichte Der Bunsenges. Für Phys. Chem. 1975, 79, 1044–1049. [Google Scholar] [CrossRef]
- Noithong, P.; Pakkong, P.; Naemchanthara, K. Color change of Spodumene gemstone by Electron beam irradiation. Adv. Mater. Res. 2013, 770, 370–373. [Google Scholar] [CrossRef]
- Czaja, M.; Lisiecki, R.; Kądziołka-Gaweł, M.; Winiarski, A. Some complementary data about the spectroscopic properties of manganese ions in spodumene crystals. Minerals 2020, 10, 554. [Google Scholar] [CrossRef]
- Rehman, H.U.; Martens, G.; Tsai, Y.L.; Chankhantha, C.; Kidkhunthod, P.; Shen, A.H. An X-ray Absorption Near-Edge Structure (XANES) Study on the Oxidation State of Chromophores in Natural Kunzite Samples from Nuristan, Afghanistan. Minerals 2020, 10, 463. [Google Scholar] [CrossRef]
- Ito, A.S.; Isotani, S. Optical Absorption and Electron Spin Resonance in Natural, Irradiated and Heated Spodumene; Sao Paulo Univ. (Brazil). Inst. de Fisica: Sao Paulo, Brazil, 1983. [Google Scholar]
- Ito, A.S.; Isotani, S. Heating effects on the optical absorption spectra of irradiated, natural spodumene. Radiat. Eff. Defects Solids 1991, 116, 307–314. [Google Scholar] [CrossRef]
- Graham, J. Some notes on α-spodumene, LiAlSi2O6. Am. Mineral. J. Earth Planet. Mater. 1975, 60, 919–923. [Google Scholar]
- Filip, J.; Novák, M.; Beran, A.; Zbořil, R. Crystal chemistry and OH defect concentrations in spodumene from different granitic pegmatites. Phys. Chem. Miner. 2006, 32, 733–746. [Google Scholar] [CrossRef]
- Wen, L.; Liang, W.; Zhang, Z. Mineral Infrared Spectroscopy; Chongqing University Press: Chongqing, China, 1989. (In Chinese) [Google Scholar]
- Kuang, G.; Chen, Z.B.; Guo, H.; Li, M.H. Lithium extraction mechanism from α-spodumene by fluorine chemical method. Adv. Mater. Res. 2012, 524, 2011–2016. [Google Scholar] [CrossRef]
- Bowey, J.; Hofmeister, A.; Keppel, E. Infrared spectra of pyroxenes (crystalline chain silicates) at room temperature. Mon. Not. R. Astron. Soc. 2020, 497, 3658–3673. [Google Scholar] [CrossRef]
- Sharma, S.K.; Simons, B.; Yoder, H. Raman study of anorthite, calcium Tschermak’s pyroxene, and gehlenite in crystalline and glassy states. Am. Mineral. 1983, 68, 1113–1125. [Google Scholar]
- Prencipe, M.; Mantovani, L.; Tribaudino, M.; Bersani, D.; Lottici, P.P. The Raman spectrum of diopside: A comparison between ab initio calculated and experimentally measured frequencies. Eur. J. Mineral. 2012, 24, 457–464. [Google Scholar] [CrossRef]
- Buzatu, A.; Buzgar, N. The Raman study of single-chain silicates. Analele Stiintifice Univ. AI Cuza Din Iasi. Sect. 2 Geol. 2010, 56, 107. [Google Scholar]
- Sharma, S.K.; Simons, B. Raman study of crystalline polymorphs and glasses of spodumene composition quenched from various pressures. Am. Mineral. 1981, 66, 118–126. [Google Scholar]
- Souza, S.; Ferraz, G.; Watanabe, S. Effects of Mn and Fe impurities on the TL and EPR properties of artificial spodumene polycrystals under irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2004, 218, 259–263. [Google Scholar] [CrossRef]
- Souza, S.; Watanabe, S.; Lima, A.; Lalic, M. Thermoluminescent mechanism in lilac spodumene. Acta Phys. Pol. A 2007, 112, 1001–1006. [Google Scholar] [CrossRef]
- Burns, R.G. Mineralogical Applications of Crystal Field Theory; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Oliveira, R.A.P.; Mello, A.C.S.; Lima, H.R.B.R.; Campos, S.S.; Souza, S.O. Radiation detection using the color changes of lilac spodumene. In Proceedings of the International Nuclear Atlantic Conference (INAC 2009), Rio de Janeiro, RJ, Brazil, 27 September–2 October 2009. [Google Scholar]
- Farges, F.; Panczer, G.; Benbalagh, N.; Riondet, G. THE GRAND SAPPHIRE OF LOUIS XIV AND THE RUSPOLI SAPPHIRE: HISTORICAL AND GEMOLOGICAL DISCOVERIES. Gems Gemol. 2015, 51, 392–409. [Google Scholar] [CrossRef]
- Burns, R.G. Crystal field spectra and evidence of cation ordering in olivine minerals. Am. Mineral. J. Earth Planet. Mater. 1970, 55, 1608–1632. [Google Scholar]
- Chen, L.; Yang, W.; Fu, H.; Liu, W.; Shao, G.; Tang, B.; Zheng, J. Mn2+-doped Cs2NaInCl6 double perovskites and their photoluminescence properties. J. Mater. Sci. 2021, 56, 8048–8059. [Google Scholar] [CrossRef]
- Zhu, S.-Y.; Zhao, D.; Liu, W. A broad emission band of phosphor Cs2Zn3(P2O7)2:Mn2+ induced by multi-sites of Mn2+. Inorg. Chem. Commun. 2023, 150, 110397. [Google Scholar] [CrossRef]
- Czaja, M.; Lisiecki, R.; Kądziołka-Gaweł, M.; Winiarski, A.; Krzykawski, T. The afterglow effect of Mn-bearing natural LiAlSi2O6 spodumene crystals. Opt. Mater. 2019, 96, 109321. [Google Scholar] [CrossRef]
- Song, E.; Ding, S.; Wu, M.; Ye, S.; Xiao, F.; Zhou, S.; Zhang, Q. Anomalous NIR Luminescence in Mn2+-Doped Fluoride Perovskite Nanocrystals. Adv. Opt. Mater. 2014, 2, 670–678. [Google Scholar] [CrossRef]
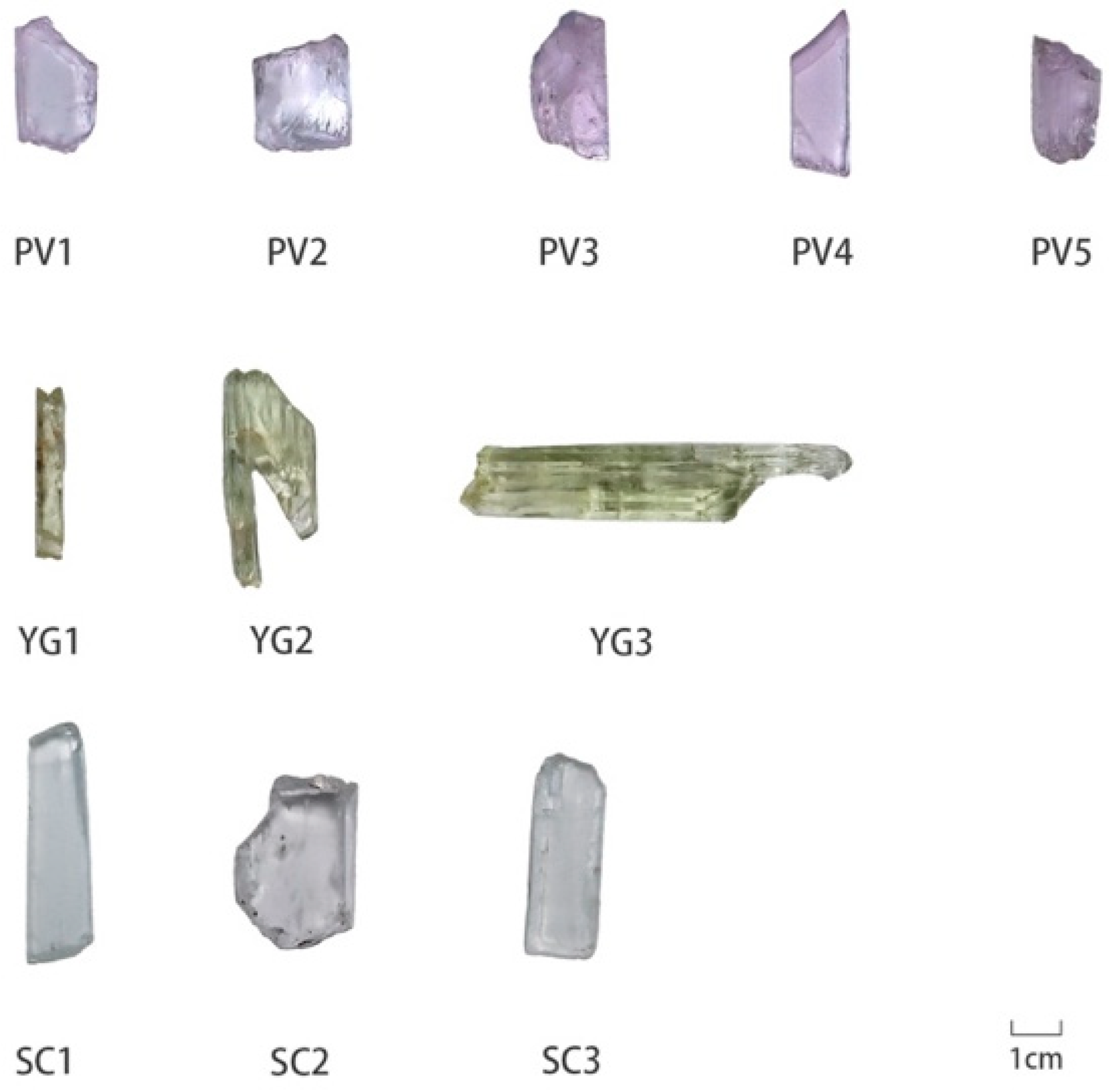
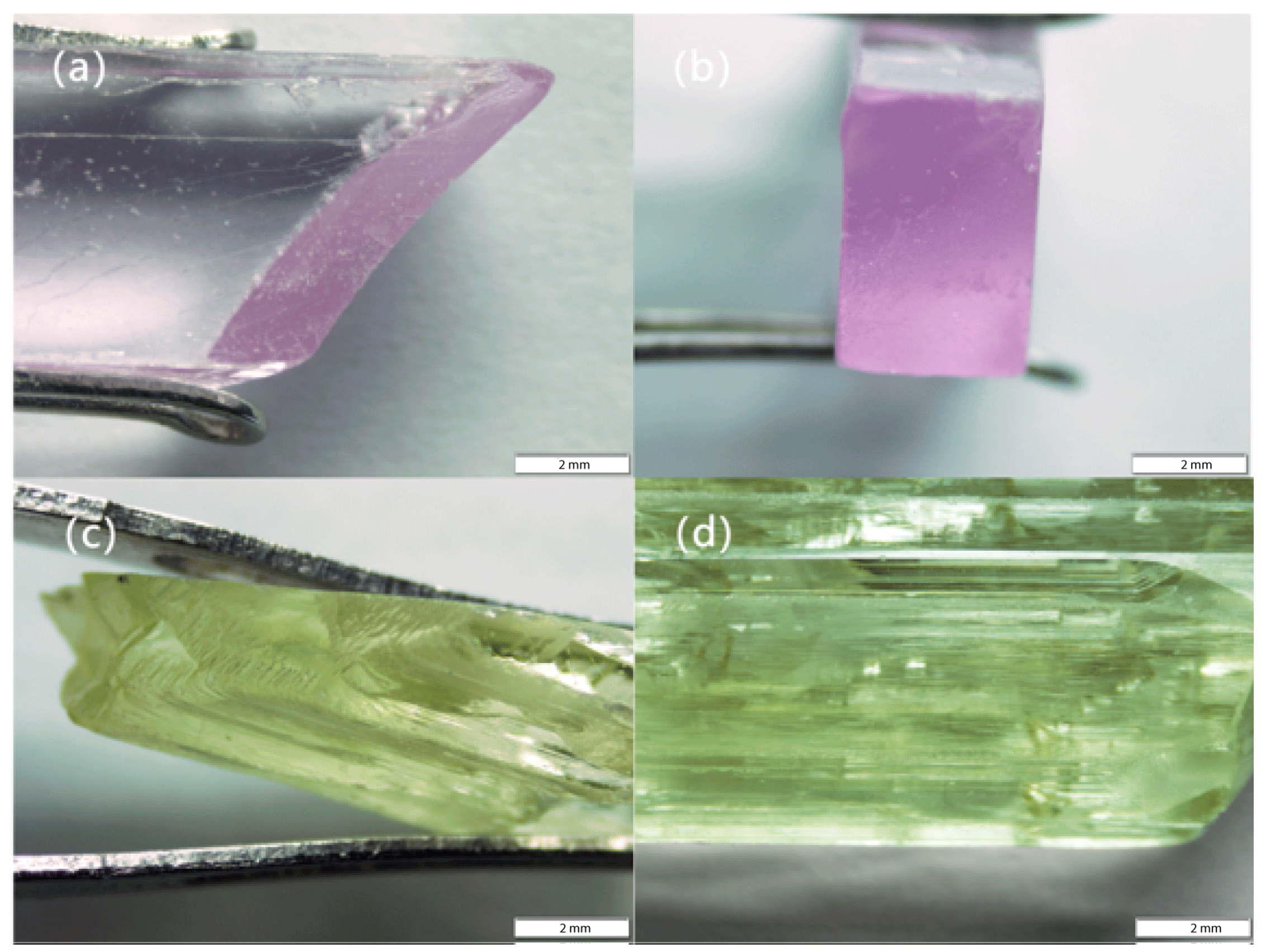

| Sample | Color | Transparency | Birefringence | Refractive Index | Weight (ct) | Specific Gravity |
|---|---|---|---|---|---|---|
| PV1 | pink to violet | semitransparent | 0.015 | 1.662–1.668 | 0.55 | 3.18 |
| PV2 | pink to violet | semitransparent | 0.014 | 1.668–1.673 | 1.49 | 3.17 |
| PV3 | pink to violet | semitransparent | 0.015 | 1.662–1.670 | 1.33 | 3.19 |
| PV4 | pink to violet | transparent | 0.015 | 1.663–1.671 | 0.89 | 3.18 |
| PV5 | pink to violet | semitransparent | 0.016 | 1.665–1.673 | 1.08 | 3.18 |
| YG1 | yellow-green | semitransparent | 0.015 | 1.663–1.668 | 0.31 | 3.22 |
| YG2 | yellow-green | transparent | 0.015 | 1.662–1.670 | 0.97 | 3.16 |
| YG3 | yellow-green | semitransparent | 0.016 | 1.671–1.676 | 2.27 | 3.20 |
| SC1 | colorless | transparent | 0.015 | 1.663–1.671 | 0.82 | 3.15 |
| SC2 | colorless | transparent | 0.015 | 1.662–1.670 | 1.21 | 3.18 |
| SC3 | colorless | transparent | 0.014 | 1.663–1.668 | 0.54 | 3.18 |
| a | b | c | alpha | beta | gamma | Volume | Rwp | Rp | χ2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Value | 9.475689 | 8.400311 | 5.226055 | 90.000 | 110.176 | 90.000 | 390.462 | 11.38% | 8.67% | 3.450 |
| Sigmas | 0.000095 | 0.000052 | 0.000082 | 0.000 | 0.001 | 0.000 | 0.008 |
| Si-O Stretching Vibrations and M-O Bending Vibrations | Si-O-Si Bending Vibrations | Si-O Stretching Vibrations | |
|---|---|---|---|
| Infrared Characteristic peak/cm−1 | 590 cm−1 543 cm−1 485 cm−1 450 cm−1 | 667 cm−1 | 1200 cm−1 1097 cm−1 860 cm−1 |
| M-O Bending Vibration | O-Si-O Bending Vibration | Si-Obr Stretching Vibrations | Si-Onbr Asymmetric Stretching Vibration | |
|---|---|---|---|---|
| Raman Characteristic peak/cm−1 | 248 cm−1 298 cm−1 355 cm−1 394 cm−1 438 cm−1 | 522 cm−1 583 cm−1 | 706 cm−1 978 cm−1 | 1017 cm−1 1072 cm−1 |
| Sample | Color | Characteristic Peak | Assignment |
|---|---|---|---|
| PV1–5 | pink to violet | 540 nm | Mn3+ (5E → 5T2) |
| YG1–3 | yellow-green | 433 nm | Fe3+ (6A1 → 4E + 4A1) |
| 507 nm | Fe2+ (5T2 → 5E) | ||
| 580 nm | |||
| SC1–3 | colorless | 433 nm | Fe3+ (6A1 → 4E + 4A1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Liu, J.; He, K.; Yang, B.; Rao, Y. Comparative Study of Gemological and Spectroscopic Features and Coloration Mechanism of Three Types of Spodumene. Crystals 2025, 15, 109. https://doi.org/10.3390/cryst15020109
Zhou Z, Liu J, He K, Yang B, Rao Y. Comparative Study of Gemological and Spectroscopic Features and Coloration Mechanism of Three Types of Spodumene. Crystals. 2025; 15(2):109. https://doi.org/10.3390/cryst15020109
Chicago/Turabian StyleZhou, Zijia, Jing Liu, Kui He, Biao Yang, and Yinghua Rao. 2025. "Comparative Study of Gemological and Spectroscopic Features and Coloration Mechanism of Three Types of Spodumene" Crystals 15, no. 2: 109. https://doi.org/10.3390/cryst15020109
APA StyleZhou, Z., Liu, J., He, K., Yang, B., & Rao, Y. (2025). Comparative Study of Gemological and Spectroscopic Features and Coloration Mechanism of Three Types of Spodumene. Crystals, 15(2), 109. https://doi.org/10.3390/cryst15020109







