Chromium Substitution Within Ruthenium Oxide Aerogels Enables High Activity Oxygen Evolution Electrocatalysts for Water Splitting
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
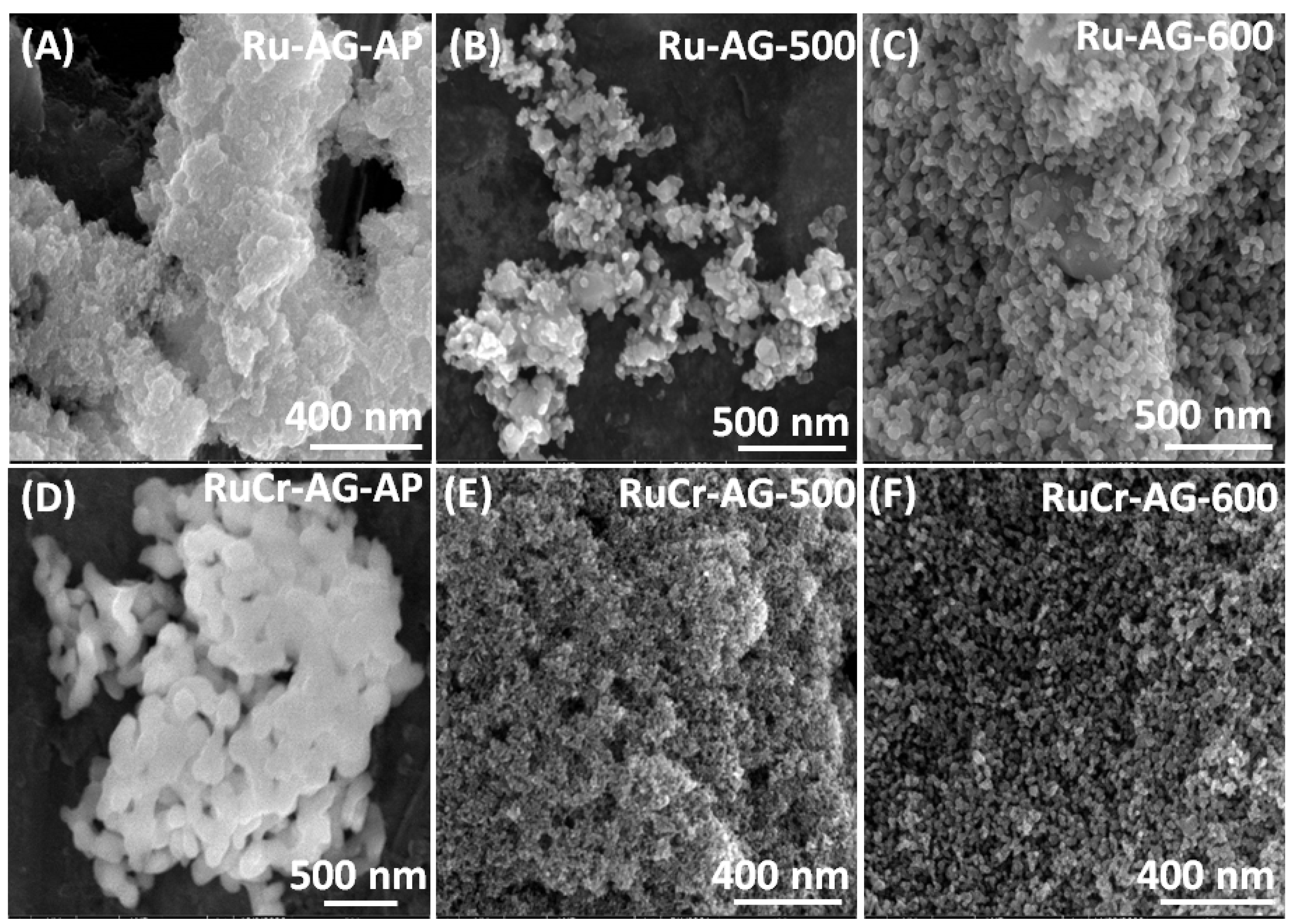
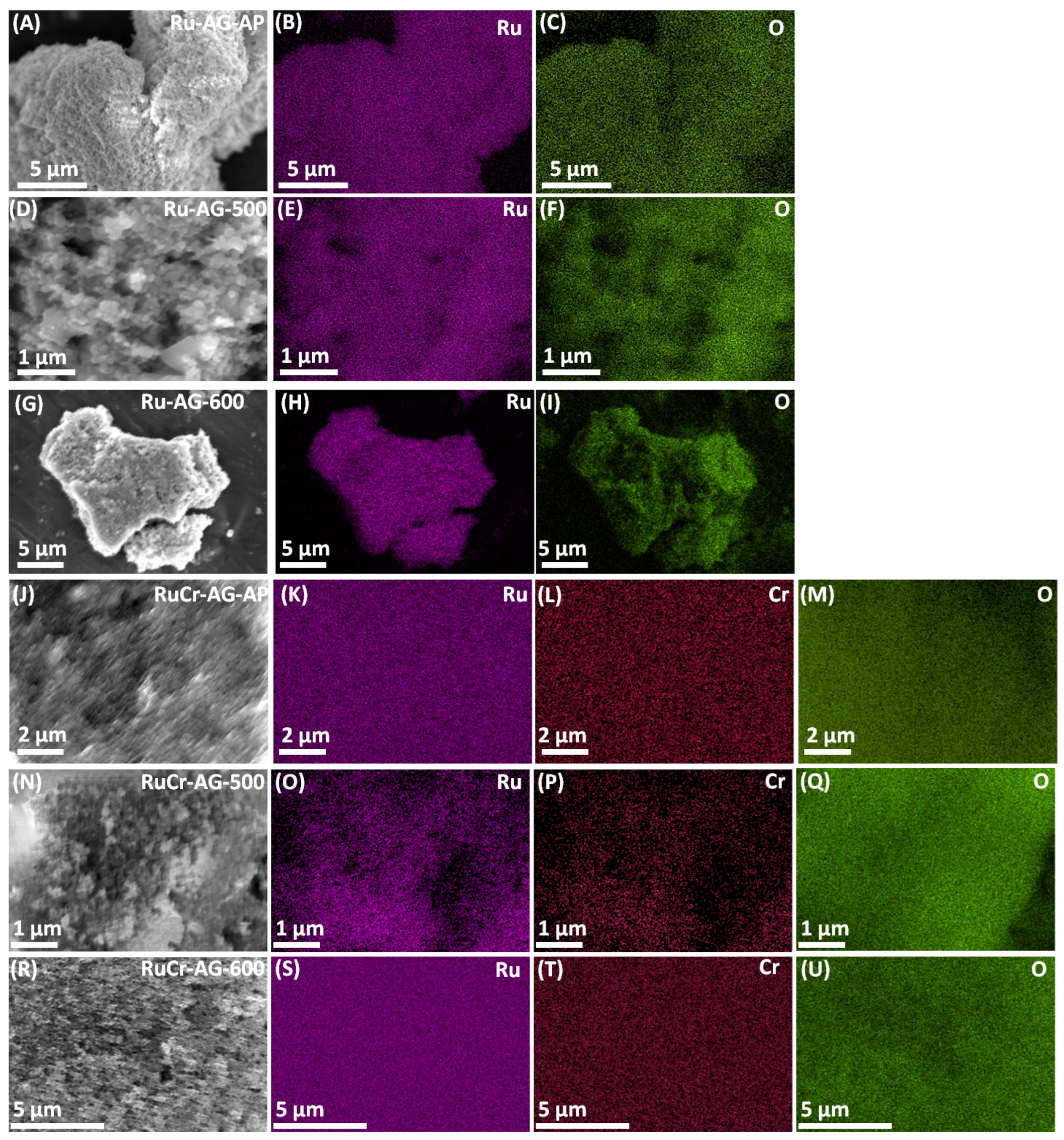
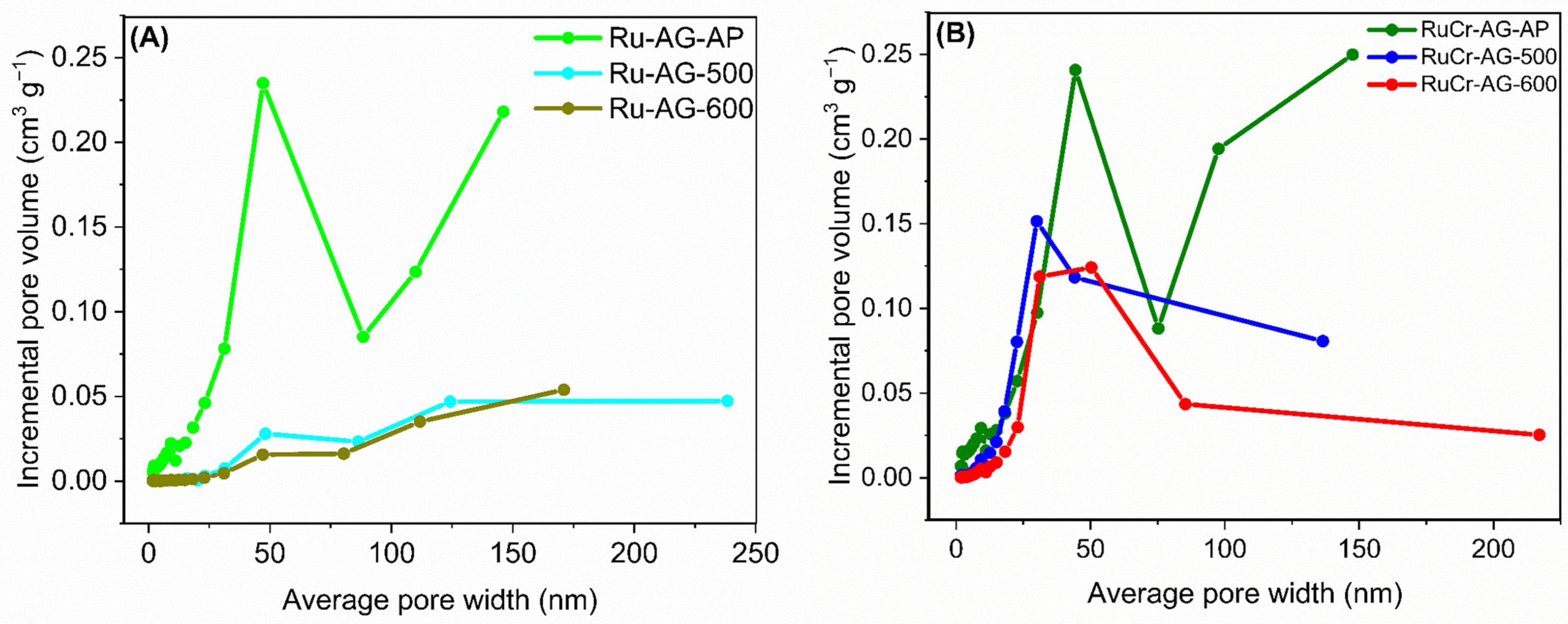
3.1. Analysis of Morphology and Elemental Composition
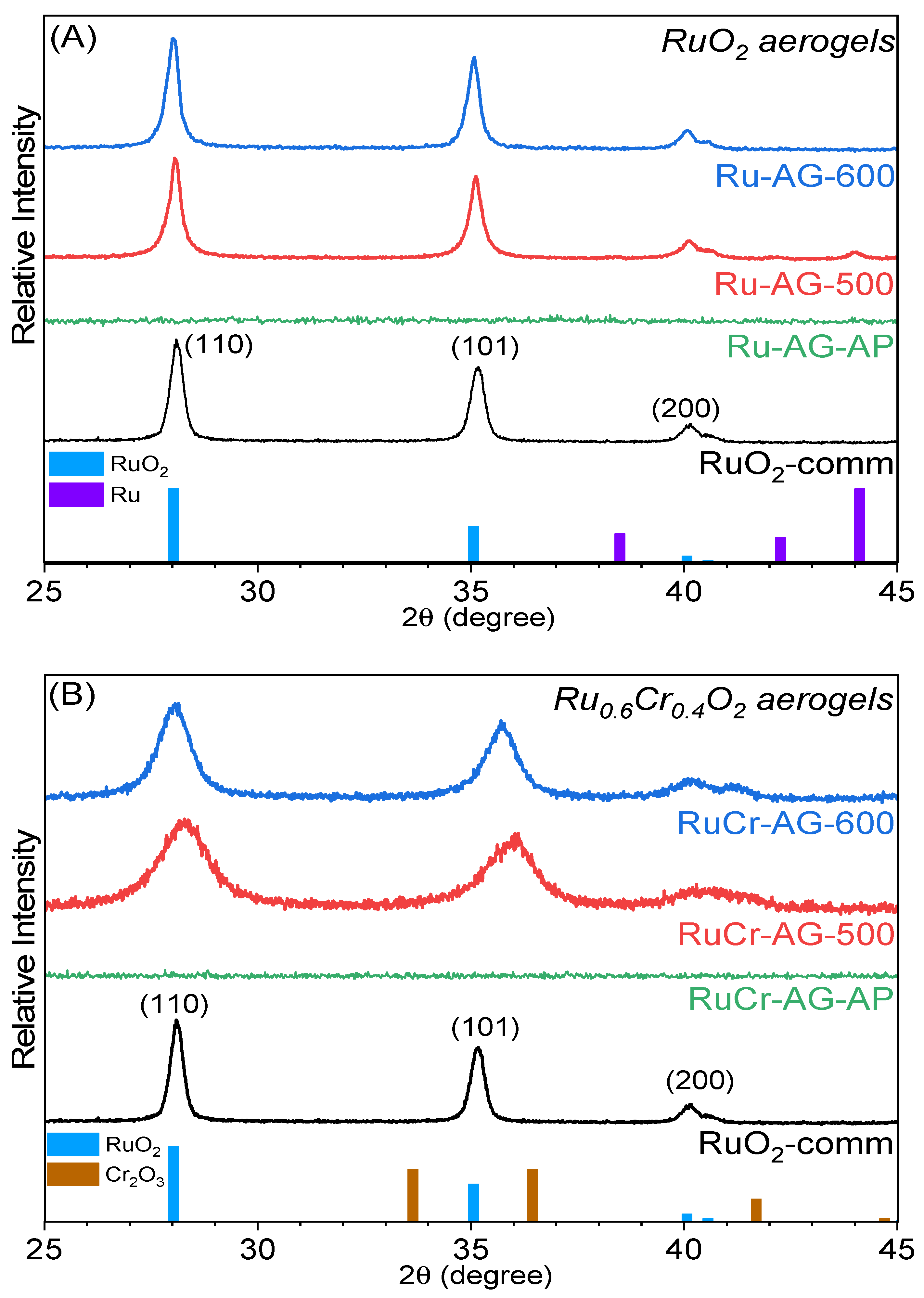
3.2. X-Ray Diffraction Characterization
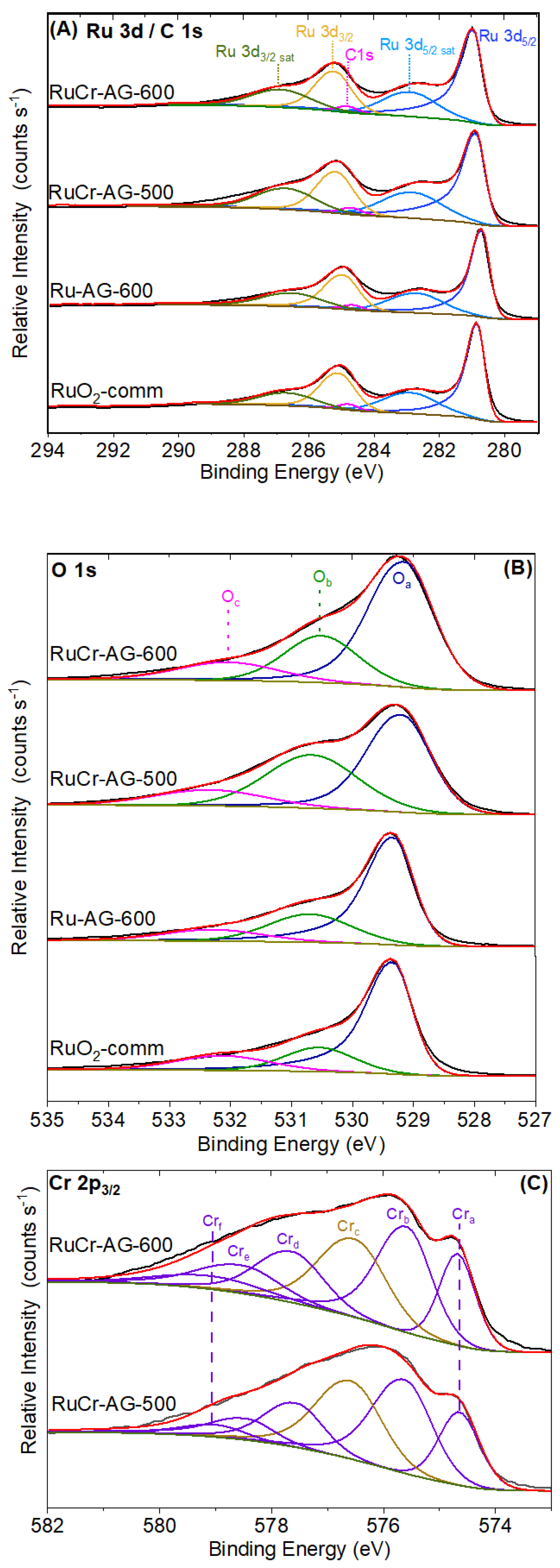
3.3. X-Ray Photoelectron Spectroscopy Characterization
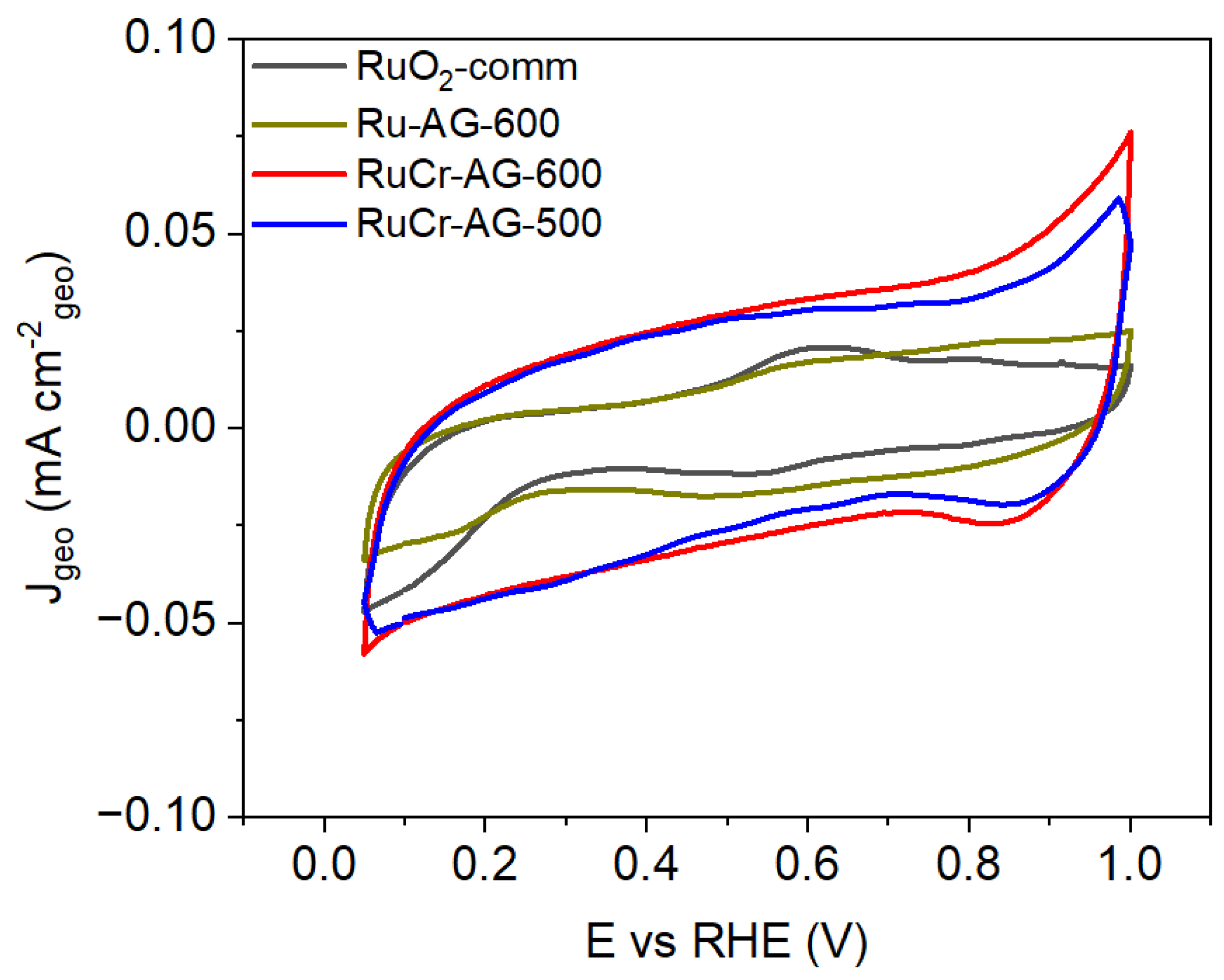
3.4. Cyclic Voltammetric Characterization
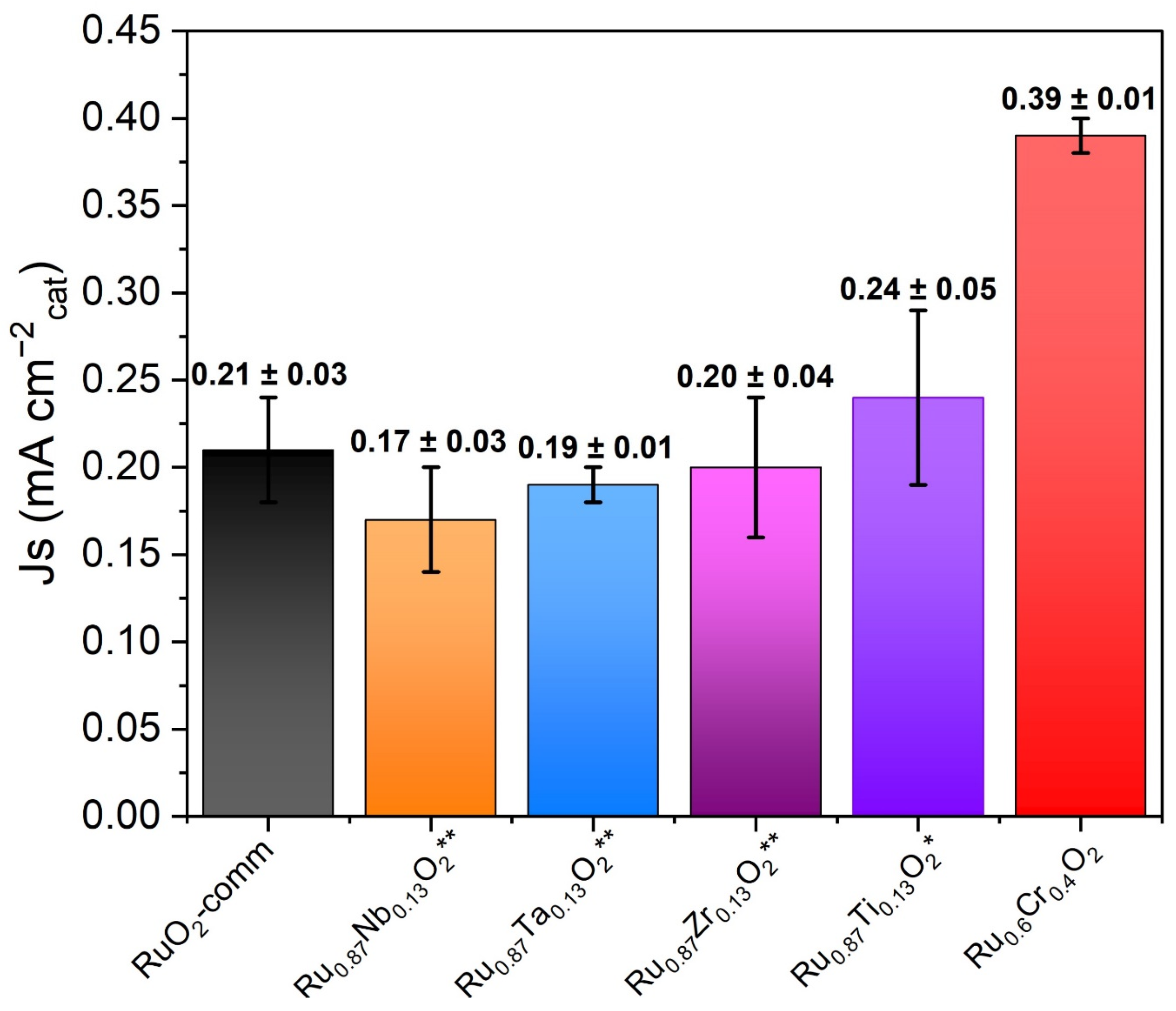
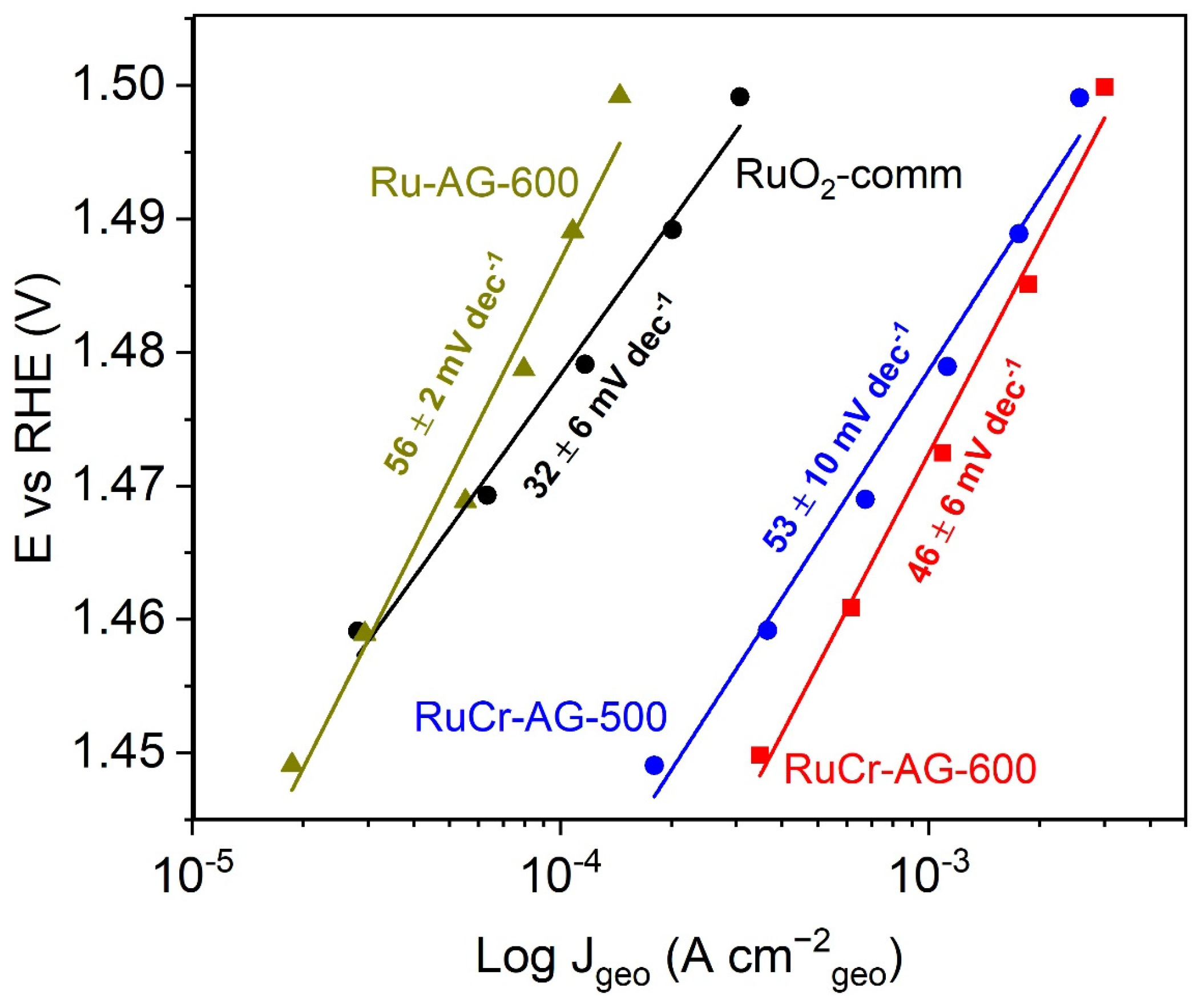
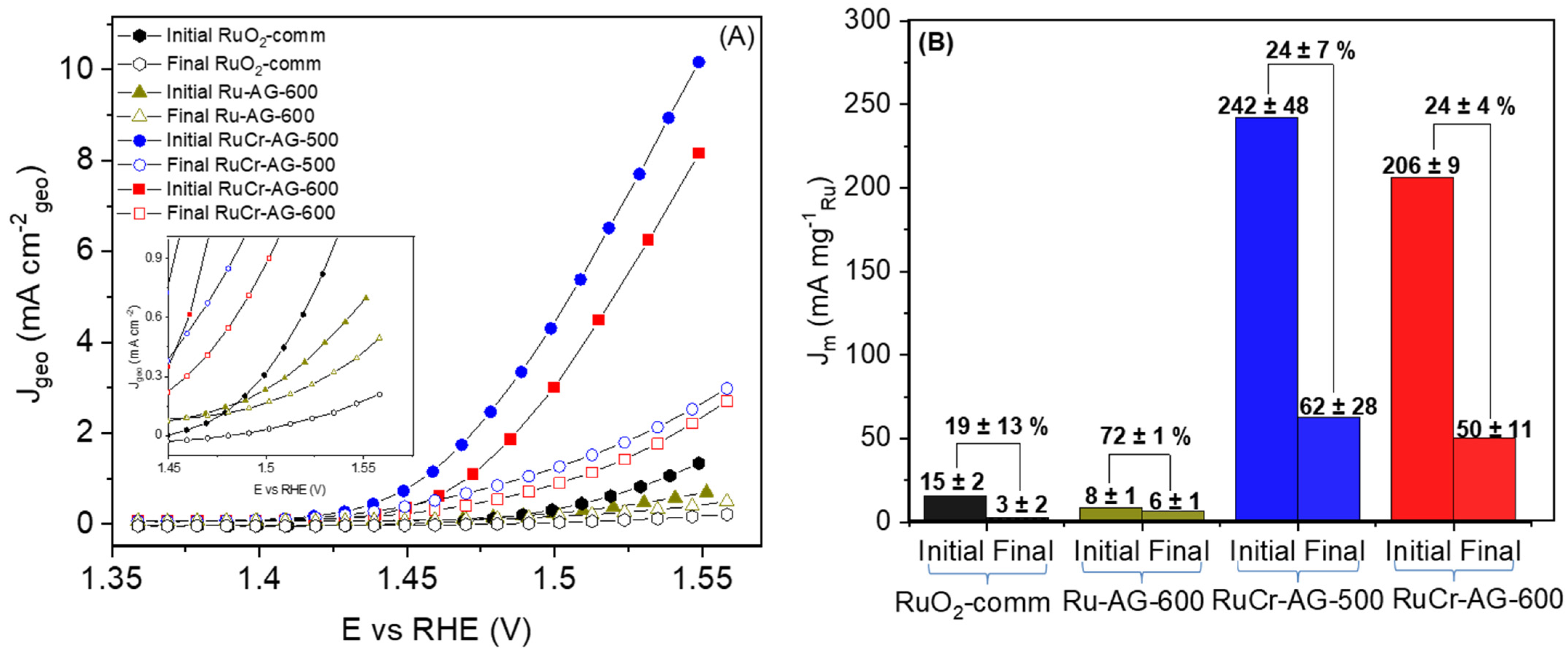
3.5. Electrochemical Oxygen Evolution Reaction Activity and Mechanism
3.6. Evaluation of Oxygen Evolution Electrochemical Stability and Dissolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ayers, K.E.; Marina, O.A. State of the Art in Low-Temperature and High-Temperature Electrolysis. MRS Bull. 2024, 49, 1226–1234. [Google Scholar] [CrossRef]
- Araújo, H.F.; Gómez, J.A.; Santos, D.M.F. Proton-Exchange Membrane Electrolysis for Green Hydrogen Production: Fundamentals, Cost Breakdown, and Strategies to Minimize Platinum-Group Metal Content in Hydrogen Evolution Reaction Electrocatalysts. Catalysts 2024, 14, 845. [Google Scholar] [CrossRef]
- Reier, T.; Nong, H.N.; Teschner, D.; Schlögl, R.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction in Acidic Environments—Reaction Mechanisms and Catalysts. Adv. Energy Mater. 2017, 7, 1601275. [Google Scholar] [CrossRef]
- Rana, M.; Mondal, S.; Sahoo, L.; Chatterjee, K.; Karthik, P.E.; Gautam, U.K. Emerging Materials in Heterogeneous Electrocatalysis Involving Oxygen for Energy Harvesting. ACS Appl. Mater. Interfaces 2018, 10, 33737–33767. [Google Scholar] [CrossRef]
- Kwak, I.; Kwon, I.S.; Kim, J.; Park, K.; Ahn, J.-P.; Yoo, S.J.; Kim, J.-G.; Park, J. IrO2–ZnO Hybrid Nanoparticles as Highly Efficient Trifunctional Electrocatalysts. J. Phys. Chem. C 2017, 121, 14899–14906. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Zhang, Y. Transition Metal Doped Graphene-Like Germanium Carbide: Screening of High Performance Electrocatalysts for Oxygen Reduction, Oxygen Evolution, or Hydrogen Evolution. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127628. [Google Scholar] [CrossRef]
- Yasin, G.; Ibrahim, S.; Ibraheem, S.; Ali, S.; Iqbal, R.; Kumar, A.; Tabish, M.; Slimani, Y.; Nguyen, T.A.; Xu, H.; et al. Defective/Graphitic Synergy in a Heteroatom-Interlinked-Triggered Metal-Free Electrocatalyst for High-Performance Rechargeable Zinc–Air Batteries. J. Mater. Chem. A 2021, 9, 18222–18230. [Google Scholar] [CrossRef]
- Chen, X.; Lin, S.; Qing, S.; Zhang, Y.; Li, X. Density Functional Theory Study of the Sulfur/Oxygen Doped CoN4-Graphene Electrocatalyst for Oxygen Reduction Reaction. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126219. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Hu, R.; Qing, S.; Zhang, H. DFT Study of C2N-Supported Ag3M (M = Cu, Pd, and Pt) Clusters as Potential Oxygen Reduction Reaction Catalysts. Chem. Eng. Sci. 2021, 239, 116642. [Google Scholar] [CrossRef]
- Nadeem, M.; Yasin, G.; Arif, M.; Tabassum, H.; Bhatti, M.H.; Mehmood, M.; Yunus, U.; Iqbal, R.; Nguyen, T.A.; Slimani, Y.; et al. Highly Active Sites of Pt/Er Dispersed N-Doped Hierarchical Porous Carbon for Trifunctional Electrocatalyst. Chem. Eng. J. 2021, 409, 128205. [Google Scholar] [CrossRef]
- Metals Daily. Live Prices. Available online: https://www.metalsdaily.com/live-prices/pgms/ (accessed on 16 December 2024).
- Kasian, O.; Geiger, S.; Stock, P.; Polymeros, G.; Breitbach, B.; Savan, A.; Ludwig, A.; Cherevko, S.; Mayrhofer, K.J.J. On the Origin of the Improved Ruthenium Stability in RuO2–IrO2 Mixed Oxides. J. Electrochem. Soc. 2016, 163, F3099. [Google Scholar] [CrossRef]
- United States Department of Energy Homepage. Hydrogen Shot Summit. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-shot-summit (accessed on 16 December 2023).
- Yu, J.; He, Q.; Yang, G.; Zhou, W.; Shao, Z.; Ni, M. Recent Advances and Prospective in Ruthenium-Based Materials for Electrochemical Water Splitting. ACS Catal. 2019, 9, 9973–10011. [Google Scholar] [CrossRef]
- Audichon, T.; Morisset, S.; Napporn, T.W.; Kokoh, K.B.; Comminges, C.; Morais, C. Effect of Adding CeO2 to RuO2–IrO2 Mixed Nanocatalysts: Activity towards the Oxygen Evolution Reaction and Stability in Acidic Media. ChemElectroChem 2015, 2, 1128–1137. [Google Scholar] [CrossRef]
- Petrykin, V.; Macounova, K.; Shlyakhtin, O.A.; Krtil, P. Tailoring the Selectivity for Electrocatalytic Oxygen Evolution on Ruthenium Oxides by Zinc Substitution. Angew. Chem. Int. Ed. 2010, 49, 4813–4815. [Google Scholar] [CrossRef]
- Gaudet, J.; Tavares, A.C.; Trasatti, S.; Guay, D. Physicochemical Characterization of Mixed RuO2−SnO2 Solid Solutions. Chem. Mater. 2005, 17, 1570–1579. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Boodts, J.F.C.; De Faria, L.A. Oxygen Evolution at RuO2(x)+Co3O4(1−x) Electrodes from Acid Solution. Electrochim. Acta 2001, 46, 1369–1375. [Google Scholar] [CrossRef]
- Macounová, K.; Jirkovský, J.; Makarova, M.; Franc, J.; Krtil, P. Oxygen Evolution on Ru1−x NixO2−y Nanocrystalline Electrodes. J. Solid State Electrochem. 2009, 13, 959–965. [Google Scholar] [CrossRef]
- Godinez-Salomon, J.F.; Ospina-Acevedo, F.; Albiter, L.A.; Bailey, K.O.; Naymik, Z.G.; Mendoza-Cruz, R.; Balbuena, P.B.; Rhodes, C.P. Titanium Substitution Effects on the Structure, Activity, and Stability of Nanoscale Ruthenium Oxide Oxygen Evolution Electrocatalysts: Experimental and Computational Study. ACS Appl. Nano Mater. 2022, 5, 11752–11775. [Google Scholar] [CrossRef]
- Ospina-Acevedo, F.; Albiter, L.A.; Bailey, K.O.; Godínez-Salomón, J.F.; Rhodes, C.P.; Balbuena, P.B. Catalytic Activity and Electrochemical Stability of Ru1–xMxO2 (M = Zr, Nb, Ta): Computational and Experimental Study of the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2024, 16, 16373–16398. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Logadottir, A.; Nørskov, J.K. Electrolysis of Water on (Oxidized) Metal Surfaces. Chem. Phys. 2005, 319, 178–184. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Qu, Z.W.; Zhu, H.; Kroes, G.J.; Nørskov, J.K. Electrolysis of Water on Oxide Surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Jamadar, A.S.; Sutar, R.; Patil, S.; Khandekar, R.; Yadav, J.B. Progress in Metal Oxide-Based Electrocatalysts for Sustainable Water Splitting. Mater. Rep. Energy 2024, 4, 100283. [Google Scholar] [CrossRef]
- Exner, K.S.; Anton, J.; Jacob, T.; Over, H. Ligand Effects and Their Impact on Electrocatalytic Processes Exemplified with the Oxygen Evolution Reaction (OER) on RuO2(110). ChemElectroChem 2015, 2, 707–713. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Jang, H.; Wei, X.; Wang, L.; Kim, M.G.; Cho, J.; Liu, X.; Qin, Q. 2D Ruthenium–Chromium Oxide with Rich Grain Boundaries Boosts Acidic Oxygen Evolution Reaction Kinetics. Small 2024, 20, 2311172. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Tian, Z.; Zhang, L.; Ma, J.; Jiang, Z.; Deibert, B.J.; Ge, R.; Chen, L. Chromium-Ruthenium Oxide Solid Solution Electrocatalyst for Highly Efficient Oxygen Evolution Reaction in Acidic Media. Nat. Commun. 2019, 10, 162. [Google Scholar] [CrossRef]
- Jeong, S.; Kwon, T.; Kim, Y.; Yang, J.H.; Kim, M.H.; Lee, Y. Bimetallic Ru1−xVxO2 and Trimetallic Ru1−x−yVxCryO2 Alloy Nanofibers for Efficient, Stable and pH-Independent Oxygen Evolution Reaction Catalysis. J. Alloys Compd. 2023, 968, 171916. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Geng, S.; Song, S.; Wang, Y. Modulating the Electronic Structure of RuO2 through Cr Solubilizing for Improved Oxygen Evolution Reaction. Small Methods 2022, 6, 2200636. [Google Scholar] [CrossRef]
- Ma, H.; Liu, C.; Liao, J.; Su, Y.; Xue, X.; Xing, W. Study of Ruthenium Oxide Catalyst for Electrocatalytic Performance in Oxygen Evolution. J. Mol. Catal. A Chem. 2006, 247, 7–13. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Scherer, G.G.; Xu, Z.J. A Facile Synthesis of Size-Controllable IrO2 and RuO2 Nanoparticles for the Oxygen Evolution Reaction. Electrocatalysis 2016, 7, 420–427. [Google Scholar] [CrossRef]
- Tsuji, E.; Imanishi, A.; Fukui, K.-i.; Nakato, Y. Electrocatalytic Activity of Amorphous RuO2 Electrode for Oxygen Evolution in an Aqueous Solution. Electrochim. Acta 2011, 56, 2009–2016. [Google Scholar] [CrossRef]
- Roy, C.; Rao, R.R.; Stoerzinger, K.A.; Hwang, J.; Rossmeisl, J.; Chorkendorff, I.; Shao-Horn, Y.; Stephens, I.E.L. Trends in Activity and Dissolution on RuO2 under Oxygen Evolution Conditions: Particles versus Well-Defined Extended Surfaces. ACS Energy Lett. 2018, 3, 2045–2051. [Google Scholar] [CrossRef]
- Iwakura, C.; Hirao, K.; Tamura, H. Preparation of Ruthenium Dioxide Electrodes and their Anodic Polarization Characteristics in Acidic Solutions. Electrochim. Acta 1977, 22, 335–340. [Google Scholar] [CrossRef]
- Galizzioli, D.; Tantardini, F.; Trasatti, S. Ruthenium Dioxide: A New Electrode Material. I. Behaviour in Acid Solutions of Inert Electrolytes. J. Appl. Electrochem. 1974, 4, 57–67. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Chang, K.C.; Chang, S.H.; Kang, Y.; Snyder, J.; Paulikas, A.P.; Strmcnik, D.; Kim, Y.T.; Myers, D.; et al. Using Surface Segregation to Design Stable Ru-Ir Oxides for the Oxygen Evolution Reaction in Acidic Environments. Angew. Chem. Int. Ed. 2014, 53, 14016–14021. [Google Scholar] [CrossRef]
- Paoli, E.A.; Masini, F.; Frydendal, R.; Deiana, D.; Malacrida, P.; Hansen, T.W.; Chorkendorff, I.; Stephens, I.E.L. Fine-Tuning the Activity of Oxygen Evolution Catalysts: The Effect of Oxidation Pre-Treatment on Size-Selected Ru Nanoparticles. Catal. Today 2016, 262, 57–64. [Google Scholar] [CrossRef][Green Version]
- Kim, J.Y.; Choi, J.; Kim, H.Y.; Hwang, E.; Kim, H.J.; Ahn, S.H.; Kim, S.K. Activity and Stability of the Oxygen Evolution Reaction on Electrodeposited Ru and its Thermal Oxides. Appl. Surf. Sci. 2015, 359, 227–235. [Google Scholar] [CrossRef]
- Dickens, C.F.; Nørskov, J.K. A Theoretical Investigation into the Role of Surface Defects for Oxygen Evolution on RuO2. J. Phys. Chem. C 2017, 121, 18516–18524. [Google Scholar] [CrossRef]
- Hess, F. Corrosion Mechanism and Stabilization Strategies for RuO2 and IrO2 Catalysts in the Electrochemical Oxygen Evolution Reaction. Curr. Opin. Electrochem. 2023, 41, 101349. [Google Scholar] [CrossRef]
- Devadas, A.; Baranton, S.; Coutanceau, C. Green Synthesis and Modification of RuO2 Materials for the Oxygen Evolution Reaction. Front. Energy Res. 2020, 8, 571704. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Chen, Y.; Ma, B. Electrochemical Behavior and Corrosion Mechanism of Ti/IrO2-RuO2 Anodes in Sulphuric Acid Solution. J. Electroanal. Chem. 2019, 837, 175–183. [Google Scholar] [CrossRef]
- Zhang, T.; Morimitsu, M. Effects of Amorphization of RuO2-Ta2O5 Catalytic Coating (Ru=30 mol%) on Oxygen Evolution in Sulfuric Acid Solution*. J. MMIJ 2015, 131, 572–576. [Google Scholar] [CrossRef][Green Version]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Yang, J.; Shao, Q.; Huang, B.; Sun, M.; Huang, X. pH-Universal Water Splitting Catalyst: Ru-Ni Nanosheet Assemblies pH-Universal Water Splitting Catalyst: Ru-Ni Nanosheet Assemblies. iScience 2019, 11, 492–504. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wei, C.; Lu, M.; Liu, H.; Chen, Y.; Scherer, G.G.; Fisher, A.C.; Xi, P.; Xu, Z.J.; Yan, C.-H. Recent Development of Oxygen Evolution Electrocatalysts in Acidic Environment. Adv. Mater. 2021, 33, 2006328. [Google Scholar] [CrossRef]
- Lee, S.W.; Baik, C.; Pak, C. Ordered Mesoporous Ruthenium Oxide with Balanced Catalytic Activity and Stability toward Oxygen Evolution Reaction. Catal. Today 2020, 358, 203–209. [Google Scholar] [CrossRef]
- Zeradjanin, A.R.; Topalov, A.A.; Van Overmeere, Q.; Cherevko, S.; Chen, X.; Ventosa, E.; Schuhmann, W.; Mayrhofer, K.J.J. Rational Design of the Electrode Morphology for Oxygen Evolution–Enhancing the Performance for Catalytic Water Oxidation. RSC Adv. 2014, 4, 9579–9587. [Google Scholar] [CrossRef]
- Baik, C.; Lee, S.; Pak, C. Control of the Pore Size Distribution Inside the RuO2 Catalyst by Using Silica Nanosphere Particle for Highly Efficient Water Electrolysis. Microporous Mesoporous Mater. 2020, 309, 110567. [Google Scholar] [CrossRef]
- Suh, D.J.; Park, T.J.; Kim, W.I.; Hong, I.K. Synthesis of High-Surface-Area Ruthenium Oxide Aerogels by Non-Alkoxide Sol–Gel Route. J. Power Sources 2003, 117, 1–6. [Google Scholar] [CrossRef]
- Walker, J.; Bruce King, R.; Tannenbaum, R. Sol–Gel Synthesis of Hydrous Ruthenium Oxide Nanonetworks from 1,2-Epoxides. J. Solid State Chem. 2007, 180, 2290–2297. [Google Scholar] [CrossRef]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H.; Hrubesh, L.W.; Simpson, R.L. New Sol-Gel Synthetic Route to Transition and Main-Group Metal Oxide Aerogels using Inorganic Salt Precursors. J. Non-Cryst. Solids 2001, 285, 22–28. [Google Scholar] [CrossRef]
- Long, J.W.; Swider, K.E.; Merzbacher, C.I.; Rolison, D.R. Voltammetric Characterization of Ruthenium Oxide-Based Aerogels and Other RuO2 Solids: The Nature of Capacitance in Nanostructured Materials. Langmuir 1999, 15, 780–785. [Google Scholar] [CrossRef]
- Maass, S.; Finsterwalder, F.; Frank, G.; Hartmann, R.; Merten, C. Carbon Support Oxidation in PEM Fuel Cell Cathodes. J. Power Sources 2008, 176, 444–451. [Google Scholar] [CrossRef]
- Khaleel, A.; Shehadi, I.; Al-Shamisi, M. Nanostructured Chromium–Iron Mixed Oxides: Physicochemical Properties and Catalytic Activity. Colloids Surf. A Physicochem. Eng. Asp. 2010, 355, 75–82. [Google Scholar] [CrossRef]
- Kaneko, K. Determination of Pore Size and Pore Size Distribution: 1. Adsorbents and Catalysts. J. Membr. Sci. 1994, 96, 59–89. [Google Scholar] [CrossRef]
- Menzel, N.; Ortel, E.; Kraehnert, R.; Strasser, P. Electrocatalysis Using Porous Nanostructured Materials. ChemPhysChem 2012, 13, 1385–1394. [Google Scholar] [CrossRef]
- Lee, D.N. Self-Diffusion in Sintering of Nonspherical Metallic Particles. In Materials Science Research; Kuczynski, G.C., Ed.; Plenum Publishing Corporation: New York, NY, USA, 1973; Volume 6, pp. 261–268. [Google Scholar]
- Puthiyapura, V.K.; Pasupathi, S.; Basu, S.; Wu, X.; Su, H.; Varagunapandiyan, N.; Pollet, B.; Scott, K. RuxNb1-xO2 Catalyst for the Oxygen Evolution Reaction in Proton Exchange Membrane Water Electrolysers. Int. J. Hydrogen Energy 2013, 38, 8605–8616. [Google Scholar] [CrossRef]
- Shannon, R. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving Ruthenium: XPS Studies of Common Ruthenium Materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Shen, J.Y.; Adnot, A.; Kaliaguine, S. An ESCA Study of the Interaction of Oxygen with the Surface of Ruthenium. Appl. Surf. Sci. 1991, 51, 47–60. [Google Scholar] [CrossRef]
- Sarma, D.D.; Rao, C.N.R. XPES Studies of Oxides of Second- and Third-Row Transition Metals including Rare Earths. J. Electron Spectrosc. Relat. Phenom. 1980, 20, 25–45. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Brown, C.; Mycroft, J.R.; Davidson, R.D.; McIntyre, N.S. X-Ray Photoelectron Spectroscopy Studies of Chromium Compounds. Surf. Interface Anal. 2004, 36, 1550–1563. [Google Scholar] [CrossRef]
- Payne, B.P.; Biesinger, M.C.; McIntyre, N.S. X-Ray Photoelectron Spectroscopy Studies of Reactions on Chromium Metal and Chromium Oxide Surfaces. J. Electron Spectrosc. Relat. Phenom. 2011, 184, 29–37. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Wang, Z.; Carrière, C.; Seyeux, A.; Zanna, S.; Mercier, D.; Marcus, P. XPS and ToF-SIMS Investigation of Native Oxides and Passive Films Formed on Nickel Alloys Containing Chromium and Molybdenum. J. Electrochem. Soc. 2021, 168, 041503. [Google Scholar] [CrossRef]
- Chalupczok, S.; Kurzweil, P.; Hartmann, H.; Schell, C. The Redox Chemistry of Ruthenium Dioxide: A Cyclic Voltammetry Study—Review and Revision. Int. J. Electrochem. 2018, 2018, 1273768. [Google Scholar] [CrossRef]
- Zheng, J.P.; Jow, T.R. A New Charge Storage Mechanism for Electrochemical Capacitors. J. Electrochem. Soc. 1995, 142, L6–L8. [Google Scholar] [CrossRef]
- McKeown, D.A.; Hagans, P.L.; Carette, L.P.L.; Russell, A.E.; Swider, K.E.; Rolison, D.R. Structure of Hydrous Ruthenium Oxides: Implications for Charge Storage. J. Phys. Chem. B 1999, 103, 4825–4832. [Google Scholar] [CrossRef]
- Babu, S.P.; Falch, A. Recent Developments on Cr-Based Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Media. ChemCatChem 2022, 14, e202200364. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; Hu, Y.; Guan, M.; Xu, L.; Li, H.; Bao, J.; Li, H. Cr-doped CoFe Layered Double Hydroxides: Highly Efficient and Robust Bifunctional Electrocatalyst for the Oxidation of Water and Urea. Appl. Catal. B Environ. 2020, 272, 118959. [Google Scholar] [CrossRef]
- Bo, X.; Li, Y.; Chen, X.; Zhao, C. High Valence Chromium Regulated Cobalt-Iron-Hydroxide for Enhanced Water Oxidation. J. Power Sources 2018, 402, 381–387. [Google Scholar] [CrossRef]
- Guerrini, E.; Trasatti, S. Recent Developments in Understanding Factors of Electrocatalysis. Russ. J. Electrochem. 2006, 42, 1017–1025. [Google Scholar] [CrossRef]
- Mattos-Costa, F.I.; de Lima-Neto, P.; Machado, S.A.S.; Avaca, L.A. Characterisation of Surfaces Modified by Sol-Gel Derived RuxIr1−xO2 Coatings for Oxygen Evolution in Acid Medium. Electrochim. Acta 1998, 44, 1515–1523. [Google Scholar] [CrossRef]
- Audichon, T.; Napporn, T.W.; Canaff, C.; Morais, C.; Comminges, C.; Kokoh, K.B. IrO2 Coated on RuO2 as Efficient and Stable Electroactive Nanocatalysts for Electrochemical Water Splitting. J. Phys. Chem. C 2016, 120, 2562–2573. [Google Scholar] [CrossRef]
- Bockris, J.O.M. Kinetics of Activation Controlled Consecutive Electrochemical Reactions: Anodic Evolution of Oxygen. J. Chem. Phys. 1956, 24, 817–827. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel Slopes from a Microkinetic Analysis of Aqueous Electrocatalysis for Energy Conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Richard, L.; Doyle, M.E.G.L. Photoelectrochemical Solar Fuel Production from Basic Principles to Advanced Devices, 1st ed.; Gimenez, S.B., Bisquert, J., Eds.; Springer: Cham, Switzerland, 2016; p. 559. [Google Scholar]
- Raveendran, A.; Chandran, M.; Dhanusuraman, R. A Comprehensive Review on the Electrochemical Parameters and Recent Material Development of Electrochemical Water Splitting Electrocatalysts. RSC Adv. 2023, 13, 3843–3876. [Google Scholar] [CrossRef]
- Kuo, D.Y.; Kawasaki, J.K.; Nelson, J.N.; Kloppenburg, J.; Hautier, G.; Shen, K.M.; Schlom, D.G.; Suntivich, J. Influence of Surface Adsorption on the Oxygen Evolution Reaction on IrO2(110). J. Am. Chem. Soc. 2017, 139, 3473–3479. [Google Scholar] [CrossRef]
- van der Heijden, O.; Park, S.; Vos, R.E.; Eggebeen, J.J.J.; Koper, M.T.M. Tafel Slope Plot as a Tool to Analyze Electrocatalytic Reactions. ACS Energy Lett. 2024, 9, 1871–1879. [Google Scholar] [CrossRef]
- Spöri, C.; Kwan, J.T.H.; Bonakdarpour, A.; Wilkinson, D.P.; Strasser, P. The Stability Challenges of Oxygen Evolving Catalysts: Towards a Common Fundamental Understanding and Mitigation of Catalyst Degradation. Angew. Chem. Int. Ed. 2017, 56, 5994–6012. [Google Scholar] [CrossRef]
- Godínez-Salomón, F.; Albiter, L.; Alia, S.M.; Pivovar, B.S.; Camacho-Forero, L.E.; Balbuena, P.B.; Mendoza-Cruz, R.; Arellano-Jimenez, M.J.; Rhodes, C.P. Self-Supported Hydrous Iridium-Nickel Oxide Two-Dimensional Nanoframes for High Activity Oxygen Evolution Electrocatalysts. ACS Catal. 2018, 8, 10498–10520. [Google Scholar] [CrossRef]
- Alia, S.M.; Shulda, S.; Ngo, C.; Pylypenko, S.; Pivovar, B.S. Iridium-Based Nanowires as Highly Active, Oxygen Evolution Reaction Electrocatalysts. ACS Catal. 2018, 8, 2111–2120. [Google Scholar] [CrossRef]
- Alia, S.M.; Rasimick, B.; Ngo, C.; Neyerlin, K.C.; Kocha, S.S.; Pylypenko, S.; Xu, H.; Pivovar, B.S. Activity and Durability of Iridium Nanoparticles in the Oxygen Evolution Reaction. J. Electrochem. Soc. 2016, 163, F3105–F3112. [Google Scholar] [CrossRef]
- Maljusch, A.; Conradi, O.; Hoch, S.; Blug, M.; Schuhmann, W. Advanced Evaluation of the Long-Term Stability of Oxygen Evolution Electrocatalysts. Anal. Chem. 2016, 88, 7597–7602. [Google Scholar] [CrossRef] [PubMed]

| Material | Ru:Cr Atomic Ratio (Synthesis) | Ru:Cr Atomic Ratio (EDS) | BET Surface Area, (m2 g−1) | Pore Diameter (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|---|---|
| RuO2-comm | − | − | 7 ± 1 | 24 ± 1 | 0.04 ±0.01 |
| Ru-AG-AP | − | − | 234 ± 7 | 18 ± 1 | 1.00 ± 0.02 |
| Ru-AG-500 | − | − | 7 ± 1 | 66 ± 20 | 0.10 ± 0.03 |
| Ru-AG-600 | − | − | 7 ± 4 | 46 ± 1 | 0.08 ± 0.05 |
| RuCr-AG-AP | Ru0.60Cr0.40 | Ru0.56Cr0.44 | 285 ± 3 | 23 ± 6 | 2.0 ± 0.6 |
| RuCr-AG-500 | Ru0.60Cr0.40 | Ru0.54Cr0.46 | 77 ± 10 | 29 ± 5 | 0.51 ± 0.03 |
| RuCr-AG-600 | Ru0.60Cr0.40 | Ru0.57Cr0.43 | 51 ± 1 | 36 ± 6 | 0.46 ± 0.07 |
| Material | d-Spacing (Å) | Lattice Parameters (Å) | Unit Cell Volume (Å3) | Crystalline Domain Size (nm) | ||
|---|---|---|---|---|---|---|
| (110) | (101) | a = b | c (Å) | |||
| RuO2-comm | 3.17 | 2.55 | 4.49 | 3.10 | 62.5 | 19.1 ± 0.4 |
| Ru-AG-500 | 3.19 | 2.56 | 4.49 | 3.10 | 62.6 | 13.4 ± 0.7 |
| Ru-AG-600 | 3.20 | 2.56 | 4.51 | 3.11 | 63.3 | 16.3 ± 4.2 |
| RuCr-AG-500 | 3.14 | 2.49 | 4.46 | 3.05 | 60.7 | 3.5 ± 1.4 |
| RuCr-AG-600 | 3.18 | 2.51 | 4.50 | 3.05 | 61.7 | 7.4 ± 0.2 |
| Region | Peak Label | Assignment | RuO2-Comm | Ru-AG-600 | RuCr-AG-500 | RuCr-AG-600 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| B.E. (eV) | Area % | B.E. (eV) | Area % | B.E. (eV) | Area % | B.E. (eV) | Area % | |||
| Ru 3d/ C 1s | Ru 3d5/2 | Ru 3d5/2 | 280.8 | 42 | 280.8 | 41 | 281.0 | 41 | 280.9 | 41 |
| Ru 3d5/2 sat | Ru 3d5/2 sat | 282.8 | 20 | 282.8 | 20 | 282.9 | 20 | 282.8 | 20 | |
| Ru 3d3/2 | Ru 3d3/2 | 285.1 | 23 | 285.1 | 23 | 285.3 | 21 | 285.2 | 22 | |
| Ru 3d3/2 sat | Ru3d3/2 sat | 286.6 | 12 | 286.7 | 14 | 286.8 | 16 | 286.7 | 15 | |
| C 1s | C 1s, C-C | 284.8 | 2 | 284.8 | 2 | 284.8 | 2 | 284.8 | 2 | |
| O 1s | Oa | O 1s, Ru-O, Cr-O* | 529.3 | 67 | 529.3 | 60 | 529.3 | 51 | 529.2 | 62 |
| Ob | O 1s, Ru-O sat, Ru-OH, *Cr-OH | 530.6 | 20 | 530.7 | 28 | 530.7 | 37 | 530.5 | 25 | |
| Oc | O 1s, C-O | 532.1 | 13 | 532.5 | 12 | 532.5 | 12 | 532.1 | 12 | |
| Cr 2p | Cra | Cr 2p3/2, Cr3+ oxide, peak 1 | − | − | − | − | 574.2 | 30.0 | 575.6 | 28 |
| Crb | Cr 2p3/2, Cr3+ oxide, peak 2 | − | − | − | − | 574.8 | 17.0 | 574.7 | 15 | |
| Crc | Cr 2p3/2, Cr (III) hydroxide | − | − | − | − | 576.7 | 24.0 | 576.5 | 24 | |
| Crd | Cr 2p3/2, Cr3+ oxide, peak 3 | − | − | − | − | 577.7 | 13.6 | 577.6 | 14 | |
| Cre | Cr 2p3/2, Cr3+ oxide, peak 4 | − | − | − | − | 578.6 | 10.4 | 578.5 | 11 | |
| Crf | Cr 2p3/2, Cr3+ oxide, peak 5 | − | − | − | − | 579.0 | 5.0 | 579.1 | 8 | |
| Material | Specific Capacitance (F/g) |
|---|---|
| RuO2-comm (Sigma Aldrich) | 1.5 ± 0.2 |
| Ru-AG-600 | 1.4 ± 0.6 |
| RuCr-AG-500 | 13 ± 1 |
| RuCr-AG-600 | 7 ± 2 |
| Anhydrous RuO2 (Alfa) * | 0.75 |
| RuO2·0.03H2O * | 29 |
| Material | Ru Dissolution (ppb) | Cr Dissolution (ppb) |
|---|---|---|
| Ru-AG-600 | 0.15 ± 0.01 | − |
| RuCr-AG-600 | 2.0 ± 0.8 | 2.2 ± 0.1 |
| RuCr-AG-500 | 7.6 ± 0.7 | 4.1 ± 0.3 |
| RuO2-comm | 0.35 ± 0.08 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adame-Solorio, J.; Kimmel, S.W.; Bailey, K.O.; Rhodes, C.P. Chromium Substitution Within Ruthenium Oxide Aerogels Enables High Activity Oxygen Evolution Electrocatalysts for Water Splitting. Crystals 2025, 15, 116. https://doi.org/10.3390/cryst15020116
Adame-Solorio J, Kimmel SW, Bailey KO, Rhodes CP. Chromium Substitution Within Ruthenium Oxide Aerogels Enables High Activity Oxygen Evolution Electrocatalysts for Water Splitting. Crystals. 2025; 15(2):116. https://doi.org/10.3390/cryst15020116
Chicago/Turabian StyleAdame-Solorio, Jesus, Samuel W. Kimmel, Kathleen O. Bailey, and Christopher P. Rhodes. 2025. "Chromium Substitution Within Ruthenium Oxide Aerogels Enables High Activity Oxygen Evolution Electrocatalysts for Water Splitting" Crystals 15, no. 2: 116. https://doi.org/10.3390/cryst15020116
APA StyleAdame-Solorio, J., Kimmel, S. W., Bailey, K. O., & Rhodes, C. P. (2025). Chromium Substitution Within Ruthenium Oxide Aerogels Enables High Activity Oxygen Evolution Electrocatalysts for Water Splitting. Crystals, 15(2), 116. https://doi.org/10.3390/cryst15020116






