Abstract
With the advancement of modern social science and technology, alloys composed solely of a single principal component are gradually unable to meet people’s needs. The concept of a new type of high-entropy alloy has been proposed. At present, high-entropy alloys are mostly prepared by vacuum arc furnace melting and casting methods. To improve this situation, this article uses plasma welding technology to prepare an AlCuCrFe2NiTi0.25 high-entropy alloy on a Q235 steel plate through multi-layer and multi-pass welding using plasma surfacing technology and adopts an appropriate solution treatment on this basis to obtain a higher-performance alloy. The conclusion drawn from different heat treatment processes is as follows: solution treatment was performed on an AlCuCrFe2Ni0.25 high-entropy alloy at a temperature of 1200 °C for 2 h, 3 h, and 4 h, respectively. After XRD phase analysis, it was found that the phase types of high-entropy alloys did not change after solution treatment. As the solution time increased, the diffraction peak intensity of the Laves phase gradually decreased. After 3 h of solid solution treatment, room temperature tensile tests were conducted to obtain the tensile strength and elongation of the AlCuCrFe2Ni0.25 high-entropy alloy at room temperature, which were 509 MPa and 23.8%, respectively, exhibiting the optimal comprehensive mechanical properties.
1. Introduction
Traditional alloys use one or two metal elements as the main element, and then add other elements as trace elements to improve the performance of the alloy. With the rapid development of science and technology, people are increasingly pursuing the exploration and development of metal materials with excellent comprehensive properties such as high strength, high hardness, high corrosion resistance, high wear resistance, and good strength plasticity matching [1]. In 2004, Taiwanese scholars Ye Junwei et al. [2] proposed the design concept of high-entropy alloys: high-entropy alloys are generally multi-principal element alloys composed of five or more elements, with the elements composed in an equiatomic ratio or close to equiatomic ratio so that all elements are the main constituent elements. Meanwhile, the content of each element in the high-entropy alloy system is controlled within the range of 5% to 35% to ensure the design flexibility of the high-entropy alloy [3,4]. At present, the main method for preparing high-entropy alloys is arc melting, and there is still a lack of research on the relationship between high-entropy alloys and welding processes. Welding technology can repair materials and endow them with surface wear resistance, good strength, and hardness [5]. Welding technology mainly includes MIG (Metal Inert Gas) welding technology, TIG (Tungsten Inert Gas) welding technology, and plasma welding technology, among which the advantages of plasma welding are simple equipment structure, dense welding layer structure, good formability, fast melting rate, low dilution rate, and high metallurgical bonding strength between the welding layer and the workpiece [6].
In this study, the Al-TMs system was selected, and the AlCrCuFe2NiTi0.25 high-entropy alloy was used as the base alloy, exhibiting a biphasic or multi-phase structure and obtaining excellent mechanical properties. Plasma welding technology is a fast and convenient repair process that melts high-entropy alloys onto the surface of the substrate, creating a good metallurgical bond between the deposited metal and the substrate, thereby obtaining special properties. Heat treatment has a significant effect on the mechanical properties and microstructure of metals, and this method is considered a scientific and efficient way to optimize the structure and properties of materials [7,8,9]. Since the concept of high-entropy alloys was proposed, the research team has mainly explored the production technology of high-entropy alloys and how the various constituent elements of the alloy affect its overall structure and performance. Due to the preparation methods of high-entropy alloys, internal stresses and various forms of defects are inevitably generated inside the alloy [10,11]. Therefore, using heat treatment to change the properties of high-entropy alloys has certain significance.
2. Experimental Process
2.1. Preparation of Hardfacing Alloy
This study selected alloy elements such as Al, Cr, Cu, Fe, Ni, and Ti to construct a high-entropy alloy system. Firstly, selected metal powder with a purity of 99.99% or higher was uniformly ground to 120 mesh using a ball mill. Subsequently, a Q235 steel plate (BAOSTEEL, Shanghai, China) was pretreated using an angle grinder to remove its surface oxide scale. We then used acetone to remove oil stains, then thoroughly cleaned with alcohol, and finally sandblasted the surface of the steel plate. Using a BX-ZH-400A plasma welding (Benxi, Shanghai, China) machine, the ground alloy powder was loaded into the powder feeder and deposited onto the surface of the pretreated Q235 steel plate to prepare high-entropy molten metal. A schematic diagram of plasma welding is shown in Figure 1. The welding process parameters are shown in Table 1 [12].
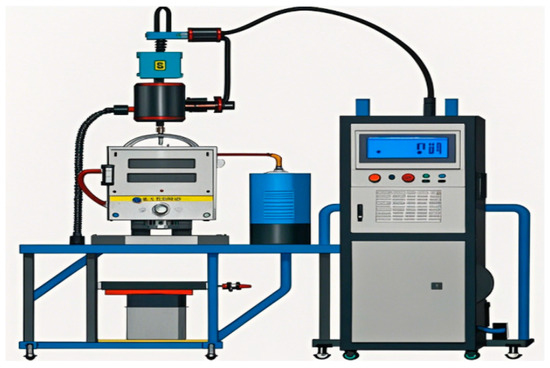
Figure 1.
Schematic diagram of plasma welding.

Table 1.
Welding process parameters.
2.2. Microstructure and Properties Analysis
Differential Scanning Calorimetry (DSC) was used to test the characteristic thermal reactions of the alloy during heating and cooling processes. We raised the temperature from room temperature to 600 °C at a rate of 10 °C/min, then raised the temperature to 1400 °C at a rate of 5 °C/min and maintained it for 10 min. When cooling down, we reduced the temperature to 600 °C at a rate of 5 °C/min, and then reduced it to room temperature at a rate of 10 °C/min. To perform solution treatment on high entropy alloys at different holding times of 2, 3, and 4 h, we took the samples out of the experimental furnace and immediately cooled them in water after the solution treatment was completed. After solution treatment, we used wire cutting for horizontal sampling to process hardness samples, tensile samples, and metallographic samples. The MTS Landmark 370.10 (MTS, Minnesota, America) microcomputer-controlled electro-hydraulic servo testing machine was used to test the mechanical properties of the alloy. The sample is shown in Figure 2. Under the radiation of pure copper target material, the tube voltage was 40 kV, the tube current was 30 mA, the scanning step was 4°/min, and the scanning range was 20~90°. The phase structure of the AlCrCuFe2NiTi0.25 high-entropy alloy was characterized by X-ray diffraction. The microstructure of the alloy was observed using a field emission GeminiSEM 300 (Zeiss, Oberkochen, Germany) with an acceleration voltage of 20 kV, an energy dispersive spectrometer (EDS), and a transmission electron microscope (JEM 2100, Tokyo, Japan) with an acceleration voltage of 2000 kV.
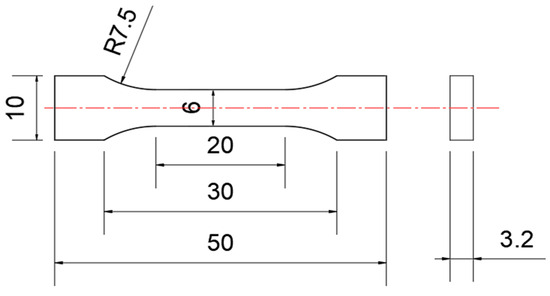
Figure 2.
Diagram of tensile specimen.
3. Results and Discussion
3.1. DSC Curve Analysis
In order to determine the temperature for the solid solution treatment of the AlCuCrFe2Ni0.25 high-entropy alloy, DSC testing was conducted on the AlCuCrFe2Ni0.25 high-entropy alloy. Figure 3a shows the differential thermal analysis curve of alloy heating. There are four distinct thermal reaction zones in the figure, corresponding to the four phase compositions in the XRD, and the liquidus temperature shown in the figure is 1329 °C, and the solidus temperature is 1273 °C. Figure 3b shows the DTA curve of high-entropy alloy cooling, where the peaks of the four phase compositions correspond to the heating curve [13]. According to the heating and cooling curves obtained from DSC testing, the initial melting temperature of the AlCuCrFe2Ni0.25 high-entropy alloy is around 1235 °C. Due to the fact that DSC testing can only detect phase transitions when they reach a certain level, the initial melting temperature measured is usually higher. Therefore, in order to maximize the efficiency of solution treatment and prevent the occurrence of initial melting and temperature fluctuations in the heat treatment furnace of high-entropy alloys, the solution treatment temperature of the AlCuCrFe2NiTi0.25 high-entropy alloy was determined to be 1200 °C. Referring to the heat treatment system of similar alloys, we set the solution time at 2 h, 3 h, and 4 h.
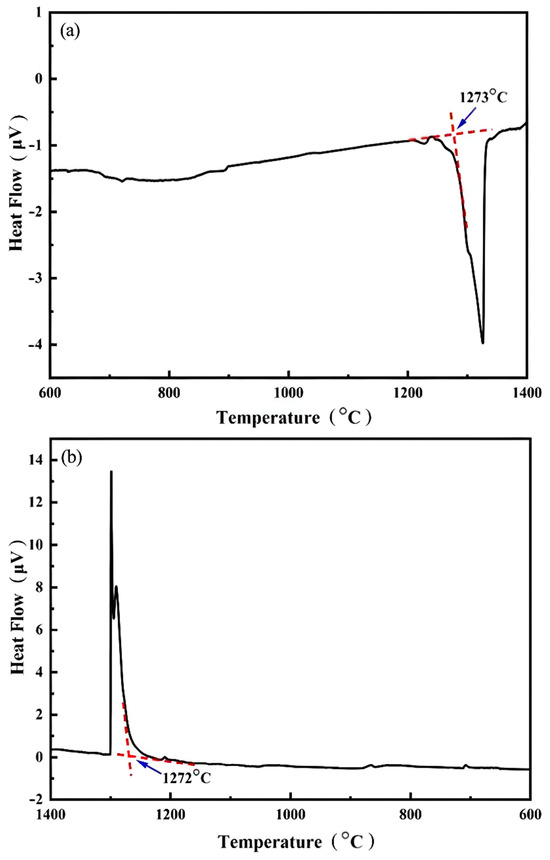
Figure 3.
Differential thermal analysis curve of AlCuCrFe2NiTi0.25 high-entropy alloy: (a) heating up; (b) cooling down.
3.2. Phase Analysis
According to Guo et al. [14], when the valence electron concentration (VEC) is greater than 8.6, the stability of the FCC solid solution is relatively good. When the valence VEC is less than 6.87, the BCC solid solution is relatively stable. When the valence VEC is between the two, there exists a two-phase solid solution of FCC and BCC. Dong et al. [15] used two parameters, electronegativity difference (∆χ) and d-orbital energy level (Md), to predict the formation of the topologically dense packed phase (TCP). TCP precipitation occurs when ∆χ > 0.117 and Md > 0.90. The specific calculation method for the above parameters is as follows:
In the formula, Ci is the atomic percentage of the i-th component, and the calculation results of the above formula are shown in Table 2. The results show that the AlCuCrFe2NiTi0.25 high-entropy alloy forms a BCC+FCC dual-phase solid solution internally, and at the same time, the Laves phase is critically precipitated. This dual-phase solid solution can regulate the comprehensive mechanical properties of high-entropy alloys by adjusting the relative amount and morphology of the two phases.

Table 2.
Calculation results of empirical parameters.
Figure 4 shows the XRD patterns of the AlCuCrFe2NiTi0.25 high-entropy alloy solid solution at 1200 °C for 2 h, 3 h, and 4 h. As shown in the figure, the phase composition of the high-entropy alloy did not change after three different solid solution treatments and still consisted of FCC, BCC1, BCC2, and Laves phases. Through comparative analysis of PDF cards in Jade 6.0 software, it can be concluded that the FCC solid solution is rich in Cu phase, the BCC1 solid solution has an Fe-Cr phase structure, the BCC2 solid solution has an AlNi phase structure, and the Laves phase is a Fe2Ti phase. The intensity difference in the BCC and FCC diffraction peaks in the AlCuCrFe2NiTi0.25 high-entropy alloy after solid solution treatment is not significant, which means that the content changes of the BCC and FCC phases are not obvious, and the number of Laves phases is reduced to a certain extent [16]. At the same time, as the solution treatment time increases, the intensity of the Laves phase diffraction peak gradually decreases. The occurrence of this phenomenon is due to the gradual dissolution of the Laves phase from between dendrites and inside the crystal after solid solution treatment, leading to a decrease in its quantity.
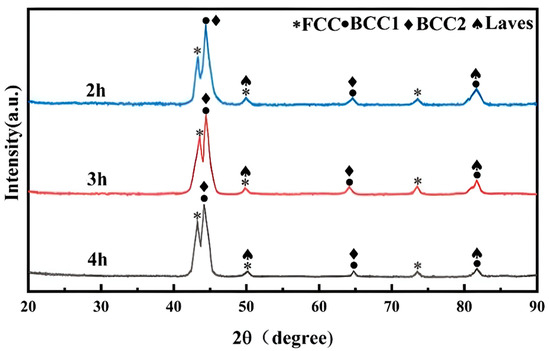
Figure 4.
X-ray diffraction patterns of AlCuCrFe2NiTi0.25 high-entropy alloy after solid solution treatment at 1200 °C for 2, 3, and 4 h.
3.3. Microstructure
Figure 5 shows the SEM microstructure of the AlCuCrFe2NiTi0.25 high-entropy alloy after solid solution treatment at 1200 °C for 2 h, 3 h, and 4 h. The microstructure of the AlCuCrFe2NiTi0.25 high entropy surfacing alloy exhibits a typical dendritic structure, with gray dendritic (DR) regions and black interdendritic (ID) regions. From Figure 5a, it can be seen that after 2 h of solid solution treatment, there are some black particles distributed on the surface of the alloy, and relatively small dendritic crystals can still be observed. From Figure 5b,c, it can be seen that as the solution treatment time increases, the distribution of black dots becomes more uniform and their number gradually decreases. When the solution treatment time reaches 4 h, the black-dot-like substance gradually disperses into the interior of the particles, and the distribution becomes more uniform. The decrease in the number of black-dot-like substances indicates that the second phase dissolves back into the matrix after solid solution treatment [17].
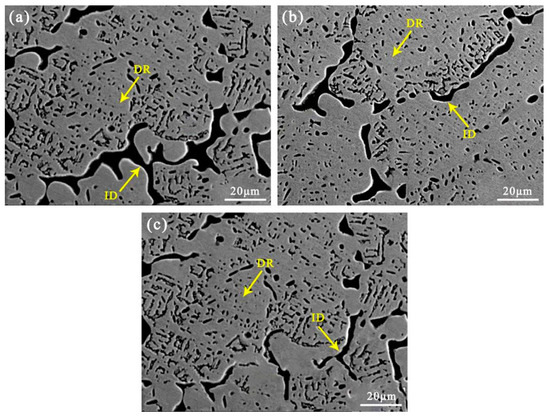
Figure 5.
SEM images of AlCuCrFe2NiTi0.25 high-entropy alloy with different solution treatment times: (a) 2 h; (b) 3 h; (c) 4 h.
Figure 6 shows the elemental surface scan of the AlCrCuFe2NiTi0.25 high-entropy alloy. According to the EDS results, the elements in the high-entropy alloy exhibit segregation. In the ID region, the Laves phase begins to precipitate and stably exists, identified as Fe2Ti. The ordered B2 phase precipitates in the same ID region as the AlNi phase, while Fe and Cr elements are enriched in the DR region. Due to the positive mixing enthalpy of Cu with other elements, the mixing enthalpy between Fe and Cr is relatively negative. Therefore, the binding force between Fe and Cr elements is significantly better than that with Cu elements, and the Cu rich phase is displaced and aggregates in the interdendritic region, resulting in the formation of an Fe-Cr phase with BCC structure in the DR region.
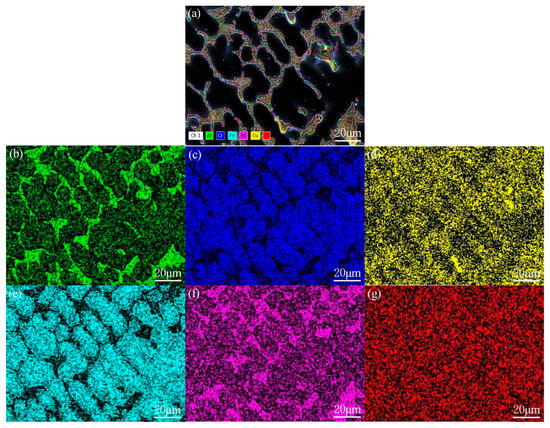
Figure 6.
Element surface scan of AlCrCuFe2NiTi0.25 high-entropy alloy: (a) microstructure; (b) Al; (c) Cr; (d) Cu; (e) Fe; (f) Ni; (g) Ti.
3.4. Mechanical Properties
Figure 7 shows the cross-section microhardness curve of the AlCuCrFe2NiTi0.25 high-entropy alloy. As the solution treatment time increases, the cross-section microhardness of high-entropy alloys gradually decreases from the top to the base metal. The total thickness of the weld overlay is about 5 mm, and the surface microhardness is about twice that of the substrate. The fusion zone is located in the transition zone between the hardfacing alloy and the base metal. Due to the high heat flux during welding, the base metal is diluted, resulting in the microhardness of the fusion zone always being between the hardfacing alloy and the base metal [18].
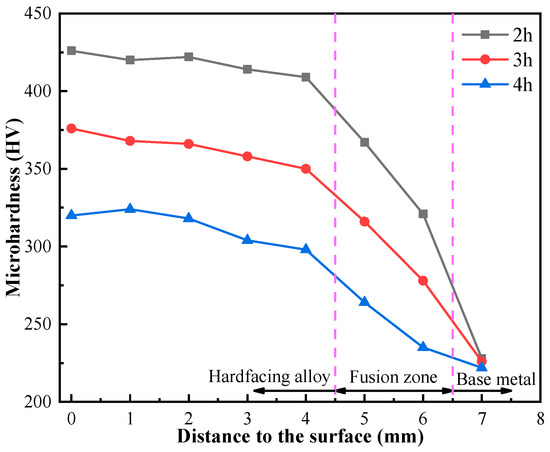
Figure 7.
Cross-section microhardness curve of AlCuCrFe2NiTi0.25 high-entropy alloy.
Figure 8 shows the average surface hardness of the AlCuCrFe2NiTi0.25 high-entropy alloy after 2 h, 3 h, and 4 h of solid solution treatment at 1200 °C. It can be seen from the figure that the average hardness of the alloy after 2 h, 3 h, and 4 h of solid solution treatment is 422 HV, 373 HV, and 317 HV, respectively. From this, it can be seen that with the increase in solution treatment time, the increase in atomic activity at grain boundaries leads to a tendency for grain growth, and the hardness of AlCuCrFe2NiTi0.25 high-entropy alloy gradually decreases. According to XRD results, as the solution treatment time increases, the number of BCC phases gradually decreases, the number of FCC phases gradually increases, and the Laves phase re-dissolves into the matrix, resulting in a continuous decrease in its quantity. Among them, the BCC phase and Laves phase belong to the hard phase, and the FCC phase belongs to the soft tough phase [19]. Therefore, with the increase in solution treatment time, the hardness of the alloy continues to decrease. The change in hardness is consistent with the results obtained from XRD. The microstructure of the high-entropy alloy under scanning electron microscopy also shows that with the increase in solution treatment time, the equiaxed grains gradually become smaller and the grain size becomes more uniform. The precipitates inside the equiaxed grains gradually dissolve back into the matrix with the increase in solution treatment time, resulting in a decrease in the hardness of the alloy [20].
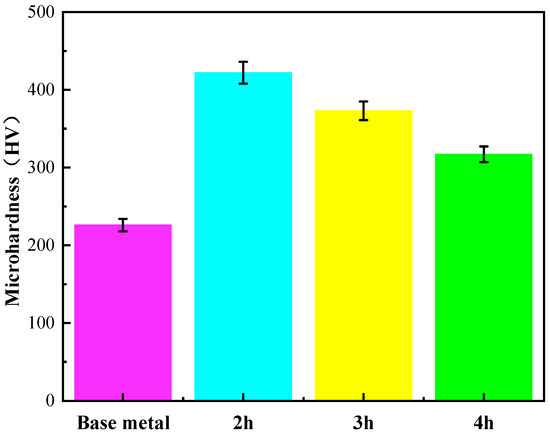
Figure 8.
Average hardness of AlCuCrFe2NiTi0.25 high-entropy alloy after solution treatment at 1200 °C for 2 h, 3 h, and 4 h.
Figure 9 shows the stress–strain curves of the AlCuCrFe2NiTi0.25 high-entropy alloy under room temperature tensile engineering at 1200 °C for 2 h, 3 h, and 4 h. After 2 h of solid solution treatment, the tensile strength of the alloy was 516 MPa and the elongation was 19.9%; after 3 h of solid solution treatment, the tensile strength and elongation of the alloy were 509 MPa and 23.8%, respectively; when the solution treatment time increased to 4 h, the tensile strength of the alloy was 454 MPa and the elongation was 24.2%. According to the data obtained from room temperature tensile experiments, as the solution treatment time increases, the tensile strength of the AlCuCrFe2NiTi0.25 high-entropy alloy decreases, while the elongation rate continues to increase. From this, it can be seen that the solution treatment system can improve the plasticity of the AlCuCrFe2NiTi0.25 high-entropy alloy. The tensile strength after 4 h of solution treatment only decreased by 62 MPa compared to 2 h, and the elongation increased by 4.3%.
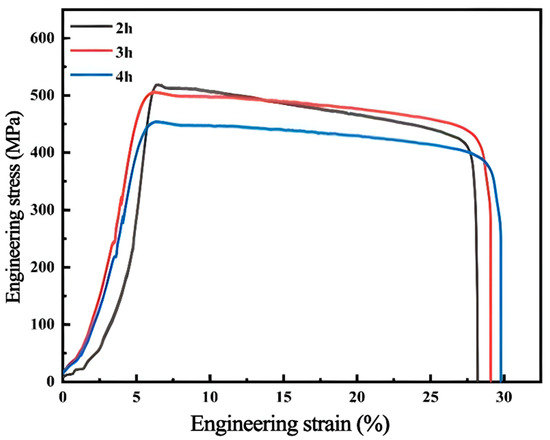
Figure 9.
Room temperature tensile stress–strain curve of AlCuCrFe2NiTi0.25 high-entropy alloy after solid solution treatment at 1200 °C.
Figure 10 shows the SEM images of the fracture morphology of the AlCuCrFe2NiTi0.25 high-entropy alloy after solid solution treatment at 1200 °C for 2 h, 3 h, and 4 h and room temperature tensile testing. From Figure 10a, it can be observed that the toughness pit depth on the fracture surface of the high-entropy alloy after 2 h of solid solution treatment and room temperature tensile testing is shallow and a flat fracture plane appears. As shown in Figure 10b, with the increase in solution time, the number and depth of toughness dimples on the fracture surface increase, and the area of the fracture plane decreases. According to Figure 10c, the solution treatment time continues to increase, the number and distribution of ductile dimples increase, and there are no obvious flat sections or tearing edges in the fracture surface [21]. The main form of fracture is ductile fracture. From the fracture morphology of the AlCuCrFe2Ni0.25 high-entropy alloy after solid solution treatment for 2 h, 3 h, and 4 h, it can be seen that with the increase in solution treatment time, the number and depth of toughness dimples on the fracture surface gradually increase, and the area of the flat fracture plane decreases. That is, as the solution treatment time of the AlCuCrFe2Ni0.25 high-entropy alloy increases, the plasticity of the high-entropy alloy is improved.
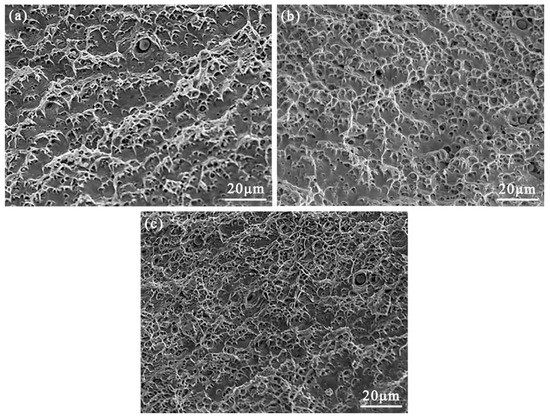
Figure 10.
Tensile fracture morphology of AlCuCrFe2NiTi0.25 high-entropy alloy at room temperature after solution treatment for 2, 3, and 4 h: (a) 2 h; (b) 3 h; (c) 4 h.
Figure 11 shows the microstructure of the AlCuCrFe2NiTi0.25 high-entropy alloy after fracture following solution treatment for 2, 3, and 4 h. As shown in the figure, the microstructure after fracture is basically similar, with increasing dislocations and entanglement. As the solution treatment time increases, the distribution of black and fine second phases in the tissue becomes more uniform, resulting in a decrease in the resistance provided during dislocation motion. Dislocations can move on multiple slip planes through slip, enhancing the plasticity of the alloy.
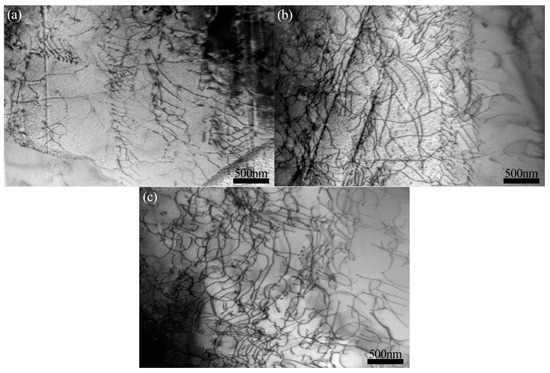
Figure 11.
Microstructure of AlCuCrFe2NiTi0.25 high-entropy alloy after fracture following solution treatment for 2, 3, and 4 h: (a) 2 h; (b) 3 h; (c) 4 h.
4. Conclusions
The AlCuCrFe2NiTi0.25 high-entropy alloy was successfully prepared on a Q235 steel plate through multi-layer and multi-pass welding using plasma surfacing technology. Based on this, appropriate solution treatment was adopted, and the high-entropy alloy’s mechanical properties were tested. The results are as follows:
- (1)
- The AlCuCrFe2NiTi0.25 high-entropy alloy underwent solid solution treatment at a temperature of 1200 °C for 2 h, 3 h, and 4 h, respectively. According to the XRD results, the type of phase composition did not change, but the quantity and morphology of the Laves phase changed. After solid solution treatment, a portion of the Laves phase dissolved back into the matrix, resulting in a decrease in its quantity. And its shape transformed from a larger rod-shaped structure to a finer spherical tissue.
- (2)
- After 3 h of solid solution treatment, room temperature tensile tests were conducted to obtain the tensile strength and elongation of the AlCuCrFe2Ni0.25 high-entropy alloy at room temperature, which were 509 MPa and 23.8%, respectively, obtaining the best comprehensive mechanical performance. As the solution treatment time increases, the number of ductile dimples on the tensile fracture surface gradually increases and the depth increases. Dislocations continuously increase in value and entangle, and can move on multiple slip planes through slip, enhancing the plasticity of the alloy.
Author Contributions
J.H.: Methodology, Writing original draft, Writing review and editing, Software. Y.S.: Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions provided in this study are included in the article. Further inquiries can be made by directly contacting the corresponding author.
Conflicts of Interest
Author Jingxuan Huang was employed by the company Shenyang Machine Tool Factory. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Gao, X.; Wang, L.; Guo, N.; Luo, L.; Zhu, G.; Shi, C.; Su, Y.; Guo, J. Microstructure and mechanical properties of multi-phase reinforced Hf-Mo-Nb-Ti-Zr refractory high-entropy alloys. Int. J. Refract. Met. Hard Mater. 2022, 102, 105723. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yan, G.; Zheng, M.; Ye, Z.; Gu, J.; Li, C.; Wu, C.; Wang, B. In-situ Ti (C, N) reinforced AlCoCrFeNiSi-based high entropy alloy coating with functional gradient double-layer structure fabricated by laser cladding. J. Alloys Compd. 2021, 886, 161252–161259. [Google Scholar] [CrossRef]
- Martin, P.; Aguilar, C.; Cabrera, J.M. A review on mechanical alloying and spark plasma sintering of refractory high-entropy alloys: Challenges, microstructures, and mechanical behavior. J. Mater. Res. Technol. 2024, 30, 1900–1928. [Google Scholar] [CrossRef]
- Liang, X.; Su, Y.; Yang, T.; Dai, Z.; Wang, Y.; Yong, X. Study on the wear resistance and mechanism of AlCrCuFe2NiTix high-entropy surfacing alloys. J. Alloys Compd. 2024, 971, 172510. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Zhao, F.; Zhang, L.; Wu, Z.; Xie, Z. corrosion wear and lubricating mechanisms of WC particles reinforced FeCoCrNiMn high entropy alloys coatings. Ceram. Int. 2024, 50, 31718–31725. [Google Scholar] [CrossRef]
- Munitz, A.; Salhov, S.; Hayun, S.; Frage, N. Heat treatment impacts the micro-structure and mechanical properties of AlCoCrFeNi high entropy alloy. J. Alloys Compd. 2016, 683, 221–230. [Google Scholar] [CrossRef]
- Qi, M.; Wu, Q.; Yang, Z.; Wang, Y.; Li, Y.; Wang, L.; Li, J.; Wang, Z.; Wang, J. Surface strengthening of Ni32-xCo30Cr10Fe10Al18Tix eutectic high-entropy alloys by laser remelting and heat treatment. Appl. Phys. A 2022, 128, 928. [Google Scholar] [CrossRef]
- Wu, M.; Li, Z.; Gault, B.; Munroe, P.; Baker, I. The Effects of carbon on the phase stability and mechanical properties of heat-treated FeNiMnCrAl high entropy alloys. Mater. Sci. Eng. A 2019, 748, 59–73. [Google Scholar] [CrossRef]
- Wu, Z.; Jia, Y.; Mu, Y.; Jia, Y.; Ji, P.; Hu, K.; Wang, Y.; Yang, D.; Ma, P.; Zhao, W.; et al. A mortise-and-tenon structure inspired high strength-ductility 3D printed high-entropy alloys with mechanically interlocked network. Mater. Sci. Eng. A 2024, 899, 146422. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Hua, K.; Cao, Y.; Song, Y.; Li, X.; Zhou, Q.; Wang, H. Microstructures and properties of FeCrAlMoSix high entropy alloy coatings prepared by laser cladding on a titanium alloy substrate. Surf. Coat. Technol. 2024, 478, 130437. [Google Scholar] [CrossRef]
- Su, Y.; Liang, X.; Liu, Y.; Dai, Z. Effect of Ti addition on the microstructure and property of FeAlCuCrNiMo0.6 high-entropy alloy. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 957–967. [Google Scholar] [CrossRef]
- Fujieda, T.; Shiratori, H.; Kuwabara, K.; Hirota, M.; Kato, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A.; Watanabe, S. CoCrFeNiTi-based high-entropy alloy with superior tensile strength and corrosion resistance achieved by a combination of additive manufacturing using selective electron beam melting and solution treatment. Mater. Lett. 2017, 189, 148–151. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.; Jiang, L.; Wang, T.; Li, T. Effects of electro-negativity on the stability of topologically close-packed phase in high entropy alloys. Intermetallics 2014, 52, 105–109. [Google Scholar] [CrossRef]
- Xin, W.; YunPeng, Z.; Jing, X. Effect of Ti addition on the microstructure and properties of AlCrCuFeNi alloy. Mater. Res. Express 2018, 6, 026541. [Google Scholar] [CrossRef]
- Huang, S.; Wu, H.; Xu, Y.; Zhu, H. Effects of heat treatment on the precipitation behaviors and mechanical properties of Nb-doped CrMnFeCoNi0.8 high-entropy alloy. Mater. Sci. Eng. A 2023, 885, 145611. [Google Scholar] [CrossRef]
- Guo, Y.J.; Li, C.G.; Zeng, M.; Wang, J.; Deng, P.; Wang, Y. In-situ TiC reinforced CoCrCuFeNiSi0.2 high-entropy alloy coatings designed for enhanced wear performance by laser cladding. Mater. Chem. Phys. 2020, 242, 122522. [Google Scholar] [CrossRef]
- Soni, V.; Gwalani, B.; Alam, T.; Dasari, S.; Zheng, Y.; Senkov, O.N.; Miracle, D.; Banarjee, R. Phase inversion in a two-phase, BCC+B2, refractory high entropy alloy. Acta Mater. 2020, 185, 89–97. [Google Scholar] [CrossRef]
- Sun, W.; Fu, Y.; Ma, H.; Wang, Y.; Gao, M.; Kong, X.; Guan, R. High-strength AlCoCrFeNi2.1 eutectic high entropy alloy skeleton with hollow brick wall structures by selective laser melting. Mater. Sci. Eng. A 2023, 887, 145757. [Google Scholar] [CrossRef]
- Tong, S.; Shen, G.; Han, Z.; Ma, Z.; Sun, Y.; Guan, S.; Zhao, H.; Ren, L.; Yan, C. Mechanical-thermal coupling structural failure and in-situ deformation mechanism of cellular high entropy alloy lattice structures. J. Mater. Res. Technol. 2024, 28, 2792–2799. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).