Boosting Charge Separation in NiS/C3N4 Type-II Heterojunction for Efficient Photoelectrocatalytic Water Reduction
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of C3N4 Photoanode
2.3. Preparation of NiS/C3N4 Photoanode
2.4. Characterization
2.5. Photoelectrochemical (PEC) Measurements
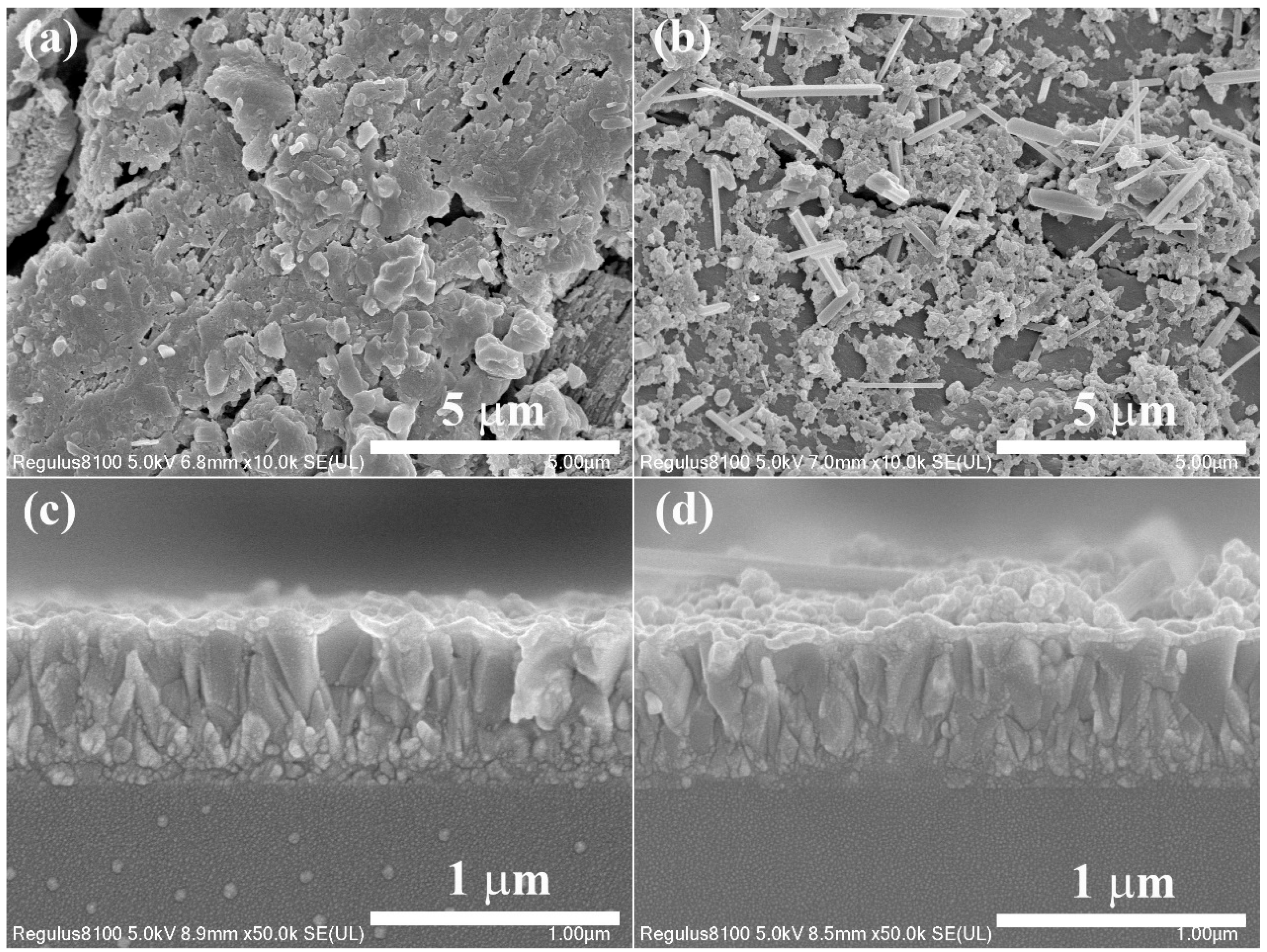
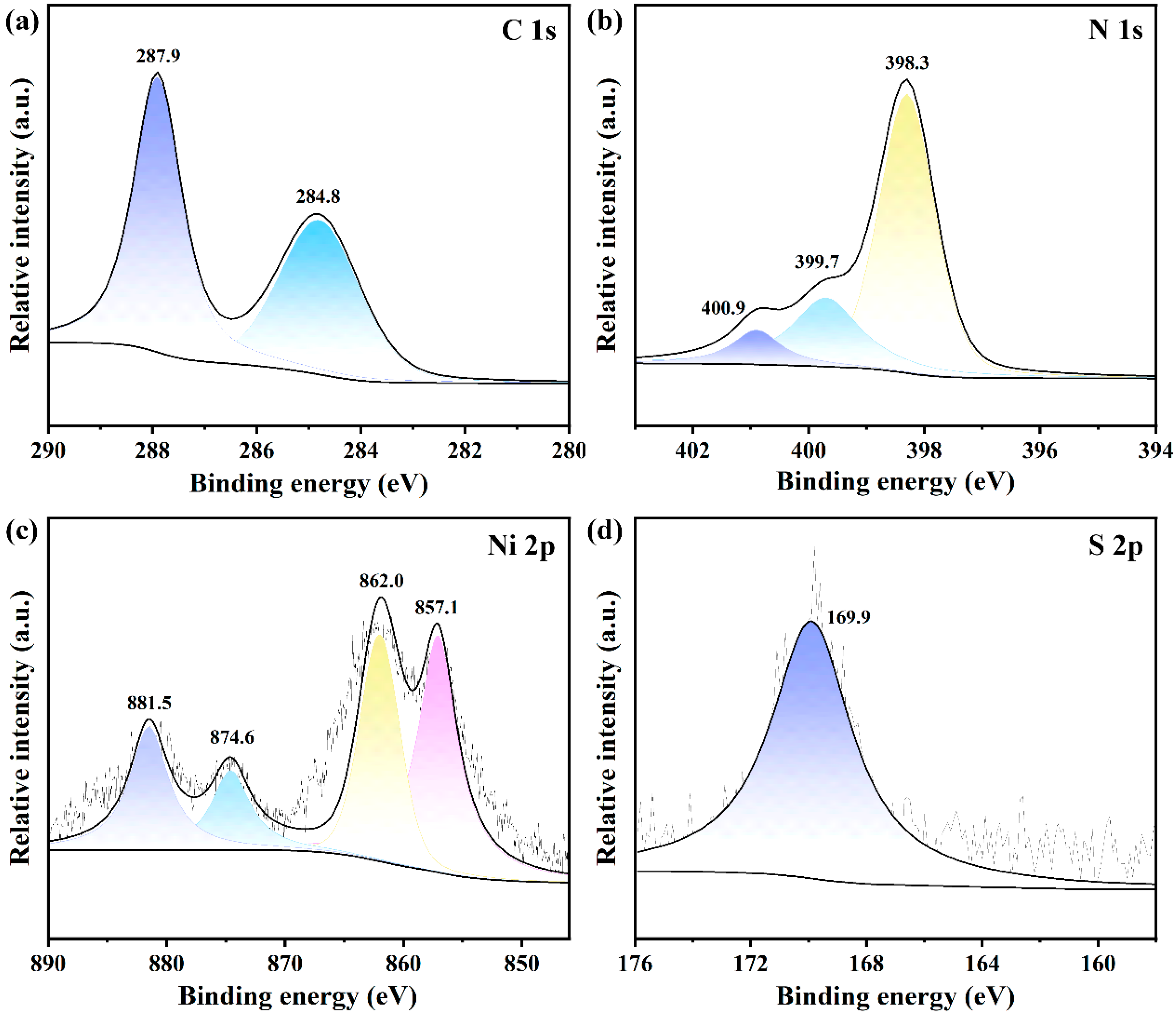
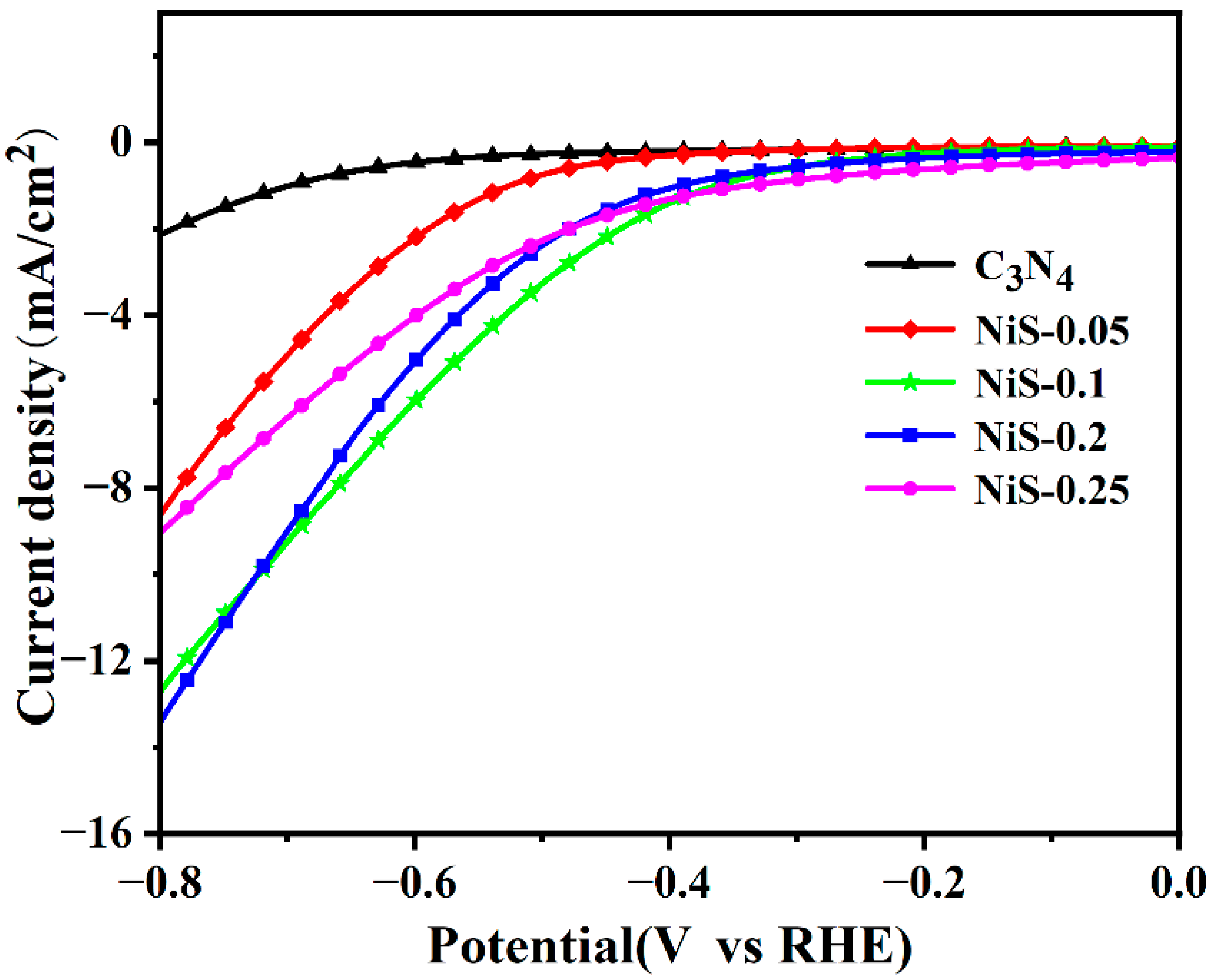
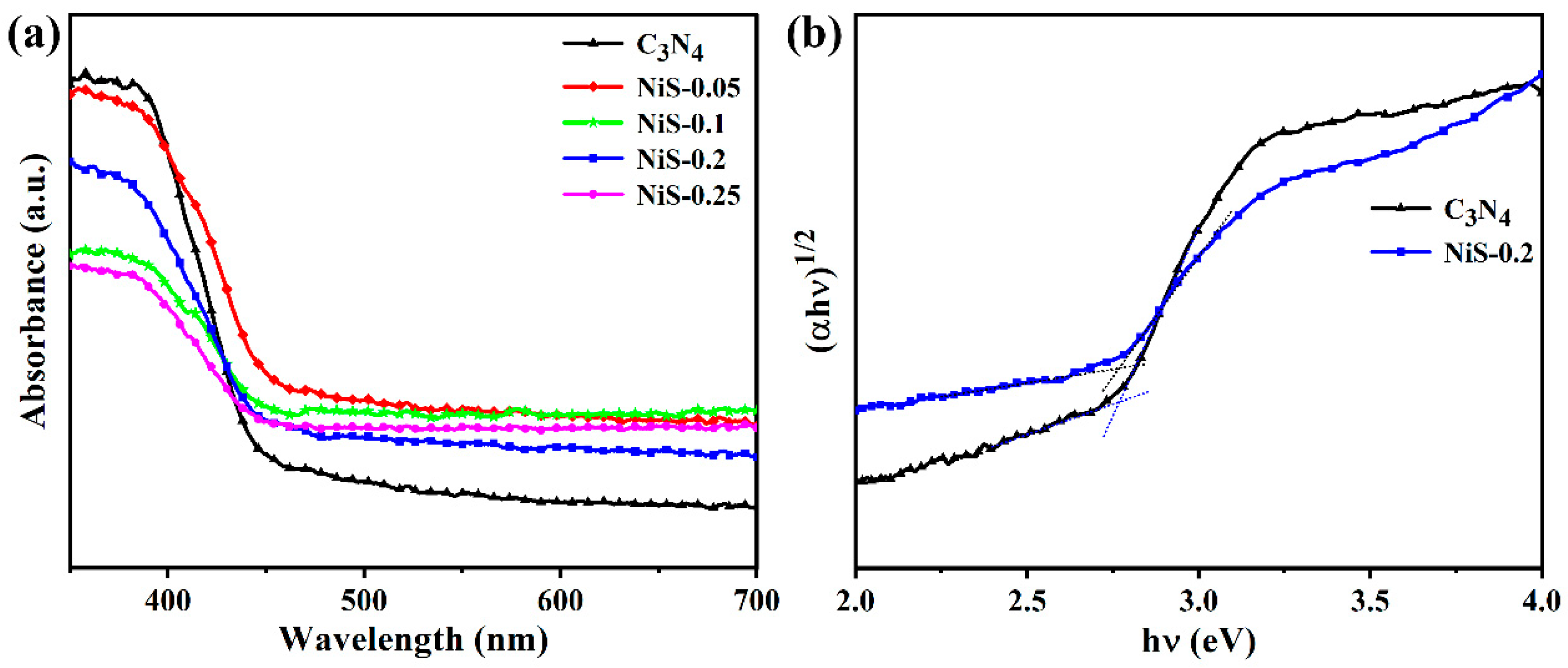
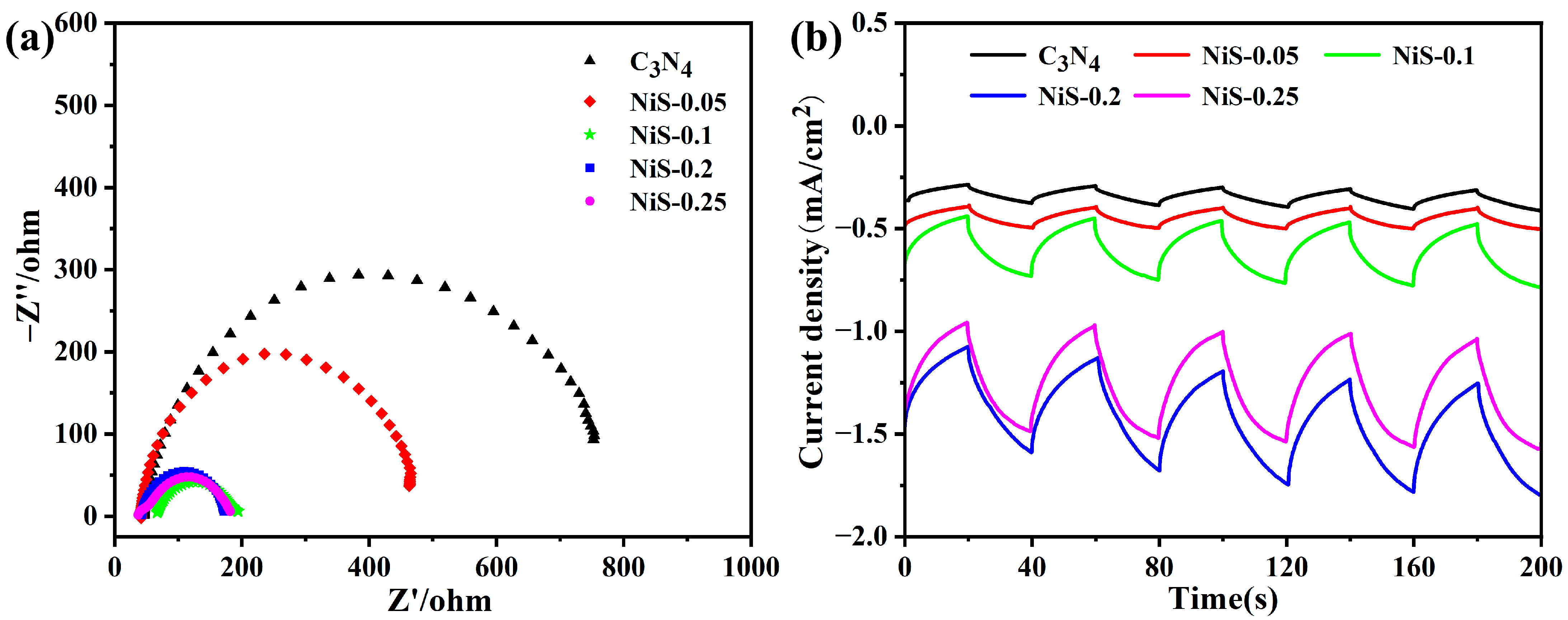
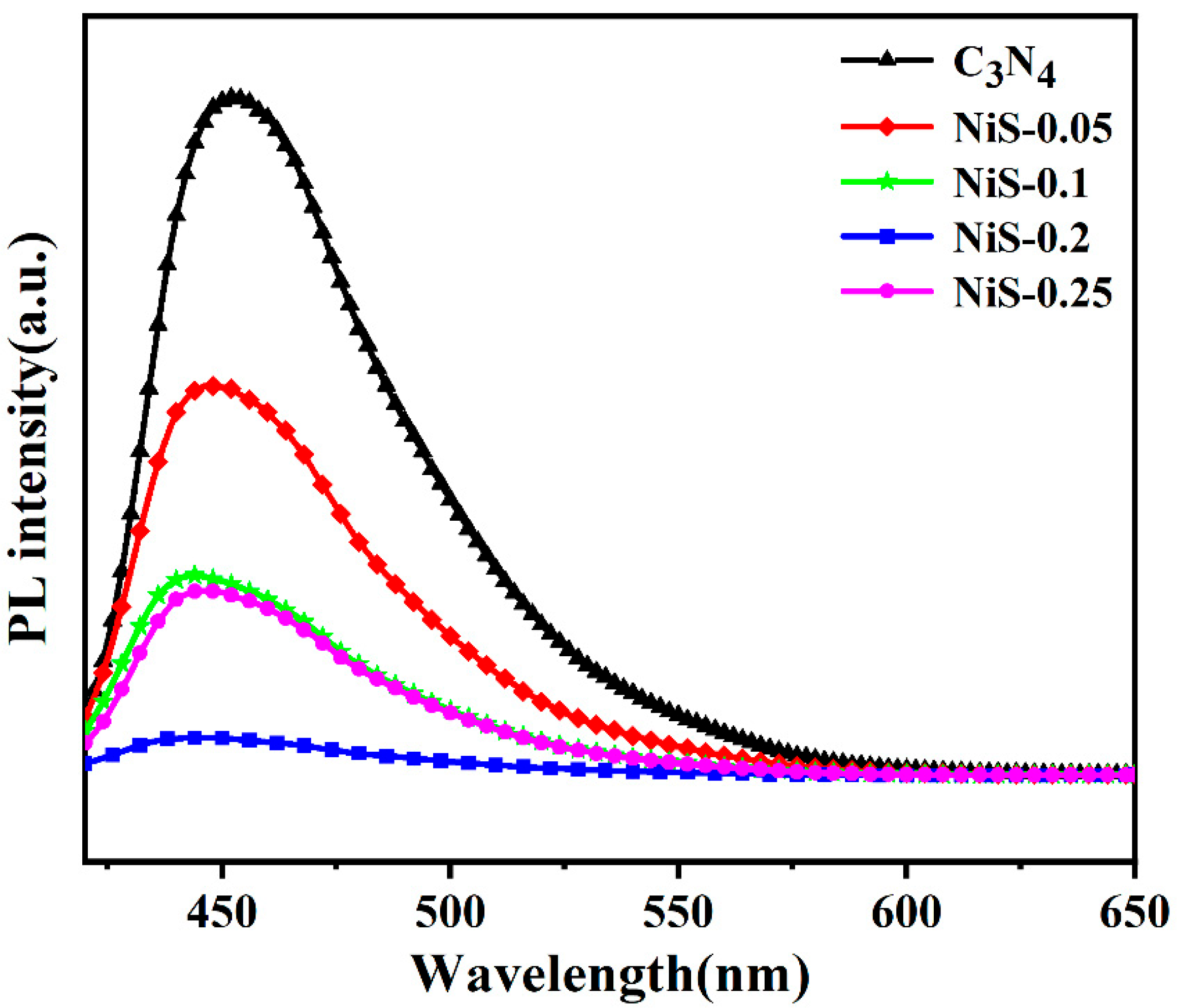
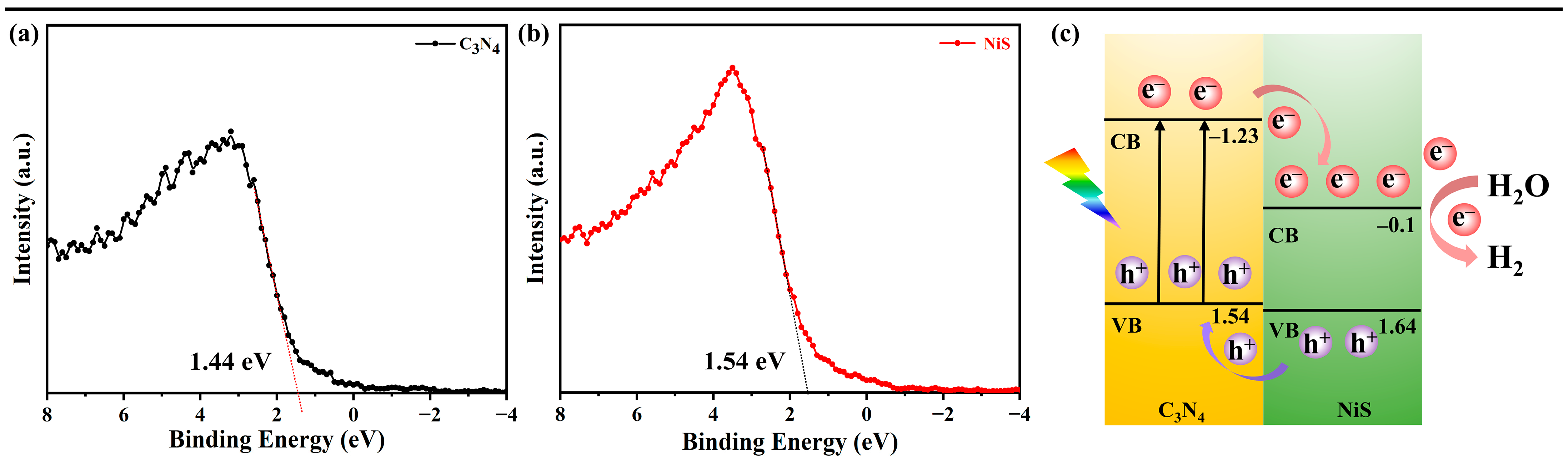
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gursoy, M.; Dincer, I. Experimental investigation and assessment of a newly designed hybrid hydrogen reactor for sustainable fuel production. Fuel 2026, 405, 136497. [Google Scholar] [CrossRef]
- Anand, C.; Chandraja, B.; Nithiya, P.; Akshaya, M.; Tamizhdurai, P.; Shoba, G.; Subramani, A.; Kumaran, R.; Yadav, K.K.; Gacem, A.; et al. Green hydrogen for a sustainable future: A review of production methods, innovations, and applications. Int. J. Hydrogen Energy 2025, 111, 319–341. [Google Scholar] [CrossRef]
- Guo, S.F.; Zhang, Y.Z.; Liu, L.N. Sorption enhanced steam reforming of biomass-based feedstocks: Towards sustainable hydrogen evolution. Chem. Eng. J. 2024, 485, 149760. [Google Scholar] [CrossRef]
- Vilanova, A.; Dias, P.; Lopes, T.; Mendes, A. The route for commercial photoelectrochemical water splitting: A review of large-area devices and key upscaling challenges. Chem. Soc. Rev. 2024, 53, 2388–2434. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Ji, S.G.; Jeong, C.S.; Kim, J.; Kwon, H.R.; Lee, T.H.; Lee, S.A.; Cheon, W.S.; Lee, S.; Lee, H.; et al. High-efficiency unbiased water splitting with photoanodes harnessing polycarbazole hole transport layers. Energy Environ. Sci. 2024, 17, 2541–2553. [Google Scholar] [CrossRef]
- He, B.; Cao, Y.; Lin, K.J.; Wang, Y.; Li, Z.; Yang, Y.K.; Zhao, Y.L.; Liu, X.Q. Strong interactions between Au nanoparticles and BiVO4 photoanode boosts hole extraction for photoelectrochemical water splitting. Angew. Chem. Int. Ed. 2024, 63, e202402435. [Google Scholar] [CrossRef]
- Najafli, E.; Ratso, S.; Ivanov, Y.P.; Gatalo, M.; Pavko, L.; Yörük, C.R.; Walke, P.; Divitini, G.; Hodnik, N.; Kruusenberg, I. Sustainable CO2-derived nanoscale carbon support to a platinum catalyst for oxygen reduction reaction. ACS Appl. Nano Mater. 2023, 6, 5772–5780. [Google Scholar] [CrossRef]
- Lacarbonara, G.; Chini, S.; Ratso, S.; Kruusenberg, I.; Arbizzani, C. A MnOx–graphitic carbon composite from CO2 for sustainable Li-ion battery anodes. Mater. Adv. 2022, 3, 7087–7097. [Google Scholar] [CrossRef]
- Zhang, H.R.; Zhang, B.Q.; Wang, X.L.; Zou, L.L.; You, J.; Lin, S.W. Effective charge separation in photoelectrochemical water splitting: A review from advanced evaluation methods to materials design. Sustain. Energy Fuels 2024, 8, 2357–2382. [Google Scholar] [CrossRef]
- Abouelela, M.M.; Kawamura, G.; Matsuda, A. Metal chalcogenide-based photoelectrodes for photoelectrochemical water splitting. J. Energy Chem. 2022, 73, 189–213. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Xiao, Y.; Li, Y.; Delaunay, J.-J. Earth-abundant Cu-based metal oxide photocathodes for photoelectrochemical water splitting. Energy Environ. Sci. 2020, 13, 3269–3306. [Google Scholar] [CrossRef]
- Shi, L.; Benetti, D.; Li, F.Y.; Wei, Q.; Rosei, F. Design of MOF-derived NiO-carbon nanohybrids photocathodes sensitized with quantum dots for solar hydrogen production. Small 2022, 18, 2201815. [Google Scholar] [CrossRef] [PubMed]
- Moakhar, R.S.; Hosseini-Hosseinabad, S.M.; Masudy-Panah, S.; Seza, A.; Jalali, M.; Fallah-Arani, H.; Dabir, F.; Gholipour, S.; Abdi, Y.; Bagheri-Hariri, M.; et al. Photoelectrochemical water-splitting using CuO-based electrodes for hydrogen production: A review. Adv. Mater. 2021, 33, 2007285. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.Q.; Cheng, Y.Y.; Qiang, Y.; Fu, X.Y.; Jia, Y.J. Boosting the performance of Cu2O/Si hybrid photocathodes for solar-driven H2 generation: From charge dynamics to thermodynamic factors. ACS Appl. Mater. Interf. 2025, 17, 22519–22528. [Google Scholar] [CrossRef]
- Li, S.J.; Yang, G.L.; Ge, P.; Lin, H.W.; Wang, Q.; Ren, X.H.; Luo, S.Q.; Philo, D.; Chang, K.; Ye, J.H. Engineering heterogeneous NiS2/NiS cocatalysts with progressive electron transfer from planar p-Si photocathodes for solar hydrogen evolution. Small Method. 2021, 5, 2001018. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Ali, R.; Khan, I.; Ullah, N.; Ahmad, M.S.; Kida, T.; Wooh, S.; Tremel, W.; Schwingenschlögl, U.; Tahir, M.N. Bandgap engineering of melon using highly reduced graphene oxide for enhanced photoelectrochemical hydrogen evolution. Adv. Mater. 2023, 35, 2301342. [Google Scholar] [CrossRef]
- Ajmal, S.; Bui, T.D.H.; Bui, Q.V.; Yang, T.; Shao, X.D.; Kumar, A.; Kim, S.G.; Lee, H. Accelerating water reduction towards hydrogen generation via cluster size adjustment in Ru-incorporated carbon nitride. Chem. Eng. J. 2022, 429, 132282. [Google Scholar] [CrossRef]
- Zeb, W.; Altaf, A.; Aamir, M.; Baig, N.; Baig, I.; Nafady, A.; Sharif, M.; Sher, M.; Sohail, M. Enhanced photoelectrochemical performance of P-doped g-C3N4/Zn0.5Cd0.5S heterojunction photocathode for water splitting. J. Saudi Chem. Soc. 2022, 26, 101542. [Google Scholar] [CrossRef]
- Li, T.; Wang, S.; Li, L.; Zhu, H.; Yang, Y.; Liu, G. Constructing porous carbon nitride nanosheets for efficient visible-light-responsive photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 628, 214–221. [Google Scholar] [CrossRef]
- Zhang, S.S.; Yan, J.; Yang, S.Y.; Xu, Y.H.; Cai, X.; Li, X.; Zhang, X.C.; Peng, F.; Fang, Y.P. Electrodeposition of Cu2O/g-C3N4 heterojunction film on an FTO substrate for enhancing visible light photoelectrochemical water splitting. Chin. J. Catal. 2017, 38, 365–371. [Google Scholar] [CrossRef]
- Zhang, T.; Shang, E.; Liao, Z.; Xu, Z.; Luo, J.; Wang, C.; Ni, C.; Ni, J. Simulated solar-driven photo-assisted anodic oxidation of sulfadiazine by C3N4 modified Ti3+ self-doping TiO2 nanotube arrays. Sep. Purif. Technol. 2024, 334, 126055. [Google Scholar] [CrossRef]
- Lin, F.F.; Tian, R.R.; Dong, P.; Jiang, G.F.; He, F.T.; Wang, S.J.; Fu, R.B.; Zhao, C.C.; Gu, Y.Y.; Wang, S.B. Defect-rich MoS2/NiS2 nanosheets loaded on SiNWs for efficient and stable photoelectrochemical hydrogen production. J. Colloid Interface Sci. 2023, 631, 133–142. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.F.; Guo, Z.G.; Yan, W.G.; Ruan, M.N. Decorating Cu2O photocathode with noble-metal-free Al and NiS cocatalysts for efficient photoelectrochemical water splitting by light harvesting management and charge separation design. Chem. Eng. J. 2020, 381, 122655. [Google Scholar] [CrossRef]
- Yu, W.Q.; Mo, F.J.; Guo, J.; Yang, Y.Q.; Jin, Y.S.; Fu, Y.Z. Ultrasensitive microRNA photoelectric assay based on a mimosa-like CdS-NiS/Au schottky junction. Anal. Chem. 2023, 95, 12097–12103. [Google Scholar] [CrossRef]
- Liu, Z.F.; Zhou, M. Co-Modification with cost-effective nickel oxides and nickel sulfides on CuInS2 nanosheets photocathode for enhanced photoelectrochemical performance. ACS Sustain. Chem. Eng. 2020, 8, 512–519. [Google Scholar] [CrossRef]
- Gu, C.J.; Zhou, G.Y.; Yang, J.; Pang, H.; Zhang, M.Y.; Zhao, Q.; Gu, X.F.; Tian, S.; Zhang, J.B.; Xu, L.; et al. NiS/MoS2 Mott-Schottky heterojunction-induced local charge redistribution for high-efficiency urea-assisted energy-saving hydrogen production. Chem. Eng. J. 2022, 443, 136321. [Google Scholar] [CrossRef]
- Yoo, J.; Kwak, I.H.; Kwon, I.S.; Park, K.; Kim, D.; Lee, J.H.; Lim, S.A.; Cha, E.H.; Park, J. Nickel sulfide nanocrystals for electrochemical and photoelectrochemical hydrogen generation. J. Mater. Chem. C 2020, 8, 3240–3247. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmad, T. Unveiling the bifunctional photo/electrocatalytic activity of in situ grown CdSe QDs on g-C3N4 nanosheet Z-scheme heterostructures for efficient hydrogen generation. ACS Catal. 2024, 14, 691–702. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, X.-T.; Jian, L.-J.; Abdallah, N.I.M.; Dong, X.-F.; Wang, C.-W. P-n heterostructured design of decahedral NiS/BiVO4 with efficient charge separation for enhanced photodegradation of organic dyes. Colloid Surface A 2021, 608, 125565. [Google Scholar] [CrossRef]
- Dos Santos, G.; Tian, L.; Gonçalves, R.; García, H.; Rossi, L. Boosting CO2 photoreduction efficiency of carbon nitride via S-scheme g-C3N4/Fe2TiO5 heterojunction. Adv. Funct. Mater. 2025, 35, 2422055. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, J.; Chu, D.; Cao, L.; Li, X.; Zhao, Y.; Liu, Y.; Dong, J.; Feng, L. Accelerated accelerated photogenerated charge separation driven synergistically by the interfacial electric field and work function in Z-scheme Zn-Ni2P/g-C3N4 for efficient photocatalytic hydrogen evolution. Exploration 2025, 5, 20240189. [Google Scholar] [CrossRef]
- Wang, K.; Yang, Y.; Farhan, S.; Wu, Y.; Lin, W.-F. S-scheme p-n junction Na0.6CoO2/g-C3N4 heterostructure as an efficient photocatalyst for green hydrogen production: Fabrication, characterization and mechanisms. Chem. Eng. J. 2024, 490, 151408. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, K.; Xie, C.; Yang, P. Vertically implanting MoSe2 nanosheets on superior thin C-doped g-C3N4 nanosheets towards interface-enhanced electrochemical activities. Carbon 2024, 220, 118884. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, G.; Zhang, H.; He, J.; Jia, J.; Zhang, Y.; Zhu, L.; Shi, M.; Du, Y.; Zhao, J.; et al. In-situ construction NiS, In(OH)3 and InOOH on the ZnIn2S4 nanosheet for synergistically boosting the photocatalytic H2 evolution. J. Alloys Compd. 2024, 1005, 176101. [Google Scholar] [CrossRef]
- Mahanthappa, M.; Bhat, M.P.; Alshoaibi, A.; S, V.R. Exploring the role of redox-active species on NiS-NiS2 incorporated sulfur-doped graphitic carbon nitride nanohybrid as a bifunctional electrocatalyst for overall water splitting. Int. J. Hydrogen Energy 2024, 84, 641–649. [Google Scholar] [CrossRef]
- Obiang Nsang, G.E.; Ullah, B.; Hua, S.; Shah, S.A.; Ullah, N.; Ullah, N.; Dike, F.N.; Yaseen, W.; Yuan, A.; Khan, N.; et al. NiS nanoparticle-MoS2 nanosheet core-shell spheres: PVP-assisted synthesis and efficient electrocatalyst for hydrogen evolution reaction. Energy Mater. 2025, 5, 500047. [Google Scholar] [CrossRef]
- Zhang, W.X.; Kong, S.L.; Wang, W.W.; Cheng, Y.M.; Li, Z.; He, C. Enhanced electrocatalytic performance of LCO-NiFe-C3N4 composite material for highly efficient overall water splitting. J. Colloid Interface Sci. 2025, 680, 787–796. [Google Scholar] [CrossRef]
- Zhu, X.L.; Zhou, E.L.; Tai, X.S.; Zong, H.B.; Yi, J.J.; Yuan, Z.M.; Zhao, X.L.; Huang, P.; Xu, H.; Jiang, Z.Y. g-C3N4 S-Scheme homojunction through Van der Waals interface regulation by intrinsic polymerization tailoring for enhanced photocatalytic H2 evolution and CO2 reduction. Angew. Chem. Int. Ed. 2025, 64, e202425439. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, Y.; Ge, W.; Dong, L.; Qi, Y.; Lian, C.; Zhou, X.; Liu, H.; Liu, Z.; Jiang, H.; et al. Mechanistic insights into surfactant-modulated electrode–electrolyte interface for steering H2O2 electrosynthesis. J. Am. Chem. Soc. 2024, 146, 7575–7583. [Google Scholar] [CrossRef]
- Li, B.; Chen, M.; Hu, Q.; Zhu, J.; Yang, X.; Li, Z.; Hu, C.; Li, Y.; Ni, P.; Ding, Y. Facilely tunable dodecahedral polyoxometalate framework loaded with mono- or bimetallic sites for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. Energy 2024, 346, 123733. [Google Scholar] [CrossRef]
- Lei, Z.N.; Ma, X.Y.; Hu, X.Y.; Fan, J.; Liu, E.Z. Enhancement of photocatalytic H2-evolution kinetics through the dual cocatalyst activity of Ni2P-NiS-decorated g-C3N4 heterojunctions. Acta Phys.-Chim. Sin. 2022, 38, 2110049. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, S.; Ma, W.; Qian, J.; Huang, L.; Deng, H.; Zhou, Q.; Zheng, S.; Li, S.; Du, H.; et al. Facile synthesis of direct Z-scheme PPy/NH2-UiO-66 heterojunction for enhanced photocatalytic Cr(VI) reduction, industrial electroplating wastewater treatment, and tetracycline degradation. Appl. Catal. B Environ. Energy 2024, 344, 123669. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, J.Y.; Zhong, C.G.; He, C.X.; Khan, M.; Liu, D.N.; Wang, J.L.; Yang, R.J.; Kan, M.; Wang, L.Z.; et al. High-loading Cu single-atom engineering on g-C3N4 for visible-light CO2 photoreduction. Small 2025, 21, 2503390. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Q.; Deng, F.; Huang, J.; Han, L.; He, C.; Chen, Z.; Luo, Y.; Zhu, Y. Double-defect-induced polarization enhanced OV-BiOBr/Cu2-xS high-low junction for boosted photoelectrochemical hydrogen evolution. Appl. Catal. B Environ. 2022, 314, 121502. [Google Scholar] [CrossRef]
- Mittal, S.; Khosya, M.; Khare, N. Nanostructured Ni-doped SnS2 photoanode for efficient photoelectrochemical water splitting. ACS Appl. Energy Mater. 2025, 8, 4080–4089. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Dong, L.; Chen, Y.; Qi, C.; Xu, C.; Zhang, W.; Bi, L.; Zhao, L. Boosting Charge Separation in NiS/C3N4 Type-II Heterojunction for Efficient Photoelectrocatalytic Water Reduction. Crystals 2025, 15, 1004. https://doi.org/10.3390/cryst15121004
Liang X, Dong L, Chen Y, Qi C, Xu C, Zhang W, Bi L, Zhao L. Boosting Charge Separation in NiS/C3N4 Type-II Heterojunction for Efficient Photoelectrocatalytic Water Reduction. Crystals. 2025; 15(12):1004. https://doi.org/10.3390/cryst15121004
Chicago/Turabian StyleLiang, Xiaobo, Lingdan Dong, Yanning Chen, Chunhai Qi, Chunyi Xu, Wenhao Zhang, Lingling Bi, and Liang Zhao. 2025. "Boosting Charge Separation in NiS/C3N4 Type-II Heterojunction for Efficient Photoelectrocatalytic Water Reduction" Crystals 15, no. 12: 1004. https://doi.org/10.3390/cryst15121004
APA StyleLiang, X., Dong, L., Chen, Y., Qi, C., Xu, C., Zhang, W., Bi, L., & Zhao, L. (2025). Boosting Charge Separation in NiS/C3N4 Type-II Heterojunction for Efficient Photoelectrocatalytic Water Reduction. Crystals, 15(12), 1004. https://doi.org/10.3390/cryst15121004




