Abstract
Although low-silicon Al-Si alloys have been extensively studied, further improvement in their mechanical performance remains a critical challenge. This study examines the synergistic effects of scandium (Sc) and zirconium (Zr) additions on the solidification behavior, microstructural evolution, and mechanical properties of Al-5Si-Cu-Mg alloys. The Sc/Zr additions refine the α-Al grains and modify the eutectic Si morphology, with the most uniform microstructure obtained at 0.5 wt.% due to the formation of coherent Al3(Sc,Zr) dispersoids. These additions also suppress the formation of needle-like β-Al5FeSi phases and promote the transformation to compact α-Al15(Fe,Mn)3(Si,Zr,Sc)2 intermetallics, optimizing the solidification process. The yield strength increases with Sc/Zr content owing to grain-boundary and precipitation strengthening. However, the alloy without Sc/Zr exhibits the highest ultimate tensile strength and elongation, likely due to its finer secondary dendrite arm spacing and the absence of casting-induced cracks in this investigation. Although Sc/Zr additions of 0.25–0.5 wt.% contribute to microstructural refinement, the concurrent formation of porosity and coarse intermetallic compounds leads to a deterioration in ductility. Excessive Sc/Zr additions further coarsen grains and degrade the overall mechanical integrity.
1. Introduction
Al-Si-Cu-Mg cast aluminum alloys have gained significant attention due to their balanced combination of castability, strength, and corrosion resistance, making them essential for a wide range of engineering applications. The growing demand for lightweight and durable components has driven the development of high-strength and high-ductility Al-Si-Cu-Mg cast alloys [1,2].
These alloys, especially those with higher silicon content (typically above 10 wt.%), offer improved fluidity and reduced solidification shrinkage defects. However, the increased silicon content often results in coarse, plate-like eutectic Si phases, which can deteriorate the alloy’s ductility and machinability [3,4,5]. To overcome these drawbacks, researchers have extensively explored microstructural refinement through various approaches, such as the use of grain refiners such as Al-Ti-Nb-B [6], Al-Ti-B [7,8] and Al-Ti-C [9], as well as modifying the plate-like eutectic Si into a more favorable fibrous structure using elements like Na and Sr [10,11].
Another promising direction is the development of low-silicon Al-Si-Cu-Mg alloys (typically <8 wt.% Si), which exhibit more homogeneous Si distributions and refined microstructures. These alloys offer improved elongation and higher thermal conductivity, making them particularly suitable for components requiring efficient heat dissipation, such as electronic housings and electric vehicle powertrain parts. Moreover, recent studies indicate that low-silicon Al-Si-based alloys are better suited for advanced casting processes such as high-pressure die casting (HPDC) and semi-solid metal (SSM) forming due to their lower hot-tearing susceptibility and enhanced process control flexibility [12,13].
To further improve the strength of these alloys, it crucial to innovate both the composition and manufacturing processes. Increasing the Cu and Mg contents, in combination with rapid solidification, has been demonstrated to strengthen Al-Si-based alloys through enhanced precipitation hardening increasing the Cu and Mg contents, combined with rapid cooling, has been shown to enhance precipitation hardening [14]. Li et al. further revealed that the three-dimensional network of second phases in Al-Si alloys plays a decisive role in controlling strength and ductility, particularly in high-performance piston alloys [15].
Despite these advancements, low-silicon Al-Si alloys still face challenges in castability, including poor fluidity and shrinkage porosity, which hinder their application in high-integrity cast components. One promising solution is the microalloying of trace elements such as Sc and Zr. These elements promote the formation of coherent Al3Sc and Al3(Sc,Zr) precipitates, which act as potent heterogeneous nucleation sites for α-Al grains and improve solidification behavior [13,16,17,18,19]. Specifically, Sc addition has been reported to enhance melt fluidity and reduce interdendritic shrinkage [20,21]. while interfacial energy calculations indicate that the heterogeneous nucleation potency follows the order Al3(Sc,Zr) > Al3Zr > Al3Sc [16,22]. Moreover, compared to Zr alone, the combined addition of Sc and Zr have demonstrated synergistic grain refinement effects and improved eutectic Si morphology compared with either element alone [23,24]. The Sc/Zr atomic ratio of 3:2 was proved as an optimal proportion to promote the formation of thermally stable Al3(Sc,Zr) dispersoids, which effectively refine α-Al grains and facilitate a uniform distribution of eutectic Si particles during solidification [23].
To explore this potential, the present study aims to clarify the synergistic effects of Sc and Zr co-addition in a low-silicon Al-Si-Cu-Mg-Sr alloy system, focusing on how the Sc/Zr ratio influences solidification behavior, grain refinement, and mechanical properties. Through integrated microstructural characterization, DSC analysis, and mechanical testing, this study establishes the compositional–microstructural–performance relationship in low-silicon Al-Si-Cu-Mg cast alloys, providing a scientific basis for the design of next-generation high-performance foundry alloys with enhanced castability, ductility, and strength for lightweight engineering applications.
2. Experimental Materials and Methods
2.1. Alloy Composition Design
In this study, a series of low-silicon Al-Si based casting alloys were designed and prepared using base materials including Al-Si-Cu-Mg master alloys, electrolytic aluminum, pure magnesium ingots, and master alloys of Al-10 wt. %Sr, Al-2 wt.% Sc, and Al-10 wt.% Zr. Four different alloy compositions with varying Sc and Zr contents at 0 wt.%, 0.25 wt.%, 0.5 wt.%, 0.75 wt.% were developed, and the Sc/Zr atomic ratio was fixed at 3:2 across all modified alloys. The chemical composition of the alloy samples was determined by inductively coupled plasma-optical emission spectrometry (ICP-OES, iCAP™ PRO XP ICP-OES, Thermo Scientific™, Bremen, Germany) in accordance with GB/T 20975.25-2020 (Methods for chemical analysis of aluminium and aluminium alloys—Part 25: Determination of elements content—Inductively coupled plasma atomic emission spectrometric method: Technical Committee of Nonferrous Metals Standardization, Beijing, China, 2020), and the detailed composition of each target alloy is listed in Table 1.

Table 1.
Chemical composition of the designed alloys (wt.%).
2.2. Experimental Procedures
The alloy melting process was conducted in an electric resistance furnace using a graphite crucible. The base materials were sequentially added and melted under the protection of a refined argon (Ar) gas to prevent oxidation and combustion of magnesium. Once the melt temperature stabilized at approximately 750 ± 5 °C, slag removal and degassing procedures were carried out. The melt was then held for 10 min to ensure complete alloying before being poured into a preheated permanent mold made of cast iron at 250 °C, with an estimated cooling rate of approximately 15–20 °C/s. The pouring temperature was maintained at around 720 °C. All alloy samples (A-D) were cast under the same controlled conditions to ensure experimental consistency.
2.3. Characterization Methods
The microstructures of the as-cast samples were characterized using a combination of optical microscopy (OM, Carl Zeiss, axiovert 7, Oberkochen, Germany), scanning electron microscopy (SEM, Hitachi TM4000Plus II, Tokyo, Japan) equipped with energy-dispersive X-ray spectroscopy (EDS). The samples were sectioned from the central part of the casting, ground with SiC papers up to 2000 grit, polished with diamond suspension, and etched using a 4% nitric acid solution in ethanol (4 vol% HNO3 + 96 vol% C2H5OH) for approximately 10–15 s to reveal the grain structures.
Phase identification and crystallographic analysis were performed using X-ray diffraction (XRD, Empyrean, Panalytical, Almelo, The Netherlands) with Cu-Kα radiation, operating at 40 kV and 30 mA. In addition, a simultaneous thermal analyzer (STA, Netzsch STA449F3, Selb, Germany) was employed to investigate the thermal behavior and phase transformation characteristics of the alloys. Samples weighing approximately 10 mg were heated from room temperature to 700 °C at a heating rate of 10 °C/min under a nitrogen atmosphere. The onset temperatures of phase transformations and precipitation behavior were recorded and compared among the different alloy compositions.
2.4. Mechanical Testing
Standard dog-bone shaped specimens were machined from the central region of the castings in accordance with GB/T 228.1-2021 (Metallic materials-tensile testing-Part 1: methods of test at room temperature: Technical Committee on Steel of Standardization Administration of China, Beijing, China, 2021) or ASTM E8/E8M-22 (Standard Test Methods for Tension Testing of Metallic Materials: ASTM International, West Conshohocken, PA, USA, 2022) standards. These specimens had an overall length of 75 mm, a gauge length of 28 mm, and a width of 12.5 mm (Figure 1). Tensile tests were conducted at room temperature using an electronic universal testing machine at a crosshead speed of 1 mm/min. Each test condition was repeated at least three times to ensure statistical reliability. The average values of ultimate tensile strength (UTS), yield strength (YS), and elongation to failure (EL%) were reported. Fracture surfaces of tensile specimens were examined using SEM to evaluate the fracture mechanisms and to correlate them with the microstructural features. In this study, a microhardness tester was employed to evaluate the macrohardness of the alloys. The test was conducted under a load of 100 gf with a dwell time of 10 s.
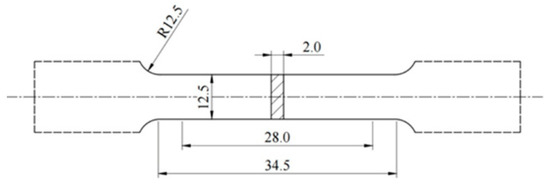
Figure 1.
A schematic showing the dimensions of the tensile sample (mm).
3. Experimental Results
3.1. Microstructures
Figure 2 presents the optical microstructures of the four target alloys with varying Sc/Zr additions. As shown in Figure 2a, the alloy without Sc/Zr additions exhibits coarse dendritic structure of the α-Al matrix, characterized by a dendritic morphology with limited cellular grains. Upon adding 0.25 wt.% Sc/Zr (Figure 2b), the number of α-Al dendrites decreases, and a greater quantity of rosette-like cellular grains emerges. The observed grain size is significantly reduced compared to that in Figure 2a, suggesting that the Sc/Zr additions promoted heterogeneous nucleation during solidification. With a further increase to 0.5 wt.% Sc/Zr (Figure 2c), the grain refinement effect is enhanced, and the number of rosette-like grains increases. However, when the Sc/Zr content reaches 0.75 wt.% (Figure 2d), a slight reduction in the number of rosette-like primary α-Al grains is observed, and these grains coarsened a little. This indicates that while Sc/Zr additions promote nucleation and the formation of rosette-like primary phases, excessive Sc/Zr may have a detrimental effect on microstructural refinement, potentially due to solute saturation or the formation of ineffective compounds [25]. Therefore, careful control of Sc/Zr addition is necessary to avoid compromising the refinement efficiency.
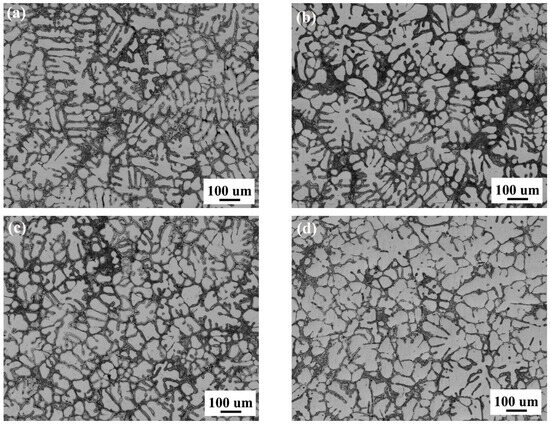
Figure 2.
Optical micrographs of Al–5Si–1.2Cu–0.5Mg–0.02Sr alloys with varying additions of Sc/Zr. (a) Alloy A00, (b) Alloy B25, (c) Alloy C50 and (d) Alloy D75.
Figure 3 presents the eutectic microstructures of the Al-5Si-Cu-0.5Mg-0.02Sr alloys with varying Sc/Zr additions. The eutectic Si phase appears dark gray, while the intermetallic particles are brighter in contrast, concentrating in the interdendritic regions. It is evident that the morphology and distribution of eutectic Si are significantly influenced by the Sc/Zr content, showing a transition from coarser and clustered structures to uniformly distributed fine particles as the addition levels increase. As shown in Figure 3a, the morphology of eutectic Si appears coarse rod-like to worm-like structures, which are densely distributed along the grain boundaries. At this stage, no Sc/Zr grain refiners were added. From Figure 3a–d, it is clear that with increasing Sc/Zr addition, the size of eutectic Si gradually decreases, and its distribution becomes scattered, with the microstructure in Figure 3c showing the most optimal morphology. However, when the Sc/Zr content reaches 0.75 wt.%, the amount of eutectic Si phase decreases a litter, and its distribution becomes more irregular. Additionally, as the Sc/Zr content increases, the gray needle-like phase transforms into rod-like phases along the grain boundaries.
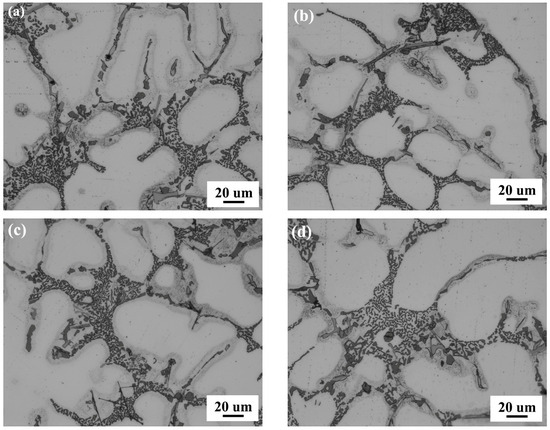
Figure 3.
Eutectic silicon morphology in Al-5Si-1.2Cu-0.5Mg-0.02Sr alloys with different Sc/Zr additions: (a) Alloy A00, (b) Alloy B25, (c) Alloy C50, (d) Alloy D75.
As shown in Figure 4, the XRD patterns of alloys A00 and D75 reveal differences in phase composition. Alloy A00 primarily consists of Al and Si phases, with a small amount of the θ-Al2Cu phase. In addition to these phases, alloy D75 also contains detectable amounts of the Al3Sc phase.
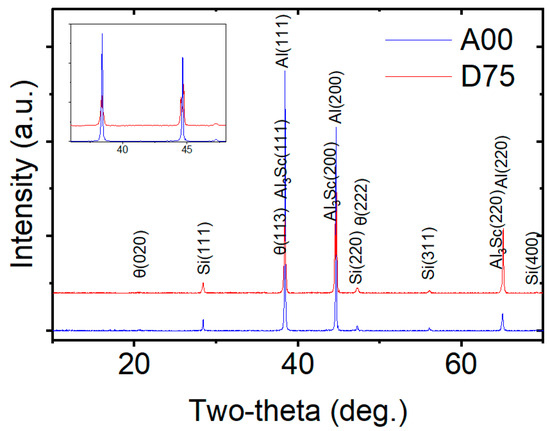
Figure 4.
X-ray diffraction (XRD) patterns of Alloy A00 and Alloy D75.
As shown in Figure 5, the SEM micrographs of alloy A00 and alloy D75 reveal typical microstructural features of an Al-Si hypereutectic alloy. In addition to the primary α-Al grains, the microstructure of the alloy also reveals the presence of eutectic Si, the θ-Al2Cu intermetallic phase, and various Fe-rich phases, which are commonly distributed along the grain boundaries and in the intermetallic regions.
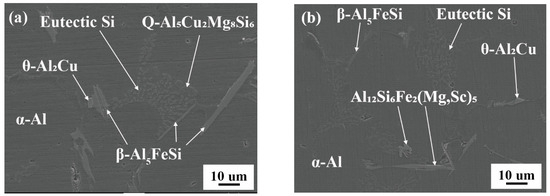
Figure 5.
Representation of the microstructure of the experimental alloys. (a) Alloy A00, (b) Alloy D75.
The chemical compositions of the phases in the microstructure of the alloys are summarized in Table 2. The EDS analysis indicates that the addition of Sc, Mn, and Zr to Al-Si-based alloys effectively suppresses the formation of the detrimental β-Al5FeSi phase while promoting the precipitation of α-Fe phases with more compact and less harmful morphologies. These α-Fe intermetallics are often observed as complex compounds, such as Al15(Fe,Mn)3(Si,Zr,Sc)2 and Al12Si6Fe2(Mg,Sc)5 [21], in which Mn, Zr, and Sc act as substitutional elements that stabilize the α-phase structure and inhibit the growth of needle-like β-phase particles. The elevated Sc and Zr content implies significant atomic substitution within the Fe-Si lattice, leading to the formation of a more complex and thermodynamically stable intermetallic phase.

Table 2.
Chemical composition of semi-quantitative compositions of intermetallic phases (wt.%).
Figure 6 presents a comparison of differential scanning calorimetry (DSC) curves for the alloy A00 and alloy D75. Exothermic peaks are observed during cooling. The peak centered around 505 °C (Peak 1 in the figure) corresponds to the formation of the Cu-rich Al2Cu phase. The peak near 570 °C (Peak 3) is attributed to the solidification of the eutectic (Si-α-Al) and Fe-containing phases, while the peak at 600 °C (Peak 4) marks the solidification of the primary α-Al phase. Notably, an additional exothermic peak of β-Fe intermetallic phase appears at around 514 °C in the D00 alloy. The disappearance of the Peak 2 in the D75 alloy suggests that the increase in Sc/Zr content to 0.75 wt.% eliminates β-Fe intermetallic phase, likely promoting the formation of additional intermetallic phases such as Al15(Fe,Mn)3(Si,Zr,Sc)2 and Al12Si6Fe2(Mg,Sc)5 [21]. The DSC thermal analysis results indicated that the β-Fe phase in the AlSi5CuMg alloys disappeared when the Sc/Zr content was increased to 0.70 wt.%, which is aligns with the SEM observations presented in Figure 5. In addition, the liquidus temperatures for the A00 and D75 alloys were measured at 607 °C and 612 °C, respectively, indicating a slight increase in the liquidus temperature with the addition of Sc/Zr.
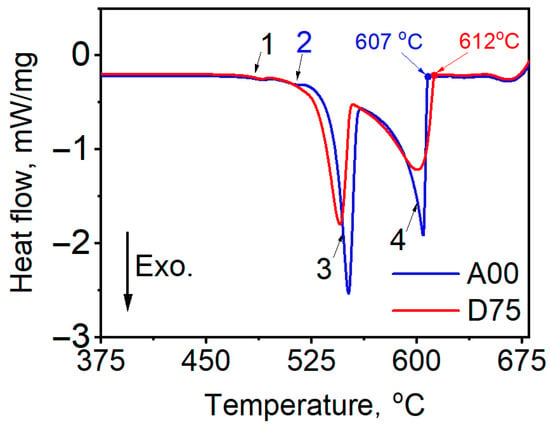
Figure 6.
Comparison of the differential scanning calorimetry (DSC) curves between A00 and D75 alloy. Peak 1 corresponds to the formation of the Cu-rich Al2Cu phase during cooling. Peak 2 is associated with the precipitation of the β-Fe intermetallic phase. Peak 3 represents the solidification of the eutectic constituent (Si + α-Al). Peak 4 corresponds to the solidification of the primary α-Al phase.
3.2. Mechanical Properties
Figure 7 shows the tensile stress–strain curves at room temperature for the four designed alloys with varying Sc/Zr additions. Alloy A00 exhibits a yield strength (YS) of 121 MPa, an ultimate tensile strength (UTS) of 241 MPa, and an elongation (EL) of 5.2%. With the addition of 0.25 wt.% Sc/Zr, Alloy B25 shows a slight increase in YS to 125 MPa, but a decrease in UTS to 209 MPa and EL to 2.7%. When the Sc/Zr content is increased to 0.5 wt.%, Alloy C50 achieves optimized mechanical performance, with a YS of 132 MPa, a UTS of 233 MPa, and an EL of 5.1%. This improvement is attributed to the optimal grain refinement and eutectic modification effects discussed previously. However, further increasing the Sc/Zr content to 0.75 wt.% in Alloy D75 results in a YS of 141 MPa, but a decrease in UTS to 216 MPa and a minimum EL of 2.3%.
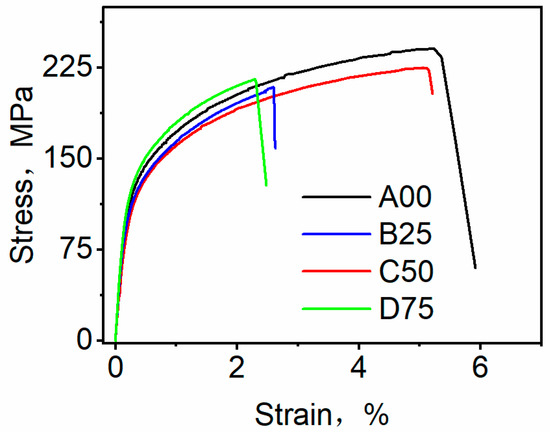
Figure 7.
Room-temperature tensile stress–strain behavior of Al-5Si-1.2Cu-0.5Mg-0.02Sr alloys as a function of Sc/Zr content.
Figure 8 shows the tensile fracture morphologies of the as-cast Al-5Si-1.2Cu-0.5Mg-0.02Sr alloys with different Sc/Zr additions. In Alloy A00 (Figure 8a) without Sc/Zr refinement, the fracture surface displays numerous cracks, large quasi-cleavage facets, and a few tear ridges and dimples, suggesting a predominantly brittle-ductile fracture. After adding 0.25 wt.% Sc/Zr (Alloy B, Figure 8b), the quasi-cleavage areas decrease while dimples and dense tear ridges become more evident, accompanied by some microporosity, indicating a mixed brittle fracture mode. For Alloy C with 0.5 wt.% Sc/Zr (Figure 8c), the fracture surface presents both quasi-cleavages and fine dimples, with visible cracks and Al2O3 inclusions, showing a transitional fracture behavior. When the Sc/Zr addition increases to 0.75 wt.% (Alloy D, Figure 8d), the fracture surface is mainly characterized by numerous small quasi-cleavage facets, a few fine cracks and fine dimples, indicating that the fracture mode has shifted toward a more brittle-ductile mechanism.

Figure 8.
SEM fractography of tensile fracture surfaces of Al-5Si-1.2Cu-0.5Mg-0.02Sr alloys with varying Sc/Zr additions: (a) A00 Alloy; (b) B25 Alloy; (c) C50 Alloy; (d) D75 Alloy.
4. Discussion
The incorporation of Sc and Zr significantly alters the solidification behavior, precipitation sequence, and mechanical response of Al-5Si-Cu-Mg alloys. These modifications arise primarily from the dual roles in promoting grain refinement and altering the stability and morphology of Fe- and Si-containing intermetallic phases.
4.1. Evolution of Solidification and Precipitation Microstructures
The introduction of scandium (Sc) and zirconium (Zr) markedly changes the solidification path and phase evolution of the base alloy. Both elements possess strong chemical affinity with Al, leading to the in-situ formation of coherent Al3(Sc,Zr) dispersoids with an ordered L12 structure. These dispersoids act as potent heterogeneous nucleation sites for primary α-Al, promoting the formation of fine equiaxed grains due to their small lattice mismatch with the Al matrix [26,27]. At moderate Sc/Zr contents (≈0.5 wt.%), the alloy exhibits refined α-Al grains and a uniformly distributed eutectic Si network (Figure 2c and Figure 3c), consistent with the findings of Lei et al. [13] and Xu et al. [23].
When the Sc/Zr content increases to 0.75 wt.%, however, the refinement effect deteriorates due to solute supersaturation and competing phase formation. Under high Si activity, Sc and Zr may preferentially react with Si to form silicides such as ScSi2 and ZrSi2, reducing the availability of Sc and Zr for Al3(Sc,Zr) formation [27,28]. The coexistence of Fe compounds further accelerates this process, as Fe promotes local Si enrichment and facilitates the reaction M + 2Si→MSi2(M = Sc, Zr), which is thermodynamically favorable [28,29]. Consequently, the precipitation of Al3(Sc,Zr) dispersoids is suppressed, and complex Al-Fe-Si-Sc-Zr intermetallics appear at elevated Sc/Zr additions [21,30].
Furthermore, the simultaneous presence of Sc, Zr, and Mn profoundly modifies the morphology and stability of Fe-rich phases. DSC and SEM analyses confirm the disappearance of the exothermic peak associated with β-Al5FeSi formation following Sc/Zr/Mn additions (Figure 5b and Figure 6). In the base alloy without Sc and Zr, a pronounced β-Al5FeSi formation peak indicates the precipitation of coarse, plate-like Fe-rich compounds. With increasing Sc/Zr content, this peak gradually weakens and eventually disappears, coinciding with the emergence of compact α-Al15(Fe,Mn)3(Si,Zr,Sc)2 and nodular Al12Si6Fe2(Mg,Sc)5 phases. These morphologies are thermodynamically more stable and mechanically less detrimental than the needle-like β-phase, as reported by Tzeng et al. [21] and Fuller et al. [26].
The suppression of β-Al5FeSi and the formation of α-Al15(Fe,Mn)3(Si,Zr,Sc)2 can be explained by the altered solidification sequence. Primary α-Al solidifies first at approximately 610–615 °C, followed by the nucleation of coherent Al3(Sc,Zr) dispersoids either at the final stage of solidification or during early solid-state cooling. As cooling proceeds to around 550–560 °C, eutectic reactions produce eutectic Si, Al2Cu, and Mg2Si within interdendritic regions. In Sc/Zr-free alloys, the β-Al5FeSi phase forms during this stage, reflected as a distinct exothermic peak in the DSC curve. However, when Sc and Zr are present, their incorporation into Fe-bearing compounds alters local thermodynamics and atomic diffusion, favoring the nucleation of compact α-type intermetallics at slightly higher temperatures. Consequently, the metastable β-Al5FeSi phase is thermodynamically suppressed, and the solidification pathway shifts toward the formation of more stable α-Al15(Fe,Mn)3(Si,Zr,Sc)2 and Al12Si6Fe2(Mg,Sc)5 phases. This transformation yields a finer and more compact Fe-rich morphology, improving mechanical compatibility with the Al matrix.
Overall, Sc/Zr additions in the range of 0.25–0.5 wt.% provide an optimal balance between nucleation efficiency and compositional stability. Below this range, Al3(Sc,Zr) formation is insufficient for significant refinement, while excessive additions deteriorate microstructural uniformity and increase porosity. Hence, precise control of Si activity and alloying sequence is essential to favor Al3(Sc,Zr) formation over competing compounds [29,31,32].
4.2. Correlation Between Microstructure and Mechanical Behavior
The evolution of microstructure induced by Sc/Zr additions exerts a pronounced influence on the mechanical behavior of the alloys. The base alloy (without Sc/Zr) exhibits moderate yield strength (121 MPa) but high ductility, attributed to its coarse α-Al grains and eutectic α-Al/Si microstructure. As Sc/Zr content increases, yield strength improves progressively due to grain-boundary strengthening and precipitation hardening. Fine α-Al grains hinder dislocation motion through the Hall-Petch effect, while coherent Al3(Sc,Zr) dispersoids impede dislocation motion [29,30].
At 0.5 wt.% Sc/Zr, the alloy achieves the best combination of strength and ductility (UTS = 233 MPa, EL = 5.1%), resulting from refined α-Al grains, homogeneous eutectic Si distribution, and effective precipitation hardening. These findings are consistent with previous studies by Lei et al. [13] and Xu et al. [23], which attribute similar property improvements to enhanced subgrain stability and reduced segregation.
However, excessive Sc/Zr addition (≈0.75 wt.%) deteriorate mechanical performance despite higher yield strength (141 MPa). Fractographic observations reveal quasi-cleavage facets and irregular dimples indicative of brittle fracture, resulting from casting defects and Al3(Sc,Zr) agglomeration. Such agglomeration increases melt viscosity, impedes feeding and degassing, and leads to porosity formation [16,33,34]. These defects act as crack initiation sites under stress, promoting premature failure.
The hardness trend (Figure 9) mirrors the microstructural evolution. The base alloy shows the lowest hardness (85.6 ± 9.3 HB), consistent with its coarse dendritic structure. The hardness increases progressively with Sc/Zr additions—89.8 ± 8.5 HB (0.25 wt.%) and 94.2 ± 10.7 HB (0.5 wt.%)—due to grain refinement and the elimination of brittle β-Fe phases. At higher Sc/Zr levels, hardness slightly decreases (92.1 ± 9.8 HB), reflecting the adverse effects of intermetallic coarsening and porosity. The fracture mode transitions from mixed brittle–ductile at low Sc/Zr contents to predominantly brittle at high contents, consistent with the findings of Fuller et al. [26] and Hegde & Prabhu [10]. Although the transformation from β-Al5FeSi to α-Al15(Fe,Mn)3(Si,Zr,Sc)2 reduces stress concentration and enhances toughness, excessive intermetallic formation ultimately embrittles the matrix.
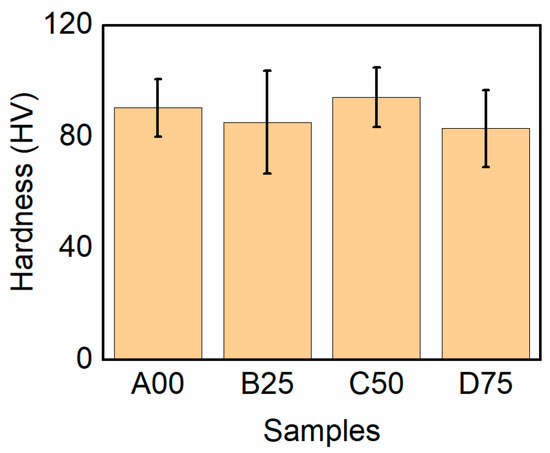
Figure 9.
Hardness of the Al–Si–Cu–Mg alloys with the addition of Sc and Zr.
In summary, the mechanical response of Sc/Zr-modified Al-Si-Cu-Mg alloys reflects a delicate balance between beneficial strengthening and detrimental defect formation. The optimal Sc/Zr addition (0.25–0.5wt.%) maximizes grain refinement and precipitation strengthening while maintaining adequate ductility and structural integrity. Beyond this range, microstructural heterogeneity and casting defects dominate, degrading overall performance. From an engineering perspective, controlling Sc/Zr additions below 0.5 wt.% offers an effective strategy to achieve high strength-to-weight ratios with reliable fracture resistance.
5. Conclusions
In this study, the effects of combined Sc and Zr additions on the microstructure and mechanical properties of Al-5Si-1.2Cu-0.5Mg-0.02Sr alloys were investigated. The following conclusions can be drawn:
- (1)
- The combined addition of scandium (Sc) and zirconium (Zr) effectively refines the α-Al grains and modifies the eutectic Si morphology in Al-5Si-1.2Cu-0.5Mg alloys. The optimal refinement occurs at 0.5 wt.% Sc/Zr, attributed to the formation of coherent Al3(Sc,Zr) dispersoids that promote heterogeneous nucleation and suppress needle-like β-Al5FeSi phases.
- (2)
- Mechanical testing shows that yield strength increases with Sc/Zr content due to grain-boundary and precipitation strengthening, while the base alloy without Sc/Zr maintains superior ductility, likely owing to its finer secondary dendrite arm spacing and absence of casting cracks.
- (3)
- Moderate Sc/Zr additions (0.25–0.5 wt.%) yield optimal mechanical balance, whereas excessive additions (>0.5 wt.%) promote silicide and Fe-rich intermetallic formation, reducing ductility and promoting brittleness. Therefore, controlled Sc/Zr additions below 0.5 wt.% are recommended to achieve an optimal combination of strength, ductility, and microstructural stability.
Author Contributions
Conceptualization, J.Z., K.W. and J.W.; Methodology, L.S. and T.L.; Formal analysis, T.L. and K.W.; Investigation, C.W., J.W. and K.W.; Writing, T.L., L.S. and K.W.; Project administration, T.L.; Funding acquisition, T.L. and K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China grant number (U24B2052, 51974050), Chongqing Natural Science Foundation Innovation and Development Joint Fund grant number (No. 2023NSCQ-LZX0153, CSTB2023NSCQ-LZX0128), and Zhejiang Province Leading Innovation and Entrepreneurship Team-Automotive Light Alloy Innovation Team grant number (2022R01018).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Tian Li, JinHua Wu, Jianming Zheng were employed by the company Zhejiang Wanfeng Precision Casting Co., Ltd. Authors Ling Shan, Chunwei Wang, JinHua Wu, Jianming Zheng were employed by the company Zhejiang Wanfeng Technology Development Co., Ltd. Author Kai Wang has no conflicts of interest to declare.
References
- Zhang, M.; Tian, Y.; Zheng, X.; Zhang, Y.; Chen, L.; Wang, J. Research Progress on Multi-Component Alloying and Heat Treatment of High Strength and Toughness Al–Si–Cu–Mg Cast Aluminum Alloys. Materials 2023, 16, 1065. [Google Scholar] [CrossRef]
- Javidani, M.; Larouche, D.; Grant Chen, X. Assessment of Post-eutectic Reactions in Multicomponent Al-Si Foundry Alloys Containing Cu, Mg, and Fe. Metall. Mater. Trans. A 2015, 46, 2933–2946. [Google Scholar] [CrossRef]
- Murakami, Y.; Furushima, R.; Shiga, K.; Miyajima, T.; Omura, N. Mechanical property prediction of aluminium alloys with varied silicon content using deep learning. Acta Mater. 2025, 286, 120683. [Google Scholar] [CrossRef]
- Liu, W.; Xiao, W.; Xu, C.; Liu, M.; Ma, C. Synergistic effects of Gd and Zr on grain refinement and eutectic Si modification of Al-Si cast alloy. Mater. Sci. Eng. A 2017, 693, 93–100. [Google Scholar] [CrossRef]
- Aguilera-Luna, I.; Castro-Román, M.J.; Escobedo-Bocardo, J.C.; García-Pastor, F.A.; Herrera-Trejo, M. Effect of cooling rate and Mg content on the Al–Si eutectic for Al–Si–Cu–Mg alloys. Mater. Charact. 2014, 95, 211–218. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Luo, Q.; Peng, L.; Li, Q. Effectively refining Al-10Si alloy via Al-Ti-Nb-B refiner with Nb2O5. J. Mater. Sci. Technol. 2024, 176, 204–210. [Google Scholar] [CrossRef]
- Greer, A.L.; Bunn, A.M.; Tronche, A.; Evans, P.V.; Bristow, D.J. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al–Ti–B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Farkoosh, A.R.; Grant Chen, X.; Pekguleryuz, M. Interaction between molybdenum and manganese to form effective dispersoids in an Al–Si–Cu–Mg alloy and their influence on creep resistance. Mater. Sci. Eng. A 2015, 627, 127–138. [Google Scholar] [CrossRef]
- Vinod Kumar, G.S.; Murty, B.S.; Chakraborty, M. Development of Al–Ti–C grain refiners and study of their grain refining efficiency on Al and Al–7Si alloy. J. Alloys Compd. 2005, 396, 143–150. [Google Scholar] [CrossRef]
- Hegde, S.; Prabhu, K.N. Modification of eutectic silicon in Al–Si alloys. J. Mater. Sci. 2008, 43, 3009–3027. [Google Scholar] [CrossRef]
- Timpel, M.; Wanderka, N.; Schlesiger, R.; Yamamoto, T.; Lazarev, N.; Isheim, D.; Schmitz, G.; Matsumura, S.; Banhart, J. The role of strontium in modifying aluminium–silicon alloys. Acta Mater. 2012, 60, 3920–3928. [Google Scholar] [CrossRef]
- Callegari, B.; Lima, T.N.; Coelho, R.S. The Influence of Alloying Elements on the Microstructure and Properties of Al-Si-Based Casting Alloys: A Review. Metals 2023, 13, 1174. [Google Scholar] [CrossRef]
- Lei, Z.; Wen, S.; Huang, H.; Wei, W.; Nie, Z. Grain Refinement of Aluminum and Aluminum Alloys by Sc and Zr. Metals 2023, 13, 751. [Google Scholar] [CrossRef]
- Bayraktar, Ş.; Alparslan, C.; Salihoğlu, N.; Sarıkaya, M. The impact of heat treatment process on the drilling characteristics of Al–5Si–1Cu–Mg alloy produced by sand casting. J. Mater. Res. Technol. 2024, 33, 2764–2772. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Wu, Y.; Wang, L.; Liu, X. Quantitative comparison of three Ni-containing phases to the elevated-temperature properties of Al–Si piston alloys. Mater. Sci. Eng. A 2010, 527, 7132–7137. [Google Scholar] [CrossRef]
- Qin, J.; Tan, P.; Quan, X.; Liu, Z.; Yi, D.; Wang, B. The effect of sc addition on microstructure and mechanical properties of as-cast Zr-containing Al-Cu alloys. J. Alloys Compd. 2022, 909, 164686. [Google Scholar] [CrossRef]
- Li, P.; Li, R.; Yang, H.; Yuan, T.; Niu, P.; Wang, M.; Li, L.; Chen, C. Selective laser melting of Al-3.48Cu-2.03Si-0.48Sc-0.28Zr alloy: Microstructure evolution, properties and metallurgical defects. Intermetallics 2021, 129, 107008. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Wang, B.; Xue, C.; Liu, X. Improving mechanical properties of Al–Si–Cu–Mg alloys by microallying Sc using thermodynamic calculations. Calphad 2022, 76, 102394. [Google Scholar] [CrossRef]
- Chuvil’deev, V.; Nokhrin, A.; Kozlova, N.; Shadrina, I.; Bobrov, A.; Kopylov, V.; Komel’kov, A.; Morozkina, E. Combined Effect of the Sc/Zr Ratio and Mg Concentration on the Intergranular Corrosion Resistance of Al–Mg–Sc–Zr Alloys: A Case of Cast Alloys and Ultrafine-Grained Alloys. Metals 2025, 15, 372. [Google Scholar] [CrossRef]
- Prukkanon, W.; Srisukhumbowornchai, N.; Limmaneevichitr, C. Influence of Sc modification on the fluidity of an A356 aluminum alloy. J. Alloys Compd. 2009, 487, 453–457. [Google Scholar] [CrossRef]
- Tzeng, Y.-C.; Wu, C.-T.; Bor, H.-Y.; Horng, J.-L.; Tsai, M.-L.; Lee, S.-L. Effects of scandium addition on iron-bearing phases and tensile properties of Al–7Si–0.6Mg alloys. Mater. Sci. Eng. A 2014, 593, 103–110. [Google Scholar] [CrossRef]
- Røyset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, W.; Zheng, R.; Hanada, S.; Yamagata, H.; Ma, C. The synergic effects of Sc and Zr on the microstructure and mechanical properties of Al–Si–Mg alloy. Mater. Des. 2015, 88, 485–492. [Google Scholar] [CrossRef]
- Marquis, E.A.; Seidman, D.N. Nanoscale structural evolution of Al3Sc precipitates in Al(Sc) alloys. Acta Mater. 2001, 49, 1909–1919. [Google Scholar] [CrossRef]
- Li, C.; Wu, S.; Lü, S.; Li, J.; Liu, L.; Xia, L. Effects of Excessive Zr Content and Ultrasonic Treatment on Microstructure and Mechanical Properties of Al-Zn-Mg-Cu Alloy. Metals 2021, 11, 632. [Google Scholar] [CrossRef]
- Fuller, C.B.; Seidman, D.N.; Dunand, D.C. Mechanical properties of Al(Sc,Zr) alloys at ambient and elevated temperatures. Acta Mater. 2003, 51, 4803–4814. [Google Scholar] [CrossRef]
- Robson, J.D. A new model for prediction of dispersoid precipitation in aluminium alloys containing zirconium and scandium. Acta Mater. 2004, 52, 1409–1421. [Google Scholar] [CrossRef]
- Srinivasan, D.; Chattopadhyay, K. Metastable phase evolution and hardness of nanocrystalline Al–Si–Zr alloys. Mater. Sci. Eng. A 2001, 304–306, 534–539. [Google Scholar] [CrossRef]
- Hirano, T.; Ohtani, H.; Hasebe, M. Thermodynamic Analysis of the Al-Si-Zr Ternary System. High Temp. Mater. Process. 2010, 29, 347–372. [Google Scholar] [CrossRef]
- Booth-Morrison, C.; Mao, Z.; Diaz, M.; Dunand, D.C.; Wolverton, C.; Seidman, D.N. Role of silicon in accelerating the nucleation of Al3(Sc,Zr) precipitates in dilute Al–Sc–Zr alloys. Acta Mater. 2012, 60, 4740–4752. [Google Scholar] [CrossRef]
- Pandee, P.; Gourlay, C.M.; Belyakov, S.A.; Patakham, U.; Zeng, G.; Limmaneevichitr, C. AlSi2Sc2 intermetallic formation in Al-7Si-0.3Mg-xSc alloys and their effects on as-cast properties. J. Alloys Compd. 2018, 731, 1159–1170. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Samuel, E.; Zedan, Y.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Intermetallics Formation during Solidification of Al-Si-Cu-Mg Cast Alloys. Materials 2022, 15, 1335. [Google Scholar] [CrossRef] [PubMed]
- Samuel, F.H.; Doty, H.; Abdelaziz, M.H.; Samuel, A.M.; Gagnon, D. Melt Treatment-Porosity Formation Relationship in Al-Si Cast Alloys. In Casting Processes and Modelling of Metallic Materials; Abdallah, Z., Aldoumani, N., Eds.; IntechOpen: London, UK, 2021. [Google Scholar]
- Samuel, A.M.; Samuel, E.; Songmene, V.; Samuel, F.H. A Review on Porosity Formation in Aluminum-Based Alloys. Materials 2023, 16, 2047. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).