Abstract
In salt lake KCl cold decomposition–direct flotation for K-Na separation, octadecylamine hydrochloride (ODA-H) acts as a collector, reducing product purity and limiting high-end applications of salt lake KCl. To study KCl purification mechanisms and optimize crystal properties, this study investigates ODA-H aqueous systems. By regulating the cooling rate, stirring rate, and ODA-H concentration, the crystallization purification of KCl and precise control of its morphology were achieved, ultimately yielding three typical crystal morphologies: cubic, spherical, and ellipsoidal. Of the three crystal morphologies, spherical crystals have the best flowability but lowest purity. Ellipsoidal crystals have better flowability than cubic ones: their purity exceeds that of cubic crystals before washing but falls below it after washing. Cubic crystals, with poorer flowability, reach the highest purity post-washing. This study provides a theoretical basis for enhancing purity via crystal morphology regulation and industrial-scale purification of salt lake KCl.
1. Introduction
Potassium chloride (KCl) is an important inorganic salt product, widely used in agriculture, medicine, food, chemical industry and many other fields [1,2,3,4]. In agriculture, KCl is the main raw material for potassium fertilizers, playing a key role in crop growth and yield improvement; in the pharmaceutical field, KCl can be used for electrolyte supplementation and pharmaceutical preparations; in the food industry, food-grade KCl can be used as a low-sodium salt substitute and additive. With the improvement of quality requirements for KCl in various industries, especially the increasing demand for high-purity KCl, the development of efficient KCl crystallization processes has important practical significance.
Potash resources from salt lakes are crucial strategic resources for global agricultural and industrial production. The world’s total potash reserves exceed 3.5 billion tons, which are mainly concentrated in these countries—Canada, Russia, Belarus and China. The cold decomposition–flotation method is one of the key processes for potash extraction, mainly applied in the processing of carnallite-type potash ores [5]. The cold decomposition–flotation method for potash purification requires the use of long-carbon-chain primary amine-based organic compound, octadecylamine hydrochloride (ODA-H) [6]. It is highly resistant to degradation in the natural environment and exerts irritant effects on the eyes, skin and respiratory system. The effective removal of ODA-H is a critical step in improving the quality of KCl production [7]. However, due to the characteristics of the flotation process, organic flotation reagents such as ODA-H inevitably remain in the produced KCl products. Test data show that the residual amount of ODA-H in finished products under traditional processes can reach 50–200 mg/kg [8]. These residues not only significantly affect the purity index of KCl, but may also change the structure of soil microbial communities during agricultural application, or cause chemical reaction interference in the application of industrial-grade products, resulting in multi-dimensional adverse effects on subsequent processing and application and even causing severe safety risks [9,10].
Numerous methods have been developed for the removal of ODA-H from KCl, including adsorption, washing, calcination, hot dissolution, redox technique and cation-regulated in situ desorption method [7,11,12]. However, the method of regulating the morphology of KCl for controlling the purity of crystalline products remains underexplored. High-purity crystals serve as a critical prerequisite for ensuring stable performance, compliance with functional requirements, and safety and reliability in fields such as scientific research, industrial manufacturing, pharmaceuticals, and electronics [13,14,15]. The presence of impurities can directly disrupt the integrity of the crystal structure and alter its intrinsic physicochemical properties [16]. In-depth research on crystal impurity removal technologies can not only systematically enhance the purity and stability of crystalline products but also break through their application limitations in high-end fields, thereby significantly broadening the application scenarios of crystalline materials. During the crystallization process in impurity-containing systems, impurities affect crystallization behavior and thereby alter crystal morphology, and in turn, the affected morphology further modifies the enrichment mechanism of impurities. This bidirectional relationship remains underexplored, yet it constitutes one of the key issues for enhancing the purity of potassium products derived from salt lakes.
There are numerous reports on the morphology control of KCl crystal particles: Jin prepared spherical KCl particles using water as the solvent by designing an additive-free cooling crystallization process, pointing out that agglomeration is a key formation step [17]; Feng regulated the morphology and microstructure of inorganic salts through the self-organization of hierarchical crystal clusters (HCC) by controlling supersaturation and crystal surface roughness in an additive-free aqueous system, successfully preparing spherical salts and multi-component functional salts [18]; Yuan controllably constructed KCl crystals with different morphologies and structures such as funnel-shaped, spherical, and hollow cubic shapes through green supersaturation regulation (adjusting parameters such as stirring speed, cooling range, cooling rate, and additional ions), proposing an environmentally friendly crystal design strategy [19]. Previous studies on the morphology control of KCl crystals have focused on how to improve the powder properties of the final crystallized products, such as enhancing fluidity, anti-caking performance, and surface loading performance; research on how to improve the purity of KCl produced by flotation processes through morphology control is almost blank. Impurities affect the morphology of crystals, and in turn, the affected morphology further modifies the enrichment mechanism of impurities. This bidirectional relationship remains underexplored, yet it constitutes one of the key issues for enhancing the purity of potassium products derived from salt lakes.
Based on previous research, we investigated the crystal morphology regulation of KCl in the presence of ODA-H at different concentrations by adjusting the cooling rate and stirring rate, and eventually obtained three different morphologies of KCl crystals: cubic, spherical, and ellipsoidal. We analyzed the purity of the KCl crystal products and conducted a comprehensive evaluation of their powder physical properties. Among the three crystal morphologies, spherical crystals exhibit superior flowability to both ellipsoidal and cubic crystals, possess a larger average particle size, yet have the lowest purity. Ellipsoidal crystals show better flowability than cubic crystals but inferior flowability to spherical ones: their purity is higher than that of cubic crystals before washing, but lower than that of cubic crystals after washing. Cubic crystals have relatively poor flowability, but achieve the highest purity following washing treatment. The core reason for these performance differences lies in impurity entrapment caused by crystal agglomeration and multi-level crystal clusters.
2. Materials and Methods
2.1. Materials and Instruments
The chemical reagents used in this study include: KCl (AR, Meryer, Tianjin, China), ODA-H (AR, Shanghai Bide Pharmaceutical Technology Co., Ltd., Shanghai, China), glacial acetic acid (GR, Macklin, Shanghai, China), sodium acetate (AR, Heowns, Tianjin, China), and ninhydrin (AR, Shanghai Bide Pharmaceutical Technology Co., Ltd., Shanghai, China).
The instruments used in this study include: crystallizer (customized by Yipujia), compact cooling bath circulation thermostat (Huber Ministat230, Offenburg, Germany).
2.2. Agglomeration and Dispersion
In the actual crystallization process, KCl crystals usually agglomerate. According to the Lifshitz-van der Waals acid-base theory [20,21], the agglomeration phenomenon of crystals in solution can be quantified through quantitative characterization and evaluation, and can be effectively inhibited by means of regulation. For two solid particles dispersed in a solution, the core driving force for their agglomeration comes from the adhesion force between the particles, and the adhesion force originates from the adhesion free energy [22]:
Here, Fadh is the adhesive force, d is the particle diameter, and ΔGSLS is the adhesion free energy.
Shear force in fluids is a key parameter describing fluid flow and intermolecular interactions. Fluid flow caused by fluid movement leads to collisions between crystal particles, as well as between them and the container walls and stirrers. Therefore, the shape of the particles is affected by the shear force to some extent. The stirring force promotes the breaking and abrasion of agglomerates, which is caused by the shear force provided by stirring [22]:
Fimp is the shear force, ρs is the crystal density, Na is the stirring rate, Dl is the diameter of the stirring paddle, and la is the acceleration distance. Generally speaking, it can be approximately considered that la = d.
2.3. Experiment
2.3.1. Crystal Natural Growth Experiment
To explore the mechanism by which ODA-H affects the growth of high-purity KCl crystals, this study employed the slow cooling method and carried out natural growth experiments of KCl crystals under different ODA-H concentration gradients.
2.3.2. Solubility Experiment
Under different ODA-H concentration levels, the solubility of KCl in aqueous solution at 70 °C was determined via the dynamic method.
2.3.3. Cooling Crystallization Experiment
All experiments were conducted in a 100 mL crystallizer equipped with an external temperature controller. First, 41.25 g of KCl was dissolved in 100 g of deionized water at 70 °C (with stirring to accelerate dissolution). After the solid was completely dissolved, 80 mL of the clear solution was obtained via atmospheric filtration (to eliminate the interference of undissolved impurities on subsequent experiments) and then transferred into the crystallizer. Next, ODA-H was added in sequence, so that the concentration of ODA-H in the system was adjusted to 1 × 10−5 mol/L, 4 × 10−5 mol/L, 6 × 10−5 mol/L, 1 × 10−4 mol/L and 4 × 10−4 mol/L, respectively. The experiments employed the cooling crystallization method, with a temperature control range of 70–20 °C; the stirring speed was maintained at 400–700 rpm during crystallization. In addition, to optimize the crystallization effect, this study designed a two-stage cooling process: for the 70–50 °C stage, a rapid cooling mode was adopted to shorten the total cooling duration; for the 50–20 °C stage, the mode was switched to slow cooling to precisely regulate the crystal growth process.
2.3.4. Morphological Characterization
The entire crystallization process was analyzed by capturing images using a scanning electron microscope (Hitachi TM3000, Tokyo, Japan), a polarizing microscope (Zeiss Axiolab5, Jena, Germany), and a stereomicroscope.
2.3.5. Product Purity Determination
ODA-H standard solutions of different concentrations were accurately pipetted into separate, clean 50 mL centrifuge tubes. To each tube, 8 mL of acetic acid–sodium acetate (HAc-NaAc) buffer solution (pH ≈ 6; to maintain system pH stability and ensure consistent color reaction conditions) and 2 mL of 2% ninhydrin solution (serving as a chromogenic reagent that undergoes a characteristic chromogenic reaction with amino compounds) were added sequentially. A suitable amount of KCl was added to supplement chloride ions, facilitating better color development. Each solution was then diluted to 20 mL with deionized water and gently shaken to mix thoroughly. After mixing, the absorbance of each standard solution was measured at a wavelength of 558 nm using an ultraviolet-visible (UV-Vis) spectrophotometer, with a blank reagent solution (containing 8 mL of HAc-NaAc buffer, 2 mL of 2% ninhydrin solution, and diluted to 20 mL with deionized water) as the reference.
An appropriate amount of KCl crystal product (with the mass adjusted based on the expected amino compound content to ensure the absorbance fell within the linear range of the standard curve) was weighed and placed in a small beaker. A small amount of deionized water was added, and the product was dissolved via ultrasonic treatment or stirring, then transferred to a volumetric flask (e.g., 25 mL or 50 mL, selected based on sample concentration). The solution was diluted to the marked volume with deionized water and shaken well to prepare the KCl sample stock solution. A certain volume (e.g., 5 mL, adjusted based on the stock solution concentration to fit the standard curve range) of this stock solution was pipetted into a 50 mL centrifuge tube. To this tube, 8 mL of HAc-NaAc buffer solution (pH ≈ 6) and 2 mL of 2% ninhydrin chromogenic agent were added in sequence. The mixture was diluted to 20 mL with deionized water, oscillated to mix thoroughly, and then placed in a constant-temperature water bath at 80 °C for 20 min (temperature and time were strictly controlled to ensure sufficient and consistent color development). After the water bath, the centrifuge tube was removed and cooled to room temperature. Wavelength scanning was then performed, using the blank reagent solution (treated under the same conditions) as the reference.
2.3.6. Particle Size Analysis
The particle size distribution (PSD) of the KCl crystals was analyzed using a laser particle size analyzer (Mastersizer 3000, Malvern Instruments Ltd., Worcestershire, UK). A suspension of KCl crystals—dispersed in ethanol (to prevent particle agglomeration and ensure accurate PSD measurement)—was introduced into the instrument’s sample dispersion unit for analysis. Each sample was measured in five replicate runs, and the final result was reported as the average value of the five measurements.
2.3.7. Flow Performance
Powder flowability is critical in various post-processing stages, including storage, transportation, formulation, mixing, compression, and packaging. To evaluate the flowability of the crystal powders, the fixed funnel method was employed to determine the angle of repose. Specifically, the funnel was positioned vertically on a laboratory workbench, and the particles were poured into the funnel to form a conical pile. The angle of repose (θ) is calculated using the formula:
θ is the angle of repose, which refers to the angle between the generatrix of the cone formed by piled powder and the horizontal plane, and it is a key indicator reflecting powder flowability. h is the height of the cone formed by piled powder. r is the base radius of the cone formed by piled powder. tan−1 is the arctangent function (also denoted as arctan), which infers the angle of repose θ through the ratio of the height h to the base radius r.
3. Results and Discussion
3.1. Crystallization of KCl in Aqueous Solution of ODA-H
3.1.1. The Effect of ODA-H Concentration on the Solubility of KCl
In this study, we chose relatively low concentrations of ODA-H as experimental variables, primarily because the critical micelle concentration (CMC) of ODA-H is only approximately 4 × 10−5 mol/L under high ionic strength. The primary purpose of this experiment is to obtain basic quantitative data on crystallization, ensuring precise control over the critical conditions for solid precipitation from the solution and avoiding arbitrariness in the experiment. Experiments were conducted to determine the solubility of KCl in systems with different ODA-H concentrations at a constant temperature of 70 °C, and the results are presented in Figure 1. The experimental data indicate that with increasing ODA-H concentration, the solubility of KCl in the system exhibits a gradual decreasing trend. To ensure that KCl could be completely dissolved and ODA-H would not precipitate at 70 °C within the ODA-H concentration range selected in this study, an initial KCl feeding ratio of 41.25 g KCl per 100 g H2O was finally determined.
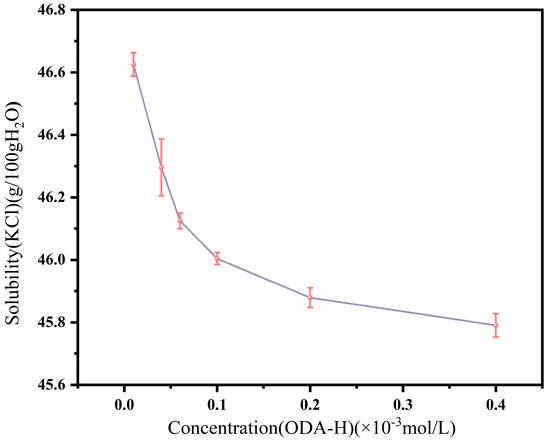
Figure 1.
Solubility of KCl at 70 °C with different ODA-H concentrations.
Existing studies have explored the spherical crystallization process for KCl and established the corresponding process parameters: 500 rpm stirring speed and 15 min/°C cooling rate [17]. Therefore, in this experiment, crystallization experiments were conducted at varying concentrations of ODA-H (i.e., 0 mol/L, 4 × 10−5 mol/L, 1 × 10−4 mol/L). The experimentally measured data of the first nucleation temperature and nucleation induction period of KCl crystals under different concentrations of ODA-H are as follows: when the concentration is 0 mol/L, the first nucleation temperature is 48.14 °C and the nucleation induction period is 327.90 min; when the concentration is 4 × 10−5 mol/L, the first nucleation temperature rises to 48.32 °C and the nucleation induction period shortens to 325.20 min; when the concentration increases to 1 × 10−4 mol/L, the first nucleation temperature further rises to 49.03 °C and the nucleation induction period significantly shortens to 314.55 min. Based on this, it can be preliminarily inferred that the addition of ODA-H can promote the nucleation of KCl crystals, and the higher its concentration, the easier the nucleation of KCl crystals (manifested by an increase in nucleation temperature and a shortening of the induction period). Previous studies have clearly shown that ODA-H has an inhibitory effect on the solubility of KCl, so it was initially speculated that its mechanism of promoting nucleation might be related to solubility inhibition. However, a contradictory phenomenon was observed in the experiment: as the concentration of ODA-H increased, its inhibitory effect on the solubility of KCl showed a decreasing trend, which did not match the result that the nucleation induction period was significantly shortened at high concentrations. Further analysis found that when the concentration of ODA-H was 1 × 10−4 mol/L, during the cooling process, when the temperature dropped to around 50 °C, the solution would exhibit an obvious liquid–liquid phase separation (LLPS) phenomenon. Based on this, it can be inferred that the acceleration of KCl nucleation under high concentrations of ODA-H does not only depend on solubility inhibition, but is more closely related to the multi-path nucleation effect caused by the LLPS process [23].
3.1.2. Effects of ODA-H on KCl Morphology and Its Mechanisms
The effect of ODA-H on the growth of KCl crystals under the experimental conditions of a rotation speed of 500 rpm and a cooling rate of 15 min/°C is shown in Figure 2. The concentration gradients of ODA-H set in the experiment are 0 mol/L (blank control group), 4 × 10−5 mol/L, and 1 × 10−4 mol/L. The setting of these concentration gradients is based on the critical micelle concentration (CMC) characteristics of ODA-H—its critical micelle concentration range is approximately 4 × 10−5~6 × 10−5 mol/L. By covering the concentration intervals of “blank control—close to the lower limit of CMC—higher than the CMC range”, the differential regulatory effects on the growth process of KCl crystals can be systematically explored in the stages of no micelle formation, initial stage of critical micelle formation, and stable existence of micelles.
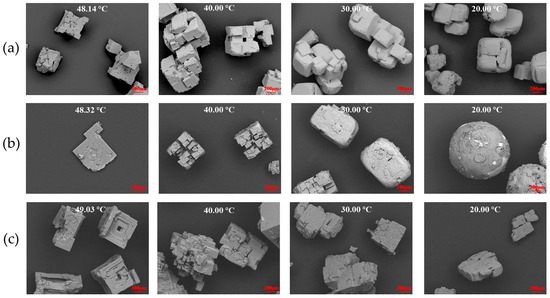
Figure 2.
Effect of ODA-H on the growth of KCl crystals under flow field conditions. ODA-H concentration: (a) 0; (b) 4 × 10−5 mol/L; (c) 1 × 10−5 mol/L.
The formation process of KCl crystals is illustrated in Figure 2. During the initial nucleation and growth stage of the crystals, they exhibit a sharp-edged morphology—this is attributed to the combined effects of their HCC growth mode and particle agglomeration. Subsequently, in the ongoing crystallization process, the stirring action induces wear and blunting of the crystal edges; meanwhile, crystal growth gradually fills in the defects. Eventually, crystal particles with relatively smooth surfaces are formed. However, surface step-like structures can be observed in high-magnification SEM images (Figure S1). In contrast, the formation mechanisms of spherical and ellipsoidal KCl crystals are similar. The fundamental difference between them lies in the degree of particle agglomeration. KCl generally exhibits agglomeration during the cooling crystallization process [17], and the experimental results of this study also confirm this characteristic.
Under the action of low-concentration ODA-H, unlike the control group (without ODA-H) where crystal agglomeration is disorderly, KCl crystals show a radial agglomeration feature from the center to the outside. Moreover, as the crystal growth process progresses, crystal particles with higher sphericity and larger particle size are finally formed. SEM shows the characteristics of the surface of spherical crystals, which have a multi-level crystal cluster structure. As the cooling process proceeds, the defects on the crystal surface will be gradually filled, and the stirring action will also blunt the edges and corners of the crystals. Under the condition of low ODA-H, crystal particles with high sphericity are formed. Theoretically, the addition of ODA-H can improve the dispersion of KCl crystal particles by modifying their surface properties. This phenomenon is closely related to the property of ODA-H to reduce the surface adsorption energy of the solvent—it regulates the interaction at the solid–liquid interface, weakens the agglomeration driving force between crystal particles, and thus improves the crystal dispersion [20]. When the concentration of ODA-H is very high, KCl crystals are difficult to form agglomerated particles, which seriously hinders the spheroidization process.
The addition of ODA-H alters the morphology of subcrystals, thereby influencing the morphology of the final crystalline product. The effect of ODA-H on the growth of KCl crystals under the condition of no flow field is shown in Figure 3. In the system without the addition of ODA-H, KCl crystals grow in a flaky form, and there are obvious overlapping and agglomeration phenomena between the flakes. With the increase in the concentration of ODA-H, the lateral growth of the crystals is significantly inhibited, and the lateral size of the crystals gradually decreases. At the same time, the edge regions of the long crystals have the characteristics of the growth of independent small crystal edges accompanied by multistage crystal clusters. When the concentration of ODA-H is further increased, the narrowing trend of the crystals intensifies: when the concentration increases to 6 × 10−5 mol/L, feathery KCl crystals can be observed; when the concentration increases to 8 × 10−5 mol/L, the KCl crystals show a typical acicular habit. Given that the core objective of this study is to develop a preparation process for high-purity KCl, and due to the limitations of the article’s length, the in-depth research results on the mechanism of action of ODA-H on the growth behavior of each crystal face of KCl will be elaborated in detail in subsequent work.

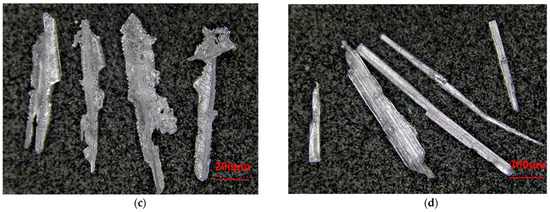
Figure 3.
Effect of ODA-H on the growth of KCl crystals under no flow field conditions. ODA-H concentration: (a), 0; (b), 4 × 10−5 mol/L; (c), 6 × 10−5 mol/L; (d), 8 × 10−5 mol/L.
3.1.3. The Influence of Cooling Rate on Crystal Morphology
The cooling rate is a key factor affecting the morphology of KCl crystals. Figure 4 intuitively shows the differences in crystal morphology corresponding to different cooling rates under the condition of a stirring speed of 400 rpm. The experimental results indicate that the faster the cooling rate, the more difficult it is for the crystal particles to form a spherical structure. In scenarios where the concentration of ODA-H is low, it can be observed that the initial growth of the crystals exhibits a radial characteristic from the center to the outside, and the overall particles have a loose texture. When the cooling rate decreases, the gaps between the branches formed by radial growth are gradually filled by the subsequently growing crystals, eventually forming spherical particles with a more compact structure.
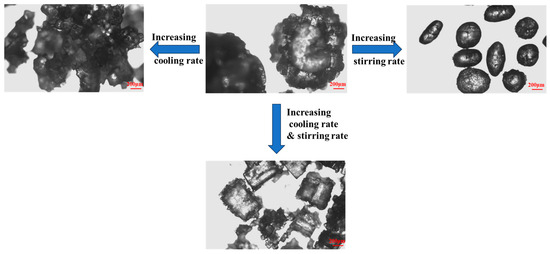
Figure 4.
Effects of stirring rate and cooling rate on crystal products. (ODA-H, 4 × 10−5 mol/L).
The fundamental reason for the differences in these crystal morphologies can be attributed to the direct regulatory effect of cooling rate on the supersaturation of the system, and supersaturation is precisely the core factor that determines the nucleation efficiency and growth behavior of crystals. Specifically, a faster cooling rate drives the system to develop a higher degree of supersaturation within a short timeframe. This elevated supersaturation further induces a substantial increase in the nucleation rate and crystal growth rate: firstly, it facilitates the formation of a greater number of crystal nuclei; secondly, the agglomeration between crystal particles and the multi-level crystal cluster growth pattern exert complex synergistic effects. Ultimately, this leads to the formation of larger-sized aggregates with complex (e.g., loose or dense) structures, rather than discrete KCl crystals [18]. However, it should be noted that under high supersaturation, the crystal growth process lacks adequate time for the ordered arrangement of KCl crystal lattices—this insufficient ordering leads to a marked decrease in crystallinity. Consequently, reduced crystallinity directly induces a sharp increase in the crystals’ specific surface area, which runs counter to the core objective of preparing high-purity KCl crystals. In contrast, under slower cooling rate conditions, the system’s supersaturation increases gradually, allowing the crystal growth process to proceed in a more fully ordered manner. This not only facilitates the formation of target spherical KCl particles with a denser surface structure but also offers the potential to enhance the purity of the final KCl product.
3.1.4. Influence of Stirring Rate on Crystal Morphology
In the process of solution crystallization, the core roles of stirring include dispersing crystal particles, enhancing mass transfer, and inducing secondary nucleation. Compared with these main functions, the impact of stirring on crystal abrasion is often underestimated [24].
In the solution crystallization process, the core functions of stirring encompass three key aspects: dispersing crystal particles (to inhibit agglomeration and ensure uniform growth), enhancing mass transfer of solute and solvents, and facilitating secondary nucleation. While these are its primary roles, the impact of stirring on crystal abrasion is often underestimated—this abrasion can generate fine crystal fragments, which may disrupt the integrity of crystal morphology and even compromise the final product’s purity.
Fluid agitation-induced wear of suspended KCl crystal particles is a complex phenomenon that integrates fluid dynamics, particle kinematics, and material wear mechanisms. This wear phenomenon primarily arises from mechanical forces exerted by fluid motion on KCl crystals, resulting in surface material loss or structural damage to the crystals. In a batch stirred crystallizer, KCl crystal particles move within the shear flow field and are subjected to fluid drag, lift, and centrifugal forces. These forces drive the particles to slide, roll, and undergo collisions and friction with the crystallizer walls, stirrer, and other KCl crystals—all of which contribute to crystal wear. When the stirring speed is low, this wear effect is extremely weak; thus, in most KCl crystallization processes, the crystals typically exhibit sharp-edged morphologies. However, once the stirring speed is elevated, KCl crystal wear intensifies and may even cause particle fragmentation, ultimately altering the KCl crystal habit.
Adhesion and shear force are in a competitive relationship [24]. When the adhesion force between KCl crystal particles is greater than the shear force in the system, the particles tend to agglomerate with each other; when the adhesion force is less than the shear force, the particles tend to disperse uniformly. Changes in adhesion force and shear force with KCl crystal size can be calculated using Equations (1) and (2). From this, it can be inferred that when the stirring speed is higher, the agglomeration of KCl crystals throughout the crystallization process can be effectively suppressed—a finding confirmed by experimental results.
The effect of stirring speed on KCl crystal products is presented in Figure 4. As the stirring speed increases, the KCl crystal products gradually form monodisperse particles or dimeric ellipsoidal particles—dark lines observable inside the crystals under a microscope allow for the identification of grain boundaries. With a further increase in stirring speed, the burrs on the surface of KCl crystals gradually diminish; this reduction in surface irregularities effectively decreases the specific surface area of the crystal particles. In turn, fewer specific surface area sites are available for the adsorption of residual ODA-H, thereby contributing to enhanced product purity. However, subsequent experiments have demonstrated that this dispersion state (and the associated ellipsoidal morphology) can also lead to impurity entrapment within the crystal structure.
Three types of crystalline products with distinct morphologies were successfully prepared in the ODA-H–water system. Figure 5 shows the simulated formation process of three types of crystals. The formation process of cubic crystalline particles can be regarded as a single crystal growth process; whereas the formation of ellipsoidal and spherical crystalline particles is the result of the synergistic effect of multiple factors, including crystal agglomeration, growth, and abrasion. Crystal agglomeration and the growth of multi-level crystal clusters are the key factors contributing to the formation of spherical and ellipsoidal crystals. Under high concentrations of ODA-H (≥1 × 10−4 mol/L, under the relative conditions of this experiment), the agglomeration behavior of KCl crystals is significantly inhibited, making it difficult to generate agglomerated particles; thus, spherical KCl crystals fail to be successfully prepared in this experiment [18].
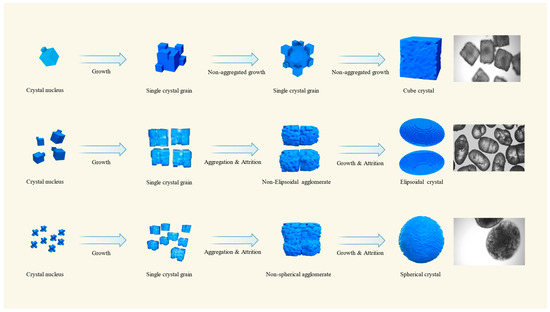
Figure 5.
Mechanism diagram of the formation of three morphological crystals.
3.2. The Relationship Between Crystal Morphology and Purity
3.2.1. Standard Curve
We used the ultraviolet spectrophotometric method to detect the ODA content [25]. First, wavelength scanning was performed on the prepared ODA-H standard solutions, and the maximum absorption wavelength (corresponding to the highest absorbance) was found to be approximately 560 nm, as presented in Figure 6a. Subsequently, the absorbance values of each ODA-H standard solution at this maximum absorption wavelength were measured and are listed in Table 1. Using the ODA-H standard concentration as the abscissa and the corresponding absorbance value as the ordinate, a standard curve was constructed via linear regression analysis, as shown in Figure 6b. This linear regression yielded a fitted line with a slope of 0.0235, an intercept of 0.0033, and a correlation coefficient (R2) of 0.99654.
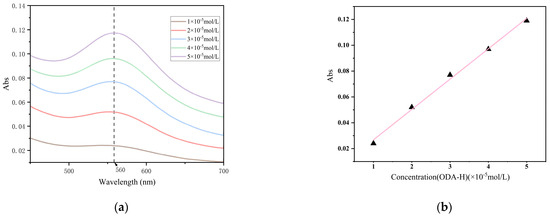
Figure 6.
(a) Absorbance of standard solution; (b) absorbance standard curve.

Table 1.
Absorbance of standard solution at 563 nm.
3.2.2. Analysis of Sample Purity
The absorbance of the KCl sample solution was measured with the UV-Vis spectrophotometer at a wavelength of about 563 nm. Subsequently, the measured absorbance value was substituted into the regression equation of the ODA-H standard curve to calculate the concentration of the target amino compound—ODA-H, the substance reacting with ninhydrin—in the sample. The content of ODA-H in the KCl crystal product was then calculated by accounting for the sample dilution factor. The purity of KCl crystals with different morphologies is presented in Table 2. The test samples in this experiment included the prepared monodisperse square KCl particles, ellipsoidal KCl particles, and large spherical KCl particles. All test samples underwent purity analysis both before and after washing; the washing process involved rinsing the samples five times with a saturated KCl solution using a Buchner funnel. For the experimental groups with added ODA-H concentrations of 4 × 10−5 mol/L and 6 × 10−5 mol/L, the post-washing detection results were below the detection limit, so these groups are not listed in the table. Additionally, at relatively high ODA-H concentrations (≥1 × 10−4 mol/L), spherical KCl particles could not be prepared; thus, no purity detection was performed on samples under this condition.

Table 2.
Sample absorbance detection data.
Experimental results indicated that without washing, among all parallel experimental groups, ellipsoidal KCl particles exhibited the highest purity—consistent with the initial hypothesis, as shown in Figure 7. Their lower specific surface area reduced the adsorption of residual ODA-H on the KCl crystal surface, thereby enhancing product purity. Notably, the purity of large spherical KCl particles was the lowest, contrary to their theoretically expected smaller specific surface area. Via optical microscopy observation and scanning electron microscopy (SEM) characterization, it was found that the surface of large spherical particles was rough with more step structures—a structural feature that actually increased their specific surface area. Furthermore, large spherical particles were formed by the agglomeration of small KCl particles, with a complex internal structure consisting of agglomerates and multi-level crystal clusters. This structure easily resulted in severe impurity entrapment—specifically, residual ODA-H being encapsulated within the crystal particles—ultimately further lowering purity. The dimeric ellipsoidal crystal products further validated this phenomenon. After washing, monodisperse square KCl particles exhibited the greatest purity improvement, with their final purity surpassing that of washed ellipsoidal KCl particles. The most notable difference between these two crystal types was the presence of grain boundaries in ellipsoidal KCl particles—a key factor contributing to impurity entrapment.
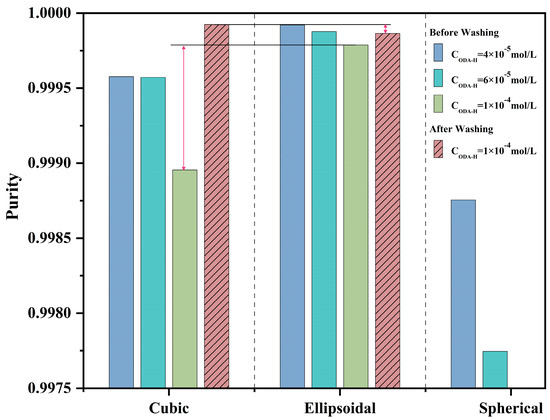
Figure 7.
Histogram of Product Purity Comparison.
Experimental results demonstrate that crystalline products with morphologies yielding excellent powder properties do not outperform those with poor powder properties in terms of purity, which challenges the understanding derived from previous cognition. For the crystalline products, the results of flowability tests are presented in Figure S2, the angle of repose data in Table S1, and the particle size distribution in Figure S3. The flowability of KCl crystals exhibits a hierarchy of spherical > ellipsoidal > cubic, which is jointly determined by crystal surface smoothness, agglomeration degree, and interparticle friction. Moreover, the flowability shows an inverse trend with post-washing purity (spherical < ellipsoidal < cubic). This result provides a key reference for industrial end-users to select crystal morphologies based on needs (e.g., prioritizing flowability for bulk processing and purity for high-end applications).
4. Conclusions
This study addresses the critical issue of octadecylamine hydrochloride (ODA-H) residue affecting potassium chloride (KCl) purity in salt lake potash processing. It systematically investigates the cooling crystallization behavior of KCl in the ODA-H–water system, with a focus on regulating key process parameters—cooling rate (two-stage control: rapid cooling at 70–50 °C, slow cooling at 50–20 °C), stirring rate (400–700 rpm), and ODA-H concentration (1 × 10−5–4 × 10−4 mol/L). The research not only achieves regulation of KCl crystal morphology across different ODA-H concentrations (1 × 10−5–4 × 10−4 mol/L) but also reveals the intrinsic mechanisms linking ODA-H’s influence on crystal morphology, crystal formation, and product purity, filling the research gap in “morphology–purity correlation” within salt lake potash flotation processes.
First, this study clarifies the multi-mechanistic regulatory effect of ODA-H on KCl crystallization, which exhibits significant concentration dependence. Second, it elucidates the differentiated formation mechanisms of three typical KCl crystal morphologies. Cubic KCl crystals form through a simple single-crystal growth process, with no significant agglomeration under specific conditions due to strong particle dispersion. In contrast, ellipsoidal and spherical crystals are products of the synergistic interaction of three processes: crystal agglomeration, oriented growth, and stirring-induced abrasion. A critical finding is that when ODA-H concentration exceeds 1 × 10−4 mol/L, its strong surface modification effect inhibits interparticle adhesion, prevents agglomeration, and thus hinders the formation of spherical crystals—confirming that agglomeration and multi-level cluster growth are prerequisites for the formation of ellipsoidal/spherical morphologies. Third, this research breaks the traditional perception that “low specific surface area = high purity” and reveals the mechanism-driven correlation between crystal morphology and purity. Spherical KCl crystals, despite their theoretically small specific surface area, have rough surfaces (with step-like structures) and internal agglomeration of small particles (forming multi-level clusters), which easily trap residual ODA-H, resulting in the lowest purity among the three morphologies. Ellipsoidal crystals, with better dispersion (fewer agglomerates), entrap fewer impurities than spherical crystals but still retain ODA-H at grain boundaries—leading to higher purity than cubic crystals before washing but lower purity after washing. Cubic crystals, as monodisperse single crystals, show no obvious agglomeration or grain boundaries; although their large specific surface area causes higher ODA-H adsorption before washing, surface impurities can be effectively removed by rinsing with saturated KCl solution. Finally, this study provides practical guidance for the industrial purification of salt lake KCl. By identifying the optimal process window (two-stage cooling; stirring rate: 500 rpm), it enables the production of high-purity KCl crystals (cubic or ellipsoidal) while balancing process efficiency. The revealed multi-factor regulatory mechanisms—including liquid–liquid phase separation (LLPS) and macroscopic agglomeration–abrasion synergy—also offer references for crystallization research in other surfactant-containing inorganic salt systems. In summary, this work not only enriches the theoretical system of KCl crystallization regulation but also lays a solid foundation for advancing the transformation of salt lake KCl production from “process-adapted manufacturing” to “multi-objective optimization (purity + powder properties).”
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst15110958/s1, Figure S1: SEM of Spherical crystal surface; Figure S2: Product Angle of Repose Test; Figure S3: Particle size distribution of potassium chloride crystals with various morphologies under different ODA-H concentrations. a, 4 × 10−5 mol/L; b, 6 × 10−5 mol/L; c, 1 × 10−4 mol/L; d, 4 × 10−4 mol/L.; Table S1: Flow Performance of Products.
Author Contributions
Conceptualization, Y.R. and M.L.; methodology, Y.R.; software, Y.R. and L.S.; validation, Y.R. and L.S.; formal analysis, Y.R. and L.S.; investigation, Y.R., H.F. and L.S.; Resources: H.F. and J.P.; data curation, Y.R.; writing—original draft preparation, Y.R. and L.S.; writing—review and editing, M.C., H.F., M.L. and J.P.; supervision: J.P.; project administration: J.P.; funding acquisition, H.F., M.C. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the funding from Qinghai Provincial Central Guiding Local Science and Technology Development Fund Project (No. 2025ZY016) and the National Natural Science Foundation of China (grant number: 22378303).
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Torabian, S.; Farhangi-Abriz, S.; Qin, R.; Noulas, C.; Sathuvalli, V.; Charlton, B.; Loka, D.A. Potassium: A Vital Macronutrient in Potato Production—A Review. Agronomy 2021, 11, 543. [Google Scholar] [CrossRef]
- Shin, J.M.; Sachs, G. Gastric H,K-ATPase as a drug target. Dig. Dis. Sci. 2006, 51, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Liu, X.C.; Xiang, J.Y.; Sun, W.Z.; Tomasevic, I. Prospects and challenges for the application of salty and saltiness-enhancing peptides in low-sodium meat products. Meat Sci. 2023, 204, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, X.; Chen, B.; He, L.; Tang, X. Effects of Different Potassium (K) Fertilizer Rates on Yield Formation and Lodging of Rice. Phyton-Int. J. Exp. Bot. 2021, 90, 815–826. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, C.; Zhong, H.; Li, L.; Guo, Z.; Yu, X.; He, G.; Han, H.; Deng, L.; Fu, W. Flotation of sylvite from potash ore by using the Gemini surfactant as a novel flotation collector. Miner. Eng. 2019, 132, 22–26. [Google Scholar] [CrossRef]
- De Silva, V.R.S.; Ranjith, P.G. Evaluation of injection well patterns for optimum fracture network generation host-rock formations: An application in in-situ leaching. Miner. Eng. 2019, 137, 319–333. [Google Scholar] [CrossRef]
- Wang, X.R.; Wu, J.G.; Xia, S.M.; Yang, K.L.; Jia, R.; Liu, X. Cation-regulated in-situ desorption of octadecylamine hydrochloride from the surface of potassium chloride in high-salt solution. Surf. Interfaces 2025, 58, 8. [Google Scholar] [CrossRef]
- Ling, L. Basic Research on Flotation Separation of Potassium Chloride and Sodium Chloride. Master’s Thesis, Central South University, Changsha, China, 2013. [Google Scholar]
- Chen, G.; Nengzi, L.-C.; Li, B.; Gao, Y.; Zhu, G.; Cheng, X. Octadecylamine degradation through catalytic activation of peroxymonosulfate by FeMn layered double hydroxide. Sci. Total Environ. 2019, 695, 133963. [Google Scholar] [CrossRef]
- Garcia-Herrero, I.; Margallo, M.; Onandía, R.; Aldaco, R.; Irabien, A. Environmental challenges of the chlor-alkali production: Seeking answers from a life cycle approach. Sci. Total Environ. 2017, 580, 147–157. [Google Scholar] [CrossRef]
- Li, H.C.; Liang, T.; Liao, W.Y.; Zhang, J.J. Purification of potassium chloride from salt lake industry using activated biological bovine bone char. Chem. Miner. Process. 2021, 50, 42–44. Available online: https://qikan.cqvip.com/Qikan/Article/Detail?id=7105137479 (accessed on 1 October 2025).
- Li, F.; Yang, Y.; Wu, Z.G.; Liu, Q.; Tian, P.J.; Shan, L.Y. Exploratory Research on the Removal Process of Amine Substances in Domestic Potassium Chloride. Salt Sci. Chem. Ind. 2024, 53, 31–34. Available online: https://qikan.cqvip.com/Qikan/Article/Detail?id=7111421442 (accessed on 1 October 2025).
- Nordstrom, F.L.; Sirota, E.; Hartmanshenn, C.; Kwok, T.T.; Paolello, M.; Li, H.Y.; Abeyta, V.; Bramante, T.; Madrigal, E.; Behre, T.; et al. Prevalence of Impurity Retention Mechanisms in Pharmaceutical Crystallizations. Org. Process Res. Dev. 2023, 27, 723–741. [Google Scholar] [CrossRef]
- Liu, M.; Wang, G.F.; Zhao, F.Y.; Li, W.F.; Zhu, G.; Liang, G.C.; Jian, W.; Liao, L.B.; Lv, G.C. Advances in purification technologies and applications of high-purity quartz resources. Prog. Nat. Sci. Mater. Int. 2025, 35, 51–64. [Google Scholar] [CrossRef]
- Sun, D.F.; Liu, L.; Wang, G.D.; Yu, J.X.; Li, Q.B.; Tian, G.; Wang, B.F.; Xu, X.G.; Zhang, L.; Wang, S.Z. Research Progress in Liquid Phase Growth of GaN Crystals. Chem. A Eur. J. 2024, 30, e202303710. [Google Scholar] [CrossRef]
- Capellades, G.; Bonsu, J.O.; Myerson, A.S. Impurity incorporation in solution crystallization: Diagnosis, prevention, and control. Cryst. Eng. Commun. 2022, 24, 1989–2001. [Google Scholar] [CrossRef]
- Jin, S.S.; Chen, M.Y.; Li, Z.F.; Wu, S.G.; Du, S.C.; Xu, S.J.; Rohani, S.; Gong, J.B. Design and mechanism of the formation of spherical KCl particles using cooling crystallization without additives. Powder Technol. 2018, 329, 455–462. [Google Scholar] [CrossRef]
- Feng, S.S.; Yao, M.H.; Guo, S.L.; Lin, J.W.; Ao, Z.X.; Yu, C.Y.; Li, K.L.; Xun, C.; Yang, L.M.; He, J.; et al. Morphology and microstructure regulation of inorganic salts in an additive-free water system via the self-organization of hierarchical crystal clusters: Mechanism, model, and applications. Chem. Eng. Sci. 2022, 262, 12. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhan, W.Q.; Zhang, W.; Yao, R.Y.; Chen, P.; Jia, F.F.; Yi, H.; Song, S.X.; Valdivieso, A.L. Controllably Constructing morphology and structure of potassium chloride crystal by green supersaturation modulation. Surf. Interfaces 2024, 52, 10. [Google Scholar] [CrossRef]
- Chen, M.Y.; Wu, S.G.; Xu, S.J.; Yu, B.; Shilbayeh, M.; Liu, Y.; Zhu, X.W.; Wang, J.K.; Gong, J.B. Caking of crystals: Characterization, mechanisms and prevention. Powder Technol. 2018, 337, 51–67. [Google Scholar] [CrossRef]
- Chen, M.; Wu, S.; Tang, W.; Gong, J. Caking and adhesion free energy of maltitol: Studying of mechanism in adhesion process. Powder Technol. 2015, 272, 235–240. [Google Scholar] [CrossRef]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Xu, S.J.; Zhang, H.M.; Qiao, B.G.; Wang, Y.F. Review of Liquid-Liquid Phase Separation in Crystallization: From Fundamentals to Application. Cryst. Growth Des. 2021, 21, 7306–7325. [Google Scholar] [CrossRef]
- Li, J.J.; Gao, Y.; Wang, J.S.; Niu, L.; Ge, Y.Z.; Zhang, Y.L.; Chen, M.Y.; Gong, J.B. Preparation of (NH4)2SO4 Spherical Particles with Functions of Sustained-Release and Anticaking by an Organic Solvent-Free Process. Ind. Eng. Chem. Res. 2024, 63, 18212–18220. [Google Scholar] [CrossRef]
- Lu, M.; Ma, L.; Ye, X.S.; Liu, H.N.; Yao, Q.L.; Wu, Z.J.; Li, M.Z. Novel Method for Rapid Determination of Octadecylamine Using UV Spectrophotometry. J. Appl. Spectrosc. 2023, 6, 1101–1106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).