Influence of Nanocrystallite Size on Magnetic Properties of Iron Nitride γ’-Fe4N
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Quantum nanoscience. Nat. Nanotechnol. 2021, 16, 1293. [CrossRef]
- Jarman, J.C.; Robinson, K.N. Introduction. In Nanomaterial Characterization; Tantra, R., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 1–24. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Wang, X. High-Entropy Materials: From Bulk to Sub-nano. Adv. Funct. Mater. 2025, 35, 2504275. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B. From Bulk to Nano: Formation, Features, and Functions of Nano-Black Carbon in Biogeochemical Processes. Environ. Sci. Technol. 2024, 58, 15910–15925. [Google Scholar] [CrossRef]
- Roumiguier, L.; Jankowiak, A.; Pradeilles, N.; Antou, G.; Maître, A. Mechanical properties of submicronic and nanometric boron carbides obtained by Spark Plasma Sintering: Influence of B/C ratio and oxygen content. Ceram. Int. 2019, 45, 9912–9918. [Google Scholar] [CrossRef]
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J. Control. Release 2019, 299, 31–43. [Google Scholar] [CrossRef]
- Pelka, R. A Method of Determining Nanoparticle Size Distribution in Iron Ammonia Synthesis Catalyst by Measuring Mass Changes During the Nitriding Process. Catal. Today 2016, 286, 118–123. [Google Scholar] [CrossRef]
- Li, P.; Yan, A.; Xue, Y. Size-dependence of melting thermodynamics of nano-Bi. J. Nanopart Res. 2025, 27, 250. [Google Scholar] [CrossRef]
- Schlexer, P.; Andersen, A.B.; Sebok, B.; Chorkendorff, I.; Schiøtz, J.; Hansen, T.W. Size-Dependence of the Melting Temperature of Individual Au Nanoparticles. Part. Part. Syst. Charact. 2019, 36, 1800480. [Google Scholar] [CrossRef]
- Acheche, A.; Nelayah, J.; Gatti, R.; Alloyeau, D.; Ricolleau, C.; Amara, H. Thermal stability of CoNiPtCuAu nanoalloys: From segregation to melting properties. Faraday Discuss. 2026; advance article. [Google Scholar] [CrossRef]
- Grigalaitis, R.; Banys, J.; Lapinskas, S.; Erdem, E.; Bottcher, R.; Glasel, H.-J.; Hartmann, E. Dielectric investigations and theoretical calculations of size effect in lead titanate nanocrystals. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Abou Taleb, M.F.; Ibrahim, M.M.; Rahman, A.U.; El-Bahy, Z.M. Magnetic response of Ho3+ doped Ni0.4Cu0.6HoyFe2-yO4 spinel ferrites and their correlation with crystallite size. Ceram. Int. 2024, 50, 37077–37084. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z.; Lin, D.; Liu, W.; Zhou, S.; Ge, S.; Su, Y.; Peng, C.; Zhang, L. Effects of particle size of dielectric fillers on the output performance of piezoelectric and triboelectric nanogenerators. J. Adv. Ceram. 2021, 10, 991–1000. [Google Scholar] [CrossRef]
- Saba, F.; Zhang, F.; Liu, S.; Liu, T. Reinforcement size dependence of mechanical properties and strengthening mechanisms in diamond reinforced titanium metal matrix composites. Compos. B Eng. 2019, 167, 7–19. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Figueiredo, R.B. An overview of the effect of grain size on mechanical properties of magnesium and its alloys. Mater. Trans. 2023, 64, 1272–1283. [Google Scholar] [CrossRef]
- Arabczyk, W.; Ekiert, E.; Pelka, R. Size-Dependent Transformation of α-Fe into γ′-Fe 4 N in Nanocrystalline the Fe–NH3–H2 System. J. Phys. Chem. C. 2016, 120, 17989–17995. [Google Scholar] [CrossRef]
- Arabczyk, W.; Ekiert, E.; Pelka, R. Hysteresis phenomenon in a reaction system of nanocrystalline iron and a mixture of ammonia and hydrogen. Phys. Chem. Chem. Phys. 2016, 18, 25796–25800. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, D.; Vauth, M.; Pezoldt, J. Size Dependent Properties of Reactive Materials. Inorganics 2022, 10, 56. [Google Scholar] [CrossRef]
- Muravev, V.; Parastaev, A.; van den Bosch, Y.; Ligt, B.; Claes, N.; Bals, S.; Kosinov, N.; Hensen, E.J.M. Size of cerium dioxide support nanocrystals dictates reactivity of highly dispersed palladium catalysts. Science 2023, 380, 1174–1179. [Google Scholar] [CrossRef]
- Arabczyk, W.; Pelka, R.; Wilk, B.; Lendzion-Bieluń, Z. Kinetics and Thermodynamics of the Phase Transformation in the Nanocrystalline Substance—Gas Phase System. Crystals 2024, 14, 129–150. [Google Scholar] [CrossRef]
- Tulinski, M.; Jurczyk, M. Nanomaterials Synthesis Methods. In Metrology and Standardization of Nanotechnology: Protocols and Industrial Innovations; Wiley: Hoboken, NJ, USA, 2017; pp. 75–98. [Google Scholar] [CrossRef]
- Bhattacharyya, S. Iron nitride family at reduced dimensions: A review of their synthesis protocols and structural and magnetic properties. J. Phys. Chem. C 2015, 119, 4, 1601–1622. [Google Scholar] [CrossRef]
- Wojciechowski, P.; Lewandowski, M. Iron Nitride Thin Films: Growth, Structure, and Properties. Cryst. Growth Des. 2022, 22, 4618–4639. [Google Scholar] [CrossRef]
- Gafner, Y.Y.; Gafner, S.L.; Nomoev, A.V.; Bardakhanov, S.P. Analysis of the Structure and Thermal Stability of Cu@Si Nanoparticles. J. Metastable Nanocryst. Mater. 2018, 30, 52–59. [Google Scholar] [CrossRef]
- Arabczyk, W.; Lendzion-Bieluń, Z.; Wróbel, R. Sposób Otrzymywania Nanomateriałów o Okreslonych Rozmiarach Krystalitów. Polish Patent PL361256A1, 24 January 2005. Available online: https://eprofil.pue.uprp.gov.pl/public/registry/view/Pat.206909?lng=pl (accessed on 31 October 2005).
- Wróbel, R.; Arabczyk, W. Solid−Gas Reaction with Adsorption as the Rate Limiting Step. J. Phys. Chem. A 2006, 110, 9219–9224. [Google Scholar] [CrossRef]
- Tan, X.; Wang, S.; Chen, Y.; Zhou, Y.; Li, Z. Design, preparation and characterization of iron nitride magnetic abrasives. J. Alloys Compd. 2019, 774, 443–450. [Google Scholar] [CrossRef]
- Feng, X.; Gu, X.; Xuan, G.; Wu, H.; Li, S. Efficient microplastic removal in aquatic environments using iron–nitrogen co-doped layered biocarbon materials. Chem. Eng. J. 2025, 506, 160095. [Google Scholar] [CrossRef]
- Mamatha, G.M.; Dixit, P.; Krishna, R.H.; Girish, K.S. Polymer based composites for electromagnetic interference (EMI) shielding: The role of magnetic fillers in effective attenuation of microwaves, a review. Hybrid. Adv. 2024, 6, 100200. [Google Scholar] [CrossRef]
- Wu, K.; Liu, J.; Saha, R.; Ma, B.; Su, D.; Peng, C.; Sun, J.; Wang, J.P. Irregularly Shaped Iron Nitride Nanoparticles as a Potential Candidate for Biomedical Applications: From Synthesis to Characterization. ACS Omega 2020, 5, 11756–11767. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.; Amorim, L.; Díez, A.G.; Ribeiro, C.; Lanceros-Mendez, S.; Martins, P. Energy-efficient electric control of magnetization in polymer-based magnetoelectrics for spintronic applications. Commun. Mater. 2025, 6, 44. [Google Scholar] [CrossRef]
- Yang, W.; Rehman, S.; Chu, X.; Hou, Y.; Gao, S. Transition Metal (Fe, Co and Ni) Carbide and Nitride Nanomaterials: Structure, Chemical Synthesis and Applications. ChemNanoMat 2015, 1, 376–398. [Google Scholar] [CrossRef]
- Saito, T.; Yamamoto, H.; Nishio-Hamane, D. Production of Rare-Earth-Free Iron Nitride Magnets (α″-Fe16N2). Metals 2024, 14, 734. [Google Scholar] [CrossRef]
- Lee, O.M.; Abbasian, M. Reducing Rare-Earth Magnet Reliance in Modern Traction Electric Machines. Energies 2025, 18, 2274. [Google Scholar] [CrossRef]
- Kartsev, A.; Feya, O.D.; Bondarenko, N.; Kvashnin, A.G. Stability and magnetism of FeN high-pressure phases. Phys. Chem. Chem. Phys. 2019, 21, 5262–5273. [Google Scholar] [CrossRef]
- Tao, Z.; Liu, S.; Fang, H.; Chen, L.; Chen, J.; Xiu, X.; Zhang, R. Magnetic and transport properties of single-phase N-rich iron nitrides. Mater. Lett. 2019, 239, 140–142. [Google Scholar] [CrossRef]
- Barba-Juan, A.; Vicente, N.; Mormeneo-Segarra, A.; Clausell-Terol, C. Microstructure-dependent magnetic permeability in ferrites from nanoparticles. Ceram. Int. 2023, 49, 21530–21537. [Google Scholar] [CrossRef]
- Upadhyay, S.; Parekh, K.; Pandey, B. Influence of crystallite size on the magnetic properties of Fe3O4 nanoparticles. J. Alloys Compd. 2016, 678, 478–485. [Google Scholar] [CrossRef]
- Arabczyk, W.; Pelka, R.; Brzoza-Kos, A.; Kocemba, I.; Rokicka-Konieczna, P.; Skulmowska-Polok, K.; Klimza, K.; Lendzion-Bieluń, Z. Studies of Phase Transformation Kinetics in the System of Nanocrystalline Iron/Ammonia/Hydrogen at the Temperature of 350 °C by Means of Magnetic Permeability In Situ Measurement. Appl. Sci. 2024, 14, 8452. [Google Scholar] [CrossRef]
- Pielaszek, R. FW15/45M method for determination of the grain size distribution from powder diffraction line profile. J. Alloys Compd. 2004, 382, 128–132. [Google Scholar] [CrossRef]
- Pielaszek, R. Analytical expression for diffraction line profile for polydispersive powders. In Applied Crystallography, Proceedings of the XIX Conference, Kraków, Poland, 1–4 September 2003; World Scientific Publishing: Singapore, 2004; pp. 43–50. [Google Scholar]
- Arabczyk, W.; Pelka, R.; Jasińska, I.; Lendzion-Bieluń, Z. Thermodynamics of Iron Ammonia Synthesis Catalyst Sintering. Crystals 2024, 14, 188. [Google Scholar] [CrossRef]
- Arabczyk, W.; Pelka, R. Studies of the Kinetics of Two Parallel Reactions: Ammonia Decomposition and Nitriding of Iron Catalyst. J. Phys. Chem. A 2009, 113, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Arabczyk, W.; Pelka, R.; Kocemba, I.; Brzoza-Kos, A.; Wyszkowski, A.; Lendzion-Bieluń, Z. Study of Phase Transformation Processes Occurring in the Nanocrystalline Iron/Ammonia/Hydrogen System by the Magnetic Permeability Measurement Method. J. Phys. Chem. C 2022, 126, 7704–7710. [Google Scholar] [CrossRef]
- Arabczyk, W.; Narkiewicz, U.; Moszyński, D. Double-Layer Model of the Fused Iron Catalyst for Ammonia Synthesis. Langmuir 1999, 15, 5785–5789. [Google Scholar] [CrossRef]
- Wilk, B.; Arabczyk, W. Investigation of nitriding and reduction processes in the nanocrystalline iron-ammonia-hydrogen system at 350 °C. Phys. Chem. Chem. Phys. 2015, 17, 20185–20193. [Google Scholar] [CrossRef]
- Lubkowski, K.; Arabczyk, W.; Grzmil, B.; Michalkiewicz, B.; Pattek-Janczyk, A. Passivation and oxidation of an ammonia iron catalyst. Appl. Catal. A Gen. 2007, 329, 137. [Google Scholar] [CrossRef]
- Nowosielecka, U.; Pelka, R.; Moszyńska, I.; Guskos, N.; Typek, J.; Żołnierkiewicz, G. Studies of magnetic properties of nanocrystalline iron of different sizes of nanocrystallites. J. Magn. Magn. Mater. 2017, 443, 324–333. [Google Scholar] [CrossRef]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Muñoz Medina, G.A.; Mudarra Navarro, A.M.; Deluque Toro, C.E.; Gil Rebaza, A.V. Structural, Thermophysical, and Magnetic Properties of the γ′-Fe4N System: Density Functional Theory and Experimental Study. Processes 2025, 13, 2402. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Yang, H. Magnetic and Electrochemical Properties of γ′-Fe4N Nanoparticles with Cuboidal and Rodlike Morphologies. J. Phys. Chem. C 2023, 127, 728–735. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Chen, J.; Ying, Y.; Yu, J.; Zheng, J.; Qiao, L.; Li, J.; Che, S. Migration of N element and evolution of microstructure in spark plasma sintered bulk γ′-Fe4N. J. Alloys Compd. 2022, 928, 167201. [Google Scholar] [CrossRef]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions; Oxford University Press: Oxford, UK, 1970; ISBN 978-0199651528. [Google Scholar]
- Gubin, S.P. Magnetic Nanoparticles; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar]
- Jakes, P.; Erdem, E. Finite size effects in ZnO nanoparticles: An electron paramagnetic resonance (EPR) analysis. Phys. Status Solidi RRL 2011, 5, 56–58. [Google Scholar] [CrossRef]
- Leniec, G.; Kaczmarek, S.M.; Macalik, L.; Ropuszyńska-Robak, P.; Hanuza, J. Magnetic properties of KY0.93Er0.05Tm0.02(WO4)2 and NaY0.97Er0.02Tm0.01(WO4)2 nanocrystals obtained using Pechini and hydrothermal methods. J. Phys. Chem. Solids 2020, 138, 109273. [Google Scholar] [CrossRef]
- Leniec, G.; Kaczmarek, S.M.; Macalik, L.; Ropuszyńska-Robak, P.; Hanuza, J. Magnetic properties of NaY1−x−yHoxYby(WO4)2: X = 0.05, y = 0.02 and KY1−x−yHoxYby(WO4)2: X = 0.02, y = 0.01 nanopowders obtained by Pechini and hydrothermal methods. Chem. Phys. Lett. 2019, 715, 360–366. [Google Scholar] [CrossRef]
- Fuks, H.; Kaczmarek, S.M.; Leniec, G.; Michalski, J.; Kucharska, B. Magnetic Properties of Steel Ball Samples, Investigated before and after Nitriding. Arch. Metall. Mater. 2018, 63, 1235–1242. [Google Scholar] [CrossRef]
- Kaczmarek, S.M.; Michalski, J.; Frączek, T.; Dudek, A.; Fuks, H.; Leniec, G. Mechanical and Magnetic Investigations of Balls Made of AISI 1010 and AISI 1085 Steels after Nitriding and Annealing. Metals 2023, 13, 1060. [Google Scholar] [CrossRef]
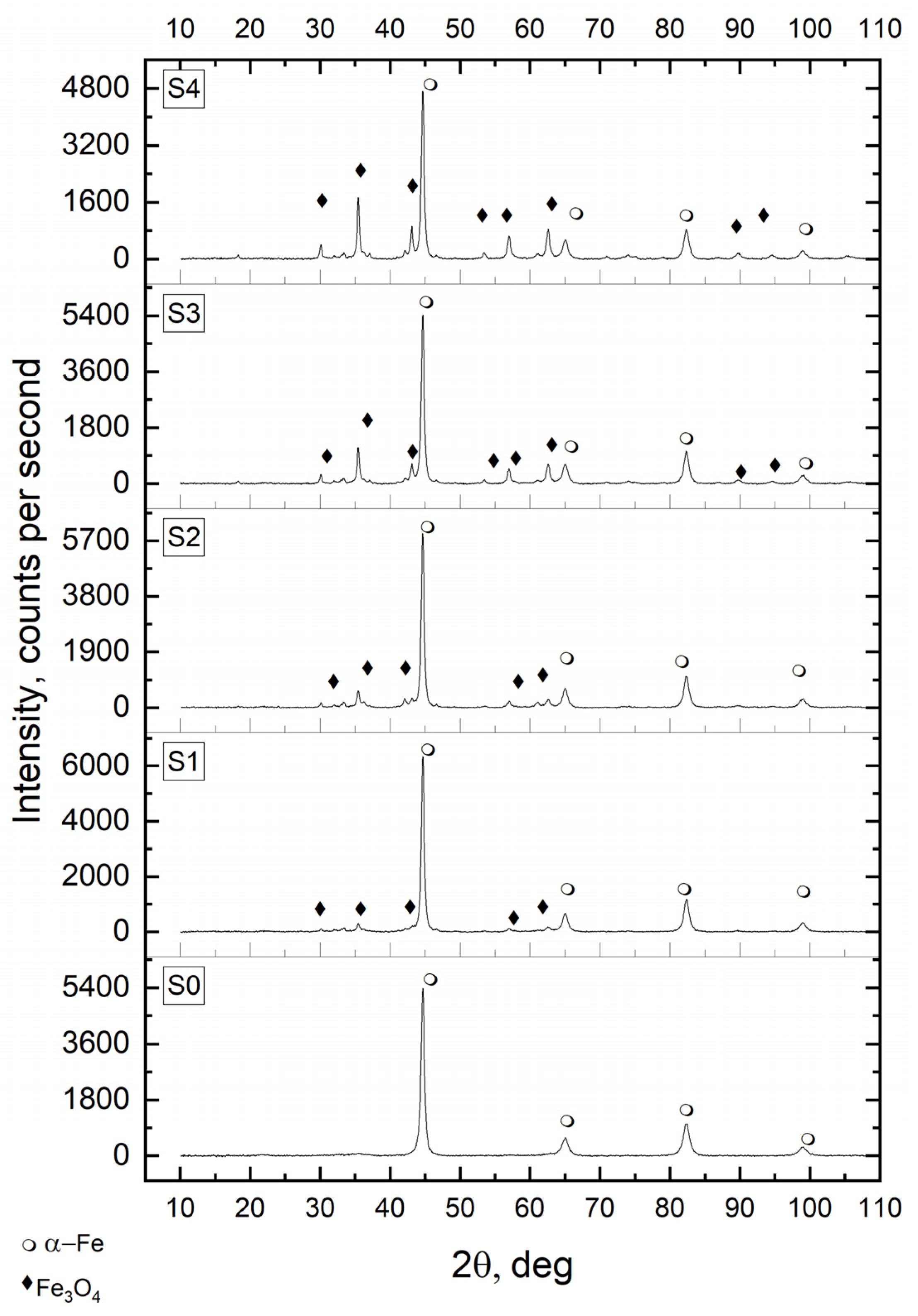
 —metallic α-iron.
—metallic α-iron.
 —metallic α-iron.
—metallic α-iron.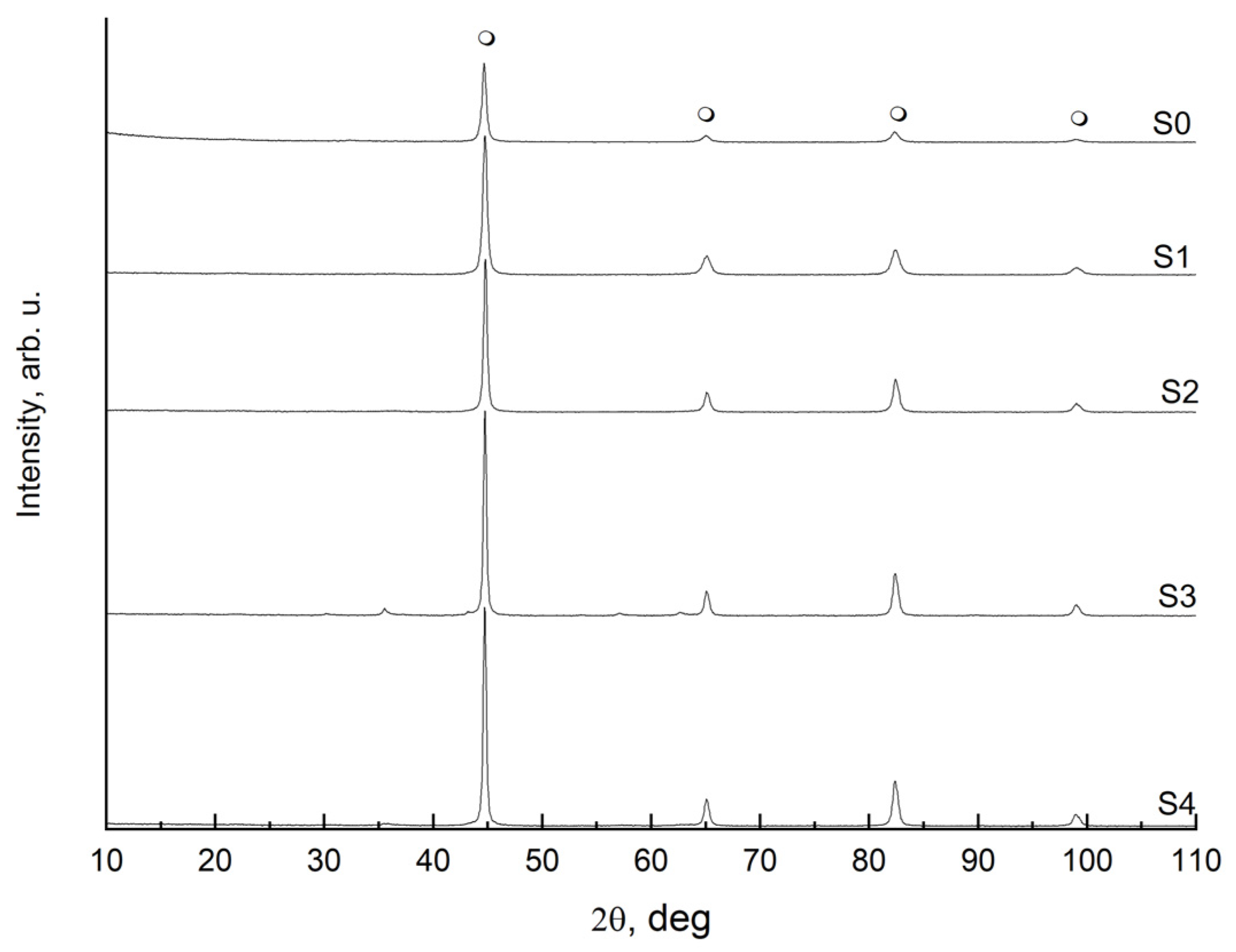

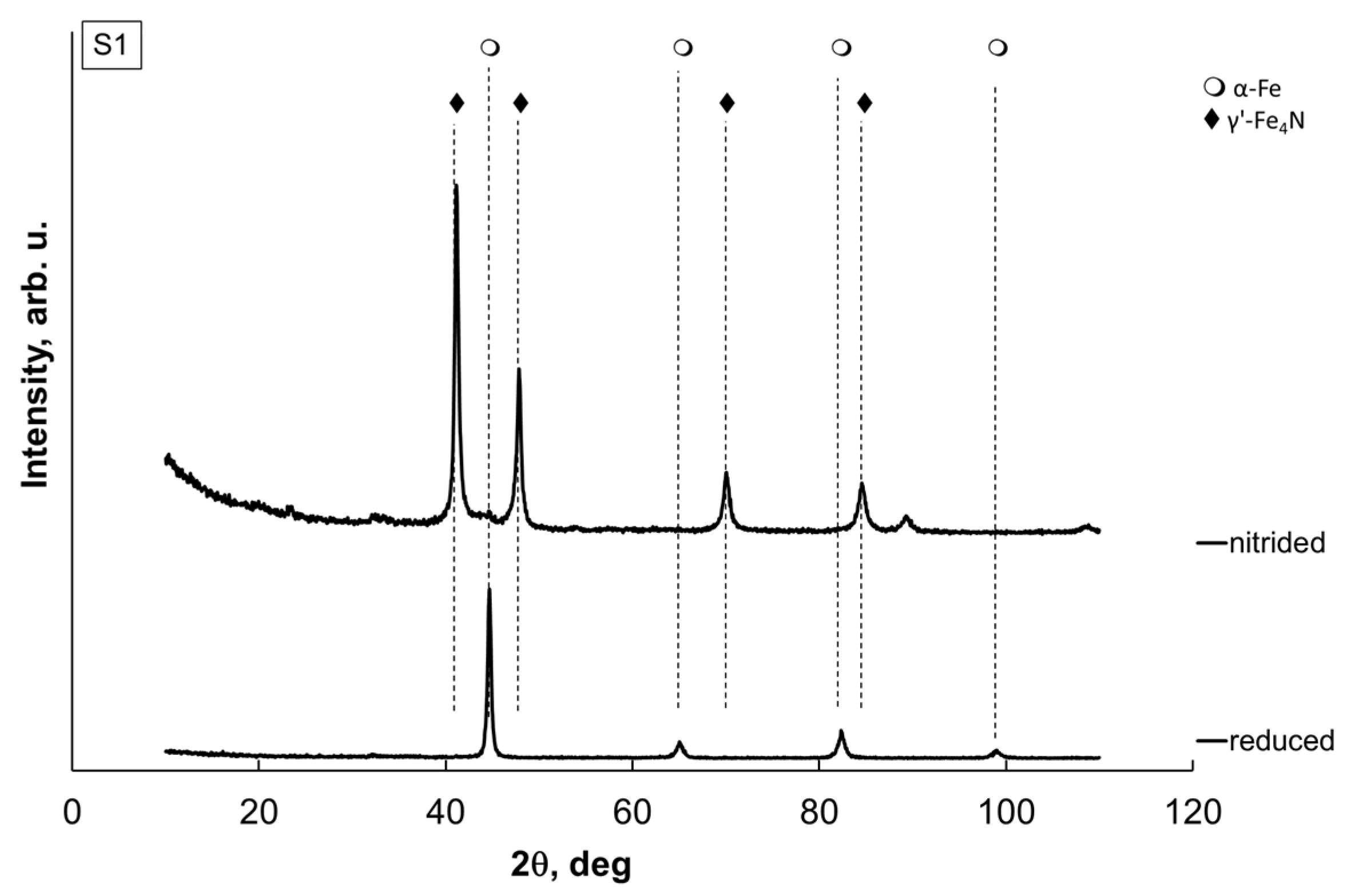
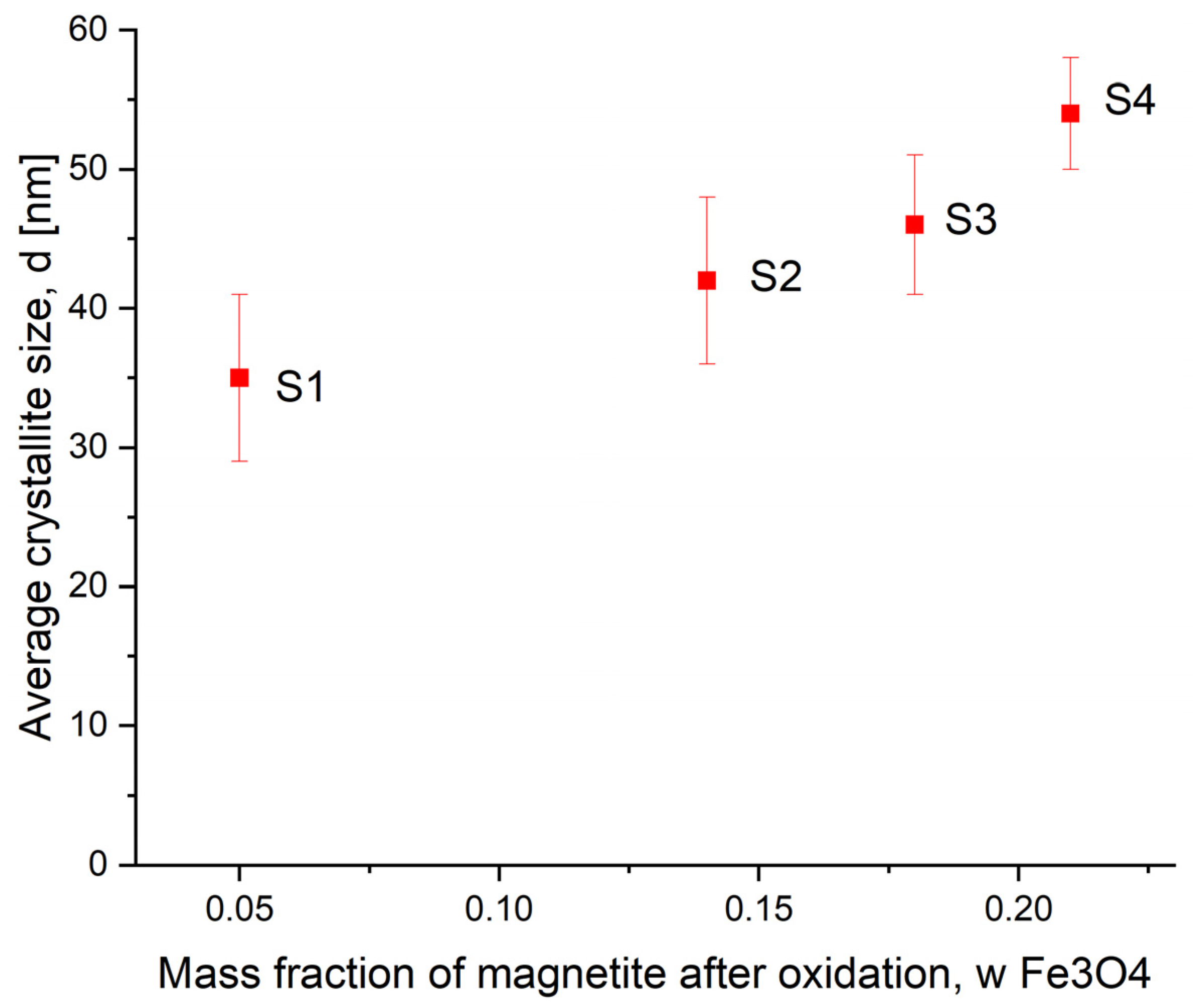

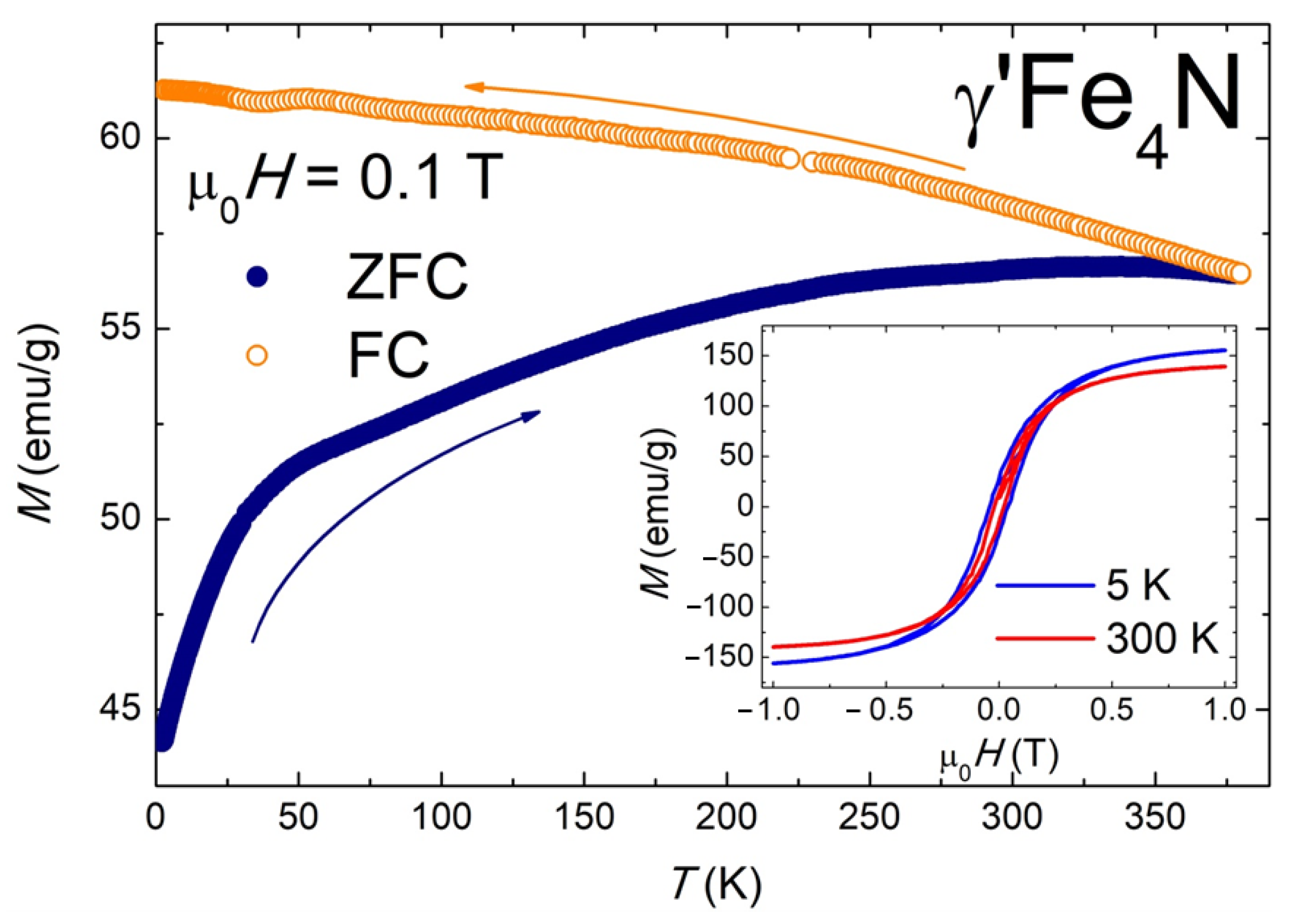
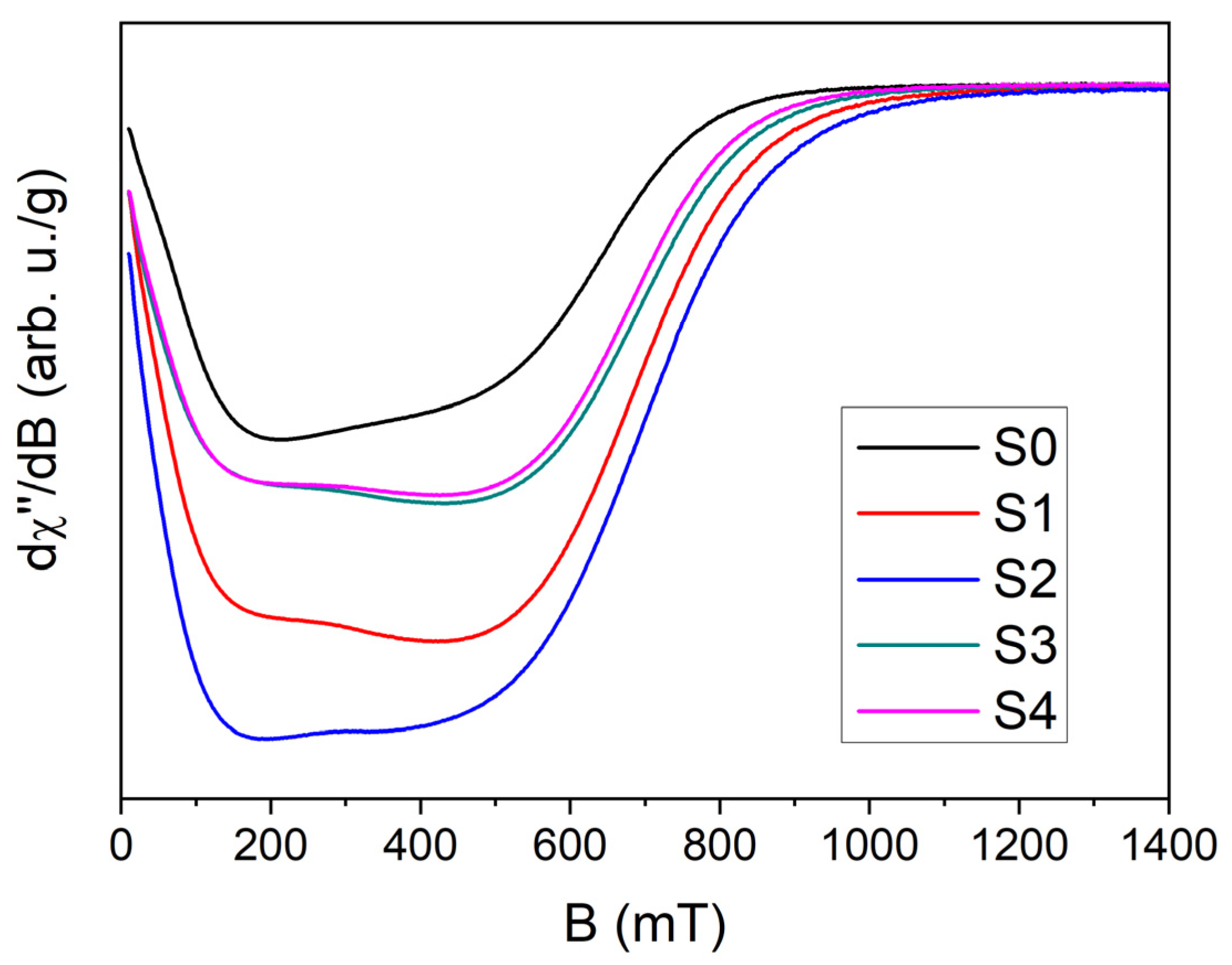
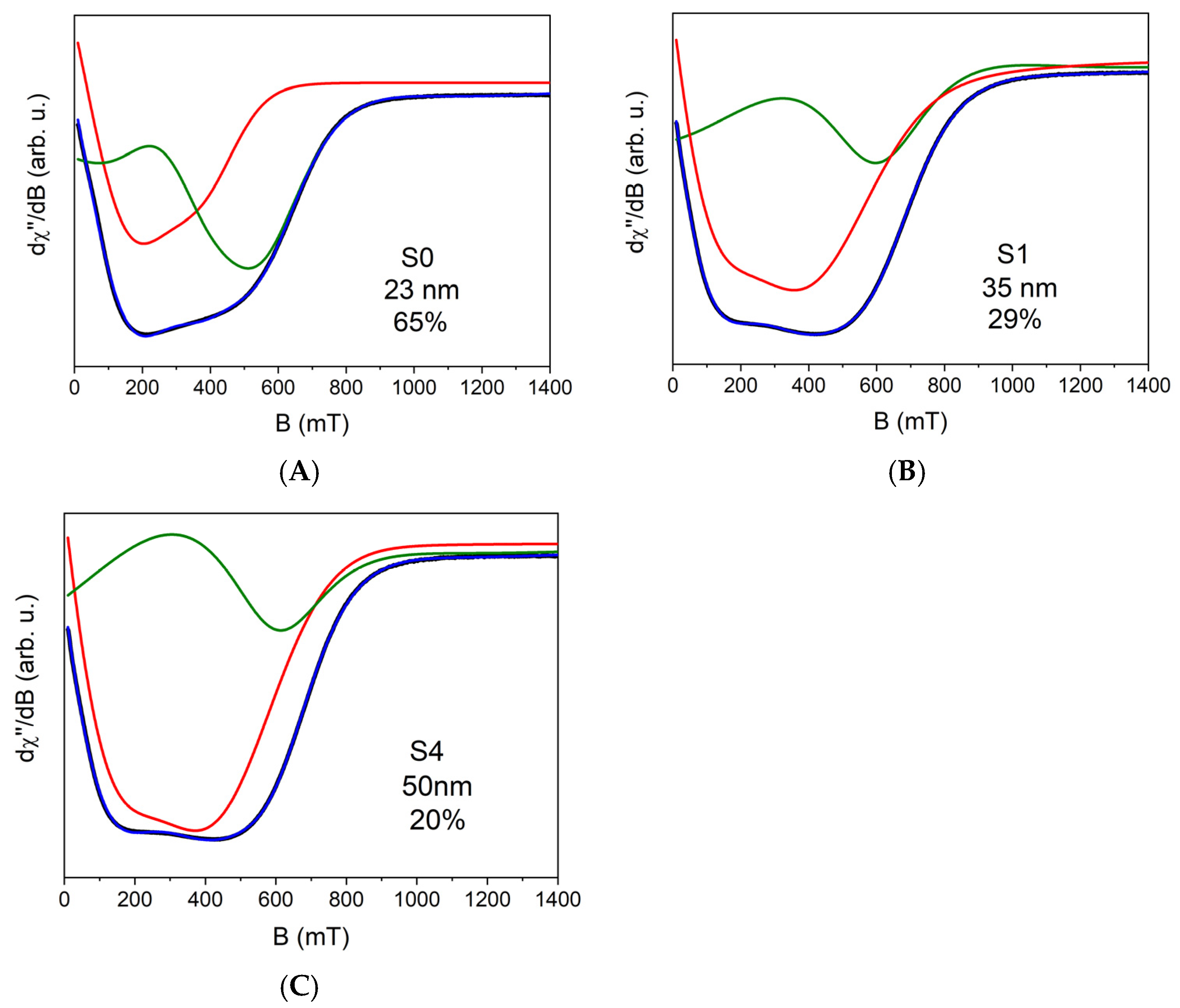

| S0 | S1 | S2 | S3 | S4 | |
|---|---|---|---|---|---|
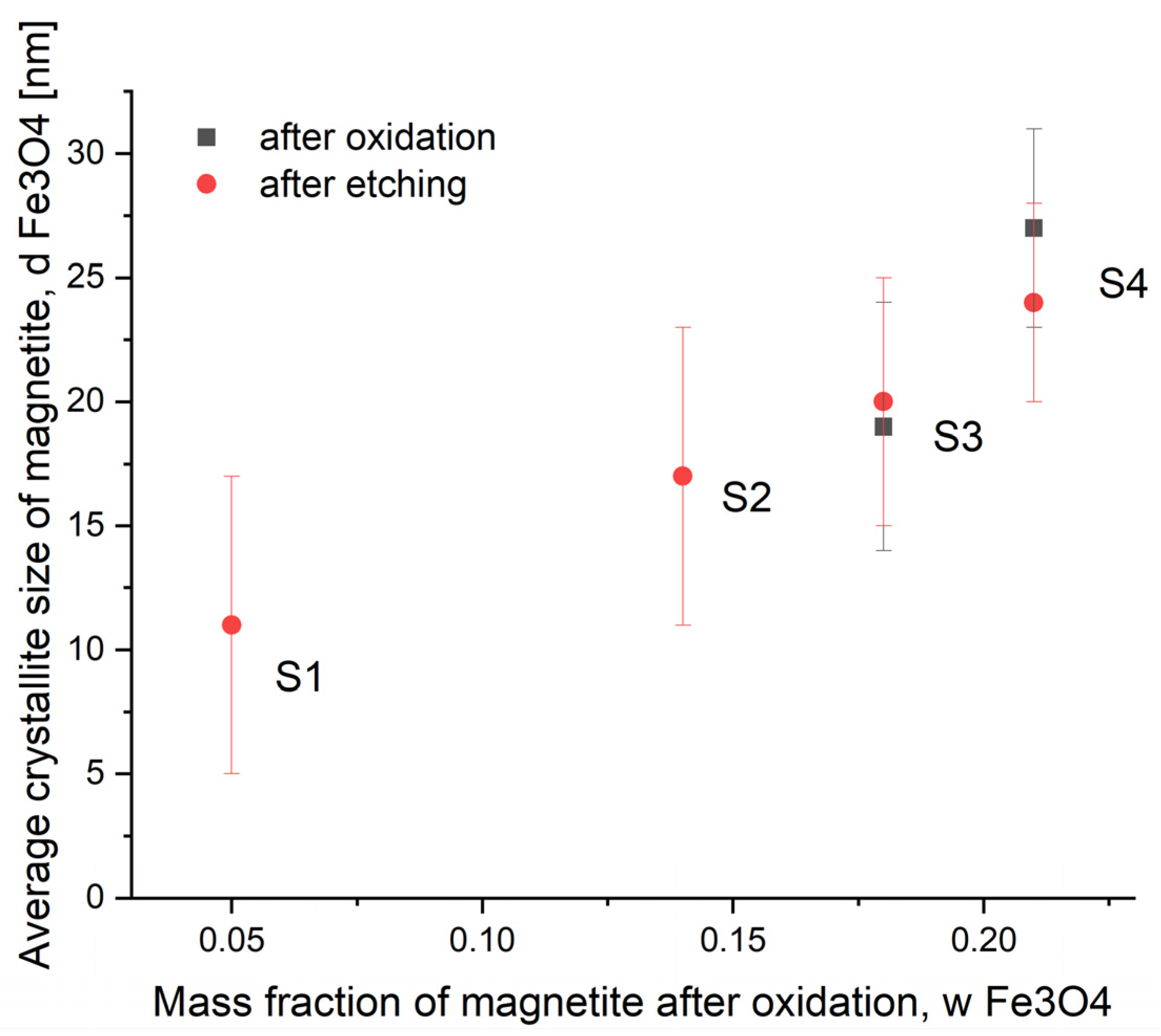
| Mean size, nm | 25 | 11 | 18 | 20 | 24 |
| Lattice strain, % | 0.430 (Fe) | 0.289 | 0.241 | 0.235 | 0.227 |
| Standard deviation, nm | 15 | 6 | 6 | 5 | 4 |
| Specific surface area, m2/g | 8.8 * | 33.0 | 29.0 | 24.0 | 16.0 |
| Surface-to-volume ratio, nm2/nm3 | 0.24 | 0.55 | 0.33 | 0.30 | 0.25 |
| Phase composition, wt. % | 100 (Fe) | 95 (Fe3O4) | 95 (Fe3O4) | 99 (Fe3O4) | 98 (Fe3O4) |
| S0 | S1 | S2 | S3 | S4 | |
|---|---|---|---|---|---|
| Mean size, nm | 23 | 35 | 42 | 46 | 54 |
| Lattice strain, % | 0.15 | 0.13 | 0.12 | 0.12 | 0.100 |
| Standard deviation, nm | 15 | 6 | 6 | 5 | 4 |
| Specific surface area, m2/g | 7.0 | 22.0 | 19.0 | 17.0 | 14.0 |
| Surface-to-volume ratio, nm2/nm3 | 0.26 | 0.17 | 0.14 | 0.13 | 0.11 |
| Phase composition, wt. % | 100 (Fe4N) | 99 (Fe4N) | 99 (Fe4N) | 99 (Fe4N) | 98 (Fe4N) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimza, K.; Leniec, G.; Synoradzki, K.; Pelka, R.; Nowosielecka, U.; Moszyńska, I.; Guskos, A.; Żołnierkiewicz, G.; Guskos, N. Influence of Nanocrystallite Size on Magnetic Properties of Iron Nitride γ’-Fe4N. Crystals 2025, 15, 956. https://doi.org/10.3390/cryst15110956
Klimza K, Leniec G, Synoradzki K, Pelka R, Nowosielecka U, Moszyńska I, Guskos A, Żołnierkiewicz G, Guskos N. Influence of Nanocrystallite Size on Magnetic Properties of Iron Nitride γ’-Fe4N. Crystals. 2025; 15(11):956. https://doi.org/10.3390/cryst15110956
Chicago/Turabian StyleKlimza, Kamila, Grzegorz Leniec, Karol Synoradzki, Rafał Pelka, Urszula Nowosielecka, Izabela Moszyńska, Aleksander Guskos, Grzegorz Żołnierkiewicz, and Nikos Guskos. 2025. "Influence of Nanocrystallite Size on Magnetic Properties of Iron Nitride γ’-Fe4N" Crystals 15, no. 11: 956. https://doi.org/10.3390/cryst15110956
APA StyleKlimza, K., Leniec, G., Synoradzki, K., Pelka, R., Nowosielecka, U., Moszyńska, I., Guskos, A., Żołnierkiewicz, G., & Guskos, N. (2025). Influence of Nanocrystallite Size on Magnetic Properties of Iron Nitride γ’-Fe4N. Crystals, 15(11), 956. https://doi.org/10.3390/cryst15110956







