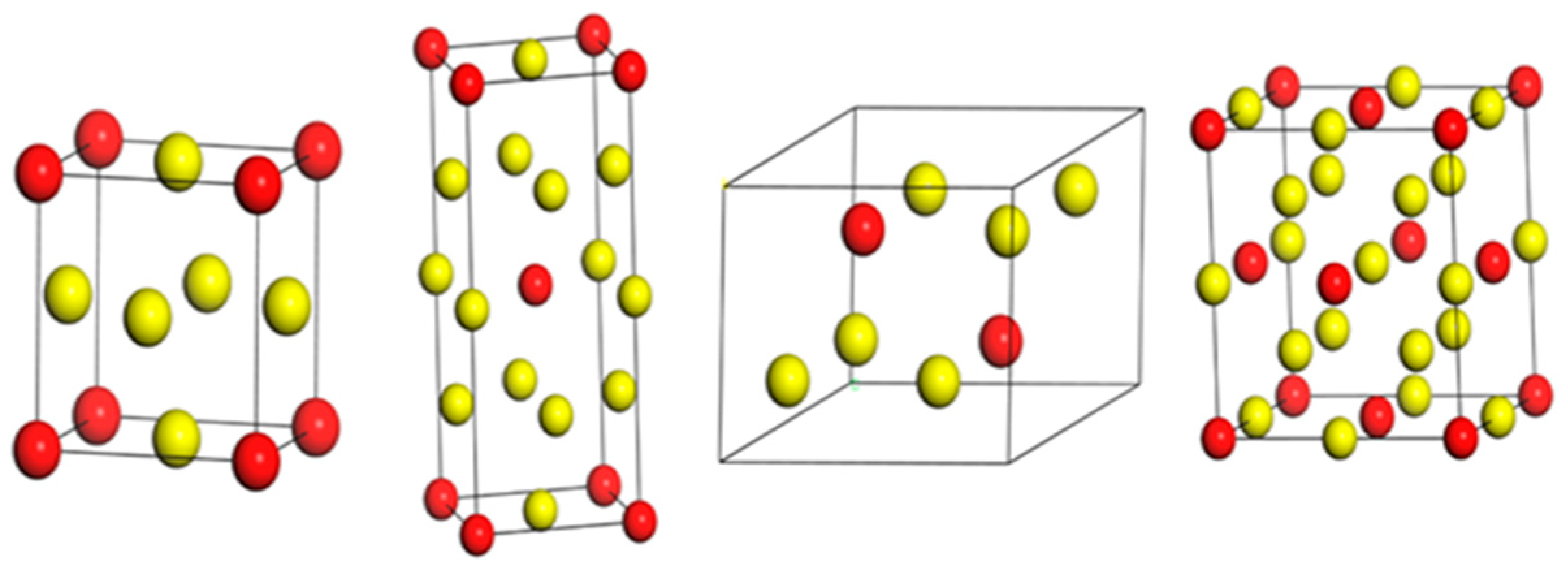
3.1. Structural Properties and Stability of In3Sc
In order to investigate the structural properties and intrinsic stability of In
3Sc, we studied its behavior in four representative crystal structures: Cu
3Au, Al
3Ti, Ni
3Sn, and BiF
3, as shown in
Figure 1. Each of these configurations represents a distinct lattice symmetry, offering insights into the interplay between atomic arrangement, electronic interactions, and mechanical response.
The Cu
3Au-type structure (Pm
m, SG 221) [
34] is established as the ground state of In
3Sc, and notably, serves as a reference for analyzing the stability of other polymorphs. It crystallizes in a simple cubic lattice with one formula unit per primitive cell. The Sc atom occupies the 1a Wyckoff position, while the three In atoms are located at the 3c sites (
Table 1). Owing to its high cubic symmetry, this structure exhibits nearly isotropic bonding, resulting in a uniform electron density distribution and minimal internal strain.
The Al
3Ti-type structure (I4/mmm, SG 139) [
35] is a body-centered tetragonal phase containing two formula units per cell. In this configuration, the Sc atom occupies the 2a site, while the In atoms are distributed over the 2b and 4d Wyckoff positions (
Table 1). The lowering of symmetry compared to the cubic phase introduces anisotropic bonding and lattice distortions, which directly affect elastic constants and lead to direction-dependent electronic band dispersion.
The Ni
3Sn-type structure (P6
3/mmc, SG 194) [
36] corresponds to a hexagonal arrangement with two formula units per unit cell. In this phase, Sc occupies the 2a site, and In atoms reside at the 6h positions (
Table 1). The layered stacking sequence produces strong in-plane bonding within the basal plane but weaker coupling along the c-axis.
Finally, the BiF
3-type structure (Fm
m, SG 225) [
37] is a high-symmetry cubic polymorph with four formula units per unit cell. Sc atoms are located at the 4a sites, while In atoms occupy the 24e sites (
Table 1). This phase is structurally more complex than the AuCu
3-type and generally appears at elevated pressures.
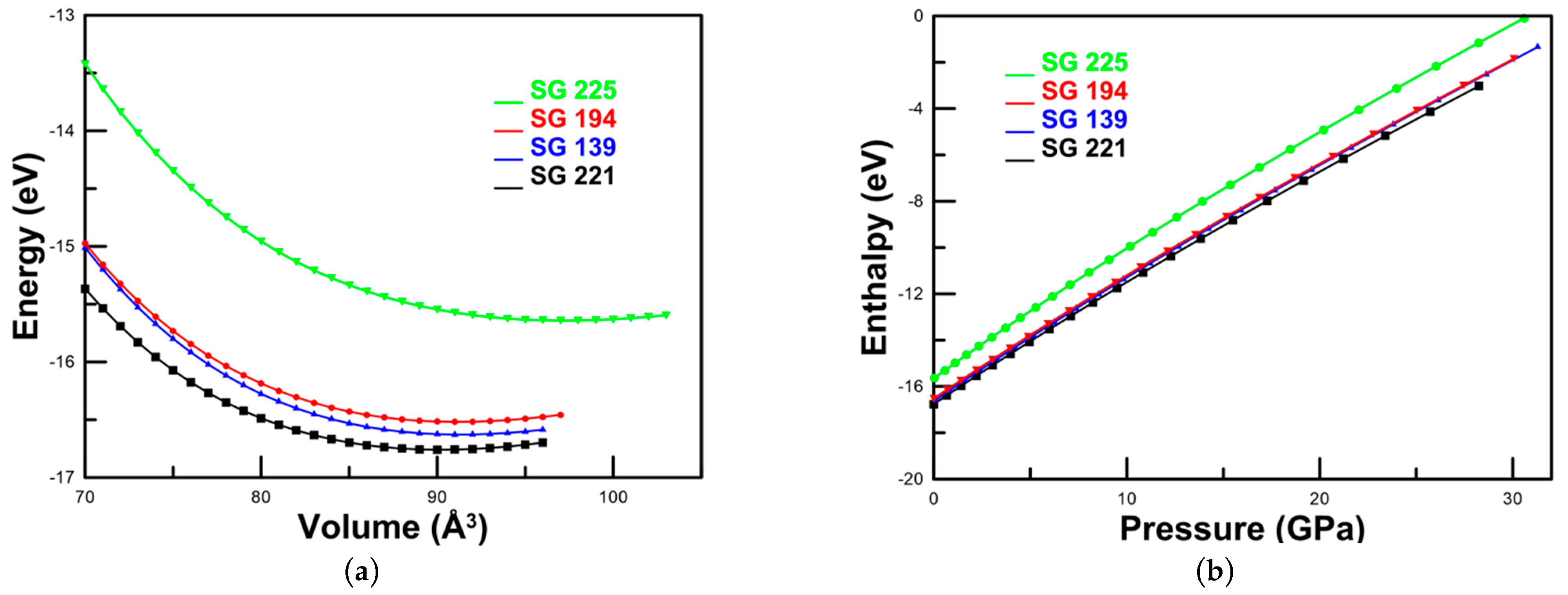
3.2. Energy-Volume and Pressure-Dependent Stability
The thermodynamic stability of each phase was analyzed through energy–volume (E–V) and enthalpy–pressure (H–P) relationships (
Figure 2a and
Figure 2b, respectively). The E–V curves identify the equilibrium volume V
0 as the point of minimum total energy. The cubic Pm
m phase exhibits the lowest energy, confirming it as the most stable configuration at ambient conditions. The tetragonal phase, however, is nearly degenerate with the cubic one, showing only a minor energy difference, which indicates that both phases are energetically favorable. Consistently, the calculated enthalpies of the cubic (SG 221) and tetragonal (SG 139) structures are almost indistinguishable across the entire investigated pressure range. This near-degeneracy suggests that the two polymorphs may coexist or compete as stable configurations under compression.
Table 2 summarizes the equilibrium lattice parameters, bulk modulus (B
0), its pressure derivative (B
0′), unit-cell volumes, total energy at equilibrium (E) and formation energy (Ef), alongside available experimental and theoretical data for In
3Sc in the four investigated phases. As shown in
Table 2, In
3Sc in the AuCu
3-type (Pm
m) structure has an equilibrium lattice parameter of 4.481 Å, which matches almost exactly the experimental value of 4.479 Å [
15]. This result also lies between other experimental determinations, namely 4.46 Å [
14] and 4.77 Å [
38]. In comparison with theoretical work, our value is slightly smaller than the lattice constant of 4.532 Å reported in Ref. [
39], reflecting possible differences in the exchange–correlation functional and computational parameters. The bulk modulus (61.15 GPa) is consistent with previous theoretical predictions ranging from 55.04 to 72.89 GPa [
40]. Relative to the other prototypes, In
3Sc-AuCu
3 is 23% stiffer than In
3Sc-BiF
3 (49.53 GPa), while differing by less than 2% from In
3Sc-Al
3Ti (62.37 GPa) and In
3Sc-Ni
3Sn (60.89 GPa). Its unit-cell volume (90.0 Å
3) is the smallest among all phases, confirming its compact lattice. The calculated pressure derivative of the bulk modulus (B
0′) for the AuCu
3-type phase is 4.933, which shows excellent agreement with the reported values in Reference [
41]. The small deviation (<2%) indicates that our fitted equation of state reproduces the computational compressibility trend reported in [
41] with high accuracy, confirming the reliability of our structural optimization.
For In3Sc in the Al3Ti-type (I4/mmm) structure, the lattice constants a = 4.335 Å and c = 9.683 Å yield a unit-cell volume of 91.3 Å3. This is ~1.4% larger than in AuCu3, consistent with a slight increase in compressibility. However, the bulk modulus (62.37 GPa) is 2% larger than in In3Sc-AuCu3 and 2.4% higher than in In3Sc-Ni3Sn, showing comparable or even slightly stronger incompressibility. Compared with In3Sc-BiF3, the tetragonal phase is 26% stiffer with ~6% smaller volume.
In the Ni3Sn-type (P63/mmc) structure, In3Sc exhibits a = 6.333 Å and c = 5.239 Å, corresponding to a unit-cell volume of 91.1 Å3—virtually identical to Al3Ti (difference of 0.2%). The bulk modulus (60.89 GPa) is only 2.4% smaller than Al3Ti and 0.4% smaller than AuCu3, highlighting near-equivalent stiffness. By contrast, it is 23% stiffer and ~6.4% denser than In3Sc–BiF3, making it mechanically more robust.
The BiF
3-type (Fm
m) structure of In
3Sc departs significantly from this trend. With a = 7.294 Å, its unit-cell volume reaches 97.3 Å
3 [
14], about 8% larger than In
3Sc-AuCu
3 and 6–7% larger than In
3Sc-Al
3Ti and In
3Sn-Ni
3Sn. The bulk modulus (49.53 GPa) is markedly reduced, being 19–26% smaller than in the other three prototypes, reflecting enhanced structural softness and compressibility. In comparing the total and formation energies listed in
Table 2, the AuCu
3-type structure is the most stable, followed by the Al
3Ti-type and Ni
3Sn-type structures, with the BiF
3-type being the least stable phase.
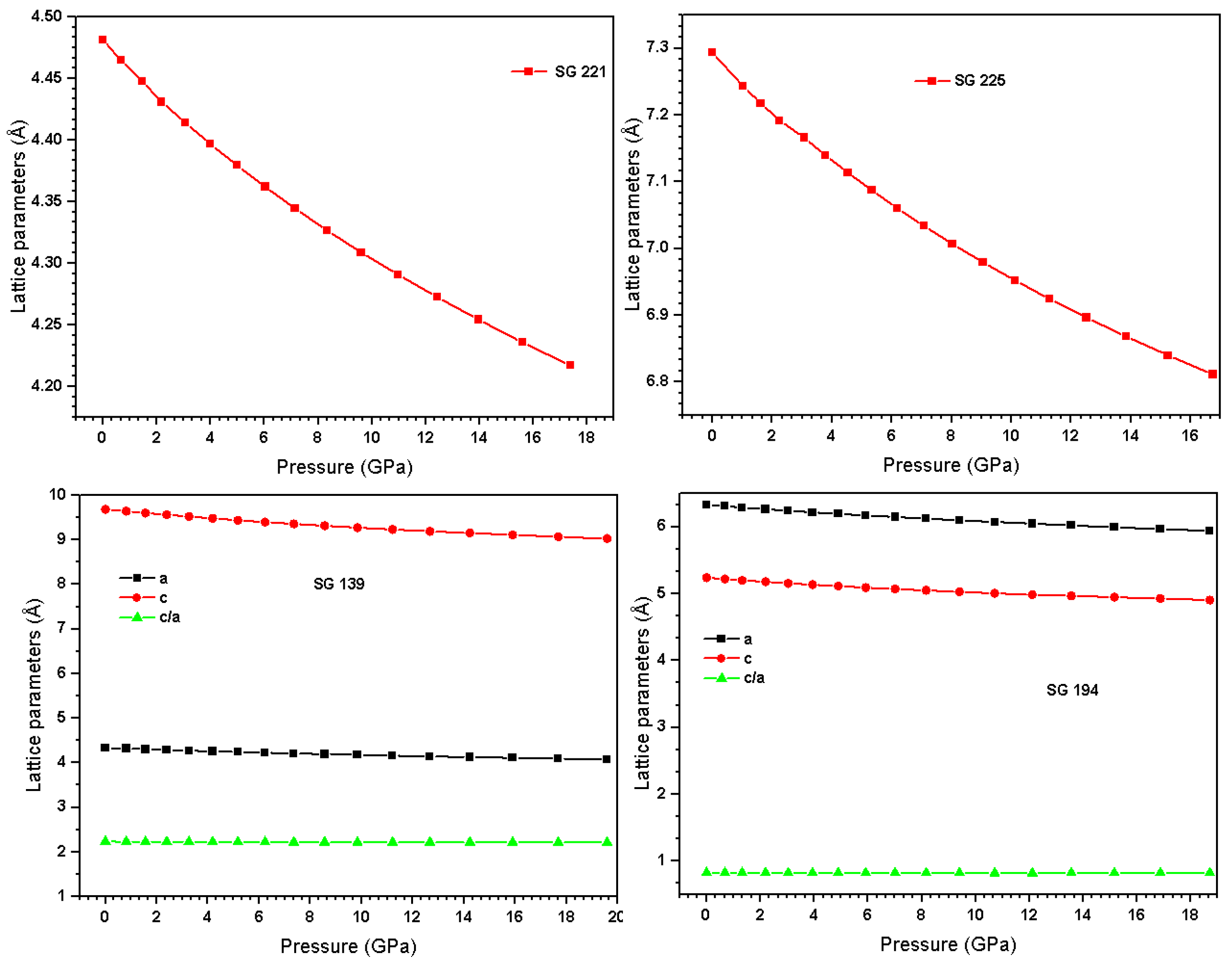
3.3. Lattice Parameters Evolution Under Pressure
The response of lattice parameters to hydrostatic pressure provides critical insights into the mechanical and electronic behavior of In
3Sc, as illustrated in
Figure 3. In this study, we focus on a pressure range of 0–20 GPa, which is relevant for typical high-pressure experiments using a Diamond Anvil Cell (DAC). Common pressure-transmitting media such as methanol-ethanol-water remain hydrostatic only up to ~8–10 GPa. Higher pressures require DACs with larger diamonds and gas-loading techniques, accessible to only a few specialized groups. This range ensures that the calculations are directly comparable to standard experimentally achievable pressures, consistent with standard practices in high-pressure research (e.g., MALTA, CRC Press) [
42,
43,
44]. For cubic structures, compression is nearly isotropic due to the equivalence of all bond directions, while lower-symmetry tetragonal and hexagonal phases exhibit anisotropic contraction along different crystallographic axes. This anisotropy arises from differences in bond stiffness, atomic packing, and electronic density distributions along distinct lattice directions.
For the cubic Pm
m (SG 221) phase, the lattice parameter evolution under pressure is described by the polynomial fit:
(Similarly, the cubic Fm
m (SG 225) phase, which has a larger unit cell and slightly lower packing efficiency, exhibits:
For the hexagonal P63/mmc (SG 194) phase, the a and c-axes compress at different rates, reflecting directional differences in bond stiffness:
The ratio of lattice parameters along the c and a-axes evolves as:
indicating that the c-axis compresses faster than the basal plane. In the tetragonal I4/mmm (SG 139) phase, a similar anisotropic trend is observed:
The linear term in each polynomial corresponds to the elastic response, indicating the compressibility along a given axis, while the quadratic term captures anharmonic effects at higher pressures, arising from nonlinear atomic interactions, electron-cloud repulsion, and deviations from Hookean behavior. Changes in the c/a ratio quantify the anisotropic mechanical response, which is crucial for predicting phase stability, pressure-induced transitions, and modifications in the electronic structure. These polynomial fits provide a quantitative framework for understanding the interplay between crystal symmetry, bond strength, and lattice deformation under external pressure, offering fundamental insights into the thermomechanical behavior of In3Sc.
3.4. Elastic Properties and Mechanical Stability
The mechanical stability of crystalline solids is fundamentally linked to their elastic constants C
ij and elastic compliance constants S
ij, which describe the lattice’s response to small deformations. According to Born (1954) [
45], the lattice is mechanically stable if the strain energy is positive for any infinitesimal distortion. Compliance constants, being inverses of C
ij, offer additional insight into the magnitude of strain produced under applied stress: lower S
ij values correspond to stiffer, less deformable lattices.
For cubic crystals (SG 221 and SG 225), mechanical stability is assessed using the Born criteria [
46]:
These conditions physically correspond to resistance to shear along [110], volumetric compression, and tetragonal shear.
Table 3 shows that SG 221 (AuCu
3) satisfies all three conditions with
C11 = 113.83 GPa,
C12 = 40.083, and C
44 = 33.25 GPa. Our calculated elastic constants C
11, C
12, and C
44 are systematically higher than those reported in theoretical work [
46], with the largest discrepancy in C
44 (+79%). Both sets of values fulfill the Born stability criteria, confirming mechanical stability in agreement with Ref. [
39]. However, our results predict a larger bulk and shear modulus and a Zener anisotropy closer to unity, indicating a stiffer and more isotropic elastic response than published in Ref. [
39]. The positive difference
C11 −
C12 = 73.75 GPa confirms strong shear resistance, while
C44 ensures stability under tetragonal distortions. The corresponding compliance constants
S11 = 0.01076 GPa
−1 and
S12 = −0.0028 GPa
−1 (
Table 4) indicate low strain per unit stress, confirming a stiff and mechanically stable lattice.
In contrast, SG 225 (BiF3) violates the first criterion (C11 − C12 = −42.49 GPa), indicating mechanical instability. Its compliance constants (S11 = 0.01349, S12 = 0.01005 GPa−1) are comparatively high, showing that even small stresses lead to large strains, consistent with the observed lattice instability.
Tetragonal crystals (SG 139) require additional criteria for mechanical stability [
45]:
As shown in
Table 3, SG 139 (Al
3Ti) satisfies all these conditions with C
11 = 109. GPa, C
12 = 52.64 GPa, C
13 = 35.45 GPa, C
33 = 114.90, C
44 = 37.20 GPa, and C
66 = 57.47 GPa. High C66 indicates strong basal-plane shear resistance, reflecting the anisotropic bonding network in the tetragonal structure. The compliance constants S
11 = 0.01244, S
12 = −0.00529, and S
33 = 0.01006 GPa
−1 (
Table 4) suggest moderate anisotropy, with slightly higher compliance along the c-axis compared to the basal plane. Hexagonal (SG 194) lattices also require shear and axial constraints for stability [
46]:
Table 3 shows that SG 194 Ni
3Sc (Ni
3Sn) satisfies all criteria with C
11 = 87.39 GPa, C
12 = 47.73 GPa, C
13 = 52.76 GPa, C
33 = 76.11 GPa, C
44 = 22.61 GPa, and C
66 = 19.83 GPa. Lower values of C44 and C66 indicate moderate shear resistance, characteristic of ductile layered structures. Compliance constants S
11 = 0.02067, S
12 = −0.00454, and S
33 = 0.02864 GPa
−1 (
Table 4) confirm higher compliance along the c-axis, consistent with the structural anisotropy of hexagonal crystals.
3.5. Mechanical Properties and Elastic Moduli
The elastic properties also link directly to other macroscopic mechanical characteristics. For example, the bulk modulus (B) describes the resistance to uniform compression, while the shear modulus (G) reflects the resistance to shape change at constant volume. The ratio B/G is often used to infer ductility or brittleness following Pugh’s criterion [
47]: values greater than 1.75 indicate ductile behavior, while smaller values correspond to brittleness. The Young’s modulus (E) can be derived from B and G as E = 9BG/(3B + G) [
48], which quantifies the stiffness of the material under tensile loading. The Poisson’s ratio (ν) is obtained from B and G as ν = (3B − 2G)/(6B + 2G) [
48], describing the transverse strain response to axial loading. Additionally, the longitudinal P-wave modulus, P = B + 3/4G [
49], links the elastic constants to wave propagation, relevant in geophysical and high-pressure studies.
Table 5 presents the calculated bulk modulus (B), shear modulus (G), Young’s modulus (E), Poisson’s ratio (ν), P-wave modulus (P), and the B/G ratio using Voigt, Reuss, and Hill (average) approximations [
50]. These parameters are crucial for understanding the intrinsic resistance of the studied phases to external mechanical deformation.
Beyond the Pugh’s ratio, the ductility and brittleness of cubic, hexagonal, and tetragonal crystals can also be effectively evaluated using the Cauchy pressure (CP), which provides a complementary and physically meaningful insight into the nature of interatomic bonding. The Cauchy pressure is defined as C
12 − C
44 for cubic structures [
51,
52], and C
13 − C
44 and C
12 − C
66 for hexagonal and tetragonal structures [
53,
54,
55], respectively. A positive Cauchy pressure indicates metallic bonding and ductile behavior, whereas a negative value suggests directional covalent bonding and hence brittleness. For the cubic AuCu
3 (SG 221) structure, C
12 − C
44 = +6.83 GPa, indicating a positive Cauchy pressure and, consequently, a ductile nature. Similarly, the hexagonal Ni
3Sn (SG 194) phase yields C
13 − C
44 = +30.15 GPa and C
12 − C
66 = +27.90 GPa, both positive, reaffirming its metallic bonding and ductility. The cubic BiF
3 (SG 225) compound also exhibits a positive Cauchy pressure of C
12 − C
44 = +52.93 GPa, further confirming its ductile behavior despite its lower overall stiffness. In contrast, the tetragonal Al
3Ti (SG 139) structure presents negative Cauchy pressures, C
13 − C
44 = −1.75 GPa and C
12 − C
66 = −4.83 GPa, indicating directional covalent bonding and thus brittleness. These Cauchy pressure results are in excellent agreement with the Pugh’s ratio (B/G) values, where phases with B/G > 1.75 (such as AuCu
3, Ni
3Sn, and BiF
3) exhibit ductility, while Al
3Ti, with B/G < 1.75, remains brittle. The strong consistency between Cauchy pressure and Pugh’s ratio demonstrates the robustness of the present mechanical analysis and provides reliable insight into the bonding and deformation characteristics of the investigated In
3Sc compounds.
In the cubic system (SG 221), the bulk modulus (B) remains consistent across Voigt, Reuss, and Hill approximations [
47] with a value of 64.660 GPa, reflecting strong isotropic resistance to compression. The shear modulus (G) is calculated as 34.654 GPa on average, showing a good resistance to shape change under applied stress. Young’s modulus (E) is given as 88.204 GPa, indicating a relatively high stiffness of the phase against uniaxial deformation. The Poisson’s ratio (ν) of 0.273 is typical of materials with mixed ionic and metallic bonding, which implies that both central and angular forces play a role in its bonding characteristics. The P-wave modulus (P), averaging 110.865 GPa, further indicates robustness of elastic wave propagation along longitudinal directions. Importantly, the B/G ratio of 1.866 is greater than the critical threshold of 1.75, signifying that this cubic phase should exhibit ductile mechanical behavior according to Pugh’s criterion. For the tetragonal structure (SG 139), the bulk modulus (B) averages to 64.397 GPa, similar to the cubic case, which suggests comparable volumetric resistance. However, the shear modulus (G) is slightly higher at 39.295 GPa, reflecting enhanced rigidity against shear deformation compared to the cubic phase. Young’s modulus (E) reaches 97.951 GPa, indicating greater stiffness under uniaxial strain, consistent with the increased shear modulus. The Poisson’s ratio (ν) averages to 0.246, a lower value than that of the cubic system, implying stronger covalent-like bonding contributions in this phase. The P-wave modulus (P) averages at 116.790 GPa, which is the highest among the systems studied, signifying superior longitudinal wave propagation properties. The B/G ratio is 1.639, which is below the ductility threshold, suggesting that the tetragonal phase tends toward brittle behavior despite its relatively high stiffness.
In the hexagonal phase (SG 194), the bulk modulus is slightly lower than the cubic and tetragonal phases, with an average value of 61.874 GPa. More significantly, the shear modulus (G) is drastically reduced to 19.087 GPa, indicating poor resistance to shear deformations. This reduction is also reflected in the relatively low Young’s modulus (E) of 51.922 GPa, highlighting the softness and reduced stiffness of this phase compared to the cubic and tetragonal structures. The Poisson’s ratio (ν) is much higher, averaging 0.360, which suggests a bonding environment dominated by central forces and strong compressibility in the lateral directions. The P-wave modulus (P) is calculated at 87.323 GPa, lower than both cubic and tetragonal phases, consistent with the reduced stiffness. The B/G ratio is extremely high at 3.24, far above the ductility threshold, indicating a very ductile mechanical nature. However, such high ductility is often accompanied by weak shear rigidity, which limits structural stability under shear loads.
The cubic system with space group 225 exhibits unusual behavior. While the bulk modulus (B) is moderate at 50.474 GPa, the Voigt shear modulus (G) takes a negative value (−1.477 GPa), leading to a negative Young’s modulus (E = −4.476 GPa). This indicates mechanical instability within the Voigt framework, as negative shear and Young’s moduli are unphysical. However, the Reuss and Hill approximations correct this anomaly, giving positive values: G = 30.864 GPa (Reuss) and 14.693 GPa (Hill), with corresponding Young’s moduli of 76.914 GPa and 36.219 GPa, respectively. The Poisson’s ratio also varies significantly: 0.515 in Voigt (indicative of near-incompressibility), 0.246 in Reuss (normal covalent-ionic bonding range), and 0.380 in Hill (intermediate). The P-wave modulus ranges between 48.505 and 91.626 GPa, averaging 70.065 GPa, again reflecting intermediate mechanical stiffness. The B/G ratio is high (3.435 in Hill average), which suggests ductility; nevertheless, the presence of negative elastic constants in the Voigt approximation signals potential elastic instability and raises doubts about the reliability of this phase.
Fracture toughness (KIC) is a key mechanical parameter that quantifies a material’s ability to resist crack initiation and propagation, thus providing a direct measure of structural integrity and failure tolerance. For intermetallic compounds, where brittleness is a common limitation, evaluating KIC offers crucial insight into their potential for structural and high-performance applications. While parameters such as the bulk modulus (B), shear modulus (G), and Pugh’s ratio (B/G) can indirectly describe ductility, fracture toughness serves as a more comprehensive indicator of a material’s capacity to absorb and dissipate energy under stress. However, it is important to mention that this property cannot be obtained directly using only the elasticity of the material, since the fracture toughness depends on the energy required to break the bonds at the crack tip, which can involve plastic deformation and other mechanisms not accounted for by the elastic constants.
In this study, the fracture toughness of the investigated In
3Sc-based compounds was estimated using the empirical model proposed by Niu et al. [
56,
57,
58], defined as
where
is the equilibrium atomic volume, E is the Young’s modulus, and G is the shear modulus. This model effectively links the elastic behavior to fracture resistance, allowing accurate estimation of K
IC from first-principles data.
Using the calculated elastic parameters, the obtained fracture toughness values for the considered structures follow the order Al3Ti (SG 139) > AuCu3 (SG 221) > Ni3Sn (SG 194) > BiF3 (SG 225), corresponding approximately to 7.2, 6.8, 5.5, and 4.1 , respectively. The relatively high KIC value for Al3Ti reflects its superior resistance to crack propagation, consistent with its high shear and Young’s moduli. Similarly, AuCu3 exhibits significant fracture toughness, indicating good ductility and metallic bonding characteristics as supported by its positive Cauchy pressure. In contrast, Ni3Sn and particularly BiF3 display lower KIC values, signifying greater brittleness and a tendency toward crack propagation under mechanical stress.
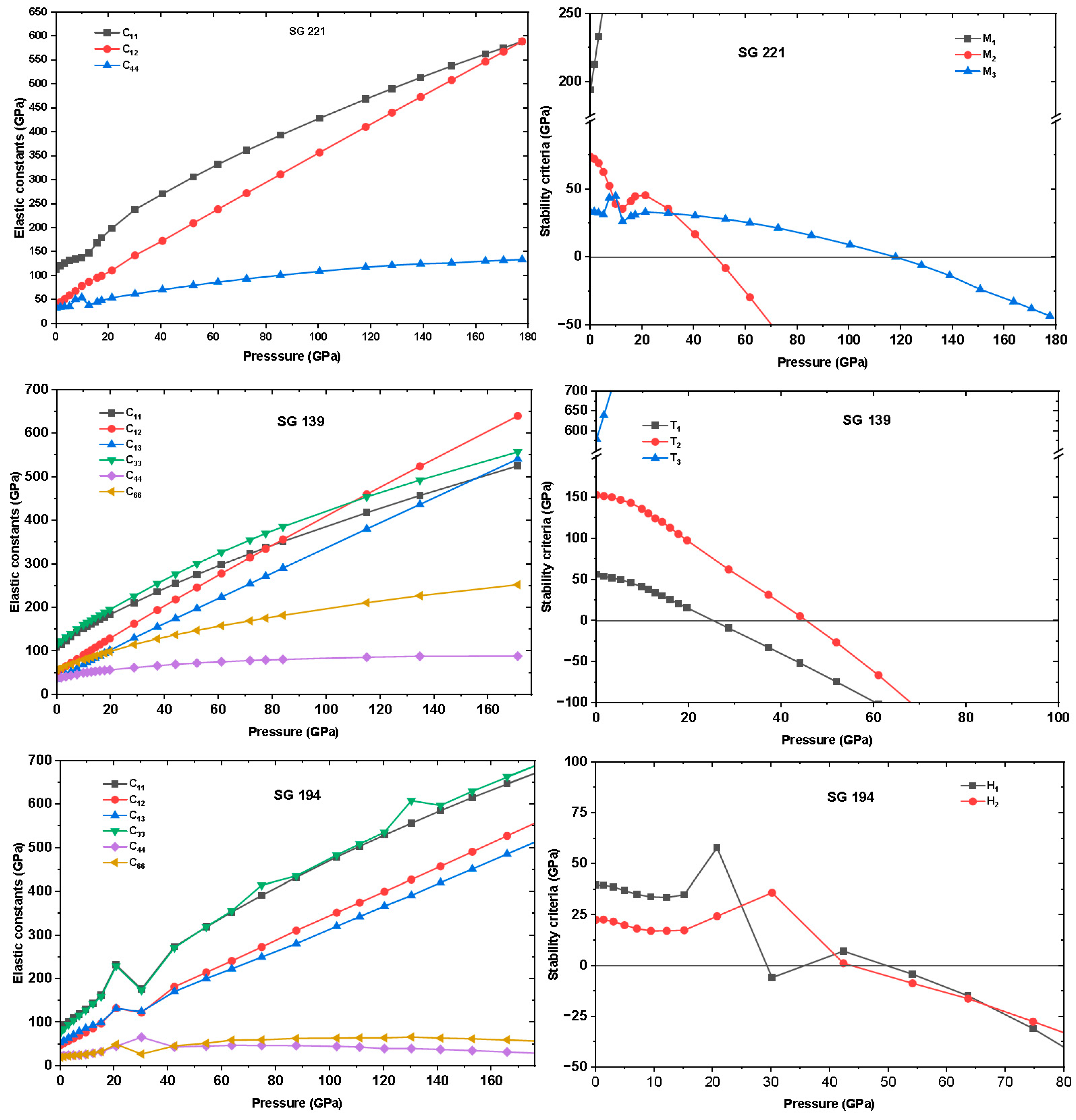
3.6. Elastic Constants and Mechanical Stability Under Pressure
Figure 4 presents the pressure dependence of the elastic constants and stability criteria for cubic, tetragonal, and hexagonal In
3Sc. In the cubic phase, the elastic constants C
11, C
12, and C
44 exhibit the expected monotonic stiffening with pressure. Both C
11 and C
12 increase steadily, reflecting enhanced resistance to longitudinal compression and interatomic bond shortening under hydrostatic loading. Similarly, C
44, associated with shear on {100} planes, remains positive and rises with pressure, suggesting robust shear rigidity at moderate compression. However, mechanical stability is not governed by these raw values but by their pressure-corrected forms:
,
, and
.
The cubic Born stability conditions are:
Up to ~50 GPa, all criteria are satisfied, ensuring elastic stability. Beyond ~52 GPa, however, the M2 criterion becomes negative despite the continued increase in C11 and C12. This shear-driven instability arises because the difference between longitudinal and transverse responses narrows, preventing the lattice from sustaining shear along ⟨110⟩ directions. Physically, this reflects a competition between bond-stretching and bond-bending forces: while bonds resist further compression, their ability to withstand shear collapses. Consequently, the cubic phase is stable only up to ≈49.09 GPa, beyond which it transforms into a lower-symmetry structure, likely tetragonal.
In the tetragonal phase, the response to compression is anisotropic due to inequivalent axial directions. Both C
11 and C
33 increase with pressure, though at different rates, revealing stronger stiffening in the basal plane than along the c-axis. The shear constants C
44 and C
66 remain positive and grow moderately, maintaining finite shear resistance in both axial and basal modes. The corresponding Born criteria are:
together with,
(α = 1, 3, 4, 6). At very low pressure, the T
2 condition is violated, indicating weak coupling between in-plane and out-of-plane compressions. As pressure increases, interlayer bonding strengthens, and above ~25.25 GPa, all stability criteria are fulfilled. This pressure-induced stabilization implies that the tetragonal distortion, initially unfavorable at low density, becomes energetically preferred once orbital overlap along the ccc-axis is enhanced. The wide stability range above this threshold suggests that the tetragonal structure acts as a pressure-stabilized intermediate between cubic and hexagonal phases.
The hexagonal phase shows a distinct elastic evolution governed by its directional bonding. The basal-plane stiffness (C
11, C
12) increases rapidly with compression, while the axial constant C
33 rises more moderately, highlighting strong anisotropy between in-plane and out-of-plane responses. The shear modulus C
44 remains positive throughout, confirming sustained rigidity against shear on prismatic planes. The Born criteria for hexagonal crystals are:
At low pressure (0–20 GPa), these criteria are satisfied, indicating that the layered network retains both axial and basal rigidity. However, near ~29.09 GPa, H1 and H2 simultaneously collapse due to the softening of C66 and excessive axial–basal coupling through C13, which signals a shear-driven instability of the basal planes coupled with axial strain. The persistent violation of the stability conditions suggests that the hexagonal lattice is not a true high-pressure host but instead a metastable configuration prone to collapse or transform once the critical shear-softening threshold is crossed.
3.7. Electronic Band Structures and Density of States of Cubic, Tetragonal, and Hexagonal In3Sc
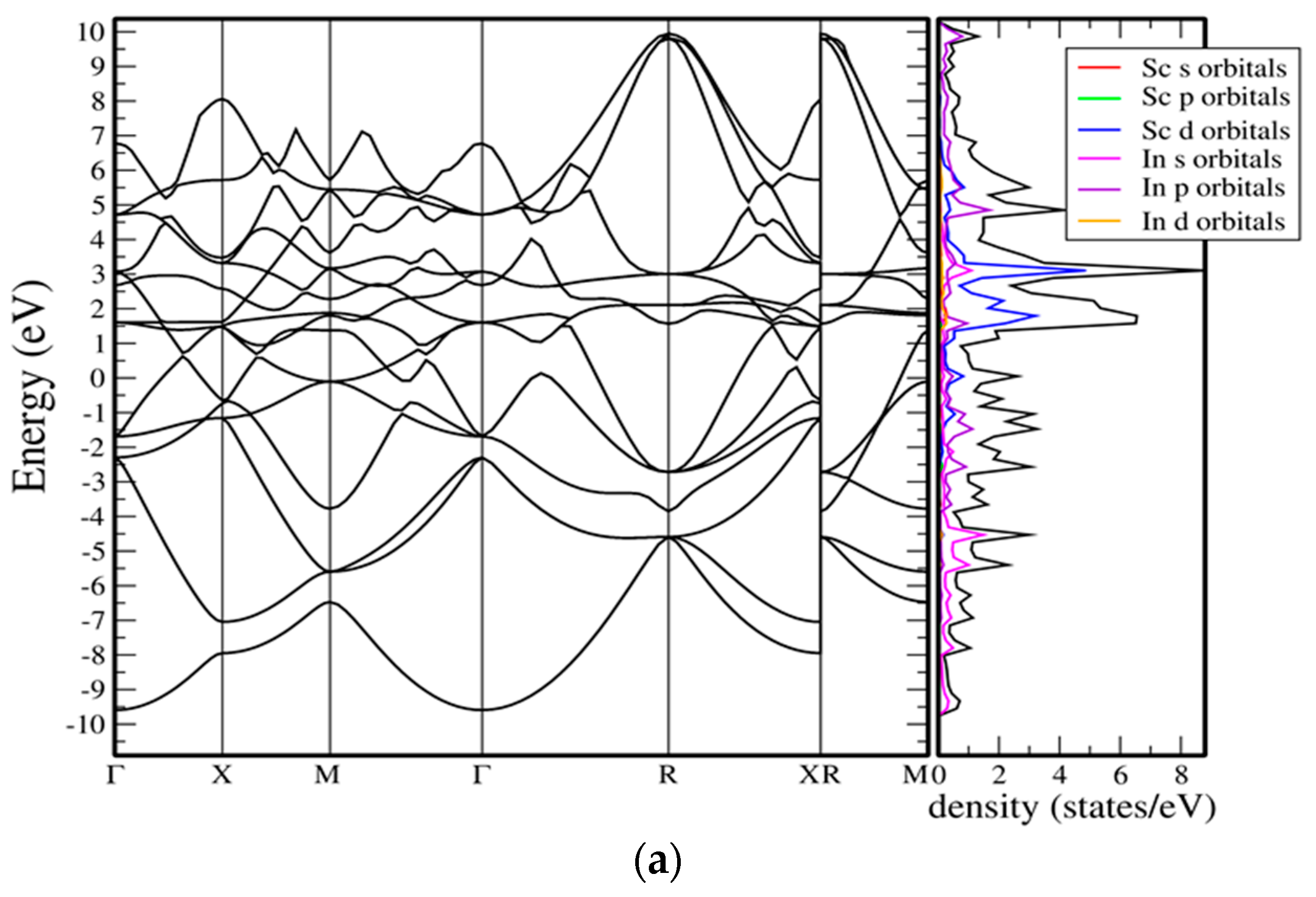
As shown in (
Figure 5a), the cubic Pm
m phase of In
3Sc exhibits a metallic band structure, characterized by multiple dispersive bands crossing the Fermi level along the high-symmetry directions. The conduction and valence bands overlap significantly, with no evidence of a gap, confirming metallicity. Near the Fermi level, the density of states (DOS) is dominated by hybridized In-5p and Sc-3d states. The Sc-d orbitals contribute sharp peaks in the vicinity of the Fermi level, reflecting relatively localized character, while the In-p orbitals form broader features that provide dispersive conduction channels. At lower energies (−5 to −8 eV), deeper In-s states are present, giving rise to pronounced DOS peaks well below the Fermi level. This orbital hybridization results in anisotropic conduction pathways, with the high DOS at
EF suggesting good metallic conductivity. The cubic symmetry ensures isotropic electronic dispersion, but the concentration of Sc-d states at the Fermi level may also predispose the phase to electron–phonon coupling effects, a factor relevant for mechanical softening and possible superconductivity.
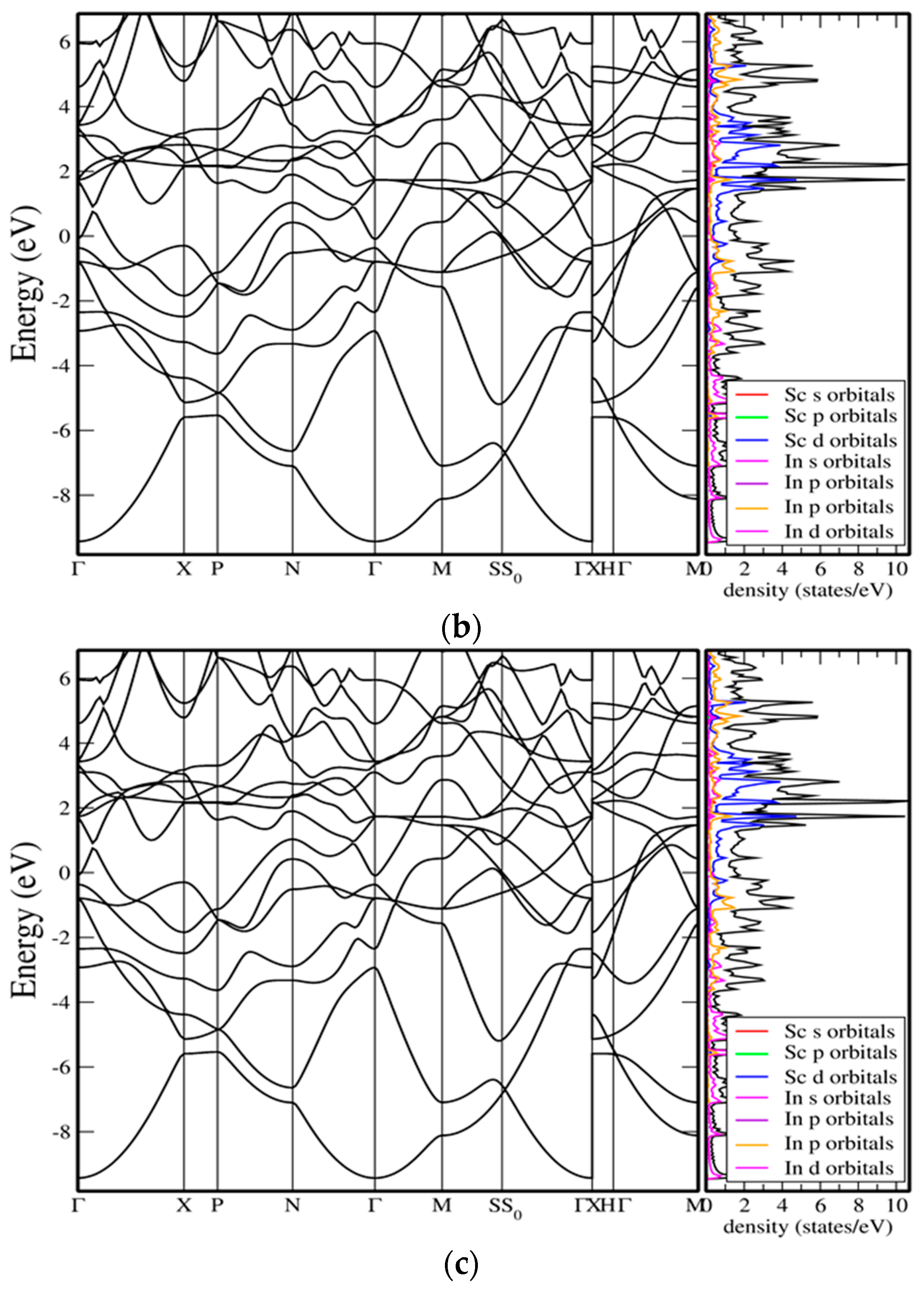
The electronic features of the tetragonal polymorph are illustrated in
Figure 5b. The phase preserves the metallic character of In
3Sc but introduces notable anisotropy into its electronic structure due to the inequivalence of in-plane and out-of-plane bonding. The band structure shows several bands crossing the Fermi level, but with reduced dispersion along the c-axis compared to the basal plane, indicating a lower carrier mobility in the out-of-plane direction. The DOS reveals that Sc-d states remain the dominant contribution near E
F, but the splitting of degeneracies compared to the cubic case results in a slightly reduced density at the Fermi level. This subtle decrease suggests that the tetragonal distortion partially lifts band overlap and redistributes electronic states across a broader energy window. In addition, the In-p orbitals continue to hybridize strongly with Sc-d states, producing broad features in the valence region and ensuring conduction remains metallic. From a physical standpoint, the tetragonal band structure indicates that while both cubic and tetragonal phases are nearly degenerate in enthalpy, the tetragonal polymorph may display direction-dependent transport properties, with enhanced in-plane conductivity relative to the c-axis. This anisotropy aligns with the structural distortion observed under pressure and points toward a pressure-stabilized metallic phase with anisotropic electronic behavior.
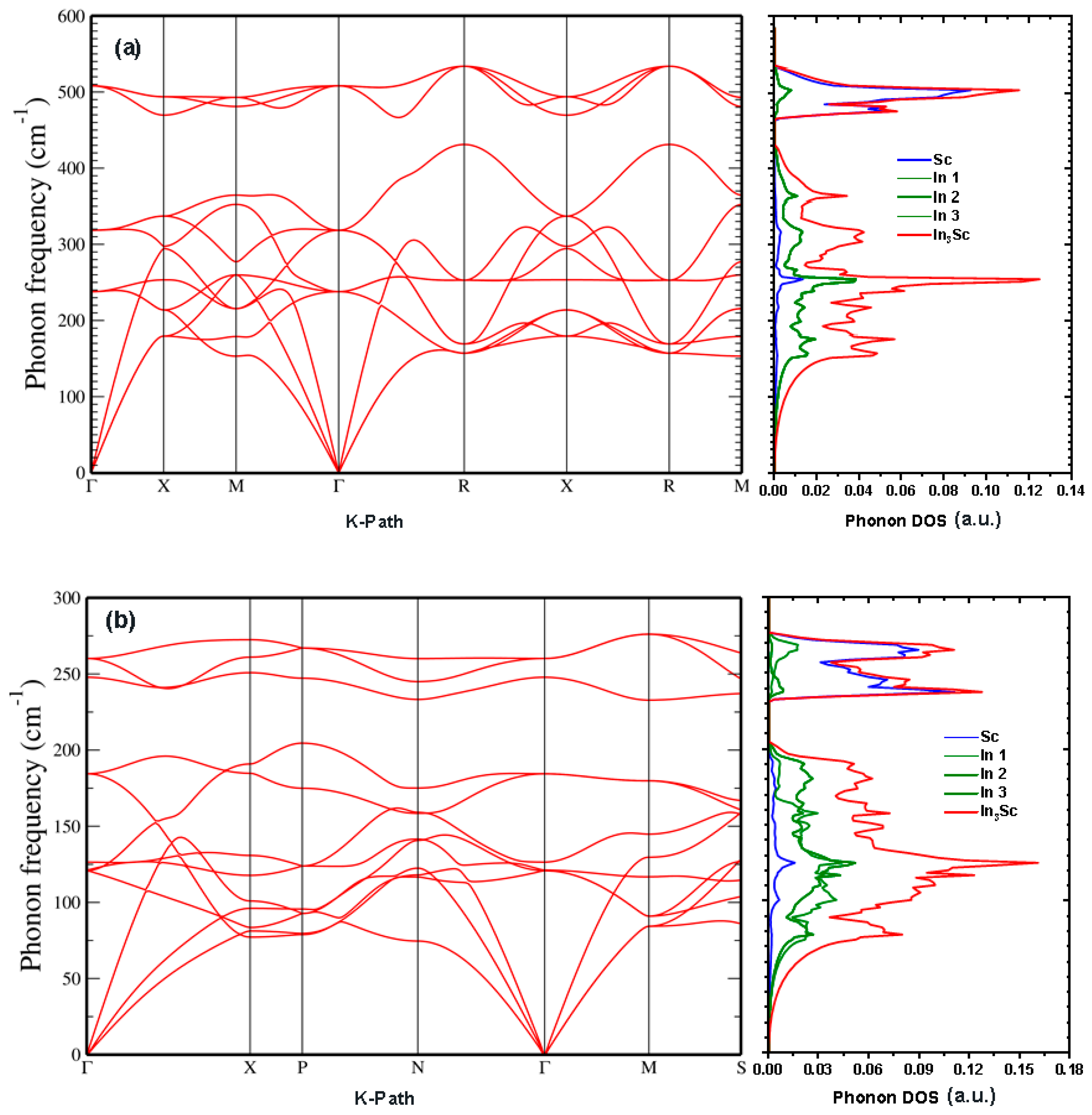
The band structure and DOS of the hexagonal phase are displayed in
Figure 5c. This phase shows the most distinct electronic features among the three polymorphs. Bands near the Fermi level are less dispersive than in the cubic or tetragonal phases, reflecting reduced electronic mobility and a stronger localization of carriers. The DOS confirms metallic behavior, with contributions at E
F arising predominantly from Sc-d orbitals. In this case, however, the hybridization with In-p orbitals is weaker and more anisotropic. A clear separation of orbital contributions is visible: In-s and In-p states dominate the deeper valence region, while Sc-d states cluster near the Fermi level. This orbital distribution leads to a narrower bandwidth and more localized conduction channels, consistent with the layered atomic arrangement of the hexagonal structure. Importantly, the DOS at E
F is lower than in both the cubic and tetragonal phases, implying that while metallicity persists, the phase may exhibit reduced electrical conductivity and weaker electron–phonon coupling. Physically, this behavior suggests that the hexagonal polymorph, although mechanically stable only over limited pressure ranges, is electronically less favorable for high carrier transport but may be stabilized by directional bonding that favors anisotropic conduction within the basal planes.
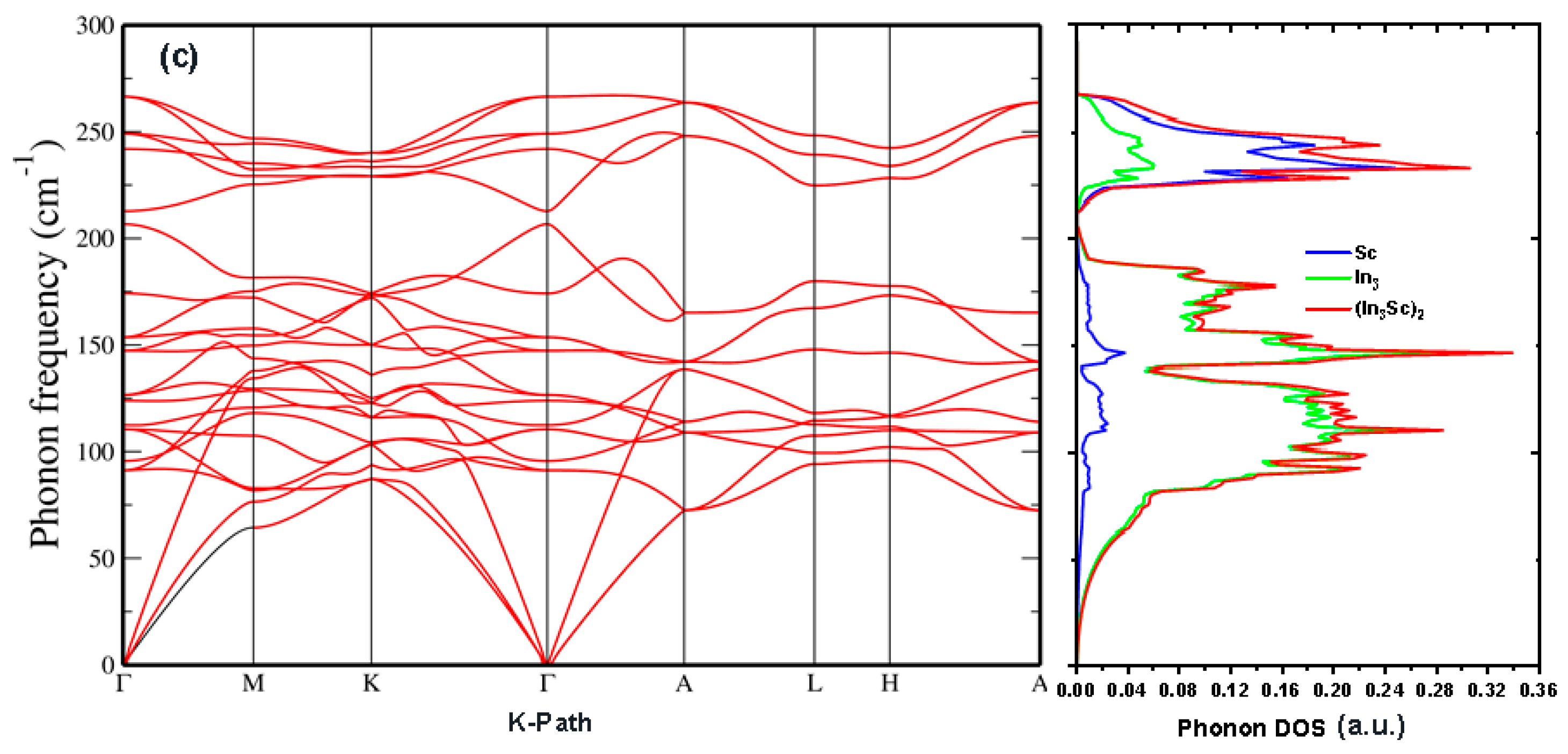
3.8. Phonon Stability and Pressure Evolution
Figure 6a–c presents the phonon spectra for cubic (SG 221), tetragonal (SG 139), and hexagonal (SG 194) phases of In
3Sc at high pressure, while (
Figure 7) and (
Table 6,
Table 7 and
Table 8) quantify the pressure evolution of individual phonon modes. A consistent feature across all phases is the absence of imaginary frequencies in the calculated dispersions, demonstrating that each structure is dynamically stable at very high compression (177.5 GPa for cubic, 17.7 GPa for tetragonal, and 16.9 GPa for hexagonal). Phonon gaps in the AuCu
3- and TiAl
3-type structures of In3Sc result from In and Sc mass differences and stronger ionicity, which split vibrational modes. In contrast, the Ni
3Sn-type structure, which has a different coordination number and weaker ionicity, exhibits a dispersion with a small gap.
In the cubic phase (SG 221), (
Table 6) shows that infrared-active T
2u and T
1u modes stiffen linearly with pressure (dω/dp = 1.4–3.1 cm
−1/GPa, γ ≈ 0.9).
Figure 7 confirms this trend, with all optic branches shifting to higher frequencies under compression. Interestingly, the Born criteria analysis indicated a shear-driven instability at ~52 GPa (violation of
M2), yet the phonon spectrum at 177.5 GPa (
Figure 6a) remains fully stable. This apparent discrepancy reflects the different sensitivities of elastic versus phonon probes: Born criteria test long-wavelength shear compliance, whereas phonon calculations assess harmonic stability across the entire Brillouin zone. The re-hardening of optic modes at very high compression suggests that short-range repulsion and orbital overlap restore lattice rigidity beyond the softening window, leading to re-entrant dynamical stability.
The tetragonal phase (SG 139) exhibits the strongest phonon hardening with pressure. As shown in
Table 7, A
2u and Eu modes have large pressure slopes (up to 3.8 cm
−1/GPa) and high Grüneisen parameters (γ ≈ 1.3–1.6), indicating strong anharmonic sensitivity to compression.
Figure 6b confirms the absence of imaginary phonons at 17.7 GPa, while (
Figure 7) shows steady stiffening across all Raman- and IR-active branches. Physically, this reflects the anisotropic bonding of the tetragonal lattice: in-plane and out-of-plane vibrations stiffen at different rates, but both remain dynamically stable. Together with the elastic results, these phonon spectra confirm that the tetragonal phase is a robust high-pressure polymorph stabilized by enhanced orbital overlap along the c-axis.
The hexagonal phase (SG194) shows a more complex vibrational response.
Table 8 reveals that Raman-active E
2g and A
1g modes have moderate slopes (dω/dp ≈ 0.9–2.3 cm
−1/GPa), while some silent modes, such as B
2g, exhibit stronger pressure dependence (≈3.0 cm
−1/GPa). Grüneisen parameters span a broad range (0.72–1.43), reflecting a mixture of stiff and relatively soft vibrational channels. Elastic stability analysis predicted a collapse of H
1 and H
2 around 30 GPa due to basal-plane shear softening. However, the phonon dispersion at 16.9 GPa (
Figure 6c) shows no imaginary frequencies, and (
Figure 7) confirms that all branches stiffen under pressure. This indicates that the hexagonal lattice undergoes a softening–recovery cycle: shear instabilities emerge in an intermediate window but are suppressed at extreme compression by strong short-range interactions. Thus, the hexagonal phase is dynamically stable at high pressure, though more pressure-sensitive than the tetragonal form.
The present study provides a comprehensive assessment of the polymorphic forms of In3Sc, elucidating their potential structural and technological applications. The AuCu3-type cubic phase (Pmm) exhibits high stability, isotropic compressibility, positive Cauchy pressure, and significant ductility (B/G > 1.75), indicating suitability for mechanically robust and electrically conductive applications, such as interconnects and contacts. The tetragonal Al3Ti-type phase, despite slight brittleness (negative Cauchy pressure, B/G < 1.75), demonstrates elevated fracture toughness and high shear and Young’s moduli, rendering it appropriate for load-bearing components requiring resistance to crack propagation. The hexagonal Ni3Sn-type phase features anisotropic compressibility, moderate ductility, and layered conduction pathways, suggesting applicability in pressure-sensitive electronics or components with direction-dependent transport properties. The BiF3-type cubic phase, although mechanically softer, retains high ductility and metallic character, potentially serving in low-stress, high-conductivity environments or as a precursor for pressure-induced phase transformations. Furthermore, pressure-dependent analyses indicate that specific polymorphs can be stabilized or tuned, enabling controlled modulation of electronic and mechanical behavior. Collectively, these findings underscore the versatility of In3Sc and provide a foundation for the design of phases with tailored mechanical, electronic, and functional properties.