Research on Synchronous Synthesis of Schwertmannite for Removal of Pb2+ from Acidic Wastewater
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Synthesis of Schwertmannite
2.3. Experimental Design
2.3.1. Initial Pb2+ Concentration Effect
2.3.2. Schwertmannite Dosage Effect
2.3.3. pH Dependence Study
2.3.4. Temperature Optimization
2.4. Analytical Methods for Lead Concentration
2.5. Characterization of Adsorbent
3. Results and Discussion
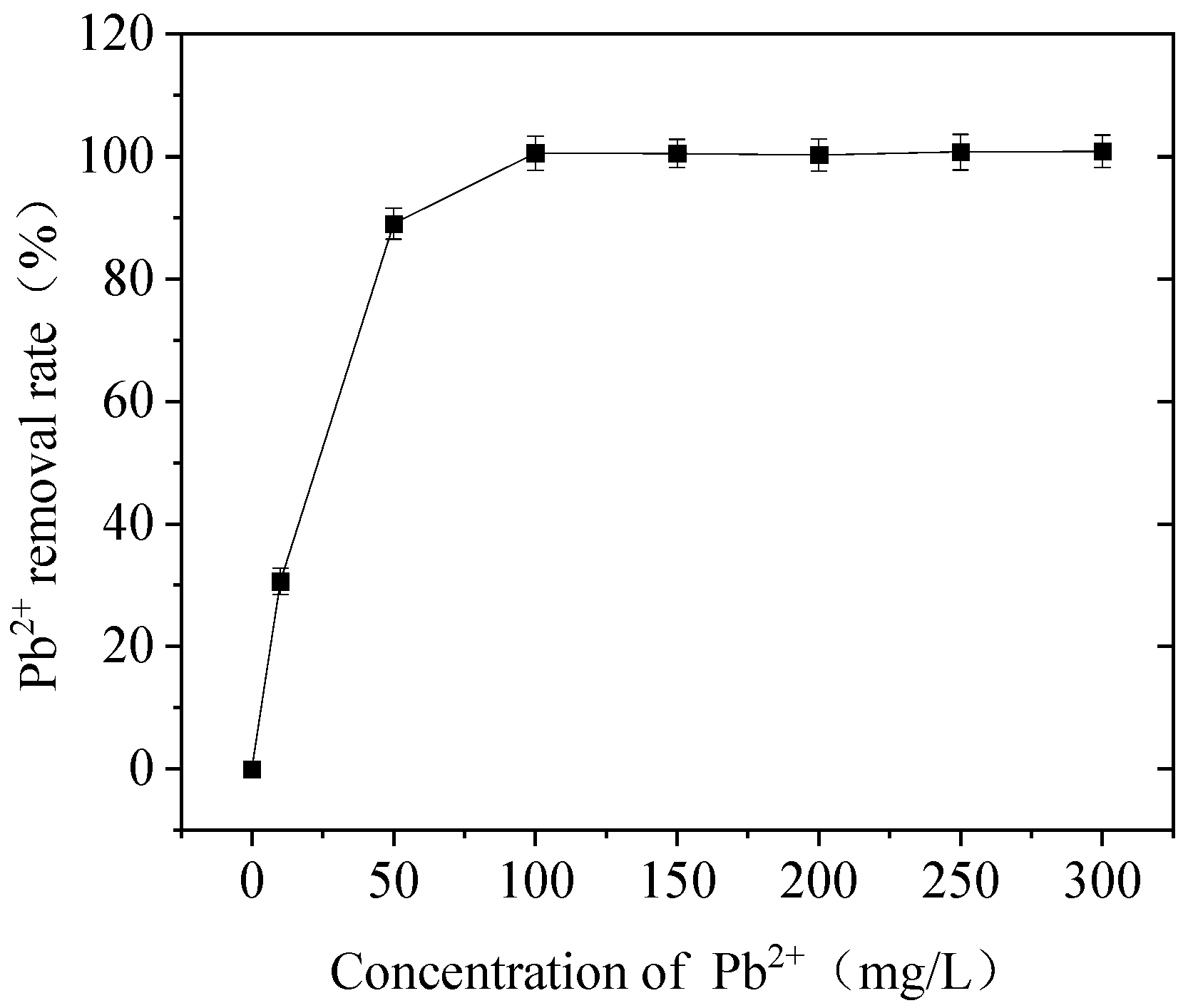
3.1. Effect of Pb Concentration
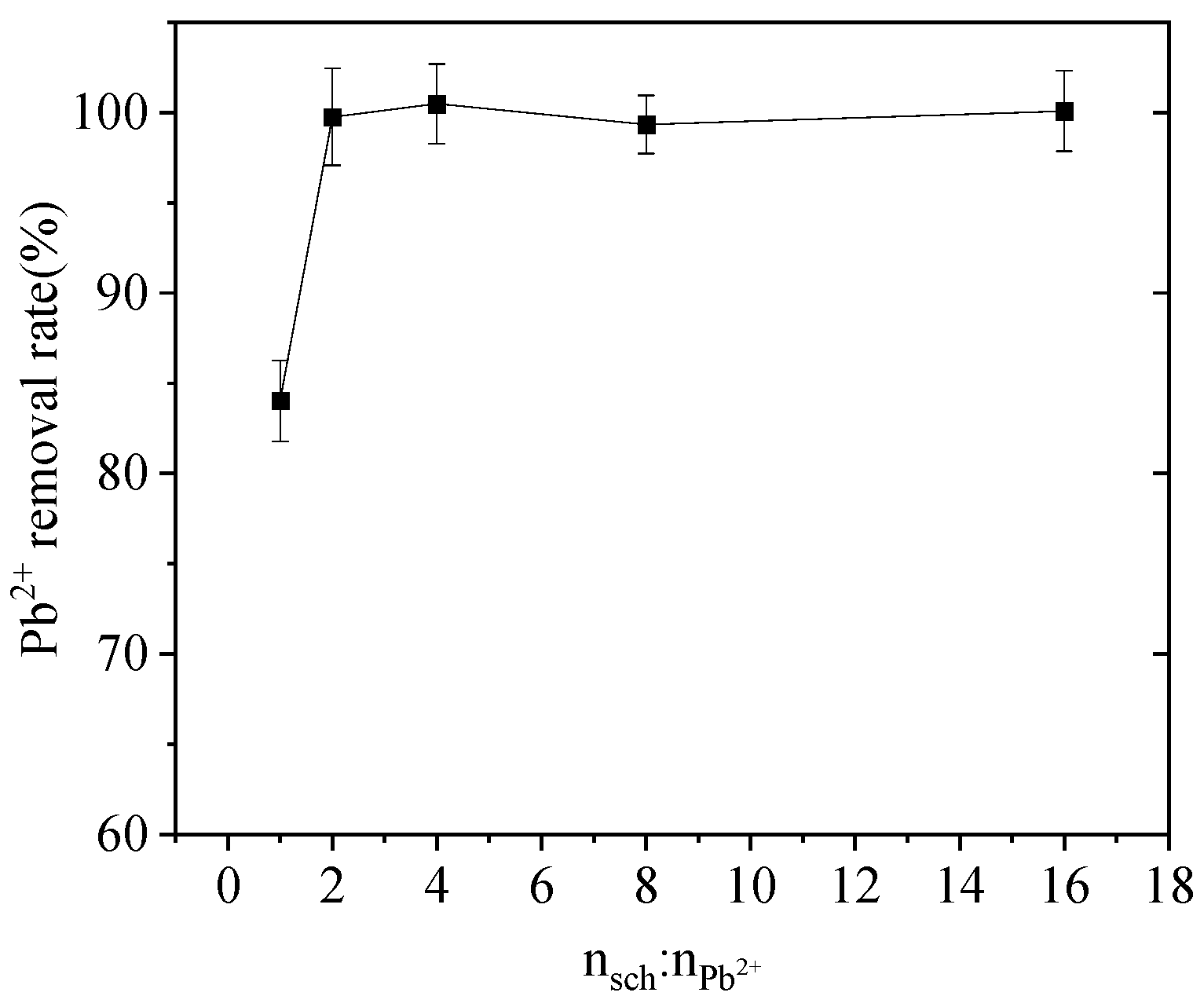
3.2. Effect of Schwertmannite Mass
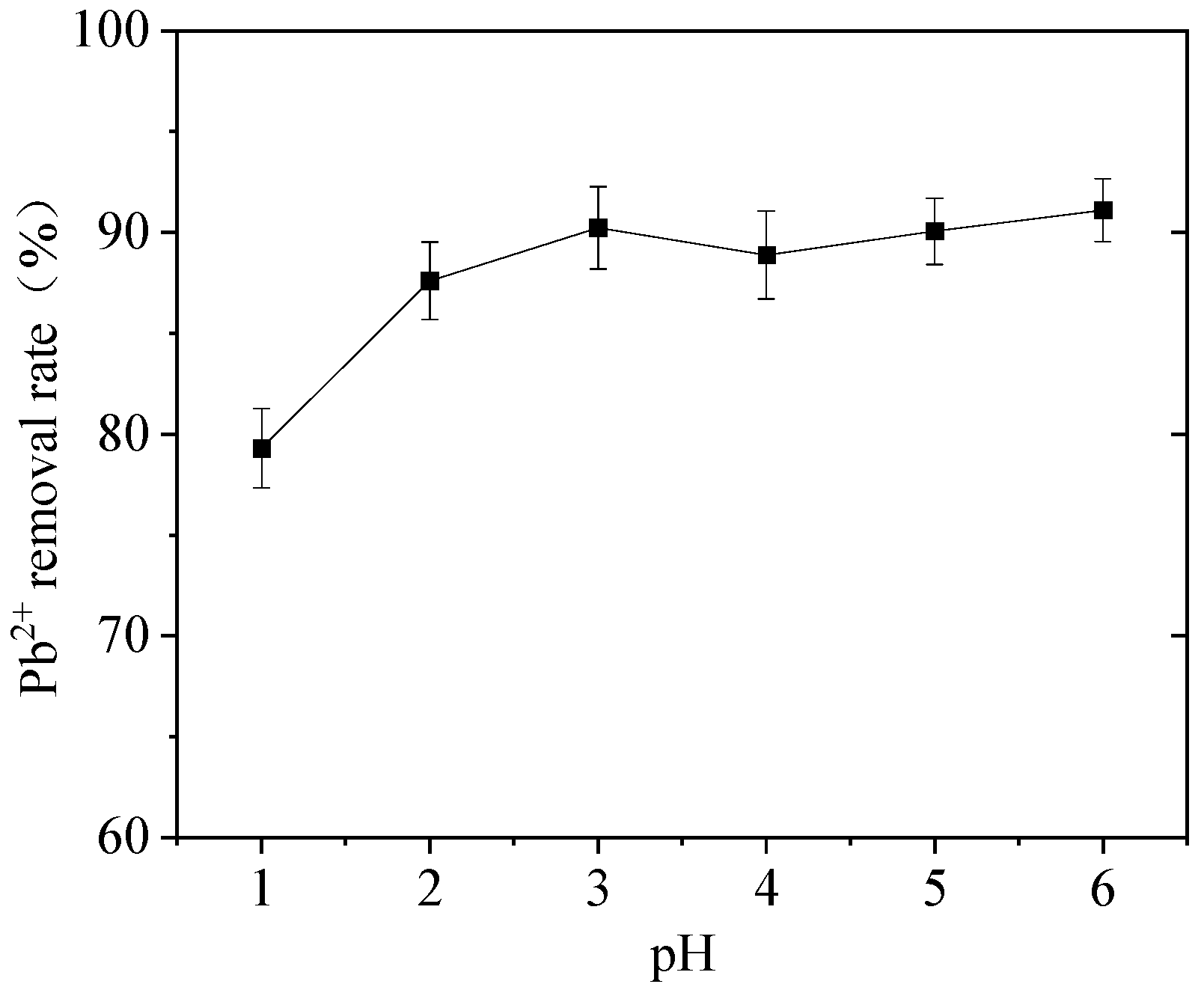
3.3. Effect of pH
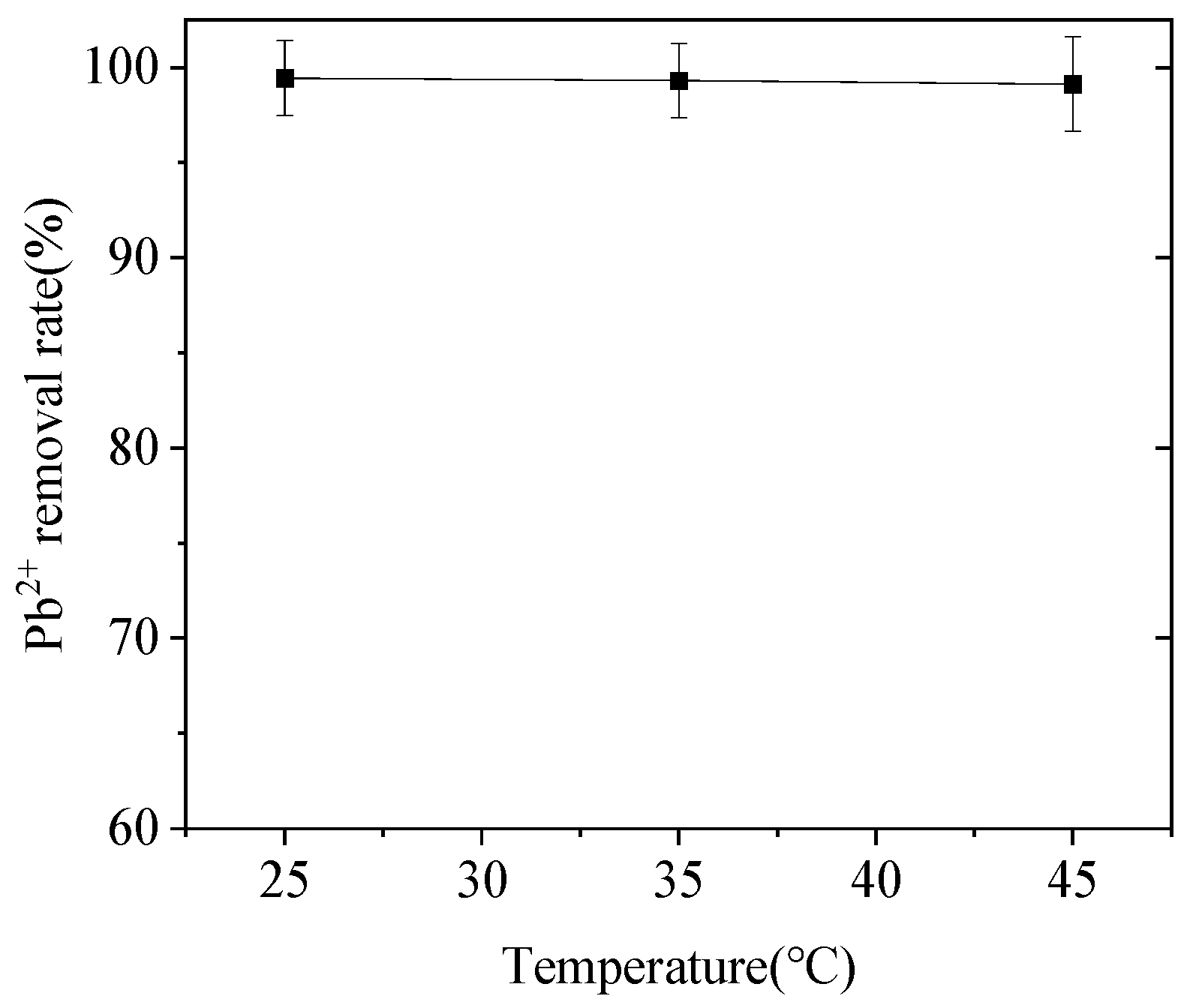
3.4. Effect of Temperature
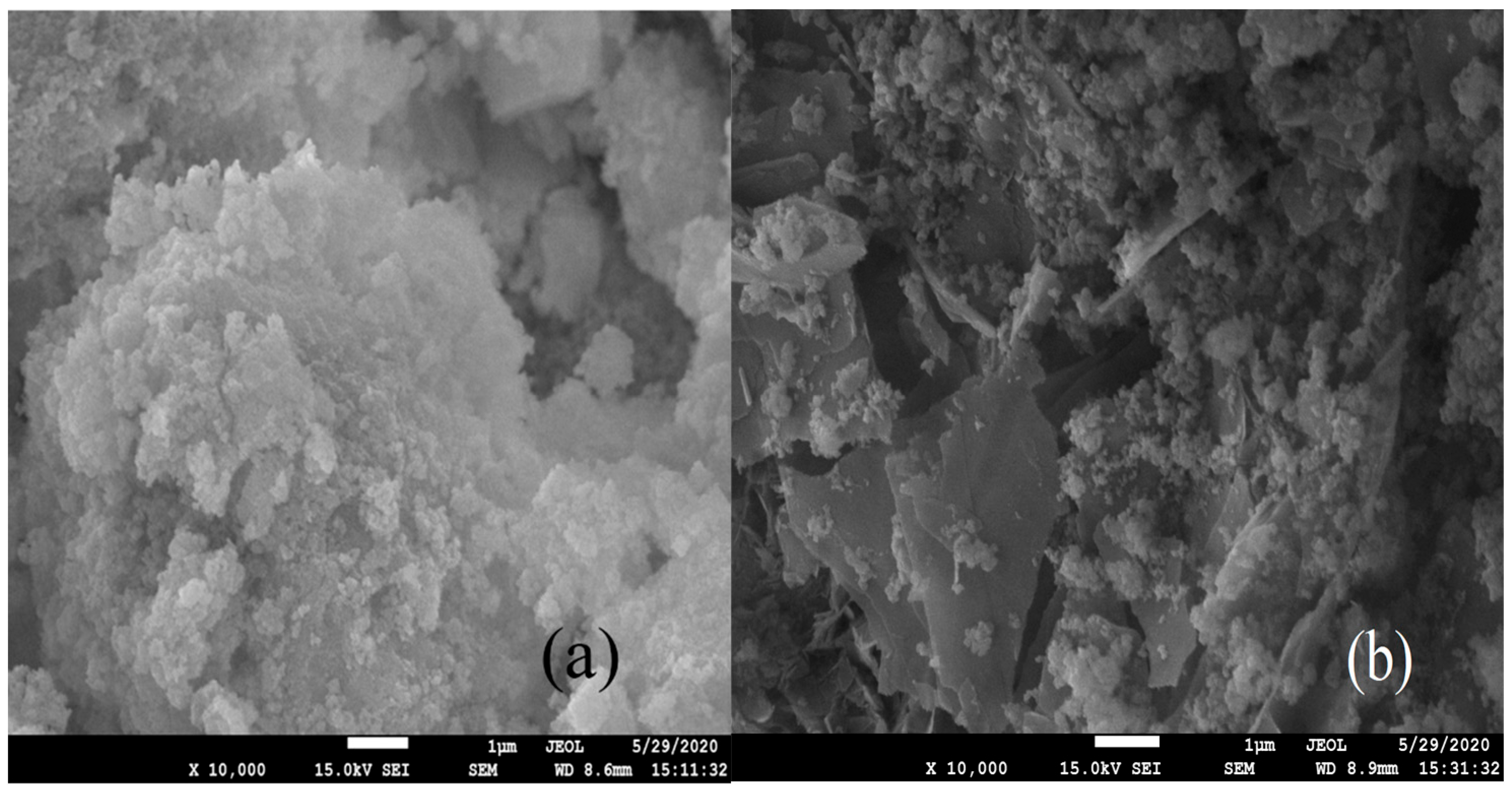
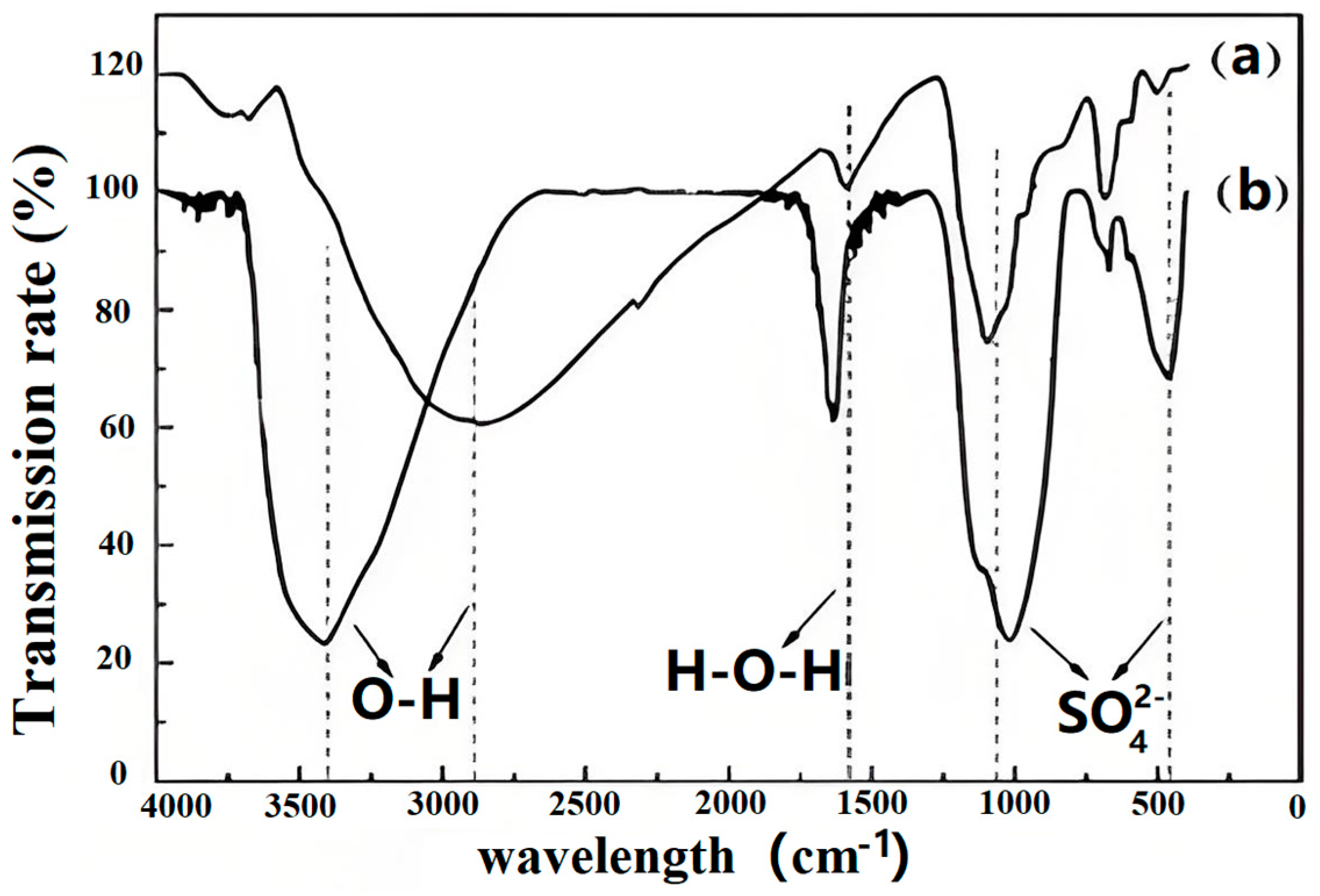
3.5. Microstructural Characterization of Schwertmannite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, J.; Ding, Z.; Bi, Y.; Li, J.; Wen, S.; Bai, S. Resource utilization of acid mine drainage (AMD): A review. Water 2022, 14, 2385. [Google Scholar] [CrossRef]
- Zheng, X.; Lu, Y.; Xu, J.; Geng, H.; Li, Y. Assessment of heavy metals leachability characteristics and associated risk in typical acid mine drainage (AMD)-contaminated river sediments from North China. J. Clean. Prod. 2023, 413, 137338. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K.; Oh, S. A critical review on lead removal from industrial wastewater: Recent advances and future outlook. J. Water Process Eng. 2022, 45, 102518. [Google Scholar] [CrossRef]
- Abbas, N.; Husnain, S.M.; Asim, U.; Shahzad, F.; Abbas, Y. A novel green synthesis of MnO2-Coal composite for rapid removal of silver and lead from wastewater. Water Res. 2024, 256, 121526. [Google Scholar] [CrossRef]
- Schoepfer, V.A.; Burton, E.D. Schwertmannite: A review of its occurrence, formation, structure, stability and interactions with oxyanions. Earth-Sci. Rev. 2021, 221, 103811. [Google Scholar] [CrossRef]
- Wang, X.; Fu, J.; Dong, Y.; Wang, L.; Zhou, L. Alkaline modification on schwertmannite promoted the simultaneous immobilization of arsenite and cadmium. Chem. Eng. J. 2023, 454, 140236. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Wu, S.; Dai, X.; Zhang, Y.; Feng, L.; Han, X. Efficient removal of arsenic and phosphate contaminants by diatomite-modified schwertmannite. J. Environ. Chem. Eng. 2022, 10, 108808. [Google Scholar] [CrossRef]
- Ke, C.; Deng, Y.; Zhang, S.; Ren, M.; Liu, B.; He, J.; Wu, R.; Dang, Z.; Guo, C. Sulfate availability drives the reductive transformation of schwertmannite by co-cultured iron-and sulfate-reducing bacteria. Sci. Total Environ. 2024, 906, 167690. [Google Scholar] [CrossRef]
- Rastegari, M.; Karimian, N.; Johnston, S.G. Doherty; S.J.; Hamilton, J.L.; Choppala, G.; Hosseinpour Moghaddam, M.; Burton, E.D. Antimony (V) incorporation into schwertmannite: Critical insights on antimony retention in acidic environments. Environ. Sci. Technol. 2022, 56, 17776–17784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Wang, L.; Hou, D. Biogeochemical interaction between thallium (Tl) and schwertmannite in acidic environment and the anti-dissolution mechanisms of Tl (I)-coprecipitated schwertmannite. J. Hazard. Mater. 2025, 484, 136764. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, C.; Zhang, L.; Liu, Y.; Wang, Y.; Li, X. Comparison of arsenate and arsenite removal behaviours and mechanisms from water by FeLa binary composite (hydr) oxides. J. Water Process Eng. 2024, 57, 104603. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, C.; Liu, Y.; Li, X.; Liu, Y.; Naidu, R.; Rahman, M.M. High efficiency of phosphate adsorption by yolk-shell Mn–La binary nanocomposites from aqueous solution: Adsorption and mechanism studies. J. Water Process Eng. 2025, 71, 107347. [Google Scholar] [CrossRef]
- Duan, J.; Chen, B.; Zhang, Y.; Cai, P.; Wang, F. Enhanced adsorption of Cr (VI) from aqueous solutions by CTAB-modified schwertmannite: Adsorption performance and mechanism. Chem. Eng. Res. Des. 2024, 208, 464–474. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, S.; Wei, Y.; Zhou, L. Schwertmannite modified with ethanol: A simple and feasible method for improving As (III) adsorption capacity. J. Environ. Chem. Eng. 2022, 10, 107412. [Google Scholar] [CrossRef]
- Fan, C.; Guo, C.; Chen, W.; Tao, L.; Yao, Q.; Lu, G.; Shen, Y.; Dang, Z. Chromate and phosphate adsorption on schwertmannite: Competition, mobilization and mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130691. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, Y.; Wang, S.; Zhu, S. Schwertmannite and akaganéite for adsorption removals of Cr (VI) from aqueous solutions. Environ. Sci. Pollut. Res. 2023, 30, 62295–62311. [Google Scholar] [CrossRef]
- Li, N.; Shi, M.; Lan, Y.; Zhang, H.; An, G.; Lin, S.; Xue, L. Efficacy and mechanism of copper removal from electroplating wastewater by schwertmannite-like mineral. J. Environ. Chem. Eng. 2024, 12, 112001. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, Y.; Hong, W.; Liu, J.; Zhu, H.; Liu, X.; Geng, Y.; Cai, Z.; Lin, S.; Ni, C. Mechanism of simultaneous lead and chromium removal from contaminated wastewater by a schwertmannite-like mineral. Environ. Sci. Pollut. Res. 2022, 29, 85364–85375. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Y.; Wu, S.; Dong, Y.; Zhou, B.; Liang, J.; Zhou, L. Synergetic interactions between zero-valent iron and schwertmannite for enhanced arsenic (III) removal: Role of morphological variations. Chem. Eng. J. 2023, 477, 146934. [Google Scholar] [CrossRef]
- Jiao, H.; Ge, X.; Wang, Q.; Rong, T.; Ruan, Z.; Li, G.; Xu, J.; Chang, X.; Lian, X.; Fang, Y. Solidification/Stabilization mechanisms of heavy metal ions in cemented paste backfill for green mine operations: A review. Int. J. Miner. Metall. Mater. 2025. [Google Scholar]
- Ji, Y.; Sun, W.; Shah, K.J.; Sun, Y. A review on application of artificial intelligence in the construction of environmental functional materials. Desalination Water Treat. 2025, 323, 101384. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Fu, J.; Zhang, Y.; Dong, Y.; Zheng, G.; Zhou, L. Enhancing coimmobilization capacity of schwertmannite for arsenic and cadmium through pH elevation after chemical oxidation. ACS EST Eng. 2024, 4, 409–418. [Google Scholar] [CrossRef]
- Carrero, S.; Fernandez-Martinez, A.; Pérez-López, R.; Cama, J.; Dejoie, C.; Nieto, J.M. Effects of aluminum incorporation on the schwertmannite structure and surface properties. Environ. Sci. Process. Impacts 2022, 24, 1383–1391. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Rzepa, G.; Bajda, T.; Wiśniewska, M.; Urban, T.; Kukowska, S.; Tomczyk, A.; Grygorczuk-Płaneta, K.; Kondracki, B. Aggregation mechanism of natural schwertmannite particles covered with two-component layers of high molecular weight tackifier and trace metal ions. J. Mol. Liq. 2022, 368, 120746. [Google Scholar] [CrossRef]
- Bari, A.S.M.F.; Choppala, G.; Lamb, D.; Hamilton, J.L.; Sathish, C.I.; Rahman, M.M.; Naidu, R.; Aughterson, R.; Burton, E.D. Is beudantite a stable host phase of arsenic and lead? New insights from molecular-scale kinetic analyses. J. Hazard. Mater. 2024, 480, 136382. [Google Scholar] [CrossRef]
- Paikaray, S. Environmental stability of schwertmannite: A review. Mine Water Environ. 2021, 40, 570–586. [Google Scholar] [CrossRef]
- Cruz-Hernández, P.; Carrero, S.; Pérez-López, R.; Fernandez-Martinez, A.; Lindsay, M.B.J.; Dejoie, C.; Nieto, J.M. Influence of As (V) on precipitation and transformation of schwertmannite in acid mine drainage-impacted waters. Eur. J. Mineral. 2019, 31, 237–245. [Google Scholar] [CrossRef]
- Liang, X.; Wei, G.; Xiong, J.; Tan, F.; He, H.; Qu, C.; Yin, H.; Zhu, J.; Zhu, R.; Qin, Z.; et al. Adsorption isotherm, mechanism, and geometry of Pb (II) on magnetites substituted with transition metals. Chem. Geol. 2017, 470, 132–140. [Google Scholar] [CrossRef]
- Liu, X.; Xue, W.; Zhang, Z.; Zhou, W.; Song, S.; Li, Y.; Benzaazoua, M.; Hu, X. Effect of cobalt isomorphic substitution on the properties of goethite and the adsorption of lead. Front. Environ. Sci. 2023, 11, 1186147. [Google Scholar] [CrossRef]
- Wang, H.; Lin, T.; Song, Z.; Huang, M.; Chai, R.; An, S.; Song, Y.F. Simultaneous mineralization of Cd (II), Pb (II) and As (V) using MgAl-NO3: Performance and mechanism. Sep. Purif. Technol. 2025, 362, 131853. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Fu, S.; Zhang, H.; Wu, X.; Han, J.; Ma, X.; Rong, J.; Chen, S.; Chen, G.; Li, Y.; et al. Research on Synchronous Synthesis of Schwertmannite for Removal of Pb2+ from Acidic Wastewater. Crystals 2025, 15, 929. https://doi.org/10.3390/cryst15110929
Zhu H, Fu S, Zhang H, Wu X, Han J, Ma X, Rong J, Chen S, Chen G, Li Y, et al. Research on Synchronous Synthesis of Schwertmannite for Removal of Pb2+ from Acidic Wastewater. Crystals. 2025; 15(11):929. https://doi.org/10.3390/cryst15110929
Chicago/Turabian StyleZhu, Huijie, Shuai Fu, Huiyong Zhang, Xi Wu, Jinyi Han, Xiaolin Ma, Jingtao Rong, Sixu Chen, Guang Chen, Yuxiang Li, and et al. 2025. "Research on Synchronous Synthesis of Schwertmannite for Removal of Pb2+ from Acidic Wastewater" Crystals 15, no. 11: 929. https://doi.org/10.3390/cryst15110929
APA StyleZhu, H., Fu, S., Zhang, H., Wu, X., Han, J., Ma, X., Rong, J., Chen, S., Chen, G., Li, Y., Man, J., & Ma, Z. (2025). Research on Synchronous Synthesis of Schwertmannite for Removal of Pb2+ from Acidic Wastewater. Crystals, 15(11), 929. https://doi.org/10.3390/cryst15110929





