Behavior of Formaldehyde Adsorption on ZnO [1011] Facets: A DFT Study
Abstract
1. Introduction
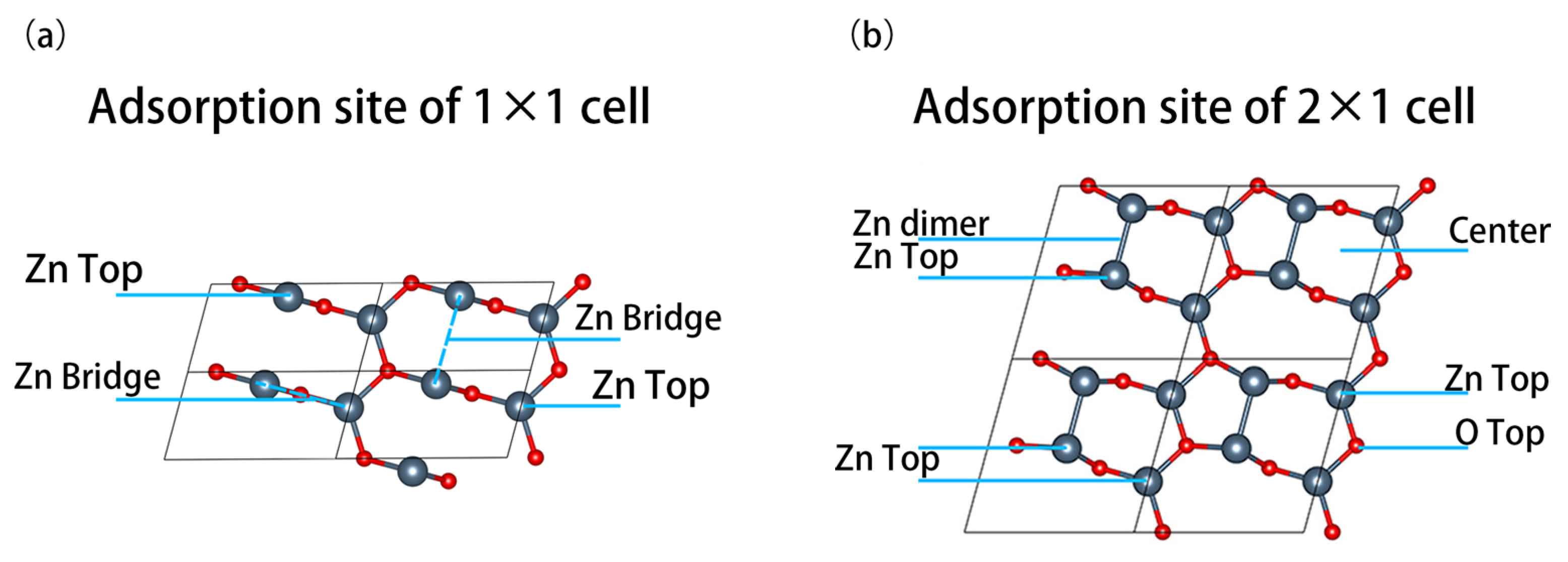
2. Computational Models and Methods
3. Results and Discussion
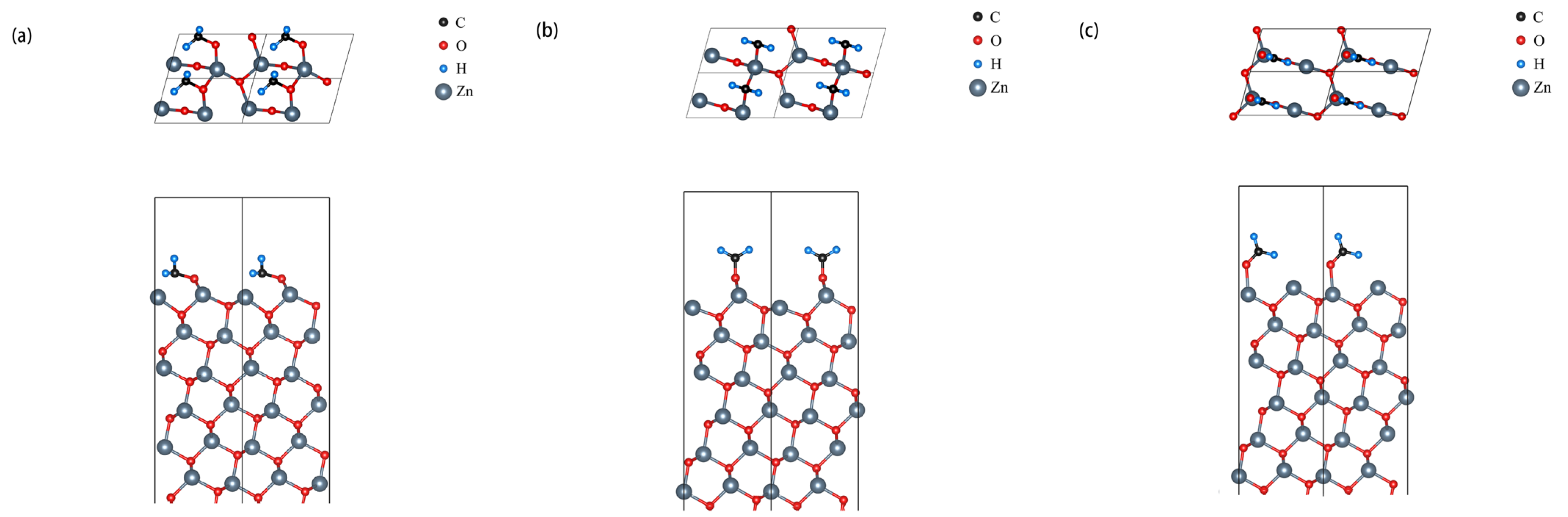
3.1. 1 ML Adsorption Behavior Study
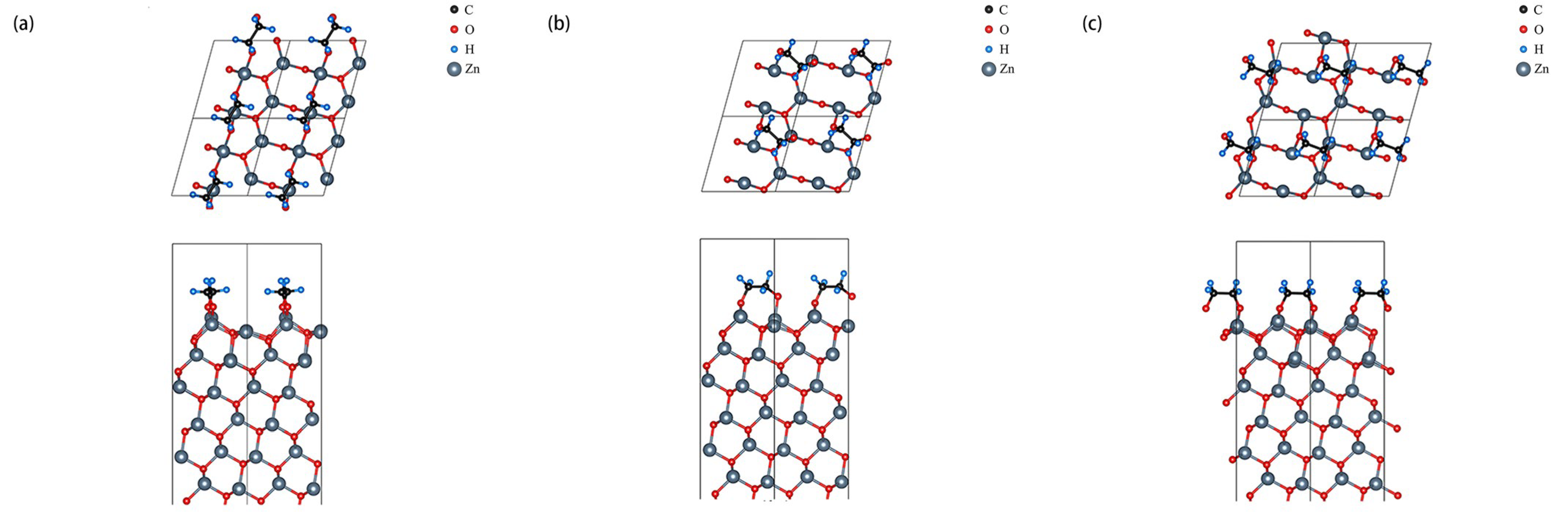
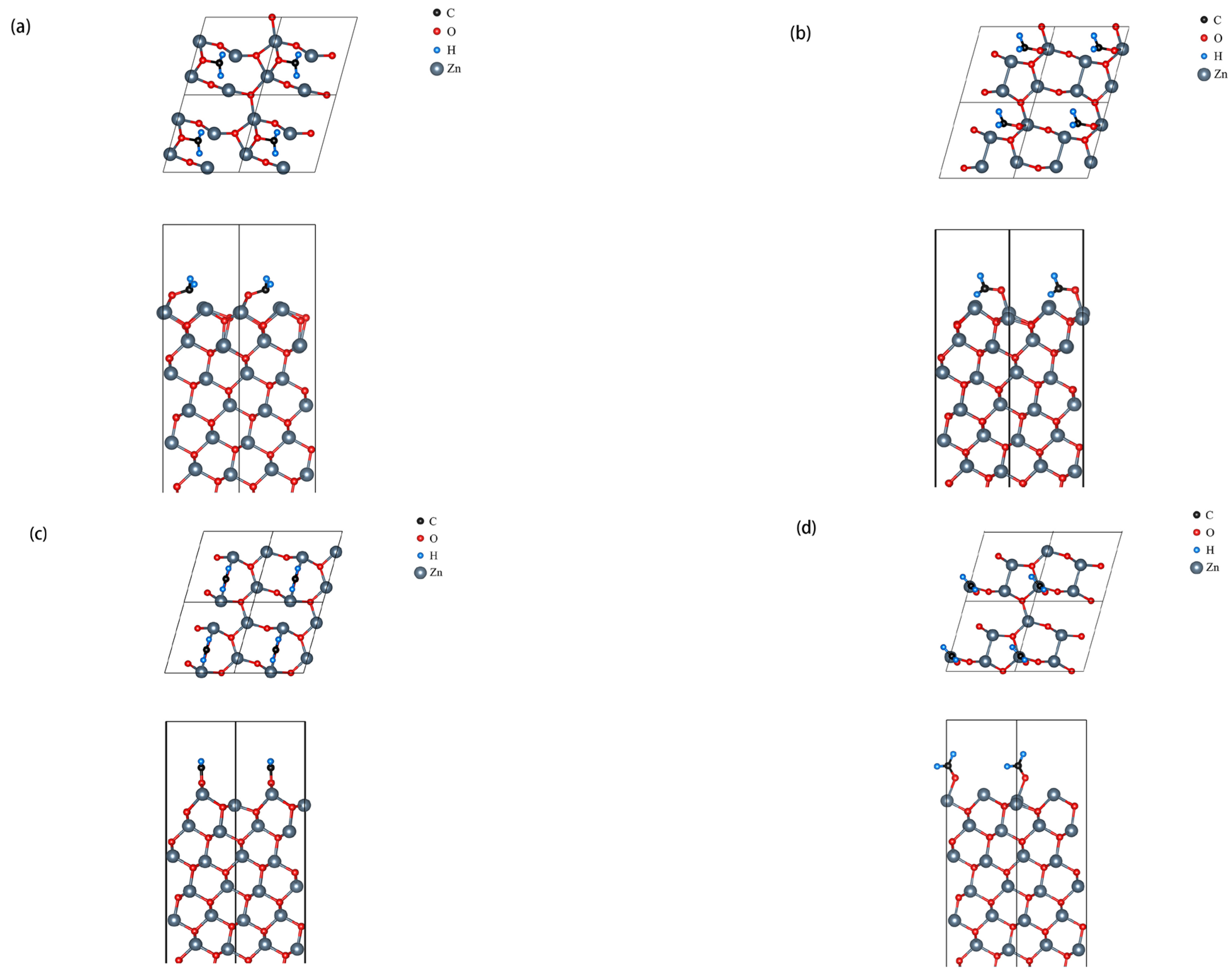
3.2. 0.5 ML Adsorption Behavior Study
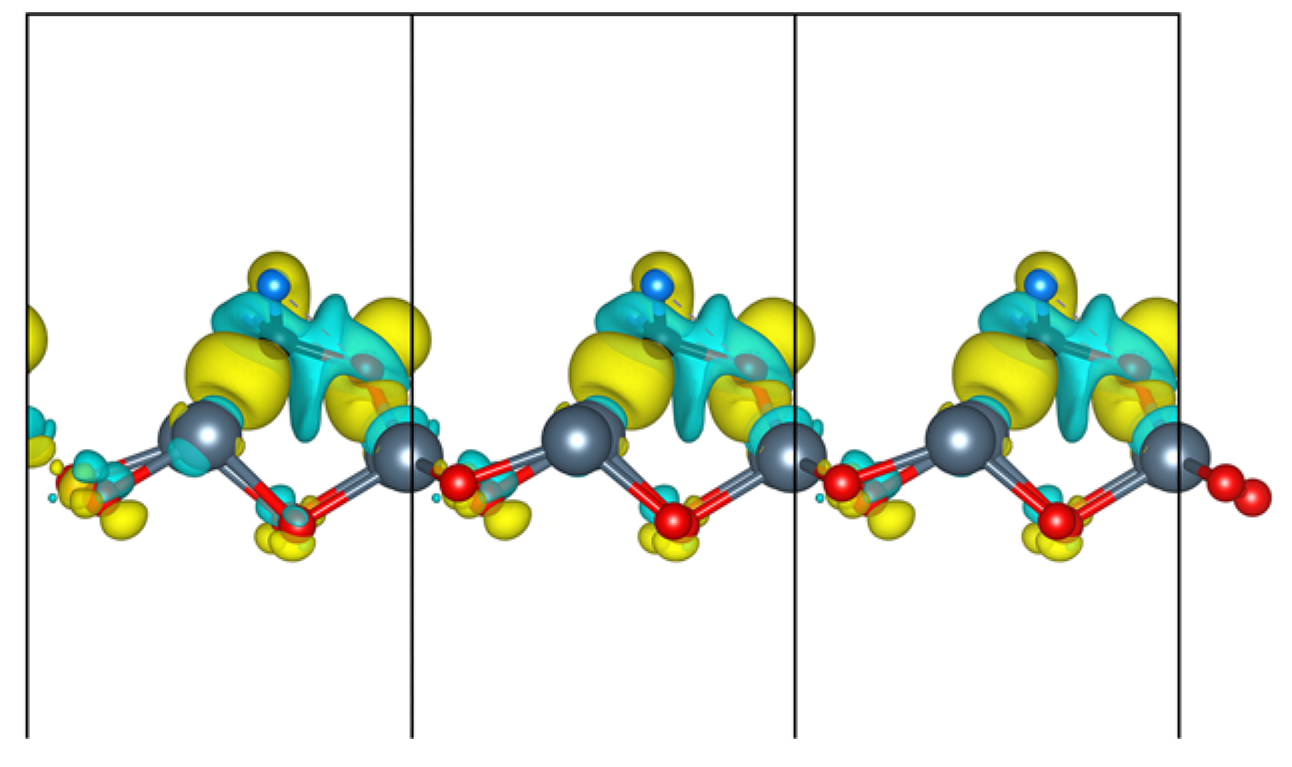
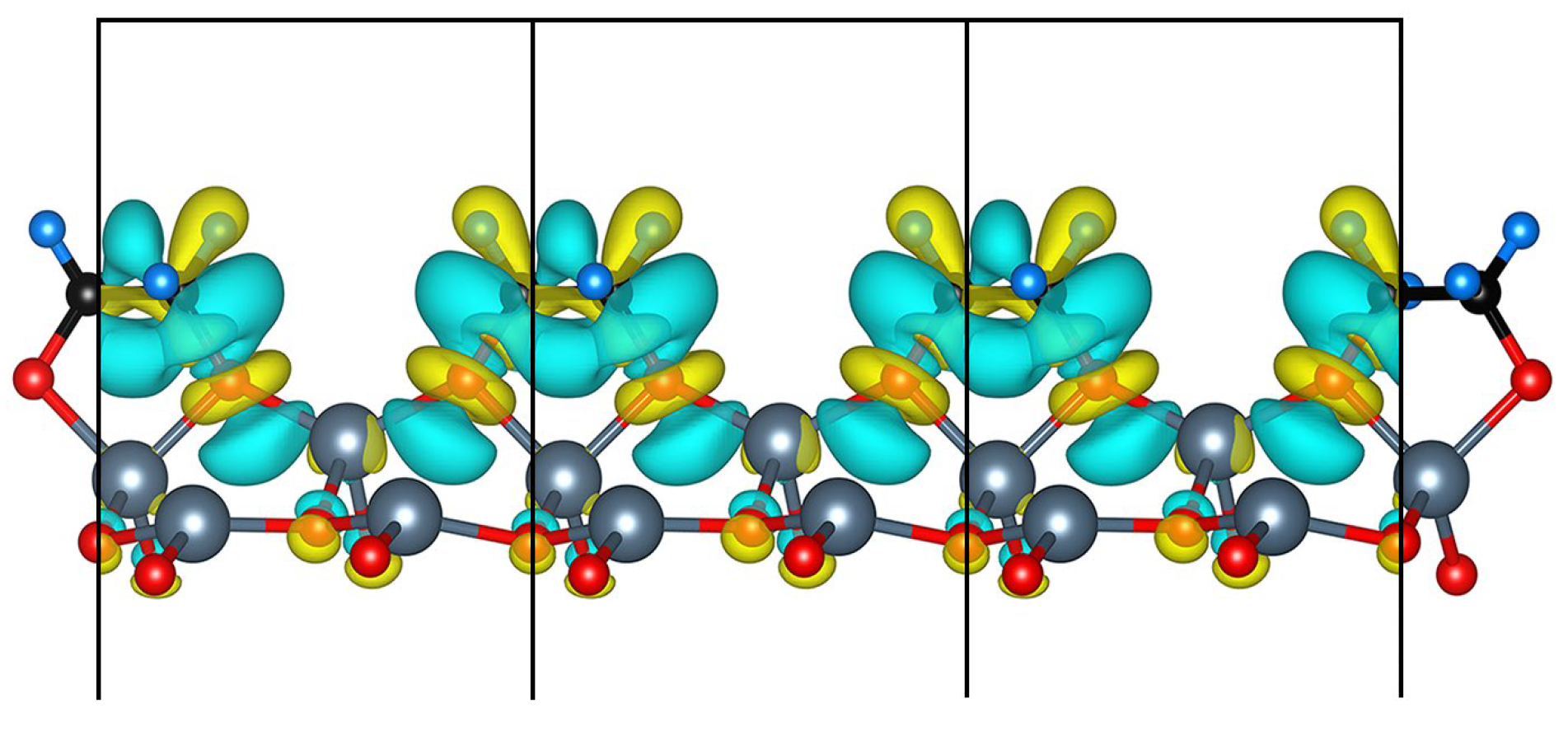
3.3. Electronic Property
3.3.1. Band Structure
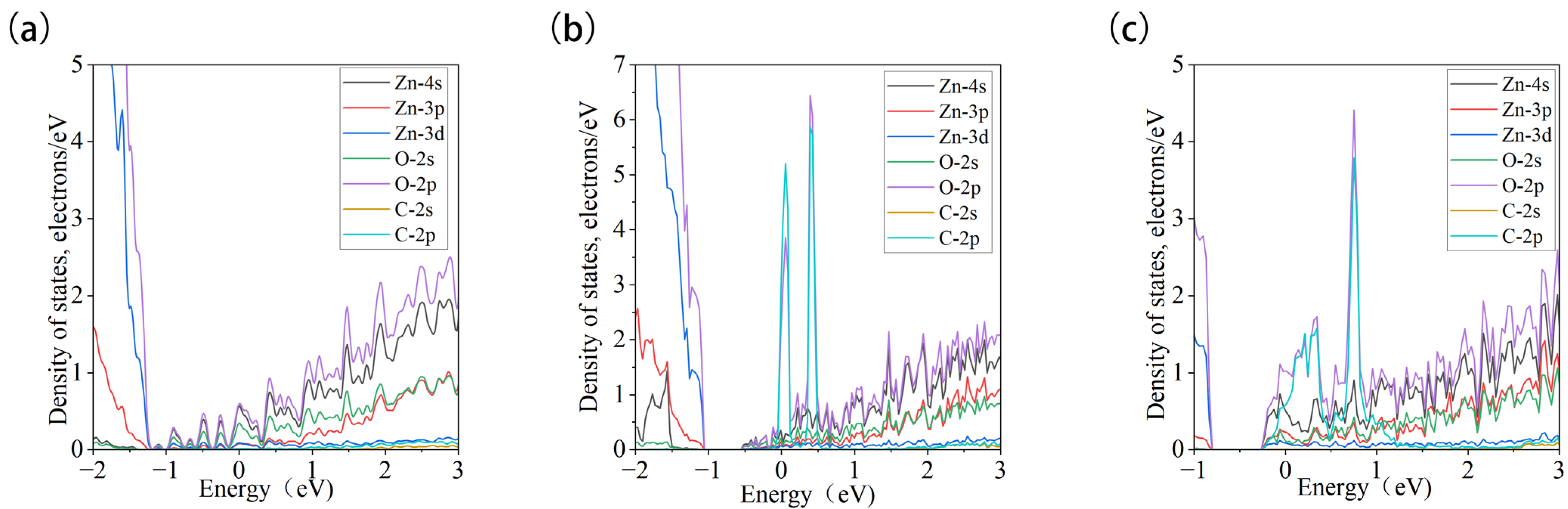
3.3.2. Projected Density of States
4. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benedict, B.; Kristensen, S.M.; Duxin, J.P. What Are the DNA Lesions Underlying Formaldehyde Toxicity? DNA Repair 2024, 138, 103667. [Google Scholar] [CrossRef]
- Rose, J.J.; Wang, L.; Xu, Q.; McTiernan, C.F.; Shiva, S.; Tejero, J.; Gladwin, M.T. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am. J. Respir. Crit. Care Med. 2017, 195, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Adiguzel, Y.; Kulah, H. Breath Sensors for Lung Cancer Diagnosis. Biosens. Bioelectron. 2015, 65, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Mo, D.; Zhang, H.; Wang, Y.; Tuo, F.; Chen, M.; Cao, Z.; Xu, Y.; Lin, L.; Liang, X.; Mmereki, D.; et al. Formaldehyde Exposure and Associated Health Burdens Apportioned to Residential and Public Places Based on Personal and Environmental Measurements. Atmosphere 2025, 16, 1165. [Google Scholar] [CrossRef]
- Cicolella, A. Les Composés Organiques Volatils (COV): Définition, Classification et Propriétés. Rev. Des Mal. Res-Piratoires 2008, 25, 155–163. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, L.; Yang, H.M.; Zhang, H. Cystic Fibrosis Transmembrane Conductance Regulator-Associated Ligand Protects Dopaminergic Neurons by Differentially Regulating Metabotropic Glutamate Receptor 5 in the Progression of Neurotoxin 6-Hydroxydopamine-Induced Parkinson’s Disease Model. NeuroToxicology 2021, 84, 14–29. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Twarużek, M.; Kosicki, R.; Marchand, G.; Viegas, S. Bioburden in Health Care Centers: Is the Compliance with Portuguese Legislation Enough to Prevent and Control Infection? Build. Environ. 2019, 160, 106226. [Google Scholar] [CrossRef]
- Mo, D.; Zhang, H.; Wang, Y.; Liu, L.; Xu, Y.; Lin, L.; Liang, X.; Mmereki, D. Sources and Reactivity of Ambient VOCs on the Tibetan Plateau: Insights from a Multi-Site Campaign (2012–2014) for Assessing Decadal Change. Atmosphere 2025, 16, 1148. [Google Scholar] [CrossRef]
- Lee, S.; Yang, S.; Shim, W.-S.; Song, E.; Han, S.; Park, S.-S.; Choi, S.; Joo, S.H.; Park, S.J.; Shin, B.; et al. Development and Validation of an Improved HPLC-MS/MS Method for Quantifying Total and Unbound Lenalidomide in Human Plasma. Pharmaceutics 2024, 16, 1340. [Google Scholar] [CrossRef]
- Witkiewicz, Z.; Wardencki, W. Mobile Gas Chromatographs Coupled with Mass and Ion Mobility Spectrometers and Their Applications. Ecol. Chem. Eng. S 2021, 28, 29–37. [Google Scholar] [CrossRef]
- Yamazoe, N. Toward Innovations of Gas Sensor Technology. Sens. Actuators B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Shlenkevitch, D.; Avraham, M.; Stolyarova, S.; Blank, T.; Nemirovsky, Y. Catalytic Gas Sensor Based on Micro Machined CMOS Transistor. In Proceedings of the 2019 IEEE International Conference on Microwaves, Antennas, Communications and Electronic Systems, COMCAS, Tel-Aviv, Israel, 4–6 November 2019. [Google Scholar]
- Hassan, M.M.; Khan, W.; Mishra, P.; Islam, S.S.; Naqvi, A.H. Enhancement in Alcohol Vapor Sensitivity of Cr Doped ZnO Gas Sensor. Mater. Res. Bull. 2017, 93, 391–400. [Google Scholar] [CrossRef]
- Jiang, Z.; Cheng, B.; Zhang, L.; Zhang, Z.; Bie, C. A Review on ZnO-Based S-Scheme Heterojunction Photocatalysts. Chin. J. Catal. 2023, 52, 32–49. [Google Scholar] [CrossRef]
- Helal, H.; Ben Arbia, M.; Pakdel, H.; Zappa, D.; Benamara, Z.; Comini, E. Enhanced NO2 Detection in ZnO-Based FET Sensor: Charge Carrier Confinement in a Quantum Well for Superior Sensitivity and Selectivity. Chemosensors 2025, 13, 358. [Google Scholar] [CrossRef]
- Jiang, W.; Xia, Y.; Pan, A.; Luo, Y.; Su, Y.; Zhao, S.; Wang, T.; Zhao, L. Facet-Dependent Gas Adsorption Selectivity on ZnO: A DFT Study. Chemosensors 2022, 10, 436. [Google Scholar] [CrossRef]
- Wu, X.-L.; Liu, S.; Li, Y.; Yan, M.; Lin, H.; Chen, J.; Liu, S.; Wang, S.; Duan, X. Directional and Ultrafast Charge Transfer in Oxygen-Vacancy-Rich ZnO@Single-Atom Cobalt Core-Shell Junction for Photo-Fenton-Like Reaction. Angew. Chem.-Int. Edit. 2023, 62, e202305639. [Google Scholar] [CrossRef]
- Jasmi, K.K.; Anto Johny, T.; Siril, V.S.; Madhusoodanan, K.N. Influence of Defect Density States on NO2 Gas Sensing Perfor-Mance of Na: ZnO Thin Films. J. Sol-Gel Sci. Technol. 2023, 107, 659–670. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, W.; Tang, M.; Wang, D.; Yu, S.; Mi, Q.; Pan, Q.; Hu, Y. Diversiform Gas Sensors Based on Two-Dimensional Nanomaterials. Nano Res. 2023, 16, 11959–11991. [Google Scholar] [CrossRef]
- Saniz, R.; Sarmadian, N.; Partoens, B.; Batuk, M.; Hadermann, J.; Marikutsa, A.; Rumyantseva, M.; Gaskov, A.; Lamoen, D. First-Principles Study of CO and OH Adsorption on in-Doped ZnO Surfaces. J. Phys. Chem. Solids 2019, 132, 172–181. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, B.; Zhang, Z.; Deng, Y.; Li, X. The Adsorption Properties of Environmentally Friendly Insulation Gas C4F7N on Zn (0001) and ZnO () Surfaces: A First-Principles Study. Appl. Surf. Sci. 2020, 509, 144854. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Y.; Yuan, Y.J. Investigation of Formaldehyde Adsorption on ZnO(0001) Surface by Density Functional The-Ory. Russ. J. Phys. Chem. 2020, 94, 423–428. [Google Scholar] [CrossRef]
- Jin, W.; Chen, G.; Duan, X.; Yin, Y.; Ye, H.; Wang, D.; Yu, J.; Mei, X.; Wu, Y. Adsorption Behavior of Formaldehyde on ZnO Surface: A First Principles Study. Appl. Surf. Sci. 2017, 423, 451–456. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, B.-A.; Xue, P.; Tian, N.; Zhou, Z.-Y.; Lin, X.; Lin, W.-F.; Sun, S.-G. High-Index-Facet- and High-Surface-Energy Nanocrystals of Metals and Metal Oxides as Highly Efficient Catalysts. Joule 2020, 4, 2562–2598. [Google Scholar] [CrossRef]
- Lim, T.; Mirabedini, P.S.; Jung, K.; Alex Greaney, P.; Martinez-Morales, A.A. High-Index Crystal Plane of ZnO Nanopyramidal Structures: Stabilization, Growth, and Improved Photocatalytic Performance. Appl. Surf. Sci. 2021, 536, 147326. [Google Scholar] [CrossRef]
- Chang, J.; Ahmed, R.; Wang, H.; Liu, H.; Li, R.; Wang, P.; Waclawik, E.R. ZnO Nanocones with High-Index Facets for Enhanced Energy Conversion Efficiency of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 13836–13844. [Google Scholar] [CrossRef]
- Mehta, S.; Joshi, K. From Molecular Adsorption to Decomposition of Methanol on Various ZnO Facets: A Periodic DFT Study. Appl. Surf. Sci. 2022, 602, 154150. [Google Scholar] [CrossRef]
- Sun, L.; Tan, D.; Liu, Y.; Jiang, C. Photoelectrical Properties of High-Index Facets ZnO Nanobelts. Chem. Lett. 2021, 50, 1045–1048. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, W.; Li, Y. Computational Study of Surface Orientation Effect of Rutile TiO2 on H2S and CO Sensing Mech-Anism. Appl. Surf. Sci. 2019, 495, 143619. [Google Scholar] [CrossRef]
- Batyrev, E.D.; van den Heuvel, J.C. Modification of the ZnO(0001)–Zn Surface under Reducing Conditions. Phys. Chem. Chem. Phys. 2011, 13, 13127–13134. [Google Scholar] [CrossRef]
- Duan, X.; Ma, C.; Jin, W.; Ma, X.; Guo, L.; Wei, S.-H.; Yu, J.; Wu, Y. The Stabilization Mechanism and Size Effect of Nonpo-Lar-to-Polar Crystallography Facet Tailored ZnO Nano/Micro Rods via a Top-down Strategy. Phys. Chem. Chem. Phys. 2018, 20, 18455–18462. [Google Scholar] [CrossRef]
- Juang, F.-R.; Sung, W.-C. Boosting CO2 Sensitivity via Multiphase ZnO Heterojunctions and Surface-Engineered Silicon Pyramids. Sens. Actuators B Chem. 2026, 447, 138895. [Google Scholar] [CrossRef]
- Duan, X.; Chen, G.; Li, C.; Yin, Y.; Jin, W.; Guo, L.; Ye, H.; Zhu, Y.; Wu, Y. Tailoring the Surface of ZnO Nanorods into Corrugated Nanorods via a Selective Chemical Etch Method. Nanotechnology 2016, 27, 295601. [Google Scholar] [CrossRef] [PubMed]
- Roosen, A.R.; McCormack, R.P.; Carter, W.C. Wulffman: A Tool for the Calculation and Display of Crystal Shapes. Comput. Mater. Sci. 1998, 11, 16–26. [Google Scholar] [CrossRef]
- Ma, C.; Jin, W.; Duan, X.; Ma, X.; Han, H.; Zhang, Z.; Yu, J.; Wu, Y. From the Absolute Surface Energy to the Stabilization Mechanism of High Index Polar Surface in Wurtzite Structure: The Case of ZnO. J. Alloys Compd. 2019, 772, 482–488. [Google Scholar] [CrossRef]
- Lejaeghere, K.; Bihlmayer, G.; Björkman, T.; Blaha, P.; Blügel, S.; Blum, V.; Caliste, D.; Castelli, I.E.; Clark, S.J.; Dal Corso, A.; et al. Reproducibility in Density Functional Theory Calculations of Solids. Science 2016, 351, aad3000. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kim, E.M.; Kim, J.; Fichthorn, K.A. A Study of Cl Adsorption on Pt(111) and Pt(100) Using Ab Initio Grand-Canonical Monte Carlo. Surf. Sci. 2025, 752, 122647. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, Q.-D.; Liu, D.; Liang, J. Effectively Improving the Accuracy of PBE Functional in Calculating the Solid Band Gap via Machine Learning. Comput. Mater. Sci. 2021, 198, 110699. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A User-Friendly Interface Facilitating High-Throughput Com-Puting and Analysis Using VASP Code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Decremps, F.; Datchi, F.; Saitta, A.M.; Polian, A.; Pascarelli, S.; Cicco, A.; Itié, J.P.; Baudelet, F. Local Structure of Condensed Zinc Oxide. Phys. Rev. B 2003, 68, 104101. [Google Scholar] [CrossRef]
- Yan, Y.; Al-Jassim, M.M. Structure and Energetics of Water Adsorbed on the ZnO () Surface. Phys. Rev. B 2005, 72, 235406. [Google Scholar] [CrossRef]
- Ma, C.; Jin, W.; Ma, X.; Han, H.; Yu, J.; Wu, Y. Water Adsorption Behaviors of High Index Polar Surfaces in ZnO. Appl. Surf. Sci. 2019, 498, 143898. [Google Scholar] [CrossRef]
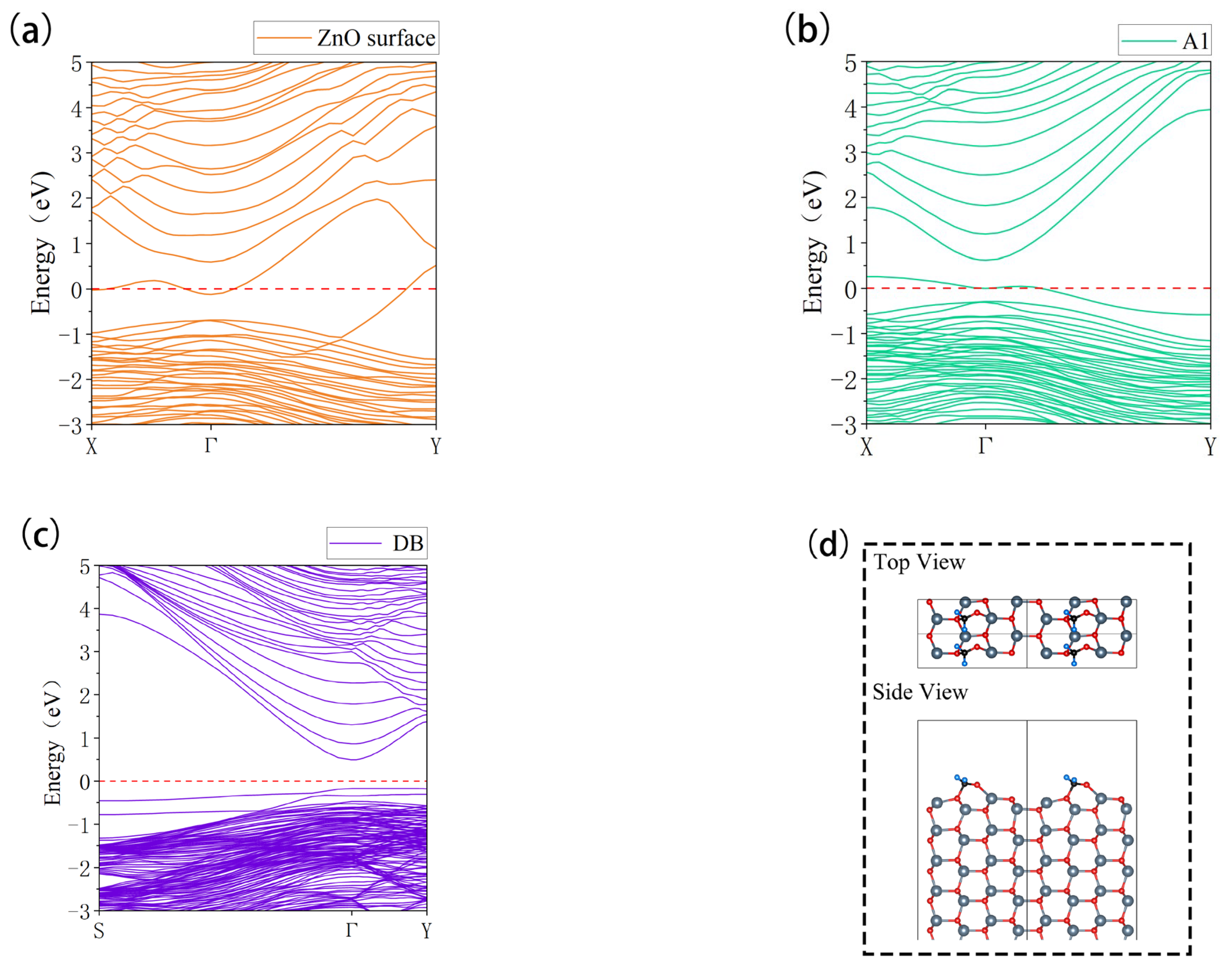
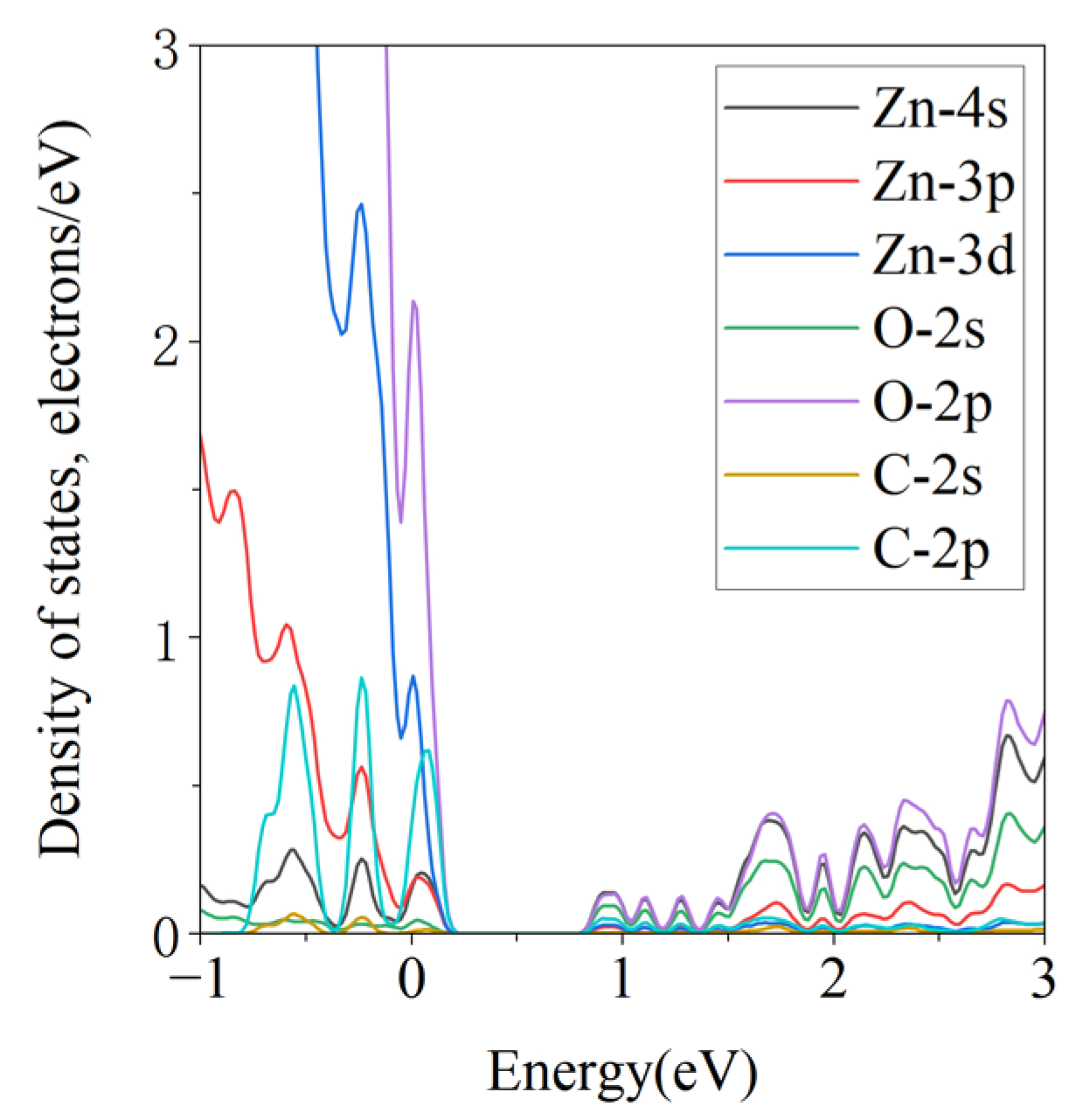
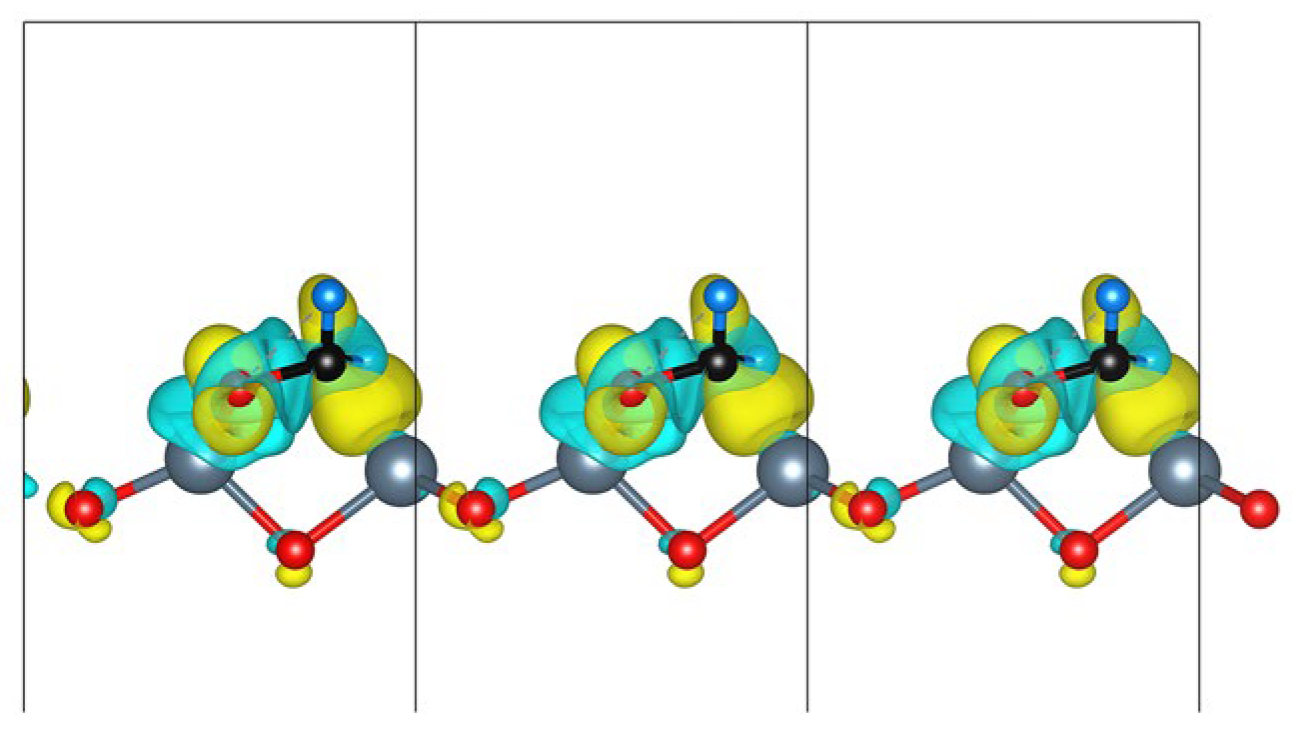
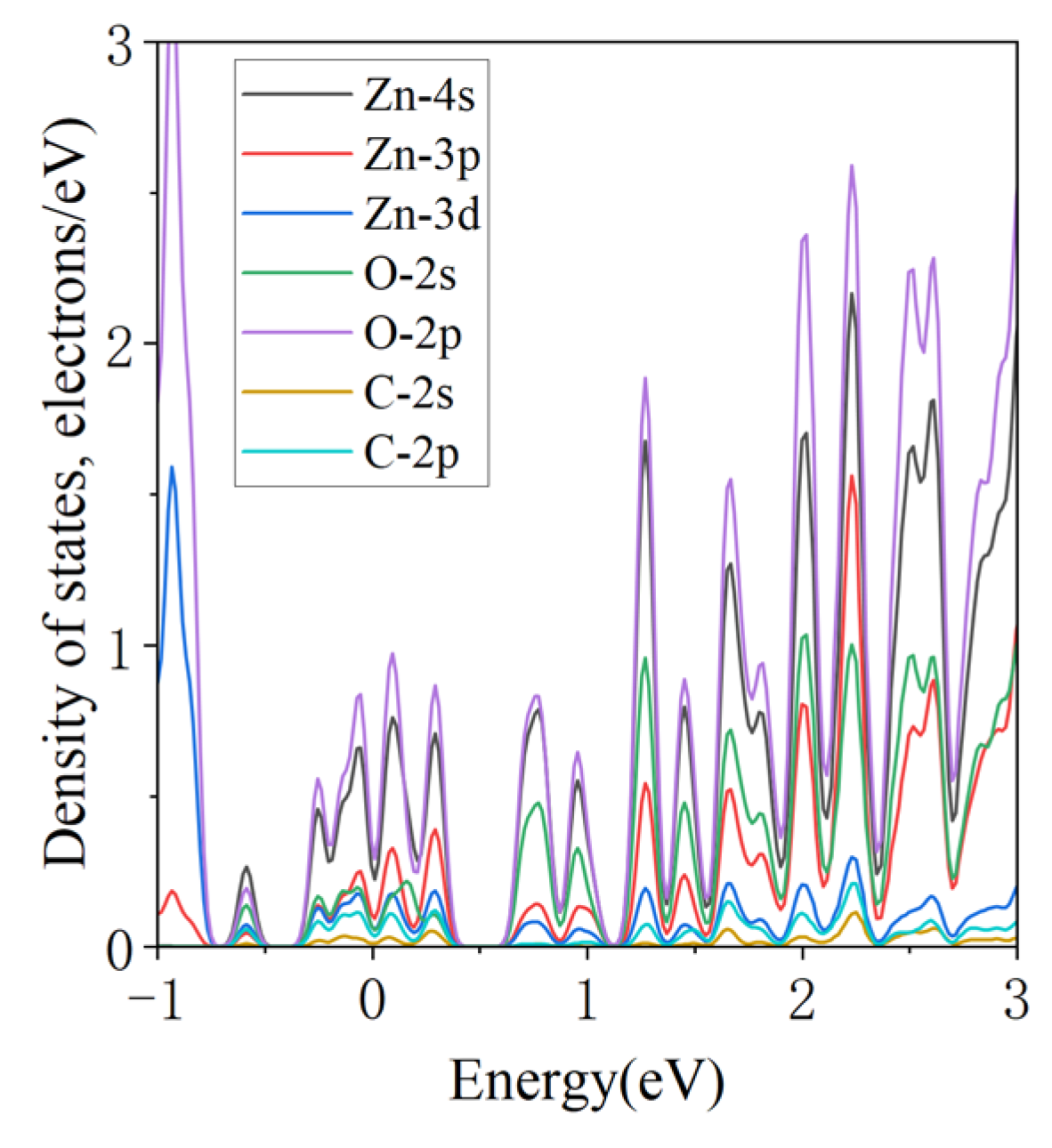
| Configuration | dO-Zn (Å) | Eads (eV) | Coverage | Size |
|---|---|---|---|---|
| A1 | 2.03 | −1.09 | 1 | 1 × 1 |
| A2 | 2.03 | −0.87 | 1 | 1 × 1 |
| A3 | 2.09 | −0.73 | 1 | 1 × 1 |
| B1 | 2.07 | −1.43 | 0.5 | 2 × 1 |
| B2 | 1.20 | −1.39 | 0.5 | 2 × 1 |
| B3 | 2.24 | −1.16 | 0.5 | 2 × 1 |
| B4 | 2.24 | −0.95 | 0.5 | 2 × 1 |
| S1 | 2.24 | −2.19 | 1 | 2 × 1 |
| S2 | 1.99 | −1.96 | 1 | 2 × 1 |
| S3 | 1.99 | −1.79 | 1 | 2 × 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Yao, J.; Ding, L.; Xiao, X.; Li, W.; He, Y.; Wang, M. Behavior of Formaldehyde Adsorption on ZnO [1011] Facets: A DFT Study. Crystals 2025, 15, 911. https://doi.org/10.3390/cryst15110911
Ma C, Yao J, Ding L, Xiao X, Li W, He Y, Wang M. Behavior of Formaldehyde Adsorption on ZnO [1011] Facets: A DFT Study. Crystals. 2025; 15(11):911. https://doi.org/10.3390/cryst15110911
Chicago/Turabian StyleMa, Chao, Jingze Yao, Liqin Ding, Xuefeng Xiao, Weiyin Li, Yujie He, and Meng Wang. 2025. "Behavior of Formaldehyde Adsorption on ZnO [1011] Facets: A DFT Study" Crystals 15, no. 11: 911. https://doi.org/10.3390/cryst15110911
APA StyleMa, C., Yao, J., Ding, L., Xiao, X., Li, W., He, Y., & Wang, M. (2025). Behavior of Formaldehyde Adsorption on ZnO [1011] Facets: A DFT Study. Crystals, 15(11), 911. https://doi.org/10.3390/cryst15110911





