Printable Silicone-Based Emulsions as Promising Candidates for Electrically Conductive Glass-Ceramic Composites
Abstract
1. Introduction
2. Materials and Methods
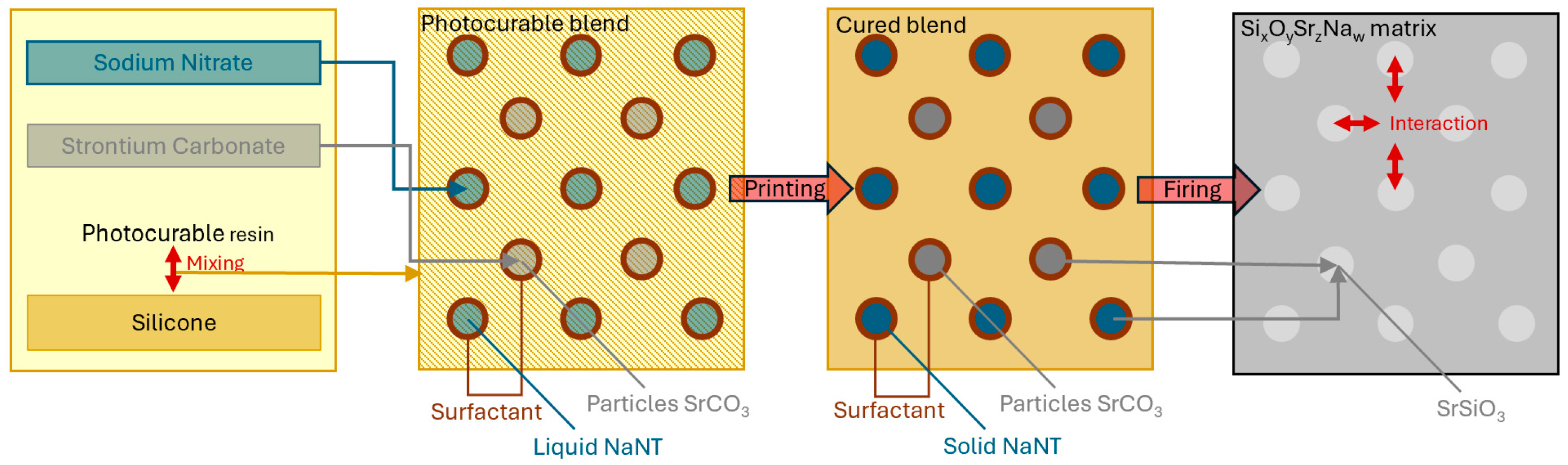
2.1. Preparation and 3D Printing of Silicone-Based Emulsions
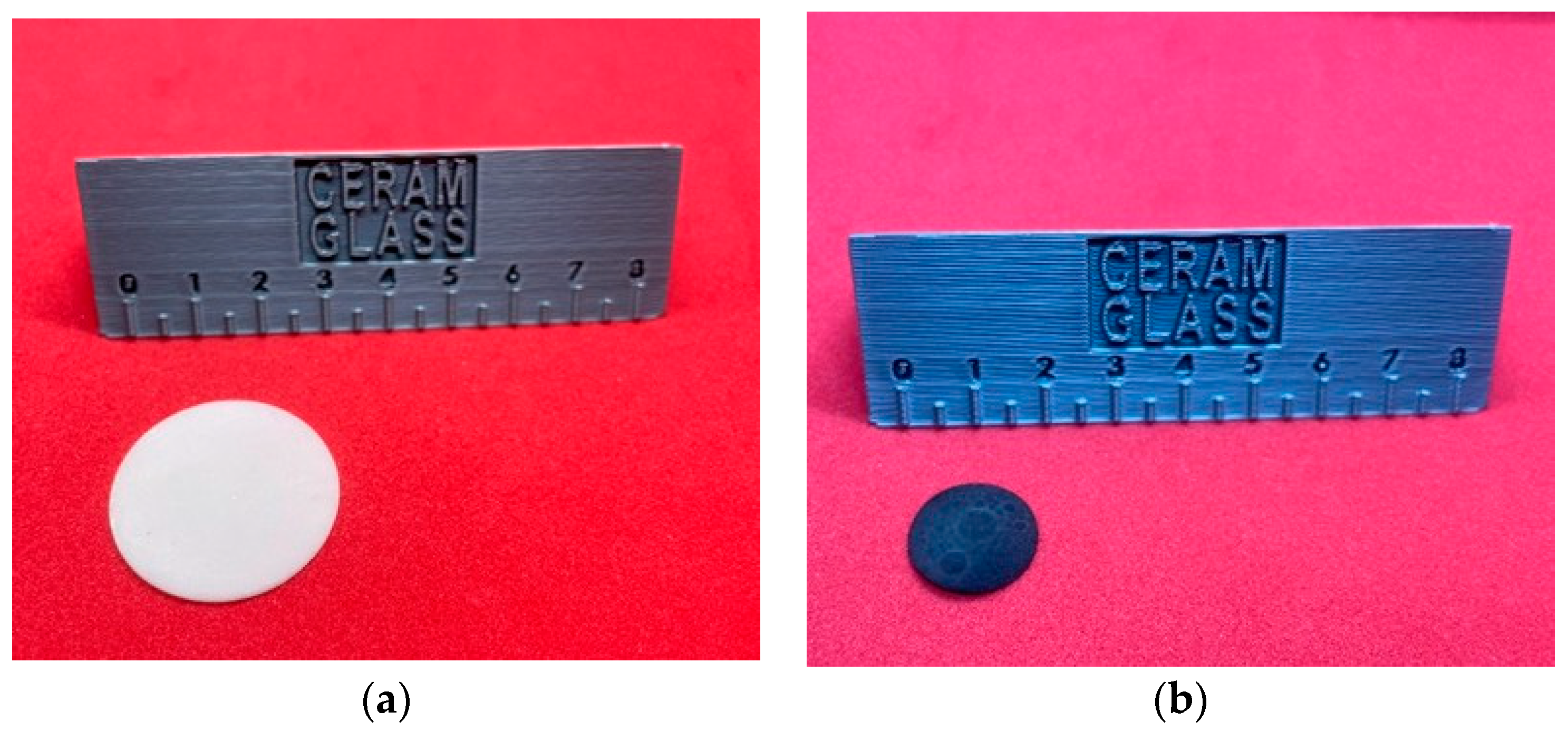
2.2. Characterization of Printed Components and Final Substrates
3. Results and Discussion
3.1. Morphological Evolution Before and After Thermal Treatment
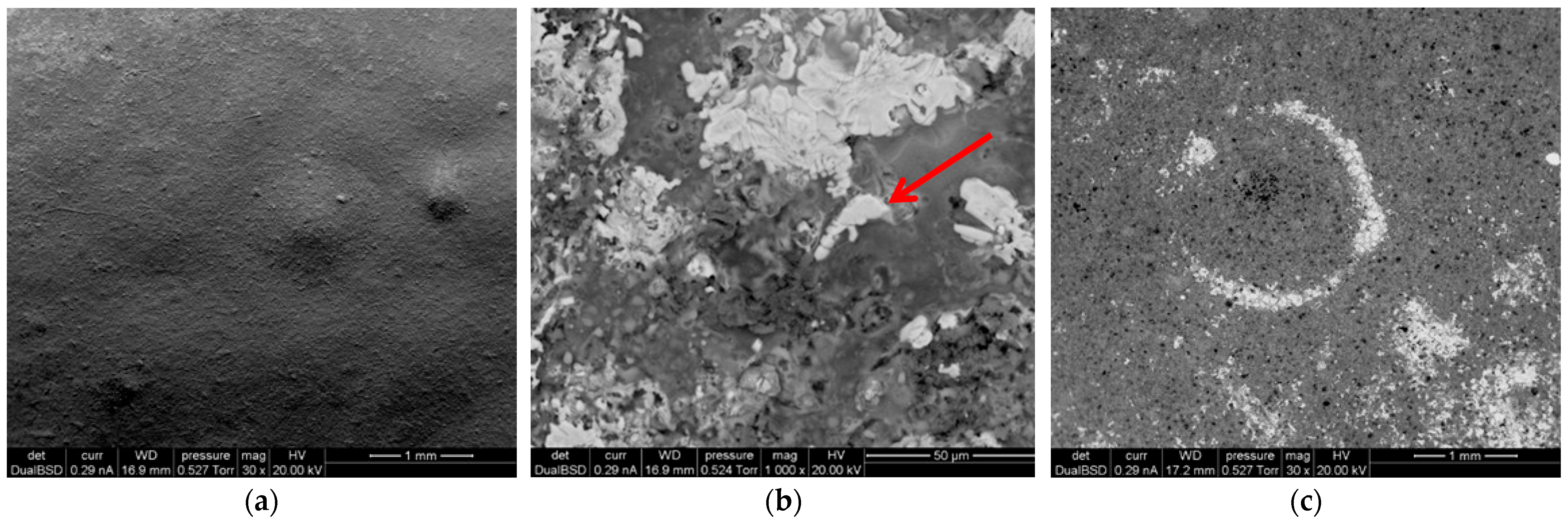
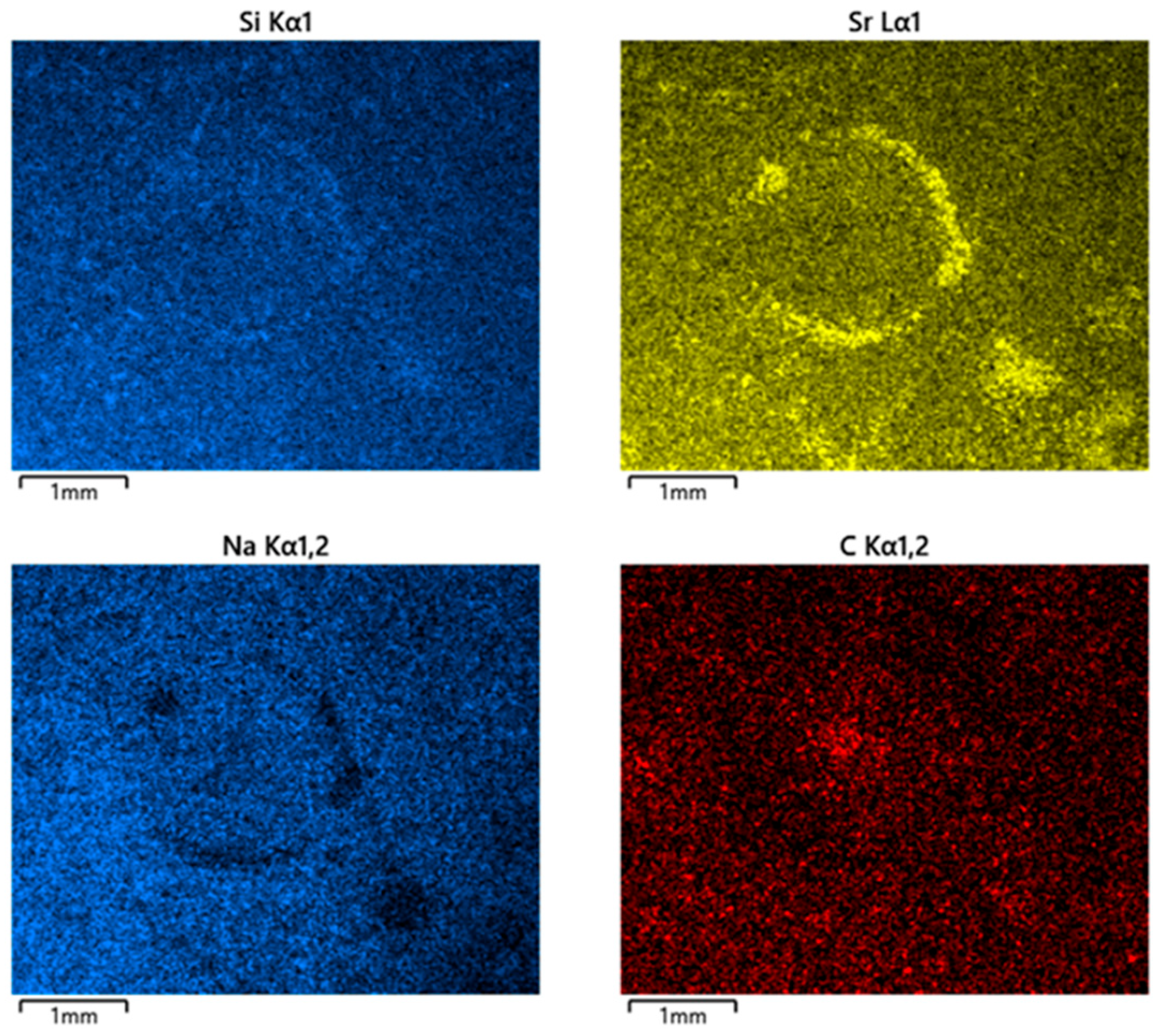
3.2. Microstructure and Elemental Distribution (SEM-EDX)
3.3. Phase Evolution by X-Ray Diffraction
3.4. Electrical Behaviour
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Pietrzak, T.K.; Wasiucionek, M.; Garbarczyk, J.E. Towards higher electric conductivity and wider phase stability range via nanostructured glass-ceramics processing. Nanomaterials 2021, 11, 1321. [Google Scholar] [CrossRef]
- Smeacetto, F.; Saffirio, S.; Salvo, M.; Palliotto, A.; Zhang, J.; De Angelis, S.; Tinti, V.B.; Esposito, V. Fast-quenched Na2Si2O5 stability and properties in crystalline composite. Materialia 2023, 33, 101968. [Google Scholar] [CrossRef]
- Singh, P.; Goodenough, J.B. Sr1-xKxSi1-γGeγO3-0.5x: A new family of superior oxide-ion conductors. Energy Environ. Sci. 2013, 6, 176–180. [Google Scholar] [CrossRef]
- Singh, P.; Goodenough, J.B. Monoclinic Sr1-xNaxSiO3-0.5x: New superior oxide ion conductors. J. Am. Chem. Soc. 2013, 135, 4258–4263. [Google Scholar] [CrossRef]
- Evans, J.S.O. Polanyi-like formulations for rapidly predicting key thermodynamic and kinetic parameters. Chem. Mater. 2015, 27, 8485–8491. [Google Scholar] [CrossRef]
- González Puente, P.M.; Song, S.; Cao, S.; Rannalter, L.Z.; Pan, Z.; Xiang, X.; Shen, Q.; Chen, F. Garnet-type solid electrolyte: Advances of ionic transport performance and its application in all-solid-state batteries. J. Adv. Ceram. 2021, 10, 933–972. [Google Scholar] [CrossRef]
- Wu, H.; Zhuo, D.; Kong, D.; Cui, Y. Improving battery safety by early detection of internal shorting with a bifunctional separator. Nat. Commun. 2014, 5, 5193. [Google Scholar] [CrossRef]
- Gao, L.; Li, Q.; Sun, L.; Zhang, X.; Huo, L.; Zhao, H.; Grenier, J.-C. A novel family of Nb-doped Bi0.5Sr0.5FeO3−δ perovskite as cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 2017, 371, 86–95. [Google Scholar] [CrossRef]
- Ti Jee, Y.; Zhao, X.; Huang, K. On the cause of conductivity degradation in sodium strontium silicate ionic conductor. Chem. Commun. 2015, 51, 9640–9642. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, E.D. A bright future for glass-ceramics. Am. Ceram. Soc. Bull. 2010, 89, 19–27. [Google Scholar]
- Jee, Y.; Zhao, X.; Lei, X.; Huang, K. Phase relationship and ionic conductivity in Na–SrSiO3 ionic conductor. J. Am. Ceram. Soc. 2016, 99, 324–330. [Google Scholar] [CrossRef]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-based polymer-derived ceramics: Synthesis, properties and applications—A review. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Bernardo, E.; Fiocco, L.; Parcianello, G.; Storti, E.; Colombo, P. Advanced Ceramics from Preceramic Polymers Modified at the Nano-Scale: A Review. Materials 2014, 7, 1927–1956. [Google Scholar] [CrossRef]
- Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Filbert-Demut, I.; Schlier, L.; Schlordt, T.; Greil, P. Additive manufacturing of ceramic-based materials. Adv. Eng. Mater. 2014, 16, 729–754. [Google Scholar] [CrossRef]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Günster, J. Additive manufacturing of ceramics: Issues, potentialities, and opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Schmidt, J.; Colombo, P. Digital light processing of ceramic components from polysiloxanes. J. Eur. Ceram. Soc. 2018, 38, 57–66. [Google Scholar] [CrossRef]
- Chaudhary, R.P.; Parameswaran, C.; Idrees, M.; Rasaki, A.S.; Liu, C.; Chen, Z.; Colombo, P. Advanced ceramics from polymer-derived ceramics: Synthesis, properties, and applications. Prog. Mater. Sci. 2022, 128, 100969. [Google Scholar] [CrossRef]
- Eckel, Z.C.; Zhou, C.; Martin, J.H.; Jacobsen, A.J.; Carter, W.B.; Schaedler, T.A. Additive manufacturing of polymer-derived ceramics. Science 2016, 351, 58–62. [Google Scholar] [CrossRef]
- Elsayed, H.; Stabile, F.M.; Savio, G.; Bernardo, E. Masked stereolithography of wollastonite-diopside glass-ceramics from novel silicone-based liquid feedstock. Open Ceram. 2023, 16, 100474. [Google Scholar] [CrossRef]
- Höland, W.; Beall, G.H. Glass-Ceramic Technology, 3rd ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Colombo, P. Engineering porosity in polymer-derived ceramics. J. Eur. Ceram. Soc. 2008, 28, 1389–1395. [Google Scholar] [CrossRef]
- Walters, C.C.; Kliewer, C.E.; Awwiller, D.N.; Rudnicki, M.D.; Passey, Q.R.; Lin, M.W. Influence of turbostratic carbon nanostructures on electrical conductivity in shales. Int. J. Coal Geol. 2014, 122, 105–109. [Google Scholar] [CrossRef]
- Monthioux, M. Describing carbons. Carbon Trends 2024, 14, 100325. [Google Scholar] [CrossRef]
- Lacelle, T.; Sampson, K.L.; Yazdani Sarvestani, H.; Rahimizadeh, A.; Barroeta Robles, J.; Mirkhalaf, M.; Rafiee, M.; Jakubinek, M.B.; Paquet, C.; Ashrafi, B. Additive manufacturing of polymer-derived ceramics: Materials, methods, and applications. APL Mater. 2023, 11, 070602. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-conducting oxides. Annu. Rev. Mater. Sci. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Bo, X.; Li, M.; Han, C.; Zhang, Y.; Nsabimana, A.; Guo, L. Noble metal-free electrocatalysts for the oxygen reduction reaction based on iron and nitrogen-doped porous graphene. J. Mater. Chem. A 2015, 3, 1058–1067. [Google Scholar] [CrossRef]



| % wt. | ||||
|---|---|---|---|---|
| Element | Spectrum 1 | Spectrum 2 | Spectrum 3 | Spectrum 4 |
| C | 23.2 | 21.4 | 19.2 | 24.6 |
| Sr | 8.1 | 19.1 | 26 | 7.5 |
| Na | 11.7 | 8.8 | 6.3 | 12.6 |
| Si | 13.6 | 11.6 | 11.7 | 12 |
| O | 42.7 | 38.6 | 36.9 | 42.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zilio, A.; Bernardo, E. Printable Silicone-Based Emulsions as Promising Candidates for Electrically Conductive Glass-Ceramic Composites. Crystals 2025, 15, 885. https://doi.org/10.3390/cryst15100885
Zilio A, Bernardo E. Printable Silicone-Based Emulsions as Promising Candidates for Electrically Conductive Glass-Ceramic Composites. Crystals. 2025; 15(10):885. https://doi.org/10.3390/cryst15100885
Chicago/Turabian StyleZilio, Annalaura, and Enrico Bernardo. 2025. "Printable Silicone-Based Emulsions as Promising Candidates for Electrically Conductive Glass-Ceramic Composites" Crystals 15, no. 10: 885. https://doi.org/10.3390/cryst15100885
APA StyleZilio, A., & Bernardo, E. (2025). Printable Silicone-Based Emulsions as Promising Candidates for Electrically Conductive Glass-Ceramic Composites. Crystals, 15(10), 885. https://doi.org/10.3390/cryst15100885







