Photo-Responsive Brominated Hydrogen-Bonded Liquid Crystals
Abstract
1. Introduction
2. Materials and Methods
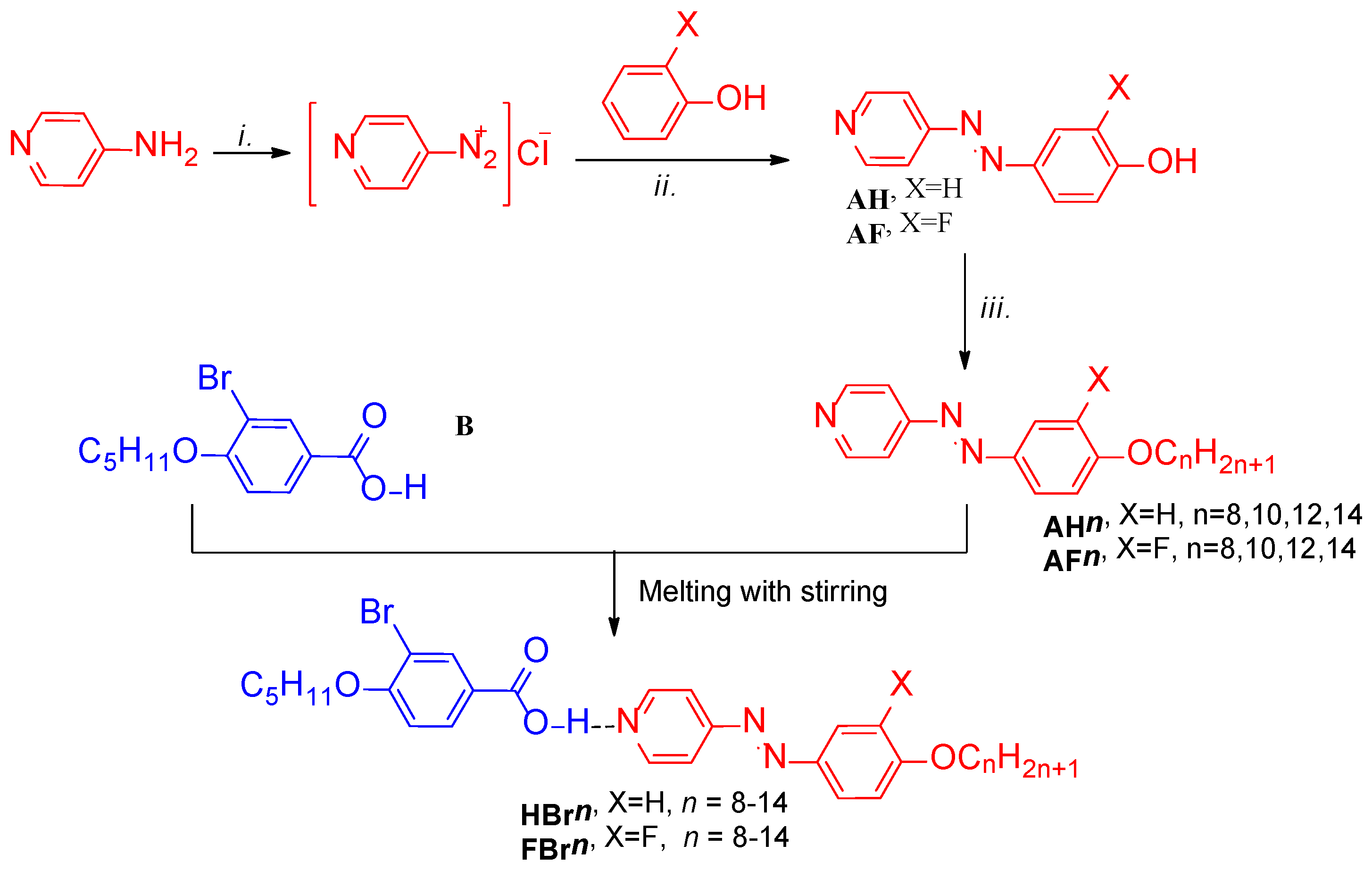
2.1. Synthesis
- 3-Bromo-4-pentyloxybenzoic acid (B): 1H NMR (402 MHz, DMSO) δ 8.04 (d, J = 2.1 Hz, Ar-H, 1H), 7.90 (dd, J = 8.6, 2.1 Hz, Ar-H, 1H), 7.16 (d, J = 8.7 Hz, Ar-H, 1H), 4.10 (t, J = 6.4 Hz, O-CH2-, 2H), 1.84–1.66 (m, OCH2CH2-, 2H), 1.50–1.24 (m, -CH2-, 4H), 0.88 (t, J = 7.2 Hz, -CH3, 3H). 13C NMR (101 MHz, DMSO) δ 166.31, 158.73, 134.22, 131.07, 124.54, 113.36, 111.15, 69.41, 40.31, 40.10, 39.89, 39.68, 39.48, 28.48, 28.01, 22.19, 14.29.
- 4-(4-Tetradeylcoxyphenylazo)pyridine (AH14): 1H NMR (600 MHz, CDCl3) δ 8.77 (d, J = 5.9 Hz, Ar-H, 2H), 7.95 (d, J = 9.0 Hz, Ar-H, 2H), 7.67 (d, J = 6.0 Hz, Ar-H, 2H), 7.02 (d, J = 9.0 Hz, Ar-H, 2H), 4.06 (t, J = 6.6 Hz, -OCH2-, 2H), 1.84–1.80 (m, -OCH2CH2-, 2H), 1.56–1.12 (m, -CH2-, 22H), 0.88 (t, J = 7.0 Hz, CH3, 3H). 13C NMR (151 MHz, CDCl3) δ 162.89, 157.51, 151.01, 146.69, 125.62, 116.10, 114.93, 68.52, 31.92, 29.70, 29.61, 29.63, 29.49, 29.52, 29.32, 29.12, 25.88, 22.60, 14.12.
- 4-(3-Fluoro-4-tetradecoxyphenylazo)pyridine (AF14): 1H NMR (500 MHz, CDCl3) δ 8.91–8.64 (m, Ar-H, 2H), 7.82 (ddd, J = 8.7, 2.4, 1.3 Hz, Ar-H, 1H), 7.72 (dd, J = 11.9, 2.3 Hz, Ar-H, 1H), 7.68–7.65 (m, Ar-H, 2H), 7.09 (t, J = 8.5 Hz, Ar-H, 1H), 4.14 (t, J = 6.6 Hz, -OCH2-, 2H), 1.98–1.77 (m, OCH2CH2-, 2H), 1.53–1.15 (m, -CH2-, 22H), 0.88 (t, J = 6.9 Hz, -CH3, 3H). 13C NMR (126 MHz, CDCl3) δ 157.07, 153.86, 151.88, 151.30, 151.24, 151.15, 146.23, 124.29, 116.15, 113.39, 107.89, 69.57, 31.90, 29.71–29.28 (m, 8C), 29.01, 25.86, 22.67, 14.09. 19F NMR (470 MHz, CDCl3) δ − 132.38 (dd, J = 11.9, 8.4 Hz).
2.2. Characterisation
3. Results and Discussion
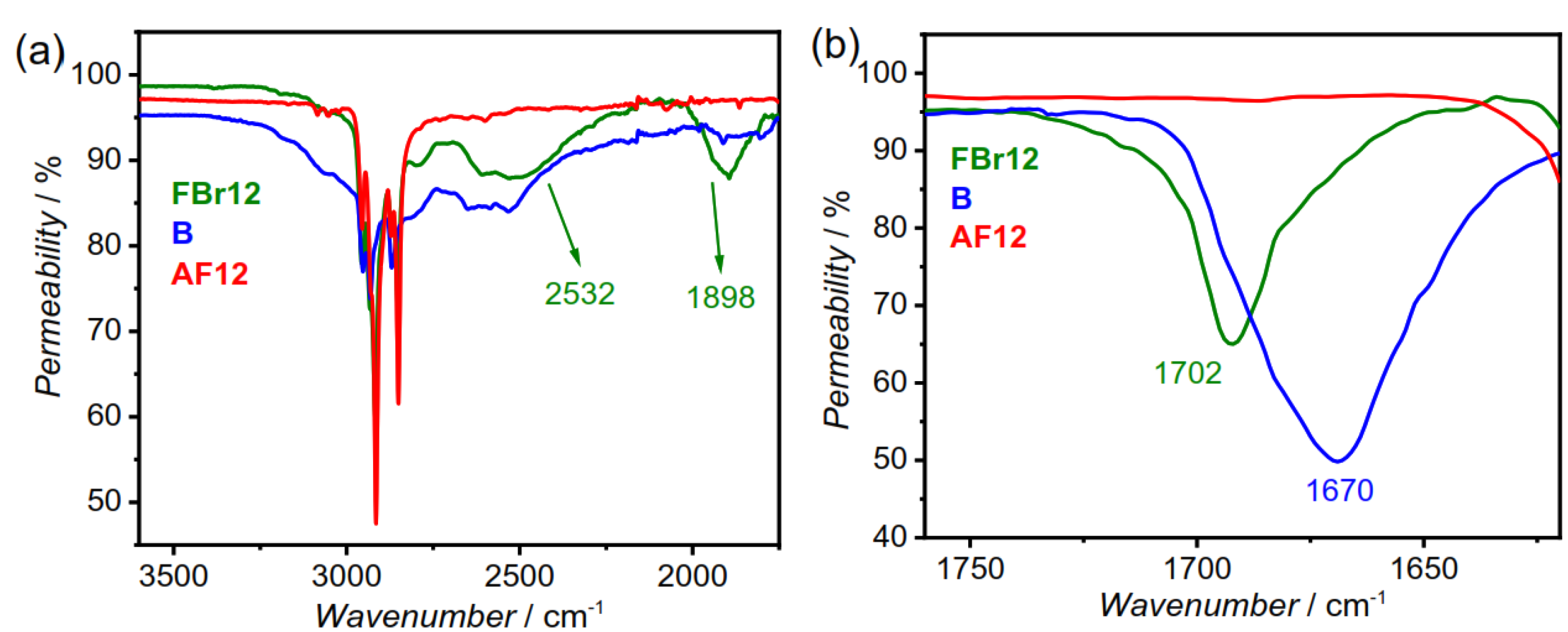
3.1. FTIR Studies
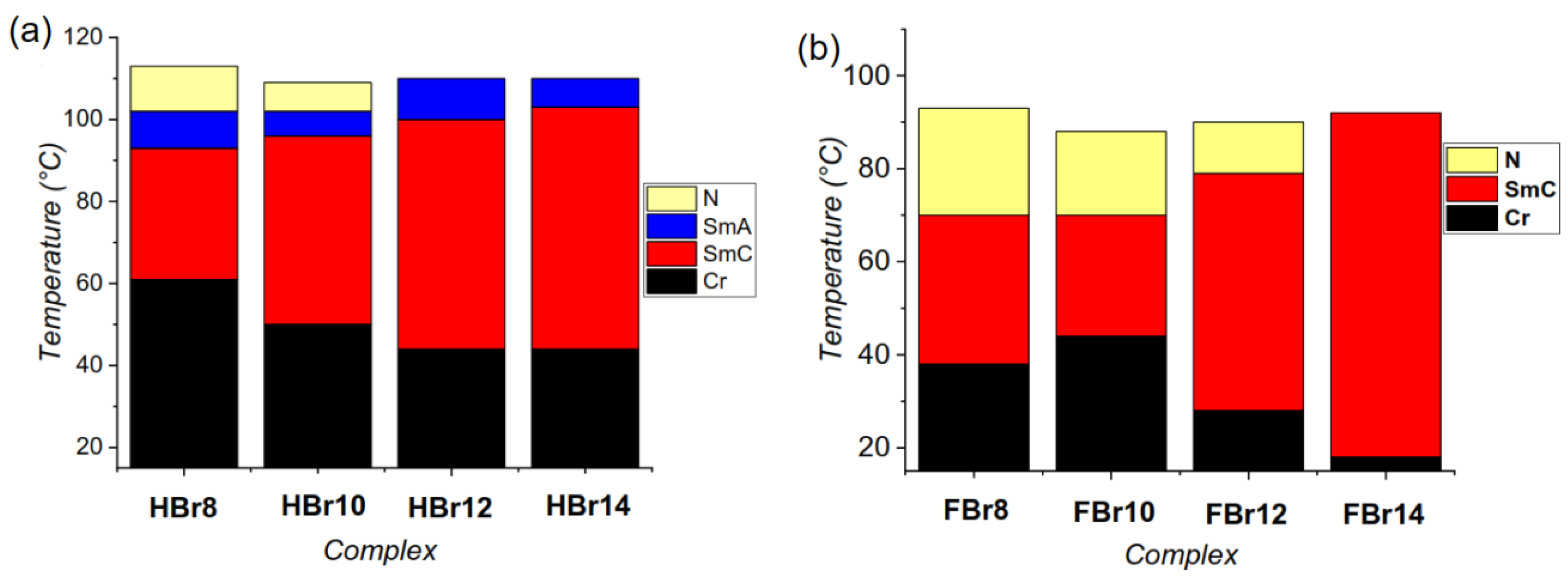
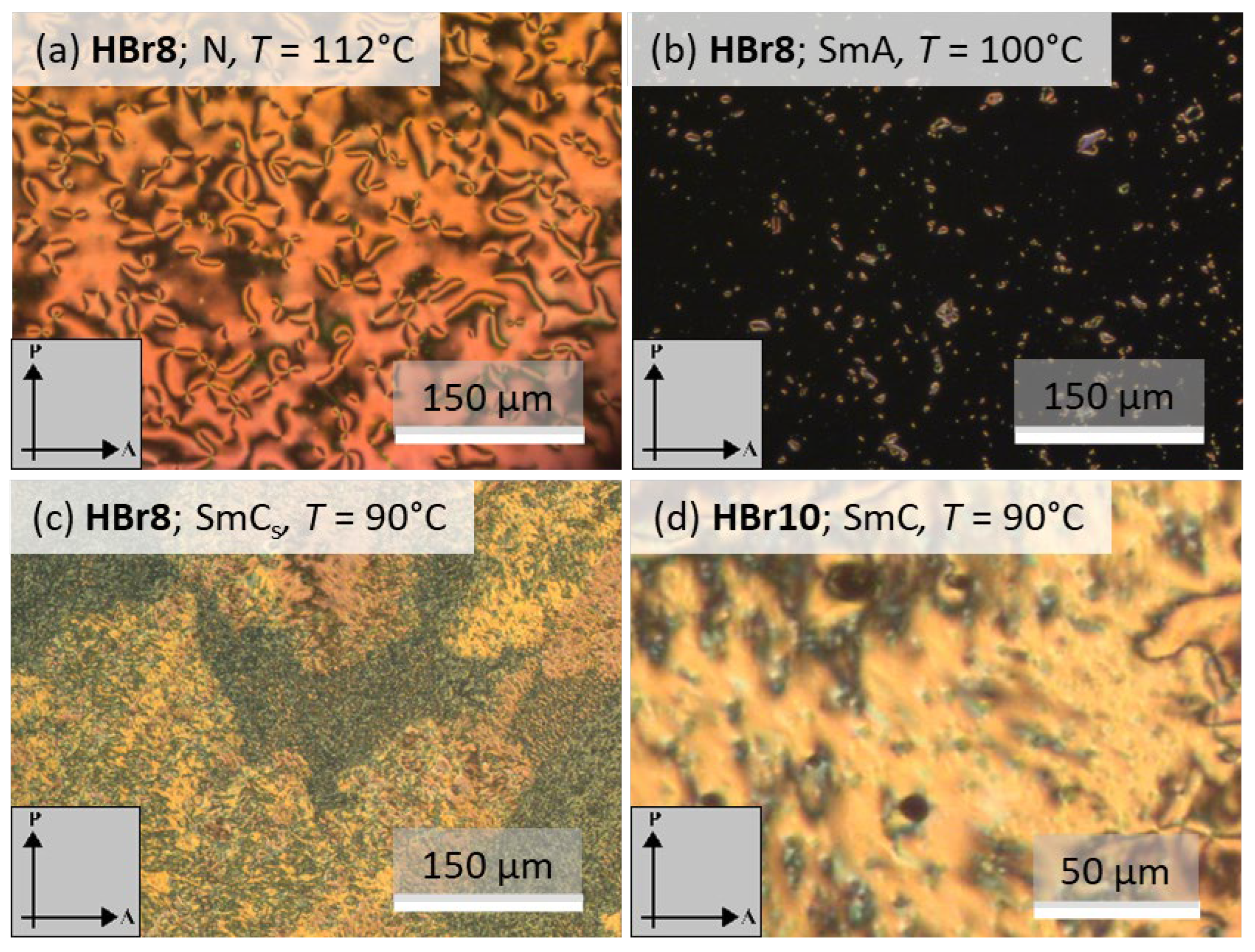
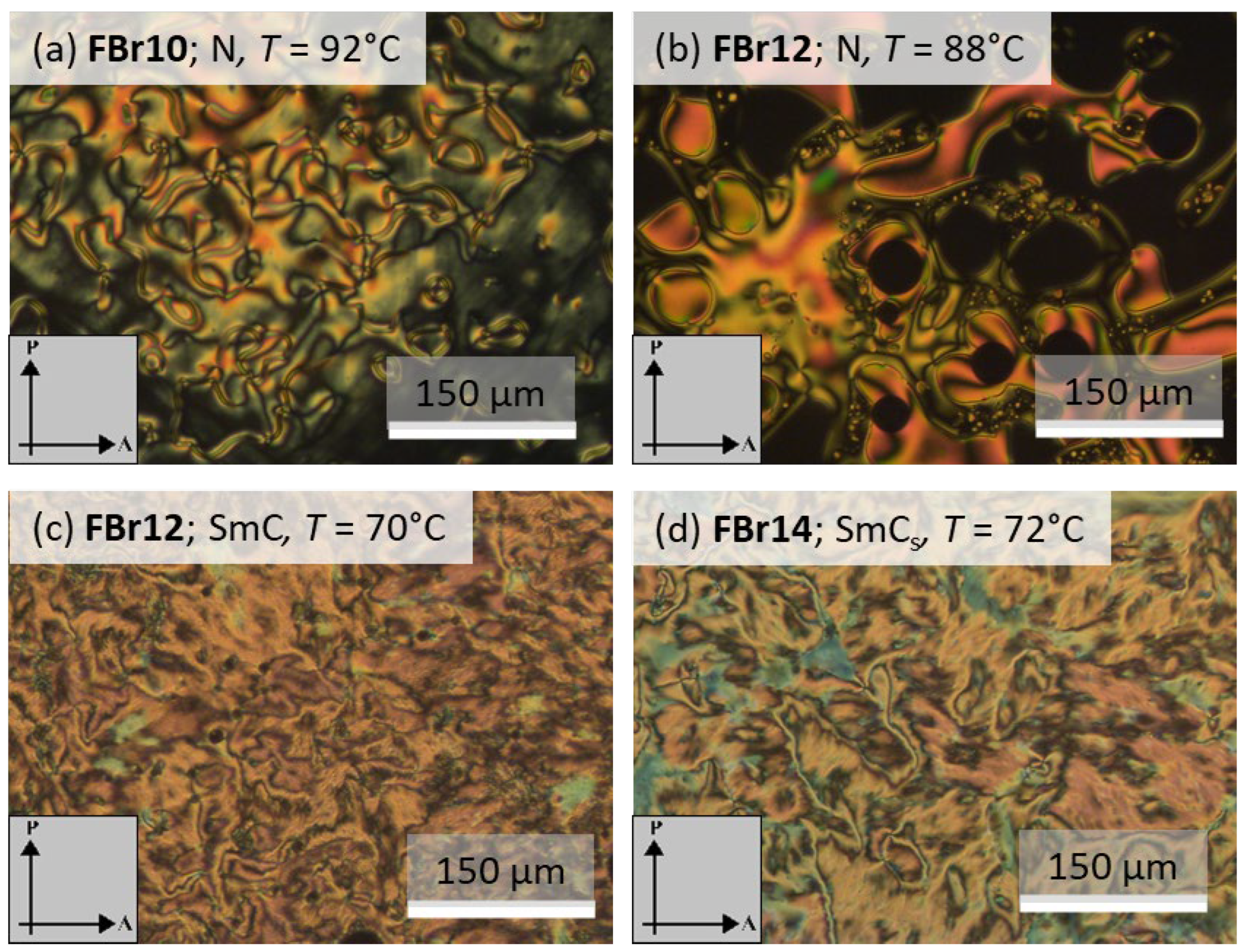
3.2. DSC and POM Investigations
3.2.1. Non-Fluorinated HBLCs (HBrn)
3.2.2. Fluorinated HBLCs (FBrn)
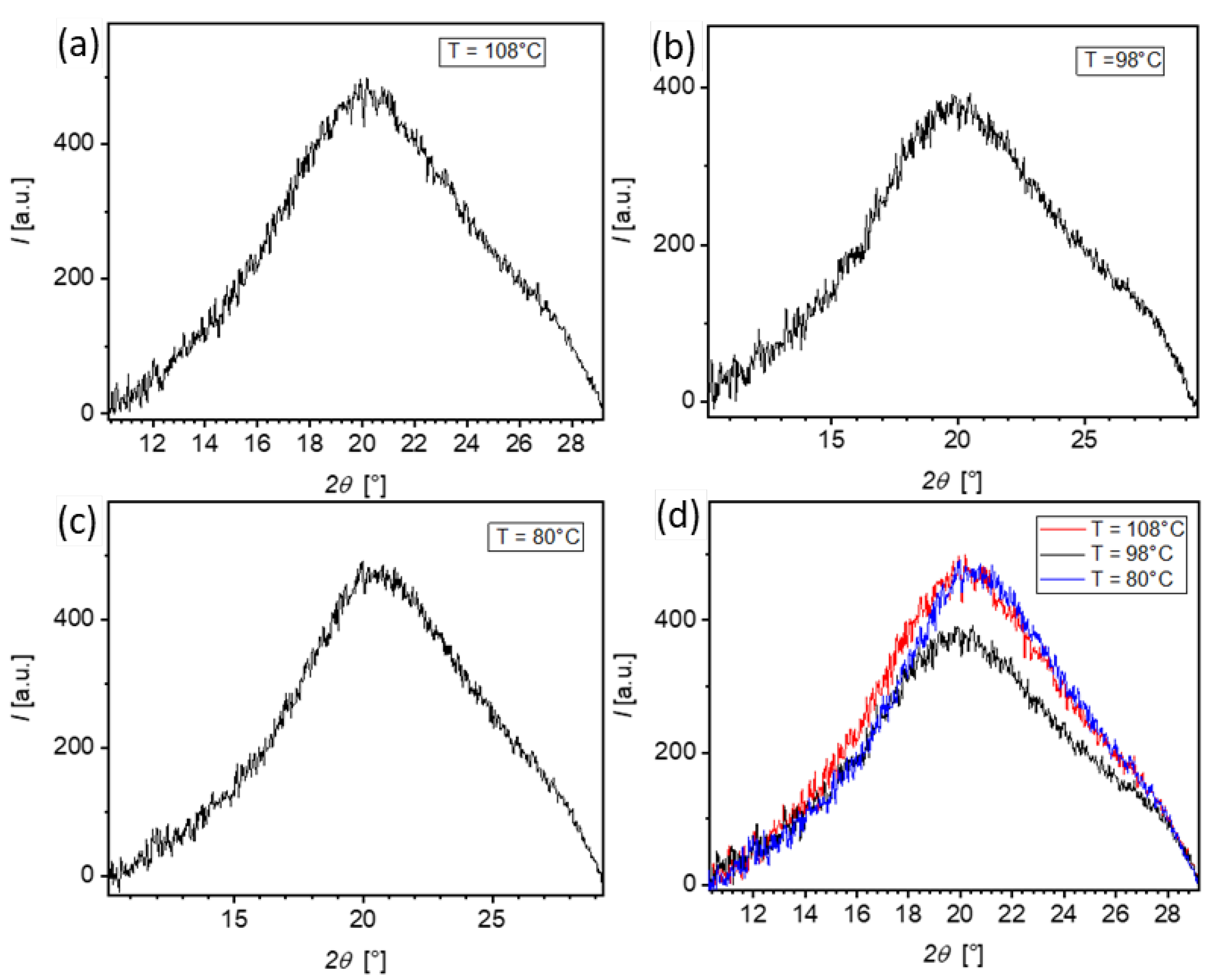
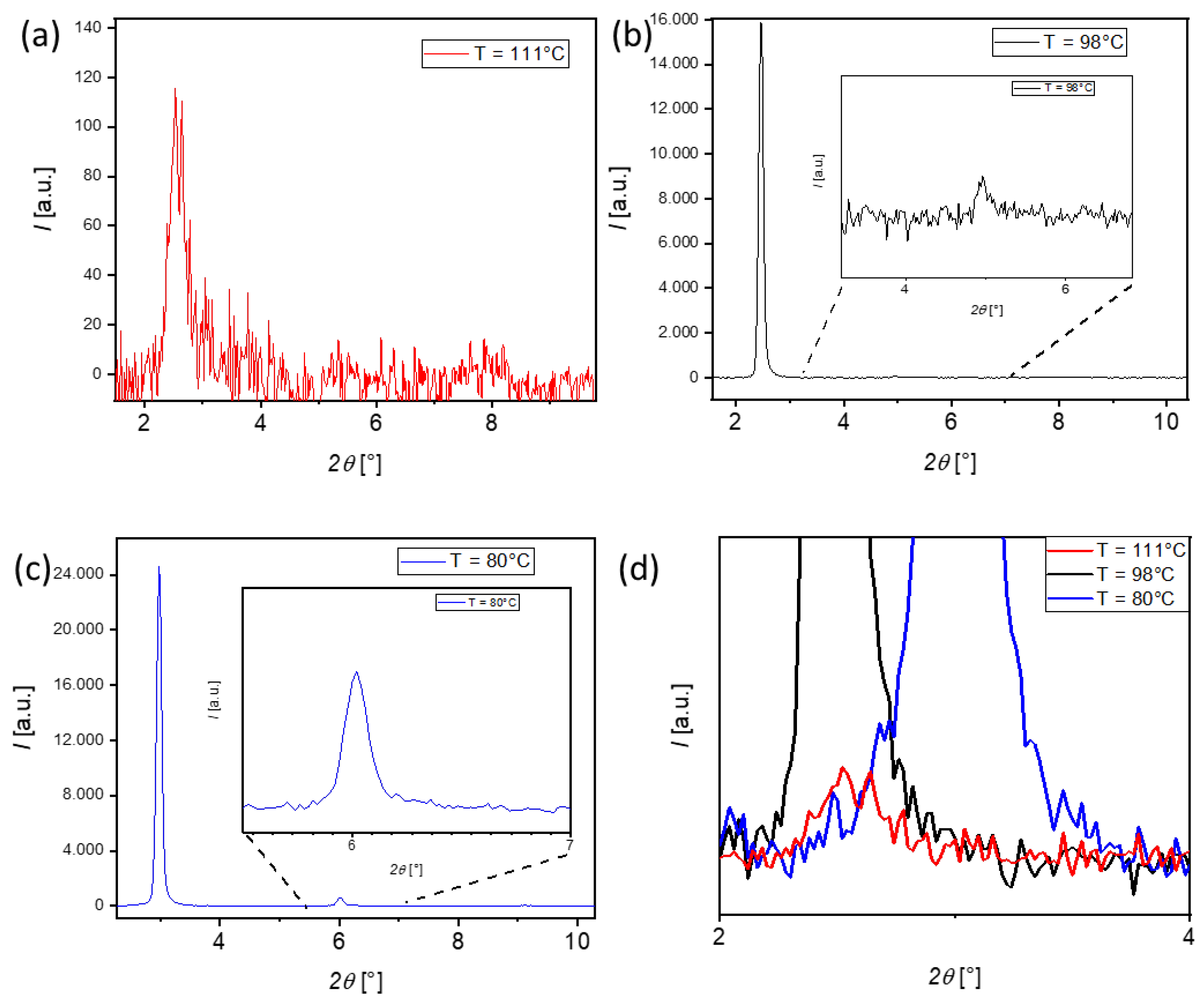
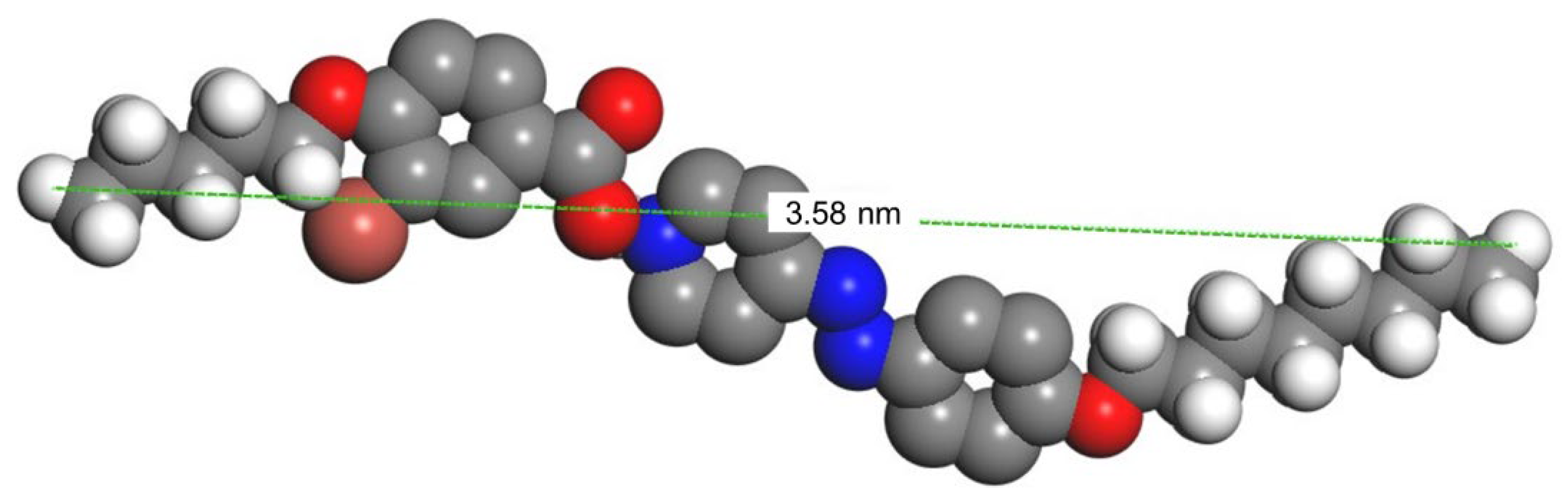
3.3. XRD Investigations
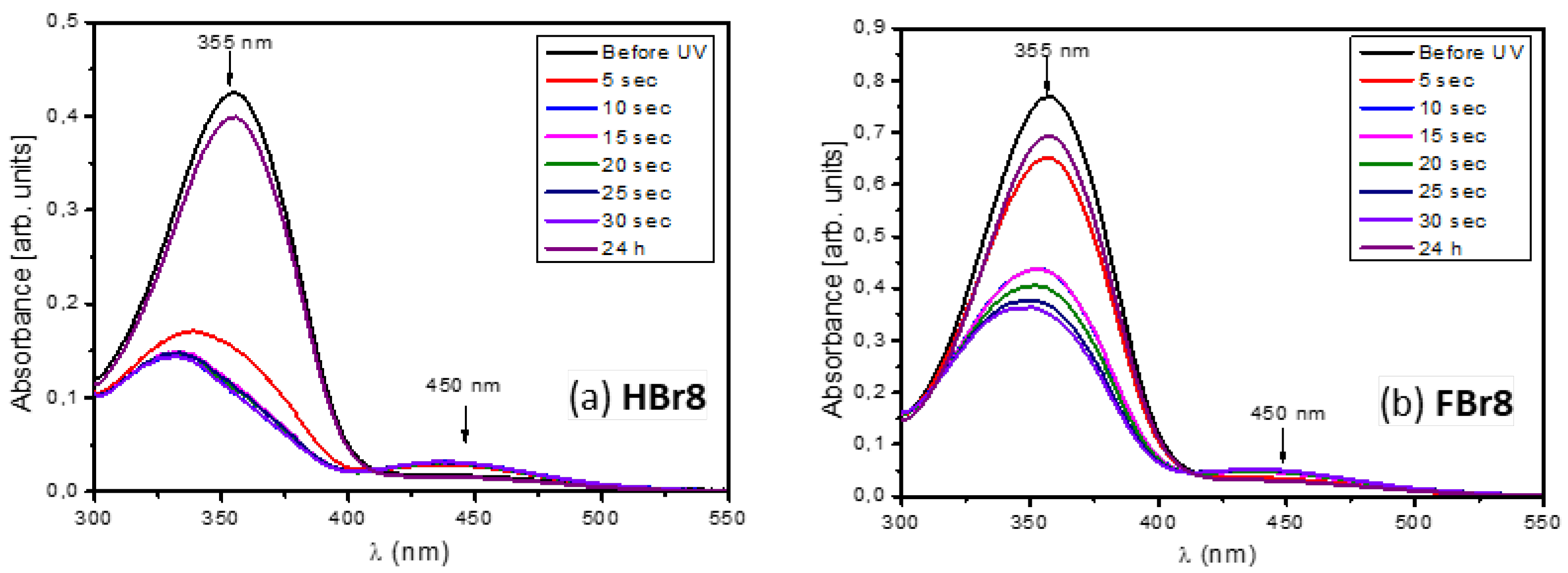
3.4. Photoisomerisation Studies
3.5. Comparison with Related Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional Liquid Crystals Towards the Next Generation of Materials. Angew. Chem. 2018, 57, 4355–4371. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Liquid Crystals: Versatile Self-Organized Smart Soft Materials. Chem. Rev. 2022, 122, 4887. [Google Scholar] [CrossRef]
- Kato, T.; Kamikawa, Y. Handbook of Liquid Crystals; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 5, pp. 513–540. [Google Scholar]
- Kato, T.; Fréchet, J.M.J. A new approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J. Am. Chem. Soc. 1989, 111, 8533–8534. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D. Supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2001, 28, 1127–1161. [Google Scholar] [CrossRef]
- Devadiga, D.; Ahipa, T.N. Recent synthetic advances in pyridine-based thermotropic mesogens. RSC Adv. 2019, 9, 23161–23228. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Konstantinov, I.I.; Vlasova, A.V.; Varfolomeeva, L.A.; Ilyin, S.O. Alkylbenzoic and Alkyloxybenzoic Acid Blending for Expanding the Liquid Crystalline State and Improving Its Rheology. Int. J. Mol. Sci. 2023, 24, 15706. [Google Scholar] [CrossRef]
- Du, M.; Li, L.; Zhang, J.; Li, K.; Cao, M.; Mo, L.; Hu, G.; Chen, Y.; Yu, H.; Yang, H. Photoresponsive Iodine-Bonded Liquid Crystals Based on Azopyridine Derivatives with a Low Phase-Transition Temperature. Liq. Cryst. 2019, 46, 37–44. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Zhang, L.; Yang, H.; Lu, Y. Photoresponsive Liquid Crystals Based on Halogen Bonding of Azopyridines. Chem. Commun. 2014, 50, 9647–9649. [Google Scholar] [CrossRef] [PubMed]
- Alaasar, M.; Poppe, S.; Tschierske, C. Photoresponsive halogen bonded polycatenar liquid crystals. J. Mol. Liq. 2019, 277, 233–240. [Google Scholar] [CrossRef]
- Kumar, V.; Mulder, D.J.; Cavallo, G.; Pilati, T.; Terraneo, G.; Resnati, G.; Schenning, A.P.H.J.; Metrangolo, P. Structural Characterization of New Fluorinated Mesogens Obtained through Halogen-Bond Driven Self-Assembly. J. Fluorine Chem. 2017, 198, 54–60. [Google Scholar] [CrossRef]
- Bruce, D.W.; Metrangolo, P.; Meyer, F.; Prasang, C.; Resnati, G.; Terraneo, G.; Whitwood, A.C. Mesogenic, Trimeric, Halogen-Bonded Complexes from Alkoxystilbazoles and 1,4-Diiodotetrafluorobenzene. New J. Chem. 2008, 32, 477–482. [Google Scholar] [CrossRef]
- Fernandez-Palacio, F.; Poutanen, M.; Saccone, M.; Siiskonen, A.; Terraneo, G.; Resnati, G.; Ikkala, O.; Metrangolo, P.; Priimagi, A. Efficient Light-Induced Phase Transitions in Halogen-Bonded Liquid Crystals. Chem. Mater. 2016, 28, 8314–8321. [Google Scholar] [CrossRef] [PubMed]
- Alaasar, M.; Tschierske, C.; Prehm, M. Hydrogen-bonded supramolecular complexes formed between isophthalic acid and pyridine-based Derivatives. Liq. Cryst. 2011, 38, 925–934. [Google Scholar] [CrossRef]
- Kappelt, A.; Giese, M. Photo-switchable Fluorescence in Hydrogen-Bonded Liquid Crystals. Chem. Eur.J. 2020, 26, 13347–13351. [Google Scholar] [CrossRef] [PubMed]
- Alaasar, M.; Tschierske, C. Nematic phases driven by hydrogen-bonding in liquid crystalline nonsymmetric dimers. Liq. Cryst. 2019, 46, 124–130. [Google Scholar] [CrossRef]
- Anders, C.; Abu Bakar, M.; Nirgude, T.; Alaasar, M. Nematic Phases in Photo-Responsive Hydrogen-Bonded Liquid Crystalline Dimers. Crystals 2025, 15, 576. [Google Scholar] [CrossRef]
- Arakawa, Y.; Sasaki, Y.; Igawa, K.; Tsuji, H. Hydrogen bonding liquid crystalline benzoic acids with alkylthio groups: Phase transition behavior and insights into the cybotactic nematic phase. New J. Chem. 2017, 41, 6514–6522. [Google Scholar] [CrossRef]
- Darweesh, A.F.; Anders, C.; Ranjitha, B.S.; Shanker, G.; Alaasar, M. On the impact of aromatic core fluorination in hydrogen-bonded liquid crystals. RSC Adv. 2025, 15, 6803–6816. [Google Scholar] [CrossRef]
- Jansze, S.M.; Martínez-Felipe, A.; Storey, J.; Marcelis, A.; Imrie, C.T. A Twist-Bend Nematic Phase Driven by Hydrogen Bonding. Angew. Chem. 2015, 54, 643–646. [Google Scholar] [CrossRef]
- Goldmann, D.; Janietz, D.; Schmidt, C.; Wendorff, J.H. Columnar liquid crystalline phases through hydrogen bonding and nanoscale segregation. J. Mater. Chem. 2004, 14, 1521–1525. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, T.-R.; Yu, W.-H.; Li, Q.-G.; Xiang, S.-K.; Cao, P.; Zhao, K.-Q.; Feng, C.; Wang, B.-Q. Hydrogen-bonding stabilized columnar mesophases in hexasubstituted triphenylene 2,3-Dicarboxamides. J. Mol. Liq. 2022, 366, 120122. [Google Scholar] [CrossRef]
- Martínez-Bueno, A.; Martín, S.; Ortega, J.; Folcia, C.L.; Termine, R.; Golemme, A.; Giménez, R.; Sierra, T. Effect of Hydrogen Bonding and Chirality in Star-Shaped Molecules with Peripheral Triphenylamines: Liquid Crystal Semiconductors and Gels. Chem. Mater. 2024, 36, 4343–4356. [Google Scholar] [CrossRef]
- Alaasar, M.; Poppe, S.; Dong, Q.; Liu, F.; Tschierske, C. Mirror symmetry breaking in cubic phases and isotropic liquids driven by hydrogen bonding. Chem. Commun. 2016, 52, 13869–13872. [Google Scholar] [CrossRef]
- Alaasar, M.; Schmidt, J.C.; Cai, X.; Liu, F.; Tschierske, C. Controlling liquid and liquid crystalline network formation by core-fluorination of hydrogen bonded supramolecular polycatenars. J. Mol. Liq. 2021, 332, 115870. [Google Scholar] [CrossRef]
- Pfletscher, M.; Wölper, C.; Gutmann, J.S.; Mezger, M.; Giese, M. A modular approach towards functional supramolecular aggregates—Subtle structural differences inducing liquid crystallinity. Chem. Commun. 2016, 52, 8549–8552. [Google Scholar] [CrossRef]
- Hird, M. Fluorinated liquid crystals: Properties and applications. Chem. Soc. Rev. 1992, 21, 147–154. [Google Scholar] [CrossRef]
- Alaasar, M.; Prehm, M.; Tschierske, C. Mirror symmetry breaking in fluorinated bent-core mesogens. RSC Adv. 2016, 6, 82890–82899. [Google Scholar] [CrossRef]
- Saccone, M.; Kuntze, K.; Ahmed, Z.; Siiskonen, A.; Giese, M.; Priimagi, A. ortho-Fluorination of azophenols increases the mesophase stability of photoresponsive hydrogen-bonded liquid crystals. J. Mater. Chem. C. 2018, 6, 9958–9963. [Google Scholar] [CrossRef]
- Karcz, J.; Herman, J.; Rychłowicz, N.; Kula, P.; Górecka, E.; Szydlowska, J.; Majewski, P.W.; Pociecha, D. Spontaneous chiral symmetry breaking in polar fluid-heliconical ferroelectric nematic phase. Science 2024, 384, 1096–1099. [Google Scholar] [CrossRef]
- Gibb, C.J.; Hobbs, J.; Nikolova, D.I.; Raistrick, T.; Berrow, S.R.; Mertelj, A.; Osterman, N.; Sebastián, N.; Gleeson, H.F.; Mandle, R.J. Spontaneous symmetry breaking in polar fluids. Nat. Commun. 2024, 15, 5845. [Google Scholar] [CrossRef]
- Inui, S.; Kitaoka, H.; Eguchi, Y.; Yasui, M.; Konno, T.; Yamada, S. Design of Near-UV Photoluminescent Liquid-Crystalline Dimers: Roles of Fluorinated Aromatic Ring Position and Flexible Linker. Crystals 2025, 15, 840. [Google Scholar] [CrossRef]
- Devadiga, D.; Ahipa, T.N. An up-to-date review on halogen-bonded liquid crystals. J. Mol. Liq. 2021, 333, 115961. [Google Scholar] [CrossRef]
- Prasang, C.; Bruce, D.W. Halogen-Bonded Liquid Crystals. Helv. Chim. Acta 2023, 106, e202300008. [Google Scholar] [CrossRef]
- Podoliak, N.; Novotná, V.; Kašpar, M.; Hamplová, V.; Glogarová, M.; Pociecha, D. Anomalous phase sequence in new chiral liquid crystalline materials. Liq. Cryst. 2014, 41, 176–183. [Google Scholar] [CrossRef]
- Podoliak, N.; Hamplová, V.; Kašpar, M.; Novotná, V.; Glogarová, M.; Pociecha, D.; Gorecka, E. High tilted smectogens with bromine-substituted molecular core. Liq. Cryst. 2013, 40, 321–328. [Google Scholar] [CrossRef]
- Takanishi, Y.; Nishiyama, I.; Yamamoto, J.; Ohtsuka, Y.; Iida, A. Remarkable effect of a lateral substituent on the molecular ordering of chiral liquid crystal phases: A novel bromo-containing dichiralcompound showing SmC* variants. J. Mater. Chem. 2011, 21, 4465–4469. [Google Scholar] [CrossRef]
- Alaasar, M.; Nirgude, T.; Anders, C. The influence of bromine substitution and linking groups on the phase behaviour of light-responsive rod-like molecules. J. Mol. Liq. 2024, 414, 126174. [Google Scholar] [CrossRef]
- Immirzi, A.; Perini, B. Prediction of density in organic crystals. Acta Cryst. 1977, A33, 216. [Google Scholar] [CrossRef]
- Dingding, G.; Keting, B.; Mingming, Z.; Yingxia, L. Design, Synthesis and Biological Evaluation of Small-Molecule Inhibitors of Signal Transducer and Activator of Transcription#br# 3 (STAT3) Signaling Pathway. Chin. J. Org. Chem. 2016, 36, 1854–1862. [Google Scholar]
- Alaasar, M.; Schmidt, J.-C.; Darweesh, A.F.; Tschierske, C. Azobenzene-based supramolecular liquid crystals: The role of core fluorination. J. Mol. Liq. 2020, 310, 113252. [Google Scholar] [CrossRef]


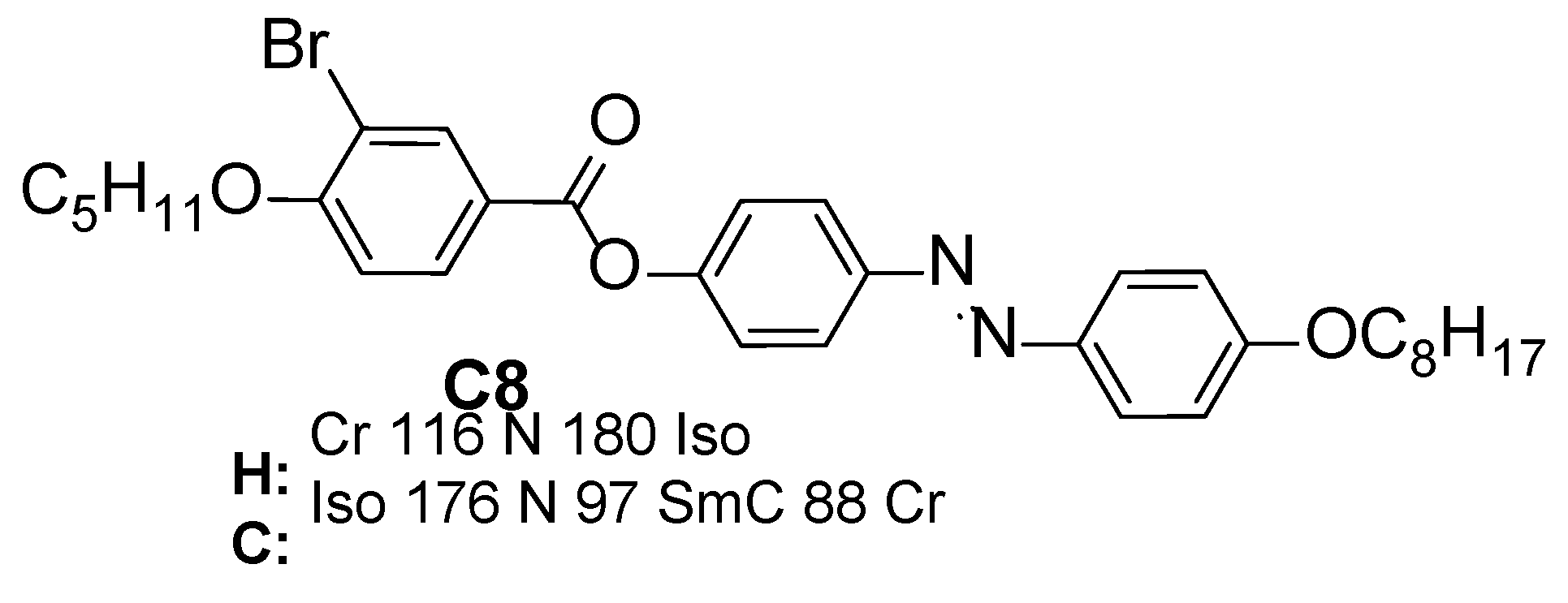
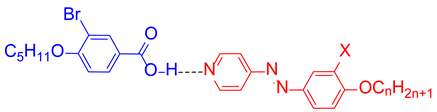 | |||
|---|---|---|---|
| Complex | n | X | Phase Sequence T/°C [ΔH/kJ mol−1] |
| HBr8 | 8 | H | H: Cr 81 [28.7] SmC 96 [0.6] SmA 104 [1.1] N 116 [2.1] Iso C: Iso 113 [−2.3] N 102 [−0.8] SmA 93 [−1.2] SmC 61 [−25.8] Cr |
| HBr10 | 10 | H | H: Cr 79 [13.3] SmC 100 [10.2]b SmA 107 [10.2]b 112 [10.2] Iso C: Iso 109 [−5.2]b N 102 [−5.2]b SmA 96 [−0.6] SmC 50 [−22.6] Cr |
| HBr12 | 12 | H | H: Cr 63 [29.1] SmC 103 [1.6] SmA 112 [6.4] Iso C: Iso 110 [−7.0] SmA 100 [−0.6] SmC 44 [−19.9] Cr |
| HBr14 | 14 | H | H: Cr 79 [46.4] SmC 106 [1.6] SmA 113 [7.8] Iso C: Iso 110 [−8.1] SmA 103 [−0.3] SmC 44 [−18.8] Cr |
| FBr8 | 8 | F | H: Cr 80 [36.9] SmC 93 [0.6] N 106 [0.8] Iso C: Iso 93 [−1.2] N 70 [−0.9] SmC 38 [−23.4] Cr |
| FBr10 | 10 | F | H: Cr 89 [46.2] N 103 [1.5] Iso C: Iso 88 [−1.4] N 70 [−0.5] SmC 44 [−22.6] Cr |
| FBr12 | 12 | F | H: Cr 77 [57.7] SmC 90 [2.5] N 105 [2.8] Iso C: Iso 90 [−3.6] N 79 [−0.3] SmC 28 [41.0] Cr |
| FBr14 | 14 | F | H: Cr 86 [72.1] SmC 105 [2.5] Iso C: Iso 92 [−4.7] SmC 18 [−37.7] Cr |
 | |||||
|---|---|---|---|---|---|
| Complex | OR | X | Y | Phase Sequence T/°C | Ref. |
| HH8 | -OC6H13 | H | H | H: Cr 106 SmA 124 N 134 Iso C: Iso 131 N 121 SmA 97 Cr | [19] |
| HBr8 | -OC5H11 | H | Br | H: Cr 81 SmC 96 SmA 104 N 116 Iso C: Iso 113 N 102 SmA 93 SmC 61 Cr | - |
| HF | -OC6H13 | F | H | H: Cr 101 N 118 Iso C: Iso 115 N 84 Cr | [19] |
| FBr8 | -OC5H11 | F | Br | H: Cr 80 SmC 93 N 106 Iso C: Iso 93 N 70 SmC 38 Cr | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anders, C.; Nirgude, T.; Darweesh, A.F.; Alaasar, M. Photo-Responsive Brominated Hydrogen-Bonded Liquid Crystals. Crystals 2025, 15, 886. https://doi.org/10.3390/cryst15100886
Anders C, Nirgude T, Darweesh AF, Alaasar M. Photo-Responsive Brominated Hydrogen-Bonded Liquid Crystals. Crystals. 2025; 15(10):886. https://doi.org/10.3390/cryst15100886
Chicago/Turabian StyleAnders, Christian, Tejal Nirgude, Ahmed F. Darweesh, and Mohamed Alaasar. 2025. "Photo-Responsive Brominated Hydrogen-Bonded Liquid Crystals" Crystals 15, no. 10: 886. https://doi.org/10.3390/cryst15100886
APA StyleAnders, C., Nirgude, T., Darweesh, A. F., & Alaasar, M. (2025). Photo-Responsive Brominated Hydrogen-Bonded Liquid Crystals. Crystals, 15(10), 886. https://doi.org/10.3390/cryst15100886









