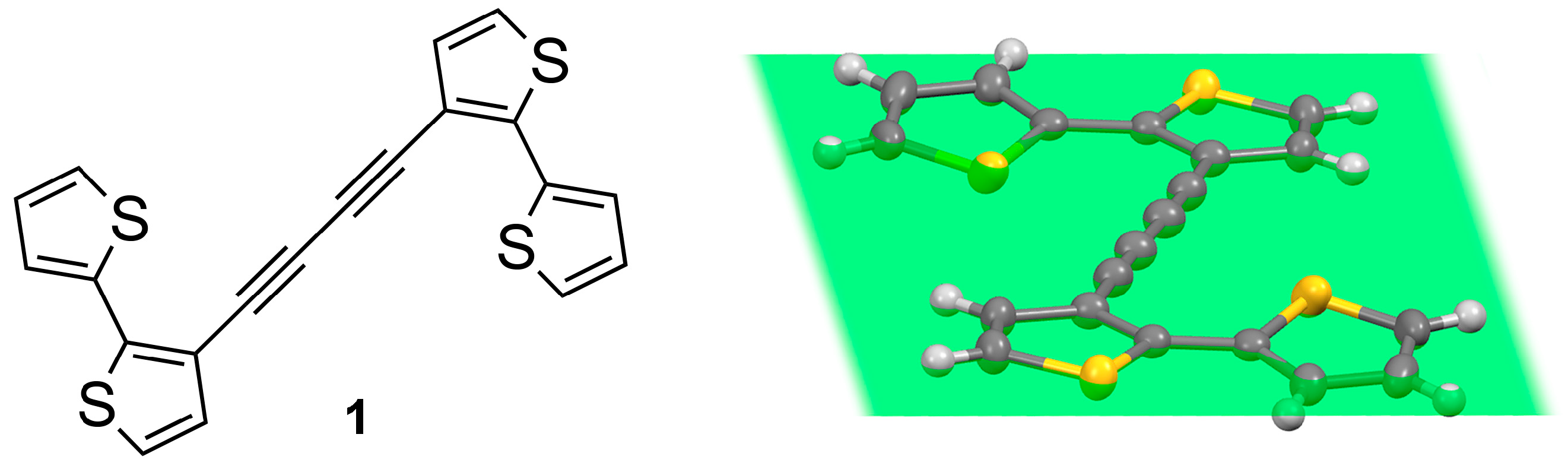
Synthesis, Crystal Structure, and Electropolymerization of 1,4-Di([2,2′-bithiophen]-3-yl)buta-1,3-diyne
Abstract
1. Introduction
2. Materials and Methods
3. Results
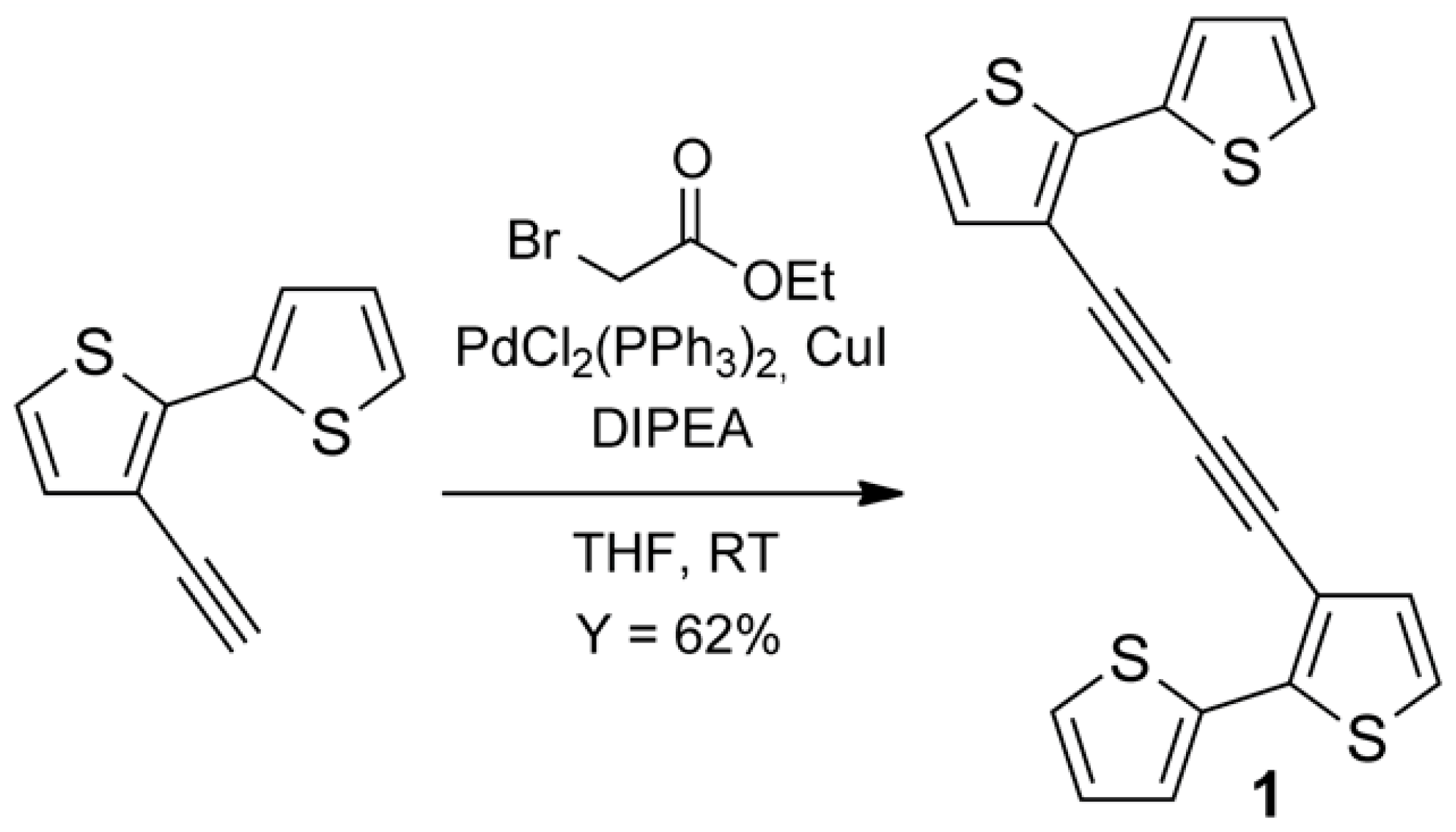
3.1. Synthesis of Tetrathiophene 1
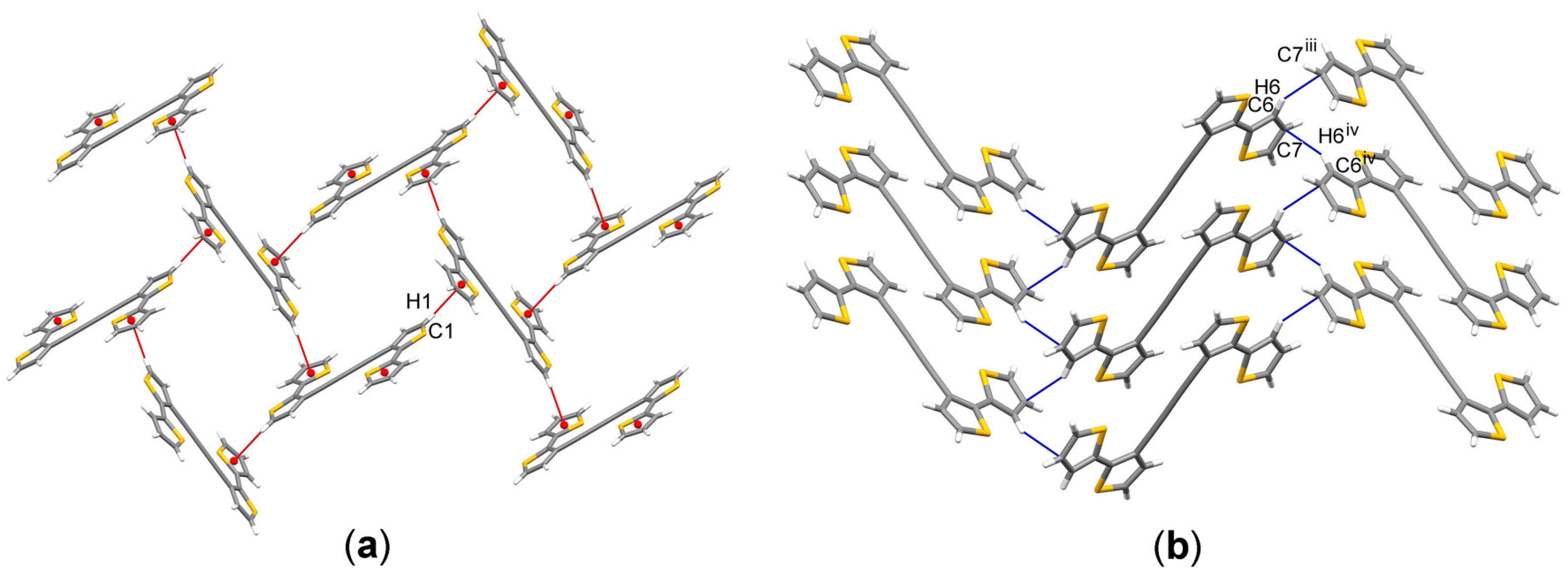
3.2. Solid-State Crystal Structure
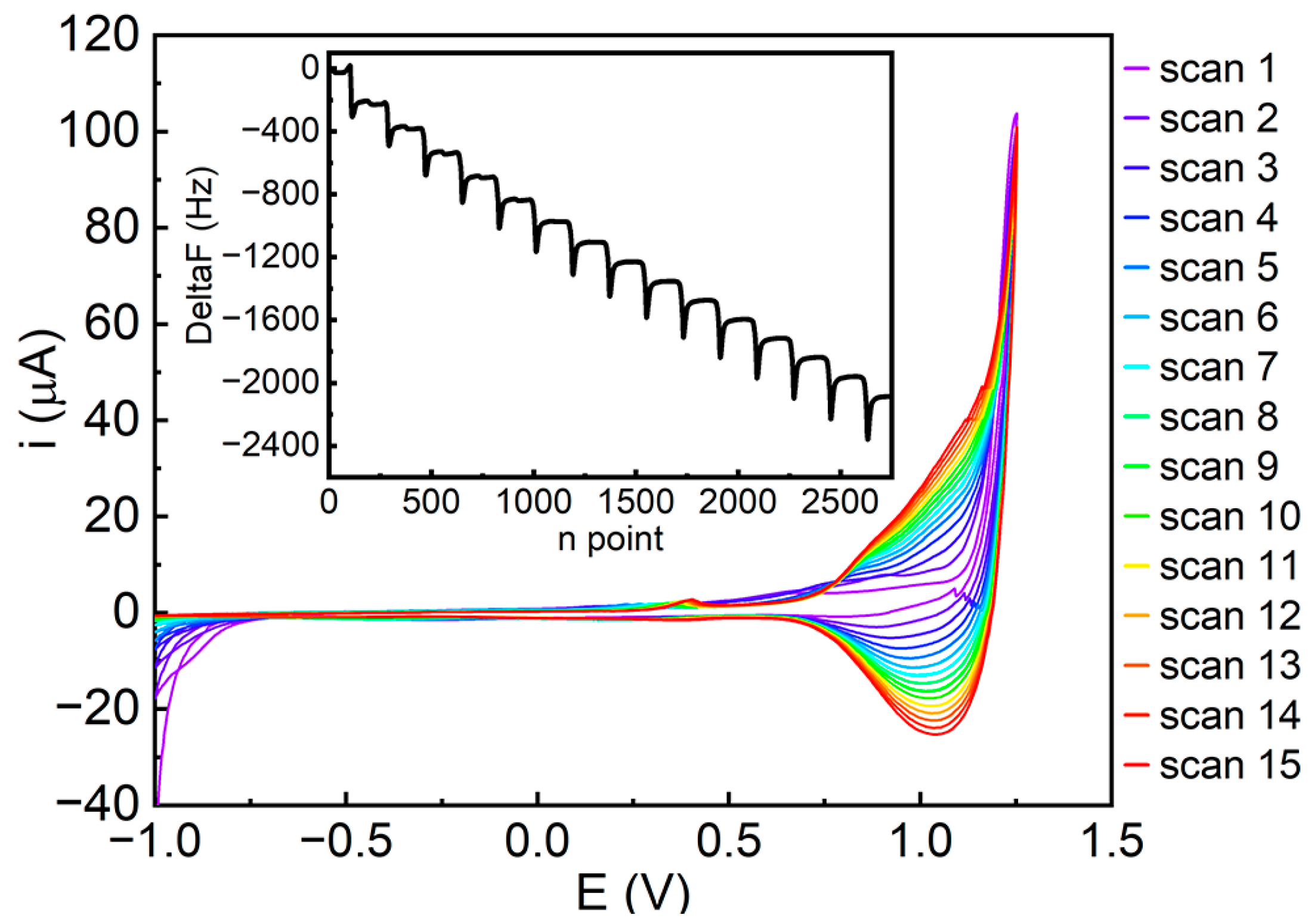
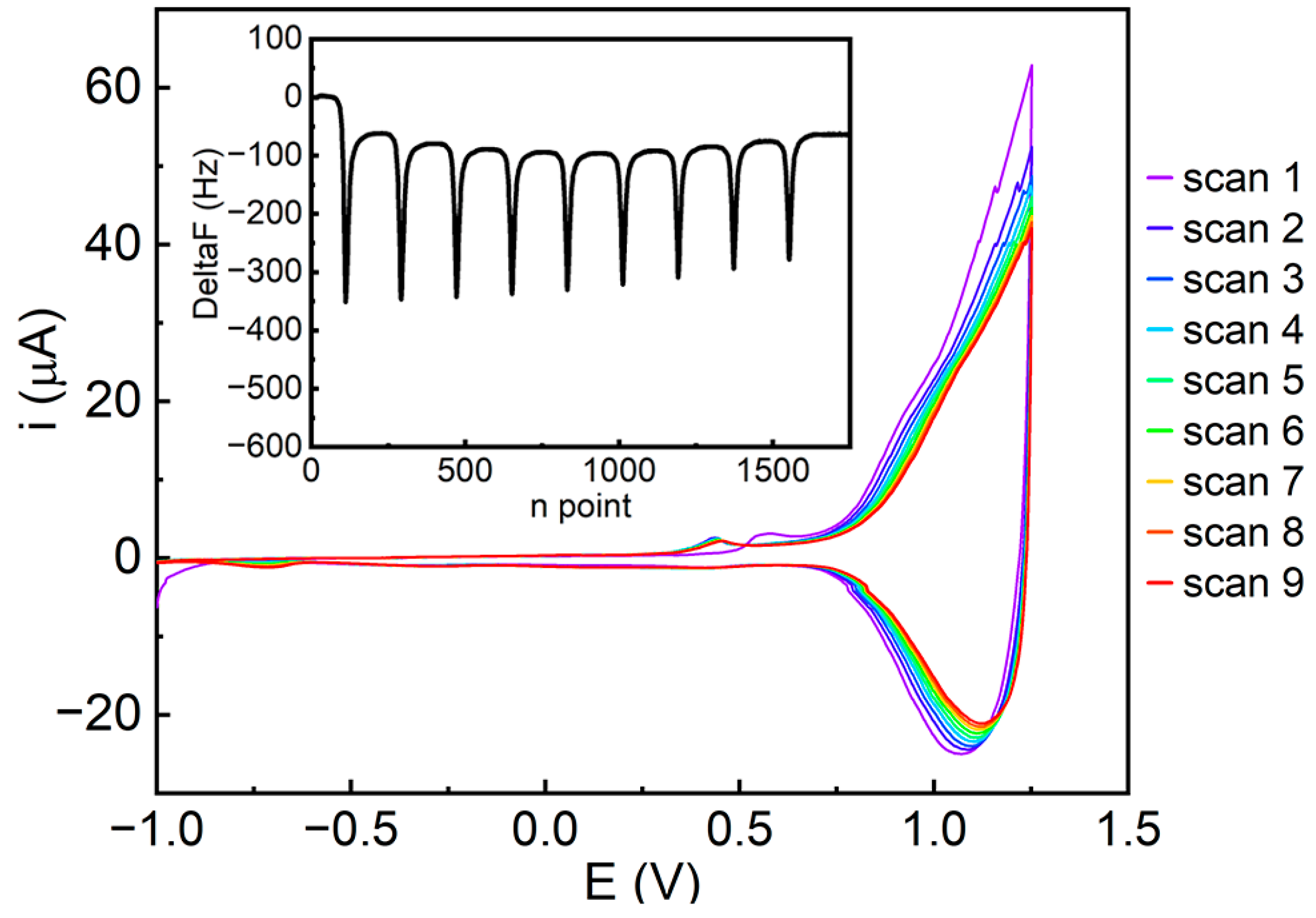
3.3. Electropolymerization Experiments
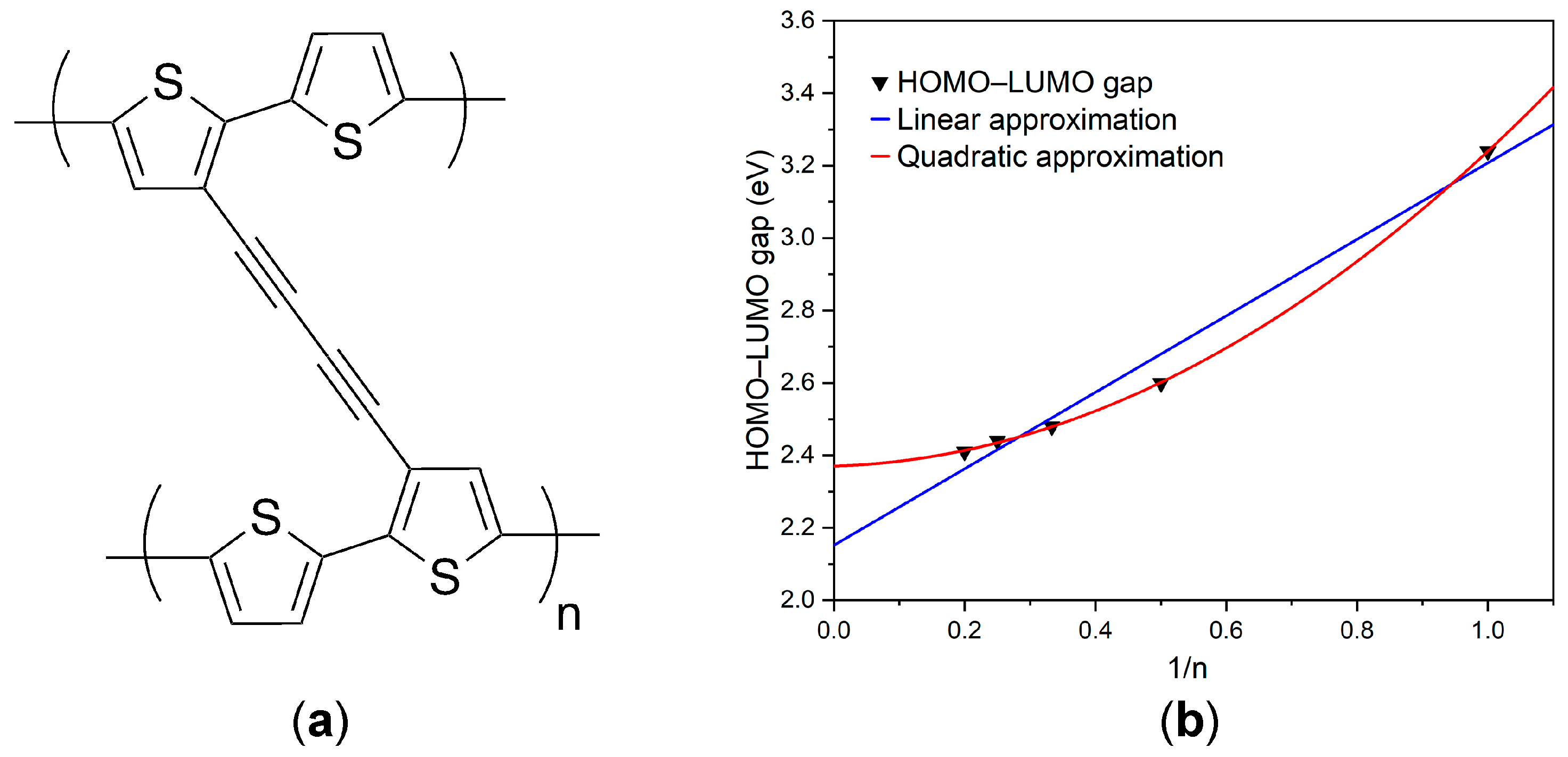
3.4. HOMO–LUMO DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perepichka, I.F.; Perepichka, D.F. Handbook of Thiophene-Based Materials; Wiley-VCH: Chichester, UK, 2009. [Google Scholar]
- Pron, A.; Gawrys, P.; Zagorska, M.; Djurado, D.; Demadrill, R. Electroactive materials for organic electronics: Preparation strategies, structural aspects and characterization techniques. Chem. Soc. Rev. 2010, 39, 2577–2632. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Wan, X.; Li, M.; Wang, Y.; Chen, Y. A–D–A small molecules for solution-processed organic photovoltaic cells. Chem. Commun. 2015, 51, 4936–4950. [Google Scholar] [CrossRef] [PubMed]
- Pathiranage, T.M.S.K.; Dissanayake, D.S.; Niermann, C.N.; Ren, Y.; Biewer, M.C.; Stefan, M.C. Role of polythiophenes as electroactive materials. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3327–3346. [Google Scholar] [CrossRef]
- Zaumseil, J.; Sirringhaus, H. Electron and Ambipolar Transport in Organic Field-Effect Transistors. Chem. Rev. 2007, 107, 1296–1323. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, A.; Afraj, S.N.; Yau, S.; Liu, C.L.; Ezhumalai, Y.; Kumaresan, P.; Chen, M.-C. Fused thiophene based materials for organic thin-film transistors. J. Chin. Chem. Soc. 2022, 69, 1253–1275. [Google Scholar] [CrossRef]
- Zhang, F.; Petr, A.; Kirbach, U.; Dunsch, L. Improved hole injection and performance of multilayer OLED devices via electrochemically prepared-polybithiophene layers. J. Mater. Chem. 2003, 13, 265–267. [Google Scholar] [CrossRef]
- Feng, Z.; Cheng, Z.; Jin, H.; Lu, P. Recent progress of sulphur-containing high-efficiency organic light-emitting diodes (OLEDs). J. Mater. Chem. C 2022, 10, 4497–4520. [Google Scholar] [CrossRef]
- Feng, K.; Xu, X.; Li, Z.; Li, Y.; Li, K.; Yu, T.; Peng, Q. Low band gap benzothiophene–thienothiophene copolymers with conjugated alkylthiothieyl and alkoxycarbonyl cyanovinyl side chains for photovoltaic applications. Chem. Commun. 2015, 51, 6290–6292. [Google Scholar] [CrossRef] [PubMed]
- Pilo, M.I.; Sanna, G.; Spano, N. Conducting Polymers in Amperometric Sensors: A State of the Art over the Last 15 Years with a Focus on Polypyrrole-, Polythiophene-, and Poly(3,4-ethylenedioxythiophene)-Based Materials. Chemosensors 2024, 12, 81. [Google Scholar] [CrossRef]
- McCullough, R.D. The Chemistry of Conducting Polythiophenes. Adv. Mater. 1998, 10, 93–116. [Google Scholar] [CrossRef]
- Ye, S.; Lotocki, V.; Xu, H.; Seferos, D.S. Group 16 conjugated polymers based on furan, thiophene, selenophene, and tellurophene. Chem. Soc. Rev. 2022, 51, 6442–6474. [Google Scholar] [CrossRef]
- Alemán, C.; Julia, L. π Conjugation in 2,2′-Bithiophene and Its Dimethyl Derivatives: Model Compounds of Organic Conducting Polymers Based on Thiophene Rings. J. Phys. Chem. 1996, 100, 1524–1529. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sato, T.; Lijima, T.; Abe, M.; Fukumoto, H.; Koizumi, T.; Usui, M.; Nakamura, Y.; Yagi, T.; Tajima, H.; et al. Highly Coplanar Polythiophenes with –C≡CR Side Chains: Self-Assembly, Linear and Nonlinear Optical Properties, and Piezochromism. Bull. Chem. Soc. Jpn. 2009, 82, 896–909. [Google Scholar] [CrossRef]
- Kurowska, A.; Kostyuchenko, A.S.; Zassowski, P.; Skorka, L.; Yurpalov, V.L.; Fisyuk, A.S.; Pron, A.; Domagala, W. Symmetrically Disubstituted Bithiophene Derivatives of 1,3,4-Oxadiazole, 1,3,4-Thiadiazole, and 1,2,4-Triazole—Spectroscopic, Electrochemical, and Spectroelectrochemical Properties. J. Phys. Chem. C 2014, 118, 25176–25189. [Google Scholar] [CrossRef]
- Slodek, A.; Filapek, M.; Szafraniec, G.; Grudzka, I.; Pisarski, W.A.; Malecki, J.G.; Zur, L.; Grela, M.; Danikiewicz, W.; Krompiec, S. Synthesis, Electrochemistry, Crystal Structures, and Optical Properties of Quinoline Derivatives with a 2,2′-Bithiophene Motif. Eur. J. Org. Chem. 2014, 2014, 5256–5264. [Google Scholar] [CrossRef]
- Debnath, S.; Bedi, A.; Zade, S.S. Thienopentathiepine: A sulfur containing fused heterocycle for conjugated systems and their electrochemical polymerization. Polym. Chem. 2015, 6, 7658–7665. [Google Scholar] [CrossRef]
- Kula, S.; Szlapa-Kula, A.; Krompiec, S.; Gancarz, P.; Filapek, M. An electrochromic behavior of novel polythiophenes obtained from unsymmetrical monomers—A comprehensive study. Synth. Met. 2019, 247, 202–211. [Google Scholar] [CrossRef]
- Zotti, G.; Vercelli, B.; Berlin, A.; Destri, S.; Pasini, M.; Hernández, V.; López Navarrete, J.T. Electrochemical, Magnetic, and Electrical Properties of α,ω-Capped Sexithiophene Films. Part 3. Conduction in Poly(bis-terthienyl-B)s (B = Ethane, Disulfide, Diacetylene, Acetylene, Ethylene). Chem. Mater. 2008, 20, 6847–6856. [Google Scholar] [CrossRef]
- Pilzak, G.S.; van Gruijthuijsen, K.; van Doorn, R.H.; van Lagen, B.; Sudhölter, E.J.R.; Zuilhof, H. Hybrid Conjugated Organic Oligomers Consisting of Oligodiacetylene and Thiophene Units: Synthesis and Optical Properties. Chem. Eur. J. 2009, 15, 9085–9096. [Google Scholar] [CrossRef]
- Krompiec, S.; Filapek, M.; Grudzka, I.; Kula, S.; Słodek, A.; Skórka, Ł.; Danikiewicz, W.; Ledwon, P.; Lapkowski, M. An Ambipolar Behavior of Novel Ethynyl-Bridged Polythiophenes—A Comprehensive Study. Synth. Met. 2013, 165, 7–16. [Google Scholar] [CrossRef]
- Meng, Q.; Gao, J.; Li, R.; Jiang, L.; Wang, C.; Zhao, H.; Liu, C.; Li, H.; Hu, W. New type of organic semiconductors for field-effect transistors with carbon-carbon triple bonds. J. Mater. Chem. 2009, 19, 1477–1482. [Google Scholar] [CrossRef]
- Meng, D.; Zheng, R.; Zhao, Y.; Zhang, E.; Dou, L.; Yang, Y. Near-Infrared Materials: The Turning Point of Organic Photovoltaics. Adv. Mater. 2022, 34, 2107330. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.L.; Stachelek, P.; Takeda, Y.; Pander, P. Recent advances in highly-efficient near infrared OLED emitters. Mater. Chem. Front. 2024, 8, 1731–1766. [Google Scholar] [CrossRef]
- Giannetto, M.; Pedrini, A.; Fortunati, S.; Brando, D.; Milano, S.; Massera, C.; Tatti, R.; Verucchi, R.; Careri, M.; Dalcanale, E. Sensing of Halogenated Aromatic Hydrocarbons in Water with a Cavitand Coated Piezoelectric Device. Sens. Actuators B Chem. 2018, 276, 340–348. [Google Scholar] [CrossRef]
- Stefani, H.A.; Guarezemini, A.S.; Cella, R. Homocoupling reactions of alkynes, alkenes and alkyl compounds. Tetrahedron 2010, 66, 7871–7918. [Google Scholar] [CrossRef]
- Lei, A.; Srivastava, M.; Zhang, X. Transmetalation of Palladium Enolate and Its Application in Palladium-Catalyzed Homocoupling of Alkynes: A Room-Temperature, Highly Efficient Route To Make Diynes. J. Org. Chem. 2002, 67, 1969–1971. [Google Scholar] [CrossRef]
- Krompiec, S.; Filapek, M.; Grudzka-Flak, I.; Slodek, A.; Kula, S.; Malecki, J.G.; Malarz, J.; Szafraniec-Gorol, G.; Penkala, M.; Schab-Balcerzak, E.; et al. Multifaceted Strategy for the Synthesis of Diverse 2,2′-Bithiophene Derivatives. Molecules 2015, 20, 4565–4593. [Google Scholar] [CrossRef] [PubMed]
- Pammer, F.; Guo, F.; Lalancette, R.A.; Jaekle, F. Synthesis, Structures, and Hydroboration of Oligo- and Poly(3-alkynylthiophene)s. Macromolecules 2012, 45, 6333–6343. [Google Scholar] [CrossRef]
- Djukic, B.; Poddutoori, P.K.; Dube, P.A.; Seda, T.; Jenkins, H.A.; Lemaire, M.T. Preparation and Magnetic Properties of Iron(3+) Spin-Crossover Complexes Bearing a Thiophene Substituent: Toward Multifunctional Metallopolymers. Inorg. Chem. 2009, 48, 6109–6116. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, C.; Terzi, F.; Seeber, R. Polythiophenes and polythiophene-based composites in amperometric sensing. Anal. Bioanal. Chem. 2013, 405, 509–531. [Google Scholar] [CrossRef]
- AL-Refai, H.H.; Ganash, A.A.; Hussein, M.A. Polythiophene and its derivatives –Based nanocomposites in electrochemical sensing: A mini review. Mater. Today Commun. 2021, 26, 101935. [Google Scholar] [CrossRef]
- Zotti, G.; Zecchin, S.; Schiavon, G.; Vercelli, B.; Berlin, A.; Dalcanale, E.; Groenendaal, L.B. Potential-Driven Conductivity of Polypyrroles, Poly-N-Alkylpyrroles, and Polythiophenes: Role of the Pyrrole NH Moiety in the Doping-Charge Dependence of Conductivity. Chem. Mater. 2003, 15, 4642–4650. [Google Scholar] [CrossRef]
- Dong, J.; Nimori, S.; Goto, H. Conjugated Polymer Films Having a Uniaxial Molecular Orientation and Network Structure Prepared by Electrochemical Polymerization in Liquid Crystals. Polymers 2021, 13, 2425. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H.P.; Izmaylov, A.F.; Bloino, J.; Zheng, G.; Sonnenberg, J.L.; et al. Gaussian 09 (Revision D.01); Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zade, S.S.; Bendikov, M. From Oligomers to Polymer: Convergence in the HOMO−LUMO Gaps of Conjugated Oligomers. Org. Lett. 2006, 8, 5243–5246. [Google Scholar] [CrossRef]
- SADABS Bruker AXS; Madison, Wisconsin, USA, 2004; SAINT, Software Users Guide, Version 6.0; Bruker Analytical X-ray Systems, Madison, WI (1999). Sheldrick, G.M. SADABS v2.03: Area-Detector Absorption Correction. University of Göttingen, Germany, 1999.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef]
- Kanazawa, K.K.; Gordon, J.G., II. Frequency of a Quartz Microbalance in Contact with Liquid. Anal. Chem. 1985, 57, 1770–1771. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrini, A.; Massera, C.; Dalcanale, E.; Giannetto, M.; Pinalli, R. Synthesis, Crystal Structure, and Electropolymerization of 1,4-Di([2,2′-bithiophen]-3-yl)buta-1,3-diyne. Crystals 2024, 14, 620. https://doi.org/10.3390/cryst14070620
Pedrini A, Massera C, Dalcanale E, Giannetto M, Pinalli R. Synthesis, Crystal Structure, and Electropolymerization of 1,4-Di([2,2′-bithiophen]-3-yl)buta-1,3-diyne. Crystals. 2024; 14(7):620. https://doi.org/10.3390/cryst14070620
Chicago/Turabian StylePedrini, Alessandro, Chiara Massera, Enrico Dalcanale, Marco Giannetto, and Roberta Pinalli. 2024. "Synthesis, Crystal Structure, and Electropolymerization of 1,4-Di([2,2′-bithiophen]-3-yl)buta-1,3-diyne" Crystals 14, no. 7: 620. https://doi.org/10.3390/cryst14070620
APA StylePedrini, A., Massera, C., Dalcanale, E., Giannetto, M., & Pinalli, R. (2024). Synthesis, Crystal Structure, and Electropolymerization of 1,4-Di([2,2′-bithiophen]-3-yl)buta-1,3-diyne. Crystals, 14(7), 620. https://doi.org/10.3390/cryst14070620







