Effect of Fluoroalcohol Chain Extension Modified HTPB Binder on the Combustion Performance of Aluminized Propellants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FPUs
2.3. Preparation of FPU0/Al/AP and FPU5/Al/AP Propellants
2.4. Characterization
3. Results and Discussion
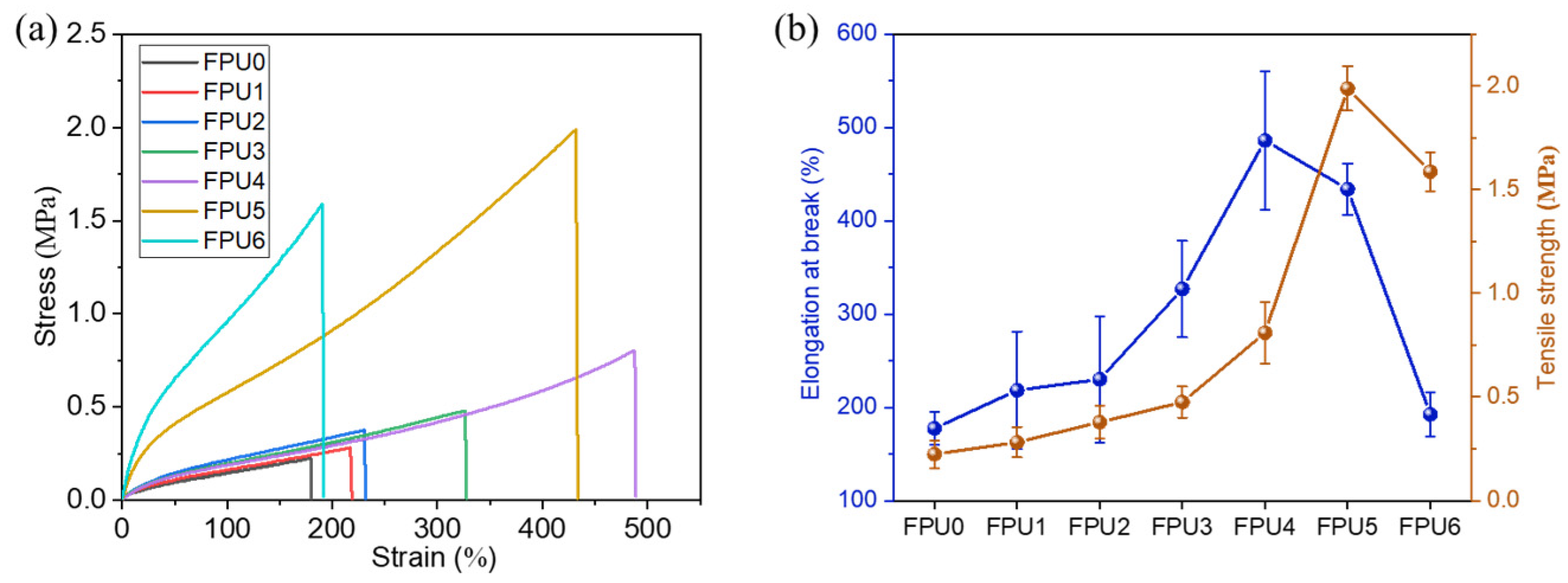
3.1. Mechanical Properties of FPUs
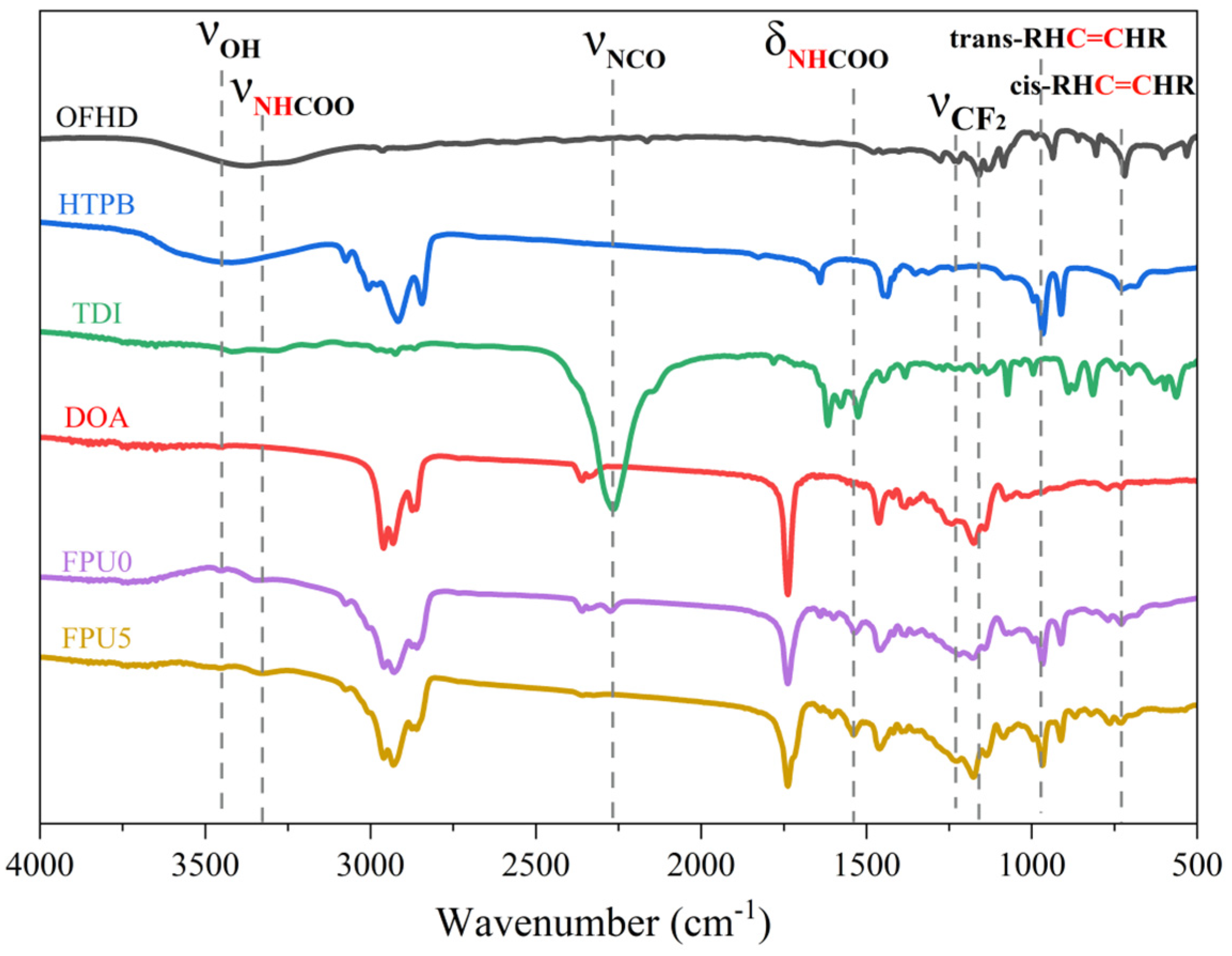
3.2. FTIR Spectra Analyses
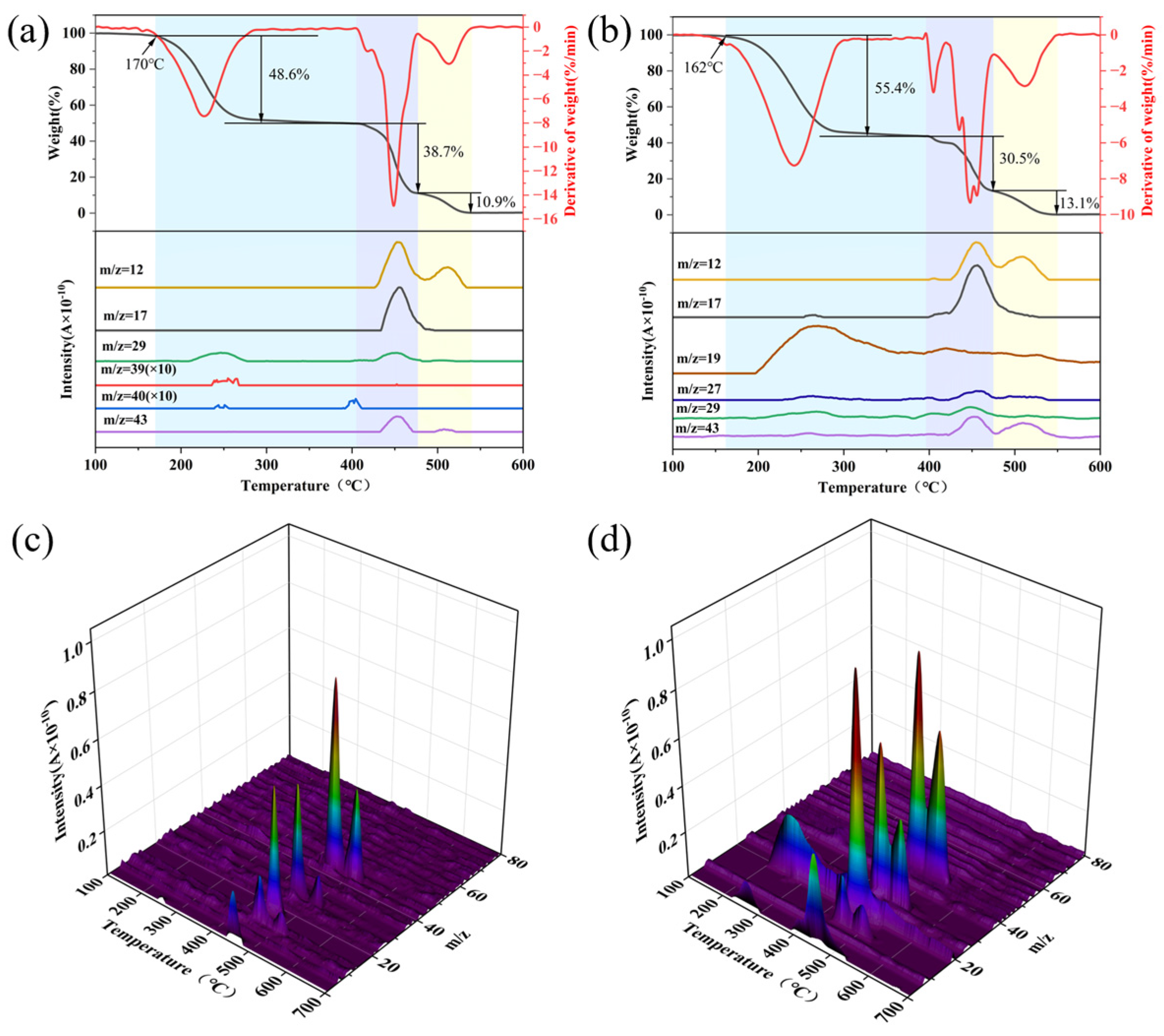
3.3. Thermal Decomposition of FPUs
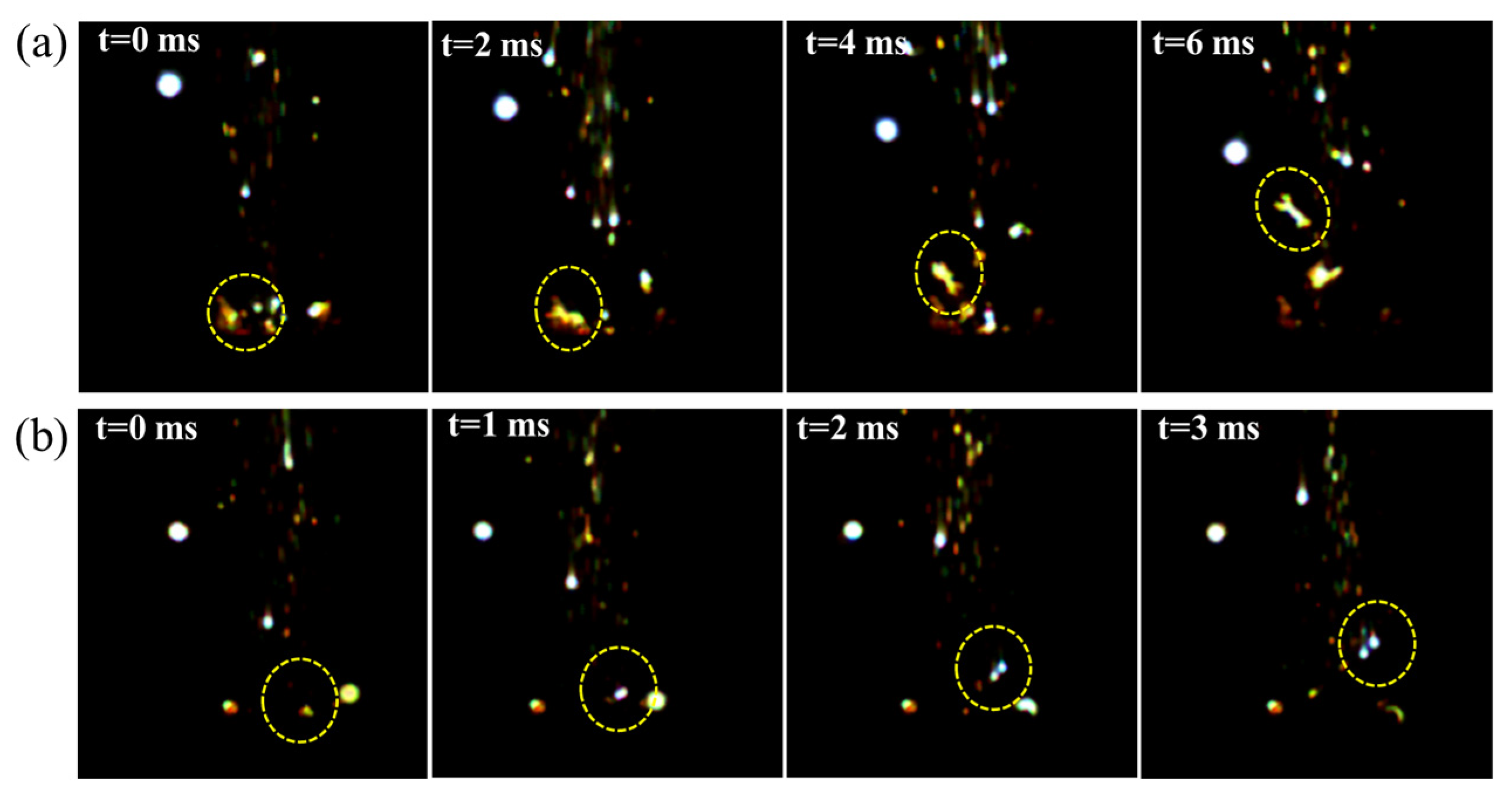
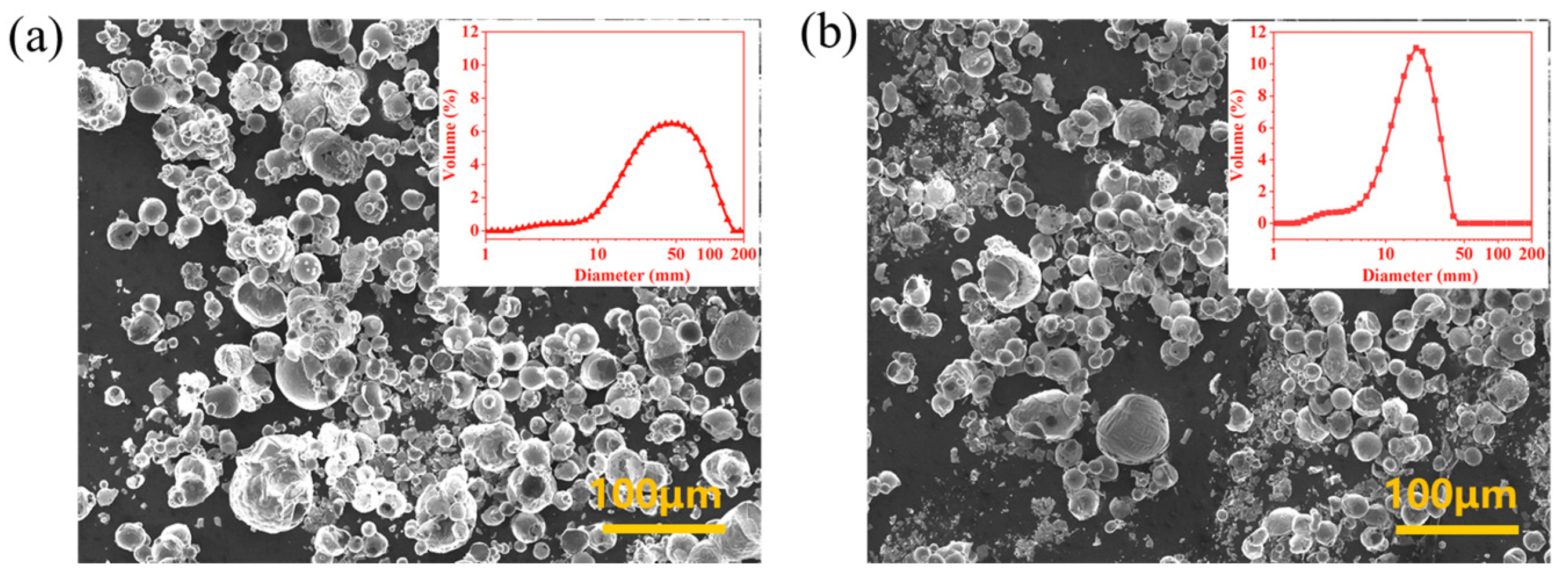
3.4. Comparison of Combustion Properties
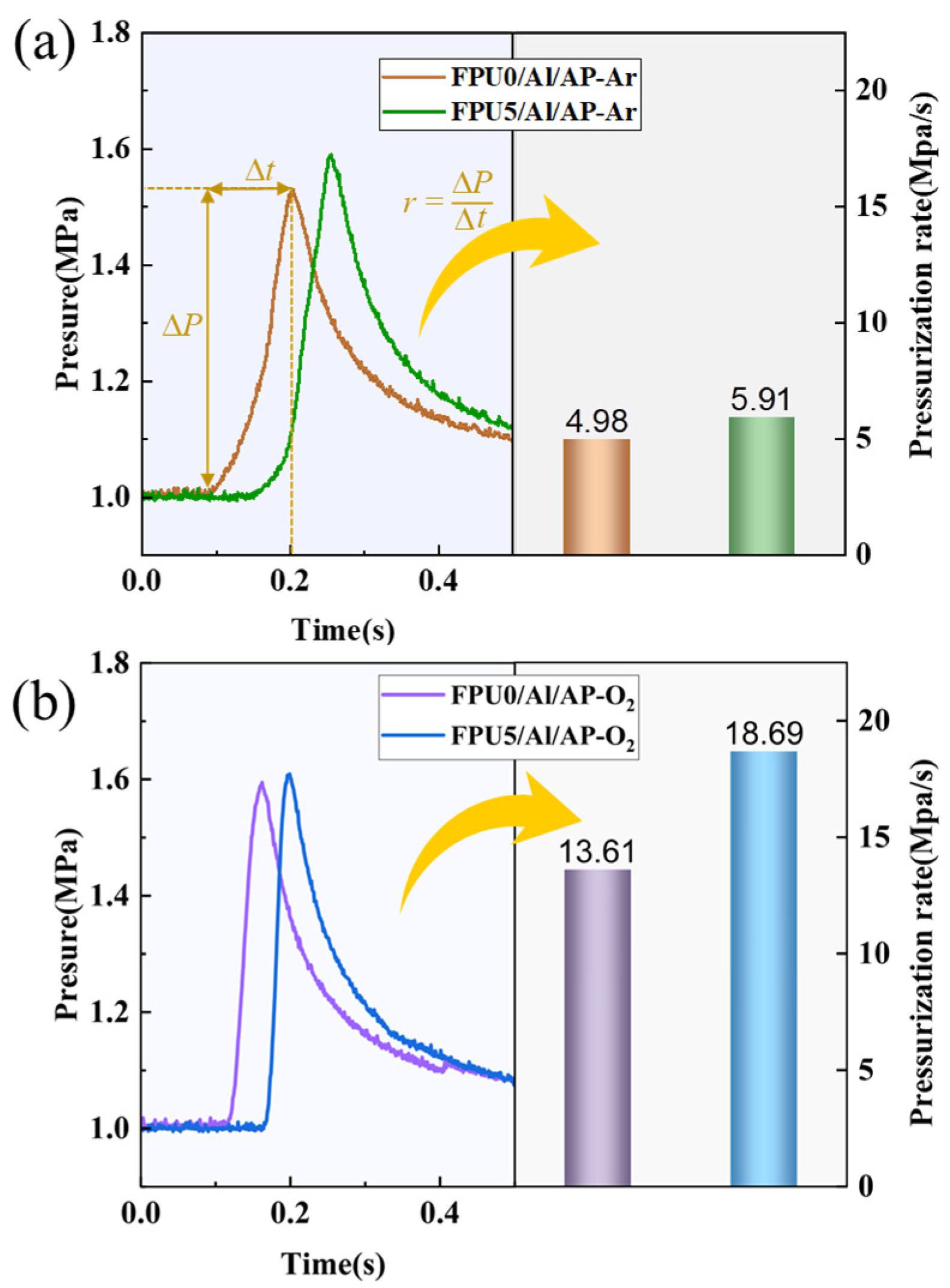
3.5. Dynamic Pressure Characteristics and Heat Release
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Choi, J.H.; Hong, I.-K.; Lee, J.W. Curing Behavior of Polyurethane as a Binder for Polymer-Bonded Explosives. J. Ind. Eng. Chem. 2015, 21, 980–985. [Google Scholar] [CrossRef]
- Liu, Z.; Nie, J.; Fan, W.; Tao, J.; Jiang, F.; Guo, T.; Gao, K. Simulation Analysis of the Safety of High-Energy Hydroxyl-Terminated Polybutadiene (HTPB) Engine under the Impact of Fragments. Crystals 2023, 13, 394. [Google Scholar] [CrossRef]
- Gopala Krishnan, P.S.; Ayyaswamy, K.; Nayak, S.K. Hydroxy Terminated Polybutadiene: Chemical Modifications and Applications. J. Macromol. Sci. Part A 2013, 50, 128–138. [Google Scholar] [CrossRef]
- Lemos, M.F.; Mendes, L.C.; Bohn, M.; Keicher, T. Application of Azide-Containing Molecules as Modifiers of HTPB. J. Therm. Anal. Calorim. 2019, 137, 411–419. [Google Scholar] [CrossRef]
- Quagliano Amado, J.C.; Ross, P.G.; Mattos Silva Murakami, L.; Narciso Dutra, J.C. Properties of Hydroxyl-Terminal Polybutadiene (HTPB) and Its Use as a Liner and Binder for Composite Propellants: A Review of Recent Advances. Propellants Explos. Pyrotech. 2022, 47, e202100283. [Google Scholar] [CrossRef]
- Yadav, A.; Pant, C.S.; Das, S. Research Advances in Bonding Agents for Composite Propellants. Propellants Explos. Pyrotech. 2020, 45, 695–704. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, G.; Du, J.; Li, J.; Li, J.; Tang, Q.; Fan, X. Investigation of Hydroxyl-Terminated Polyether Cured with Different Isocyanates: Curing Process and Mechanical Property. Propellants Explos. Pyrotech. 2020, 45, 1972–1979. [Google Scholar] [CrossRef]
- Shen, C.; Yan, S.; Ou, Y.; Jiao, Q. Influence of Fluorinated Polyurethane Binder on the Agglomeration Behaviors of Aluminized Propellants. Polymers 2022, 14, 1124. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, H.J.; Liu, D.J.; Li, Y.T. The Effect of Polyester Structures on the Damping Property of Polyurethane Elastomers. Adv. Mater. Res. 2012, 581–582, 710–714. [Google Scholar] [CrossRef]
- Yetter, R.A.; Risha, G.A.; Son, S.F. Metal Particle Combustion and Nanotechnology. Proc. Combust. Inst. 2009, 32, 1819–1838. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, K.; Liu, H.; Hu, C. Incorporating Fluoropolymer-Coated Micron-Sized Aluminum with Enhanced Reactivity into Aluminized Explosives to Improve Their Detonation Performance. Energ. Mater. Front. 2023, 4, 103–109. [Google Scholar] [CrossRef]
- Tang, W.; Yang, R.; Zeng, T.; Li, J.; Hu, J.; Zhou, X.; Jiang, E.; Zhang, Y. Positive Effects of Organic Fluoride on Reduction of Slag Accumulation in Static Testing of Solid Rocket Motors of Different Diameters. Acta Astronaut. 2022, 194, 277–285. [Google Scholar] [CrossRef]
- Tang, G.; Wang, D.; Chen, C.; Luo, Y.; Li, X. High Performance Thermoplastic Energetic Composites Using Fluorinated Energetic Polyurethanes as Binder. Propellants Explos. Pyrotech. 2023, 48, e202200249. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, S.; Sun, Y.; Ji, Y.; Wang, J. Fluorinated Graphene Improving Thermal Reaction and Combustion Characteristics of Nano-Aluminum Powder. Thermochim. Acta 2021, 705, 179038. [Google Scholar] [CrossRef]
- Liu, M. Analysis of Agglomeration Behaviors of Aluminized Composite Propellants. Case Stud. Therm. Eng. 2023, 44, 102852. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, B.; Sun, Y. Study on the Combustion Performance of Nano/Micro-Sized Aluminum Powders Regulated by Polydopamine Interface. Combust. Flame 2022, 240, 112027. [Google Scholar] [CrossRef]
- Sippel, T.R.; Son, S.F.; Groven, L.J. Aluminum Agglomeration Reduction in a Composite Propellant Using Tailored Al/PTFE Particles. Combust. Flame 2014, 161, 311–321. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, M.; Huang, F.; Yang, R.; Guo, X. Effect of Organic Fluoride on Combustion Agglomerates of Aluminized HTPB Solid Propellant. Propellants Explos. Pyrotech. 2017, 42, 417–422. [Google Scholar] [CrossRef]
- Shen, C.; Yan, S.; Sun, X.; Tan, Y.; Ou, Y.; Jiao, Q. Oxidizability Enhancement of Hydroxyl Terminated Polyether Polyurethane Elastomers via Fluoroalcohols Grafting. Propellants Explos. Pyrotech. 2022, 47, e202200048. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Gong, L.; Xiao, F.; Yang, R.; Meng, L. Effect of Organic Fluoride on Combustion Performance of HTPB Propellants with Different Aluminum Content. Combust. Sci. Technol. 2021, 193, 702–715. [Google Scholar] [CrossRef]
- Zhao, C.C.; Lu, G.L.; Wang, B.H. Effect of Chain Extender on Mechanical Properties of HTPB/IPDI Propellants. J. Solid Rocket Technol. 2000, 23, 52–55. [Google Scholar]
- Li, J.-W.; Lee, H.-T.; Tsai, H.-A.; Suen, M.-C.; Chiu, C.-W. Synthesis and Properties of Novel Polyurethanes Containing Long-Segment Fluorinated Chain Extenders. Polymers 2018, 10, 1292. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Khan, M.B.; Ma, X.; Ul-Haq, N. The Influence of Cross-Linking/Chain Extension Structures on Mechanical Properties of HTPB-Based Polyurethane Elastomers. Arab. J. Sci. Eng. 2014, 39, 43–51. [Google Scholar] [CrossRef]
- Chen, J.K.; Brill, T.B. Chemistry and Kinetics of Hydroxyl-Terminated Polybutadiene (HTPB) and Diisocyanate-HTPB Polymers during Slow Decomposition and Combustion-like Conditions. Combust. Flame 1991, 87, 217–232. [Google Scholar] [CrossRef]
- Rastogi, A.C.; Desu, S.B. Thermal Chemical Vapor Deposition of Fluorocarbon Polymer Thin Films in a Hot Filament Reactor. Polymer 2005, 46, 3440–3451. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Z.; Yang, Y.; Shen, J.; Long, Z.; Li, Z.; Cui, X.; Yang, G. Core–Shell Al-Polytetrafluoroethylene (PTFE) Configurations to Enhance Reaction Kinetics and Energy Performance for Nanoenergetic Materials. Chem.—Eur. J. 2016, 22, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Rogulska, M.; Kultys, A.; Pikus, S. Studies on Thermoplastic Polyurethanes Based on New Diphenylethane-Derivative Diols. III. The Effect of Molecular Weight and Structure of Soft Segment on Some Properties of Segmented Polyurethanes. J. Appl. Polym. Sci. 2008, 110, 1677–1689. [Google Scholar] [CrossRef]
- El-Basuony, S.A.; Sadek, M.A.; Wafy, T.Z.; Mostafa, H.E. Thermokinetic Studies of Polyurethanes Based on Hydroxyl-Terminated Polybutadiene Prepolymer. J. Therm. Anal. Calorim. 2018, 131, 2013–2019. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, Z.; Chen, L.; Liu, P.; Jia, L.; Dong, B.; Li, H.; Xu, S. A Study on the Decomposition Pathways of HTPB and HTPE Pyrolysis by Mass Spectrometric Analysis. J. Anal. Appl. Pyrolysis 2023, 170, 105929. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.; Baek, J.; Ka, D.; Huynh, A.H.; Wang, Y.; Zachariah, M.R.; Zheng, X. Perfluoroalkyl-Functionalized Graphene Oxide as a Multifunctional Additive for Promoting the Energetic Performance of Aluminum. ACS Nano 2022, 16, 14658–14665. [Google Scholar] [CrossRef]
- Tu, C.; Chen, X.; Li, Y.; Zhang, B.; Zhou, C. Experimental Study of Al Agglomeration on Solid Propellant Burning Surface and Condensed Combustion Products. Def. Technol. 2023, 26, 111–122. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, F.; Yang, R.; Li, J.; Li, S.; Li, Z.; Zhang, W.; Jiang, E.; Zhang, Y. Properties of Agglomerate-Reduced Propellant Under Solid Rocket Motor Conditions. J. Propuls. Power 2019, 35, 352–358. [Google Scholar] [CrossRef]
- Malchi, J.Y.; Yetter, R.A.; Foley, T.J.; Son, S.F. The Effect of Added Al2O3 on the Propagation Behavior of an Al/CuO Nanoscale Thermite. Combust. Sci. Technol. 2008, 180, 1278–1294. [Google Scholar] [CrossRef]
- Tang, G.; Wang, H.; Chen, C.; Xu, Y.; Chen, D.; Wang, D.; Luo, Y.; Li, X. Thermal Decomposition of Nano Al-Based Energetic Composites with Fluorinated Energetic Polyurethane Binders: Experimental and Theoretical Understandings for Enhanced Combustion and Energetic Performance. RSC Adv. 2022, 12, 24163–24171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, W.; Jin, P.; Ding, S.; Liu, S.; Zhao, S.; Yang, W.; Liu, W.; Luo, Y. Effect of Azide Polyether Pyrolysis Property on Combustion and Heat Release of Boron-Based Fuel-Rich Propellant. Combust. Flame 2022, 245, 413–422. [Google Scholar] [CrossRef]

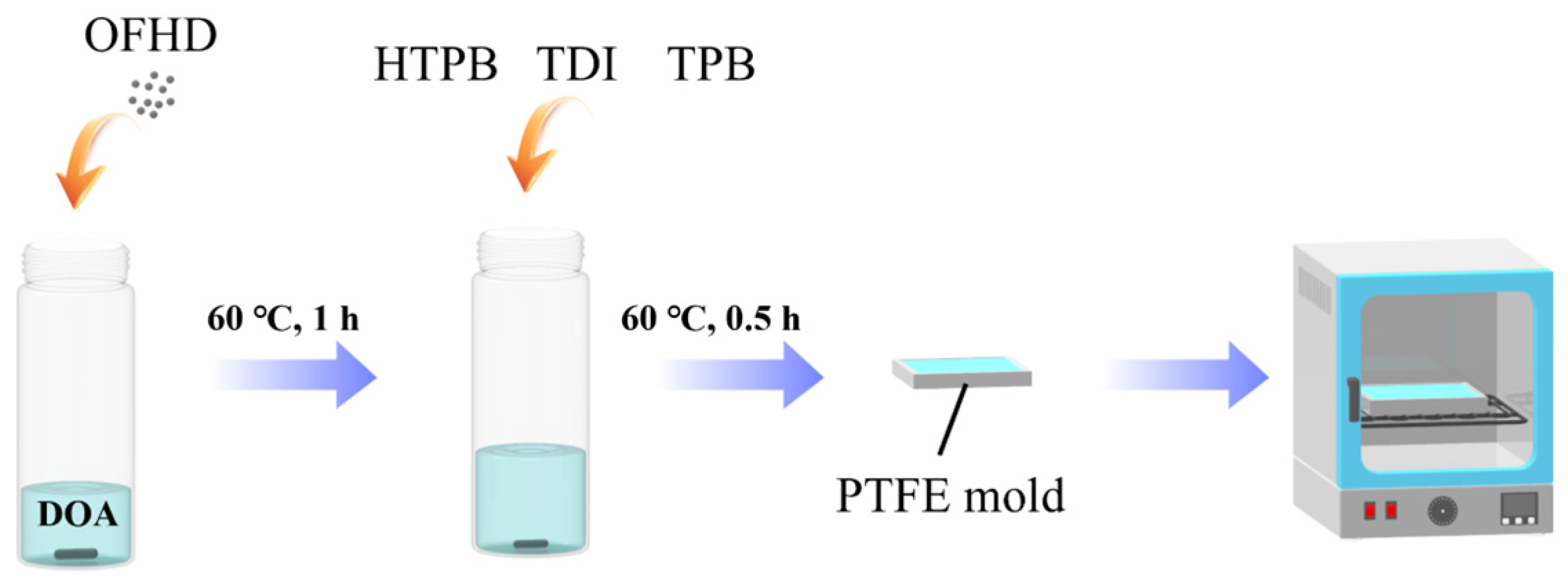

| Samples | Ratio * | HTPB/g | OFHD/g | TDI/g | DOA/g | TPB |
|---|---|---|---|---|---|---|
| FPU0 | 1/0/1 | 4.50 | 0 | 0.29 | 4.50 | 0.05% |
| FPU1 | 3/1/4 | 4.50 | 0.15 | 0.39 | 4.50 | |
| FPU2 | 2/1/3 | 4.50 | 0.22 | 0.44 | 4.50 | |
| FPU3 | 1/1/2 | 4.50 | 0.44 | 0.58 | 4.50 | |
| FPU4 | 1/2/3 | 4.50 | 0.87 | 0.87 | 4.50 | |
| FPU5 | 1/3/4 | 4.50 | 1.31 | 1.16 | 4.50 | |
| FUP6 | 1/4/5 | 4.50 | 1.75 | 1.45 | 4.50 |
| m/z | Fragment Ions | m/z | Fragment Ions |
|---|---|---|---|
| 12 | C | 29 | C2H5, CHO |
| 14 | CH2 | 30 | CH2NH2, C2H6, CHO |
| 15 | CH3 | 31 | CF, CH3NH2 |
| 16 | O, CH4 | 39 | C3H3 |
| 17 | OH, NH3 | 42 | NCO, C3H6 |
| 18 | H2O | 43 | C3H7, HNCO |
| 19 | F− | 44 | CO2, C2H4NH2 |
| 26 | C2H2 | 68 | C5H8 |
| 27 | C2H3 | 72 | C4H8O |
| Samples | The First Stage/°C | The Second Stage/°C | The Third Stage/°C |
|---|---|---|---|
| FPU0 | 170~405 | 405~477 | 477~540 |
| FPU5 | 162~396 | 396~475 | 475~550 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Chang, K.; Yao, J.; Guo, X.; Shen, C.; Yan, S. Effect of Fluoroalcohol Chain Extension Modified HTPB Binder on the Combustion Performance of Aluminized Propellants. Crystals 2024, 14, 258. https://doi.org/10.3390/cryst14030258
Huang Y, Chang K, Yao J, Guo X, Shen C, Yan S. Effect of Fluoroalcohol Chain Extension Modified HTPB Binder on the Combustion Performance of Aluminized Propellants. Crystals. 2024; 14(3):258. https://doi.org/10.3390/cryst14030258
Chicago/Turabian StyleHuang, Yanjie, Kanghua Chang, Jie Yao, Xueyong Guo, Chen Shen, and Shi Yan. 2024. "Effect of Fluoroalcohol Chain Extension Modified HTPB Binder on the Combustion Performance of Aluminized Propellants" Crystals 14, no. 3: 258. https://doi.org/10.3390/cryst14030258
APA StyleHuang, Y., Chang, K., Yao, J., Guo, X., Shen, C., & Yan, S. (2024). Effect of Fluoroalcohol Chain Extension Modified HTPB Binder on the Combustion Performance of Aluminized Propellants. Crystals, 14(3), 258. https://doi.org/10.3390/cryst14030258






