Abstract
Brushite cements (BrCs) are calcium phosphate-based materials that are being widely used in hard tissue engineering applications due to their osteoconductivity, injectability, and bioresorbability. Therefore, the goal was to evaluate the effects of Mg concentration on the phase composition, setting time, and strength of BrC. Mg, which plays a vital role in bodily functions and bone health, was added to BrC at concentrations of 0.25, 0.50, 1.00, 1.50, 2.00, and 2.50 wt.%. The results showed that Mg stabilizes the TCP structure and increases the TCP content in final BrC. The initial and final setting times of BrCs increase with higher concentrations of Mg. Although 0.25 wt.% Mg did not change the setting of BrCs significantly, a higher concentration of 1.00 wt.% increased the initial setting time from 4.87 ± 0.38 min to 15.14 ± 0.88 min. Cements with Mg concentrations of 1.5 wt.% and above did not set after 4 h. Mg addition up to 0.5 wt.% did not change the compressive strength; however, higher concentrations decreased it significantly and 2.5 Mg-BrC had the lowest strength of 0.45 ± 0.09 MPs. Together, our results show that Mg can be added up to 1.00 wt.% without any adverse effect on the physical and mechanical properties of BrC.
1. Introduction
Calcium phosphate cements (CPCs) are attractive forms of calcium phosphate ceramics (CaPs) due to properties such as injectability, moldability, osteoconductivity, and being compositionally similar to natural bone and teeth [1,2]. There are two main types of CPCs: apatite and brushite (BrC). Apatite cements have end products of hydroxyapatite (HA) or calcium-deficient hydroxyapatite (CDHA), while BrCs have dicalcium phosphate dihydrate (DCPD) as the final product. Although apatite cements have lower solubility and higher mechanical strength compared to BrCs, the solubility of BrCs allows for faster material degradation and enhanced tissue ingrowth [3]. Furthermore, BrCs have superior osteoinductivity but are mechanically weaker and more difficult to work with due to their short setting time [4]. Research to improve BrCs physical, mechanical, and biological properties is still in progress despite the cements being used commercially.
Chemical modification is a commonly used method to modify the properties of CaPs and CPCs. Elements present in small amounts in the body, trace elements, are important for a variety of physiological and metabolic processes in the body [5]. These elements can be incorporated into the structure of CPCs or applied as a coating to improve the physical, chemical, mechanical, and biological properties of the ceramics. In BrC systems, the CPC of interest in current research, iron (Fe) doping, increases the setting time and inhibits the growth of S. aureus and P. aeruginosa [6,7]. Silicon (Si) enhances the compressive strength of BrC and the addition of a high concentration of 1.1 wt.% upregulates bone formation and vasculogenesis in vivo [8]. Strontium (Sr) prolongs the initial and final setting time and improves the injectability of BrC up to 66% [9].
Among different elements, magnesium (Mg) has received significant attention due to its contribution to various functions in the body such as bone development, vitamin D activation, cell function stability, and cell repair [10,11]. Depending on age and gender, supplementary Mg with dosages of 250–1800 mg enhances bone mineral density (BMD), decreases the risk of bone fracture, and improves bone turnover in patients with osteoporosis [12,13]. However, high concentrations of Mg are associated with impaired bone mineralization by retarding collagen and calcium phosphate production [14]. Due to beneficiary effects of Mg in bone repair and regeneration through enhanced osteogenesis and angiogenesis, Mg-based biomaterials including Mg alloys, bioceramics, and biopolymers have been used for orthopedic and dental applications [15]. Among different bioceramics, Mg-substituted calcium phosphates are promising candidates in bone and dental tissue engineering applications. During substitution, Mg alters the crystal size and the degradation behavior of the ceramic. Ca2+ substitution with Mg2+ cation stabilizes the β-TCP structure, which prevents the formation of α-TCP (TCP with higher solubility than β-TCP) at lower sintering temperatures [13,14,16]. On the other hand, Mg substitution in HA increases the osteoconductivity but enhances the resorption rate as well [17]. This proves the importance of the CaP system and Mg presence in degradation behavior and tissue response to the material. Mg has been added to CPCs using various methods. Alkhraisat et al. used solid-state synthesized Mg-substituted β-TCP to fabricate Mg-BrC by mixing the Mg-TCP with monocalcium phosphate monohydrate (MCPM) and water. Mg addition up to 13.3% increases the setting time to more than 90 min and a further increase to 53.3% decreases it to 30 min. Controlled release of vancomycin from this system to treat Staphylococcus aureus infection depends on the Mg content and can be used to treat infection during bone regeneration procedures [18]. Mg-BrC has been synthesized through direct precipitation from Mg nitrate and ammonium dihydrogen phosphate to obtain TCP, followed by mixing with MCPC and water. Increases in Mg content and the ratio of Mg/(Ca + Mg) from 0.00 to 0.10 increases setting time from 4 min to 33 min and the compressive strength from 1.323 to 20.98 MPa [19]. The differences in the setting time and compressive strength show the importance of the cement fabrication method (a type of precursors used to prepare the TCP and mixing agent) in relation to the properties of the final product.
In this study, our goal was to incorporate Mg at different concentrations into the BrC crystal structure through the solid-state synthesis method and evaluate its effect on phase composition, setting behavior, and mechanical properties of the brushite. We hypothesized that the incorporation of Mg into TCP through solid-state synthesis will alter the physical and mechanical properties of the brushite cement. To validate our hypothesis, MgO was used to substitute Mg2+ for Ca2+ in the TCP lattice structure up to 2.5 wt.%. The TCP was then combined with MCPM to form the BrC end product. The phase composition, setting time, and the compressive strength were evaluated by using an X-ray diffractometer, the Gillmore needle, and the universal testing machine, respectively. To the best of our knowledge, no previous research has studied the effect of Mg incorporation through the solid-state synthesis method on the physical and mechanical properties of BrCs.
2. Materials and Methods
2.1. TCP Powder Preparation
To begin, TCP powder was prepared by using the solid-state synthesis method as explained in our previous works [20,21]. A mixture of two moles of calcium hydrogen phosphate (Alfa Aesar, ≥98.0%, Tewksbury, MA, USA) and one mole of calcium carbonate (Sigma-Aldrich, ≥99.0%, Louis, MO, USA) were ball-milled for 2 h with a milling media to a powder ratio of 4:1. The mixture was placed in a muffle furnace to calcinate for 24 h and was then cooled naturally to room temperature. Once cooled, the coarse powder was divided for further processing to produce a finer particle size. To make the fine TCP powder, coarse TCP was mixed with ethanol and milling media for 6 h with a powder/ethanol/milling media ratio of 1:1.5:5. After mixing, the ethanol was evaporated in an oven at 60 °C for 3–4 days until completely dried. To make magnesium-substituted TCP powder, relative amounts of magnesium oxide (Sigma-Aldrich, ≥99.99%, Louis, MO, USA) were added to make 0.25, 0.50, 1.00, 1.50, 2.00, and 2.50 wt.% magnesium compositions. The powders were prepared in the same manner as the pure TCP powder. The molar ratios of Ca/P and (Ca + Mg)/P were kept at 1.5 for all compositions. From now on, TCPs with 0.00, 0.25, 0.50, 1.00, 1.50, 2.00, and 2.50 wt.% of Mg will be denoted as pure, 0.25, 0.50, 1.00, 1.50, 2.00, and 2.50-Mg, respectively.
2.2. Brushite Cement Preparation
To prepare brushite cement powder, both fine TCP and coarse TCP were mixed with MCPM (Sigma-Aldrich, ≥85.0%, Louis, MO, USA) according to our previously published research [22]. Other precursors such as magnesium hydrogen phosphate trihydrate (Acros Organics, 98.0%, Pittsburgh, PA, USA), and small amounts of sodium hydrogen phosphate (Sigma-Aldrich, ≥99.0%, Louis, MO, USA) and magnesium sulphate were used to control setting time. After mixing in a mortar and pestle for 30 min, the cement powder was further mixed with a 2.0 wt.% polyethylene (PEG) solution. A powder-to-liquid ratio (P/L) of 3.33:1 was used to create a cement paste. Once the cement paste was formed, a 6 mm diameter and 12 mm height mold was filled, covered with a glass slide, and placed in an incubator at 37 °C with 100% relative humidity to set for 1 h. The samples were then removed from the mold and submerged in a phosphate-buffered solution (PBS) with a pH of 7.4 ± 0.2, then kept at 37 °C for 24 h to complete the setting reaction [22]. Samples with 0.00, 0.25, 0.50, 1.00, 1.50, 2.00, and 2.50 wt.% of Mg are denoted as pure, 0.25, 0.50, 1.00, 1.50, 2.00, and 2.50-Mg BrC, respectively. With the exception of setting time measurement, all characterizations were performed after the samples had been dried at room temperature
2.3. Phase Composition
Phase analysis of the samples was completed by X-ray diffraction (Rigaku D/MAX diffractometer, Rigaku, Japan) using a CuKα radiation source and Ni filter. For both TCP and BrC samples, a step size of 0.05° and a count of 1 sec/step was used. The intensity (after removal of the baseline) of the most intense peaks of DCPD (2θ~21°) and β-TCP (2θ~31°) were used to calculate the β-TCP/DCPD ratio of each BrC composition.
2.4. Setting Time
Initial and final setting times of BrCs were measured by using Gillmore needles according to ASTM C266. After mixing cement paste and filling the mold, a 2.12 mm diameter 113.4 g needle was used to determine the initial setting time. The final setting time was measured using a 1.06 mm diameter 453.6 g needle. Time began once the mold was properly filled and recorded once each corresponding needle no longer left an indent on the surface of the cement sample. Three samples per composition were used to record set times.
2.5. Compressive Strength
Mechanical testing of samples was measured by using universal testing machine (MTS Criterion™ Model 43, MTS, Norwood, MA, USA). A 10 kN load cell was used with a 0.01 mm/sec rate. The sample was placed in the center of the fixture and compressed until failure. Maximum applied force was recorded and used to calculate the compressive strength of the material (compressive strength = (maximum force)/(original cross section area). At least 8 samples were tested per composition.
2.6. Statistical Analysis
Setting time was measured for 3 samples per composition. Compressive strength was measured for at least 8 samples. Data for the setting time and compressive strength are presented as mean ± standard deviation. A statistical analysis was performed with Student’s t-test. p-values < 0.01 and p-values < 0.0005 were considered very significant and extremely significant, respectively.
3. Results
3.1. Mg-TCP and Mg-BrC Phase Analysis
XRD spectra of all TCP compositions after calcination at 1050 °C are presented in Figure 1. The TCP powder was used as a precursor to prepare BrCs and was analyzed to ensure that no other phases were present. The analysis indicates that the only phases present in pure and magnesium-substituted TCP (Mg-TCP) were of β-TCP, and no other peaks related to other calcium phosphate phases or magnesium-containing phases were found.
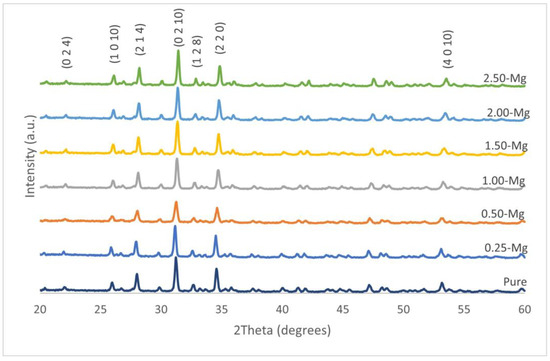
Figure 1.
Pure and Mg-TCP XRD spectra indicating that only β-TCP phase was present. No minor or secondary phases were observed with the inclusion of Mg.
In Figure 2, the XRD spectra of pure and Mg-BrCs after 24 h of immersion in PBS at 37 °C are presented. The addition of Mg did not introduce any new phases in the Mg-BrC samples, and the only two phases of DCPD (JCPDS No. 09-0077) and β-TCP (JCPDS No. 09-0169) were found in all compositions. However, the relative peak intensity of DCPD decreased significantly with Mg addition, while no specific change in β-TCP intensity was found. The β-TCP/DCPD ratios were calculated as 1.85, 2.15, 2.03, 2.42, 6.64, 8.15, and 8.24 for pure BrC, 0.25-MgBrC, 0.50-MgBrC, 1.00-MgBrC, 1.50-MgBrC, 2.00-MgBrC, and 2.50-MgBrC, respectively.
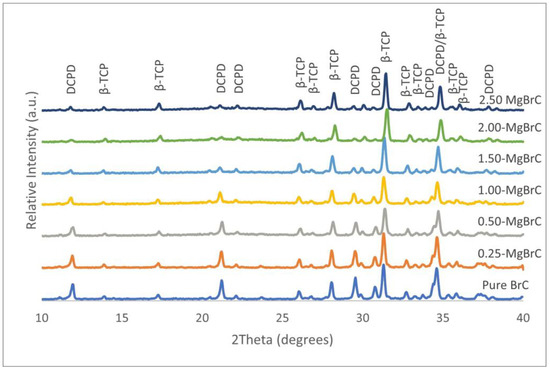
Figure 2.
Pure and Mg-BrC XRD spectra after 24 h of incubation in PBS, indicating that only β-TCP and DCPD phases were present. A decrease in intensity was noticed with the DCPD peak at ~21° with increasing Mg content.
3.2. Setting Time
The initial and final setting times of all cements are presented in Figure 3 and Figure 4, respectively. The initial setting time for pure BrC was 4.87 ± 0.38 min. Although the addition of 0.25 wt.% Mg did not affect the initial setting time of BrC, a higher concentration of 0.50 wt.% increased it to 11.13 ± 0.7 min. The initial setting time increased with increasing Mg content and the highest time of 80.59 ± 0.17 min was found for 2.5-MgBrC. The final setting times were 8.15 ± 0.52, 13.81 ± 0.69, 28.96 ± 0.44, and 82.83 ± 0.70 min for pure BrC, 0.25-MgBrC, 0.50-MgBrC, and 1.00-MgBrC, respectively. All three of the 1.50, 2.00, and 2.50-Mg BrCs showed final setting times beyond 4 h but were not measured beyond the 4 h mark due to time constraints and the ineffectiveness of the cements for biomedical applications.
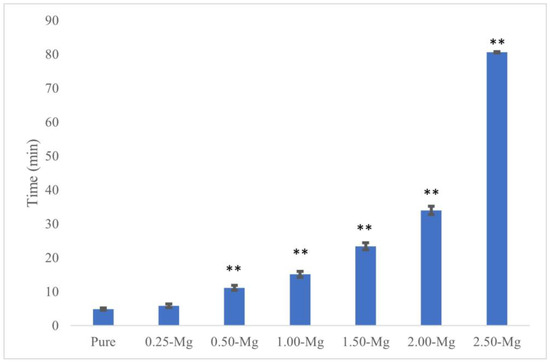
Figure 3.
Initial setting time increased with increasing Mg content of MgBrCs. An extremely significant (** p < 0.0005) increase in initial setting time was found with 0.50 wt.% Mg and above compared to pure BrC.
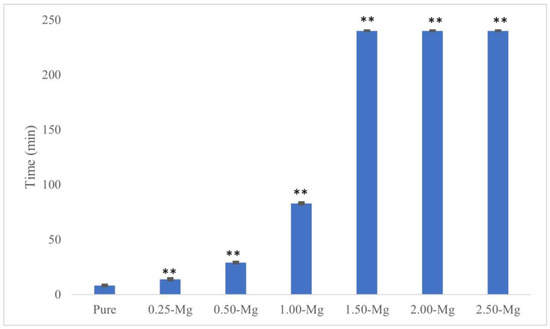
Figure 4.
Final setting time of MgBrCs increased with increasing Mg content. There was an extremely significant (** p < 0.0005) increase in final setting time of all Mg compositions compared to pure BrC.
3.3. Compressive Strength
The compressive strength of all BrC samples is presented in Figure 5. The addition of Mg at 0.25 wt.% resulted in an insignificant decrease in compressive strength from 8.22 ± 2.04 MPa to 6.95 ± 1.99 MPa. A higher concentration of 0.50 wt.% had a very significant impact on the reduction in compressive strength as a value of 6.47 ± 0.87 MPa (with p = 0.0081) was recorded. At higher concentrations such as 1.00 wt.% and beyond, there was an extremely significant decrease to 4.25 ± 0.95 MPa. The compressive strength values for 1.50-MgBrC, 2.00-MgBrC, and 2.50-MgBrC were 2.91 ± 0.92, 0.71 ± 0.16, and 0.45 ± 0.09 MPa, respectively (p < 0.0001).
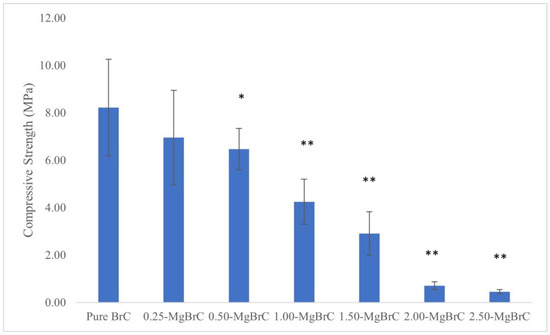
Figure 5.
Compressive strength of each composition after 24 h in PBS. The increase in Mg content in samples with 0.50 wt. % Mg results in statistically very significant decrease in compressive strength (* p < 0.01), while 1.00 wt.% Mg and beyond results in statistically extremely significant decreases in the compressive strength of BrC (** p < 0.0005).
4. Discussion
Mg-based biomaterials have received significant attention in hard tissue engineering applications. We have investigated the role of Mg deposition or incorporation in bone and dental tissue response to various biomaterials including Ti implants and calcium phosphate ceramics. Mg substitution in β-TCP enhances the proliferation and differentiation of the osteoblast cells (OBs) and mesenchymal stem cells (MSCs) [21,23]. Mg incorporation in hydroxyapatite accelerates bone remodeling and improves the osteogenic responses in MSCs/the monocyte co-culture system [24]. Our recent data show that Mg enhances the proliferation and differentiation of dental pulp stem cells in a dose-dependent manner [25]. Mg has been co-doped with other trace elements such as silver and zinc. A recent study has shown that the incorporation of silver in magnesium-doped biphasic calcium phosphate nanoparticles results in excellent cytocompatibility with human bone marrow-derived mesenchymal stem cells, and demonstrates antibacterial properties against E.coli and S. aureus bacteria [26,27]. Due to the importance of the Mg presence and concentration and the incorporation method, Mg was added to BrC and the physical and mechanical properties of the cement were evaluated. In this study, the solid-state synthesis method was used to synthesize the TCP powder, and Mg was added to the precursors, as reported in our previous studies [21,23].
The XRD spectra of the TCP powders without or with Mg up to 2.5 wt.% do not show any additional phases, with the only phase being β-TCP, which is in line with our previous data, where the addition of Mg to TCP at 1.00 wt.% did not introduce any new phase [21,23]. The current data show that Mg has a similar effect at higher concentrations when doped into TCP structures through the solid-state synthesis method. During Mg doping in TCP, Ca2+ ions with an ionic radius of 0.99 Å are substituted with Mg2+ ions with an ionic radius of 0.72 Å. The smaller size of Mg2+ ions contracts the TCP crystal structure, stabilizes the β-TCP structure, and increases the phase transformation temperature of β-TCP to α-TCP 28. A similar stabilizing effect of β-TCP is also reported while using other trace elements [7,19,28,29,30,31]. The formation of secondary phases depends on the synthesis method and Mg concentration. The increase in Mg content above 6.67 wt.% results in the formation of other Mg-substituted calcium phosphate phases [32]. The ionic substitution, the stabilization of the crystal structure, and the absence of other phases in turn affects the physical and mechanical properties of the material, such as the setting time, β-TCP/DCPD ratio, and compressive strength.
After mixing the cement powder and polymer solution, the formed paste begins a dissolution–precipitation process which continues until the cement is set. During mixing, MCPM and TCP powders begin to dissolve and release Ca2+ and PO43− ions [1]. A supersaturated gel then forms at the interface between the powder and liquid. At this interface, DCPD begins to nucleate and grow until the cement is set and hardened. With Mg incorporated in the TCP structure, the dissolution of TCP decreases due to the stabilizing effects of Mg substitution in a dose-dependent manner [33], and possibly decreases the DCPD precipitation as well as TCP dissolution, which is the first step of the setting reaction. Mg ions have also been known to inhibit the growth of brushite crystals [34] which may also affect the relative amount of β-TCP compared to DCPD. The calculated values of the β-TCP/DCPD ratios for each composition indicate that the addition of Mg increases the ratio, suggesting that the TCP dissolution rate was retarded and possibly that DCPD precipitation was delayed as well. The largest increase can be seen with 2.50-MgBrC, where the ratio increased by 4.5 times compared to pure BrC. An increase in TCP/DCPD ratio was also observed in our previous work for iron 7 and cobalt-doped BrCs [29], where the ratio increased in a dose-dependent manner up to 1.00 wt.%. In both cases, the presence of the dopant stabilized the crystal structure of the β-TCP and retarded the dissolution, which is in line with the current observation. A comparison between the Fe and Mg dopants shows the dependency of the dissolution rate on the ionic radius of the dopant. Fe with a radius of 0.64 Å is smaller than Mg with a radius of 0.72 Å. Thus, the shrinkage in β-TCP is greater with Fe substitution, leading to better stabilization of β-TCP and a higher TCP/DCPD ratio.
The setting time of BrCs is an important property to consider for clinical applications. This is also related to the dissolution–precipitation reaction. An undesirable characteristic of BrCs is how quickly the cements sets, allowing for a very limited amount of time to handle the material. Ionic substitution, P/L ratio, temperature, additional precursors, pH, and the type of liquid used to mix the cement can have a significant impact on the setting time of cements [35,36,37]. The P/L ratio and a 2 wt.% PEG solution were both utilized based on a previous study that showed optimal working consistency, as well as enhanced setting times and compressive strength [22]. The presence of Mg ions even at low concentrations increased initial and final setting times significantly. Initial and final setting times increased from 4.87 ± 0.38 min and 8.15 ± 0.52 min for pure BrC to 5.84 ± 0.55 min and 13.81 ± 0.69 min for 0.25-MgBrC, respectively. The addition of higher concentrations of Mg increased setting times for BrCs, which is in agreement with a study conducted by Saleh et al. [19]. The only difference between samples is the concentration of Mg in the TCP structure, indicating that the increase in setting time was due to the presence of Mg and its effects on TCP dissolution and/or DCPD precipitation. Another Mg-BrC system conducted by Alkhraisat et al. synthesized β-TCP through the solid-state method and used much higher concentrations of Mg compared to the current study. Newberyite (MgHPO4·3H2O) was used as the Mg source for Mg-TCP. XRD spectra of the TCP powder showed new phases such as farringtonite and stanfieldite. The setting time of the cements increased with increasing Mg content; however, cements with the new phases had similar setting times to those of pure BrC [32]. Extremely long setting times are undesirable as the wound must be kept open for an unnecessary amount of time, which could be harmful to the patient. Although the compressive strength of 1.00-Mg-BrC and 1.50-Mg-BrC is still adequate for bone substitution applications, these compounds and other Mg-BrCs with higher concentrations of Mg may not be suitable for clinical consideration due to the long initial and final setting times.
Cements should provide an adequate amount of mechanical support for both short and long-term inclusion. The compressive strength of the cements decreased most notably in Mg concentrations of 1.00 wt.% and above, with the lowest strength reaching 0.45 ± 0.09 MPa for 2.50-MgBrC. Variables that affect the mechanical strength of cements include chemical composition, formation of minor/secondary phases, the presence of polymers, ionic modifications, and TCP/DCPD ratio. In the current work, the only change during the cement preparation among the different groups was the Mg concentration. As the inclusion of Mg increases the stability and dissolution of the TCP structure [38], the mechanical properties can be expected to change as well. Concentrations of 1.00 wt.% and above of Mg produced the weakest samples, which also had higher setting times and TCP/DCPD ratios compared to 0.50-MgBrC, 0.25-MgBrC, and pure BrC. The correlation between the TCP/DCPD ratio, setting time, and compressive strength has been reported before. A prolonged setting reaction decreases the growth rate of the DCPD nuclei, and thus, the compressive strength increases [22]. Here, the addition of Mg increases both the TCP/DCPD and the setting time, showing the counteractive effect of the two parameters. As the compressive strength decreases with the addition of Mg, we can conclude that the TCP/DCPD ratio has a greater effect on the compressive strength of the Mg-substituted BrCs. Changes in the compressive strength of brushite cement with the addition of trace elements depend on the Ca/P ratio, cement formulation, the types of precursors and their ratios (β-TCP/MCPM), and the presence of polymers. Saleh et al. used the microwave-assisted wet precipitation method with a (Ca + Mg)/P molar ratio of 1.48 to prepare β-TCP and Mg β-TCP from calcium nitrate, magnesium nitrate, and diammonium hydrogen phosphate. The TCP powder was then mixed with MCPM at an equal weight ratio, and water was added to make a paste, which is different from the current method. The addition of Mg resulted in a significant increase in the compressive strength; however, the value did not change significantly in different Mg contents [19]. On the other hand, the addition of Mg to TCP through the solid-state synthesis method, followed by mixing with MCPM at an equal molar ratio and with water, resulted in more defects and lower wet diametrical tensile strength up to 40% of Mg/(Mg + Ca), where no other phase but the brushite and TCP were in the structure [32]. The addition of other trace elements such as Fe, Si, and Zn with a stabilizing effect on TCP structure has been reported to decrease the compressive strength, which is mainly due to the increase in TCP content [7,8,30].
In this current work, no new phases were formed in the TCP powder or the BrC cements, indicating that the amount of Mg present is the main variable affecting the properties of the cements. It is important to note that the differences in preparation and testing can produce significantly different results. Although the final product produced is a Mg-substituted BrC, significant differences in properties indicate that materials and methods are critical when comparing results.
5. Conclusions
In this work, the effects of Mg ionic substitution on the physical and mechanical properties of BrC were studied. Pure or Mg-doped TCP powders were made through the solid-state synthesis method and BrCs were successfully made by mixing TCP with MCPM, followed by mixing with a polymer solution. The replacement of the smaller Mg2+ ion in larger Ca2+ ion sites does not create any new phases in the β-TCP. Although the cements include only β-TCP and DCPD as the final phases, the TCP/DCPD ratio increases significantly with the increase in Mg content which is due to the stabilization of the β-TCP structure. The stabilization of the TCP structure in turn causes a longer initial and final setting time of the cements and a decrease in the strength, most noticeable in Mg concentrations of 1.00 wt.% and above. The 0.25- and 0.50-MgBrC compositions are the most optimal compositions in this work based on the setting time and compressive strength data. A comparison between our research and the literature shows the importance of precursors and cement preparation in the properties of BrCs. Our future goal is to evaluate the dissolution behavior of the BrCs and their in vitro interaction with osteoblast cells to investigate the long-term application of the cements.
Author Contributions
Conceptualization, S.V.; methodology/software/validation/formal analysis/data curation/writing—original draft preparation, S.F. and S.V.; investigation/resources/visualization/supervision/project administration/funding acquisition, S.V.; writing—review and editing, S.F. and S.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding and was solely supported by the internal resources of NIU.
Data Availability Statement
All relevant data have been used in this manuscript and are available to share with the scientific community upon request and consideration by the authors.
Acknowledgments
The authors would like to thank Paige Bothwell from the Department of Biological Sciences, Tao Xu from the Department of Chemistry and Biochemistry, and the Department of Mechanical Engineering at NIU for their help and support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ozdemir, F.; Evans, I.; Bretcanu, O. Clinical Applications of Biomaterials; Kaur, G., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 91–121. [Google Scholar]
- Graça, M.P.F.; Gavinho, S.R. Calcium Phosphate Cements in Tissue Engineering. In Contemporary Topics about Phosphorus in Biology and Materials; IntechOpen: London, UK, 2020. [Google Scholar]
- Apelt, D.; Theiss, F.; El-Warrak, A.O.; Zlinszky, K.; Bettschart-Wolfisberger, R.; Bohner, M.; Matter, S.; Auer, J.A.; Von Rechenberg, B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 2004, 25, 1439. [Google Scholar] [CrossRef] [PubMed]
- Mirtchi, A.A.; Lemaître, J.; Hunting, E. Calcium phosphate cements: Action of setting regulators on the properties of the β-tricalcium phosphate-monocalcium phosphate cements. Biomaterials 1989, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.T.; Misra, S.R.; Hussain, M. Nutritional Aspects of Essential Trace Elements in Oral Health and Disease: An Extensive Review. Scientifica 2016, 2016, 5464373. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, N.; Zhao, S.; Zhang, K.; Li, X.; Jing, A.; Liu, X.; Zhang, T. Fe-doped brushite bone cements with antibacterial property. Mater. Lett. 2018, 215, 27–30. [Google Scholar] [CrossRef]
- Vahabzadeh, S.; Fleck, S.; Marble, J.; Tabatabaei, F.; Tayebi, L. Role of iron on physical and mechanical properties of brushite cements, and interaction with human dental pulp stem cells. Ceram. Int. 2020, 46 Pt B, 11905–11912. [Google Scholar] [CrossRef] [PubMed]
- Vahabzadeh, S.; Roy, M.; Bose, S. Effects of Silicon on Osteoclast Cell Mediated Degradation, In Vivo Osteogenesis and Vasculogenesis of Brushite Cement. J. Mater. Chem. B Mater. Biol. Med. 2015, 3, 8973–8982. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Akram, M.; Jawad, Z.; Alshemary, A.Z.; Hussain, R. Strontium doped injectable bone cement for potential drug delivery applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Mederle, O.A.; Balas, M.; Ioanoviciu, S.D.; Gurban, C.V.; Tudor, A.; Borza, C. Correlations between bone turnover markers, serum magnesium and bone mass density in postmenopausal osteoporosis. Clin. Interv. Aging 2018, 13, 1383–1389. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Tartara, A.; Gasparri, C.; Perna, S.; Infantino, V.; Riva, A.; Petrangolini, G.; Peroni, G. An update on magnesium and bone health. Biometals 2021, 34, 715–736. [Google Scholar] [CrossRef]
- Sojka, J.E. Magnesium Supplementation and Osteoporosis. Nutr. Rev. 1995, 53, 71–74. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Qi, H.; Zhao, Q.; Liu, Y.; Zhang, Y. Dual Function of Magnesium in Bone Biomineralization. Adv. Healthc. Mater. 2019, 8, 1901030. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-based biomaterials as emerging agents for bone repair and regeneration: From mechanism to application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Enderle, R.; Götz-Neunhoeffer, F.; Göbbels, M.; Müller, F.A.; Greil, P. Influence of magnesium doping on the phase transformation temperature of beta-TCP ceramics examined by Rietveld refinement. Biomaterials 2005, 26, 3379–3384. [Google Scholar] [CrossRef]
- Landi, E.; Logroscino, G.; Proietti, L.; Tampieri, A.; Sandri, M.; Sprio, S. Biomimetic Mg-substituted hydroxyapatite: From synthesis to in vivo behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 239–247. [Google Scholar] [CrossRef]
- Cabrejos-Azama, J.; Alkhraisat, M.H.; Rueda, C.; Torres, J.; Pintado, C.; Blanco, L.; López-Cabarcos, E. Magnesium substitution in brushite cements: Efficacy of a new biomaterial loaded with vancomycin for the treatment of Staphylococcus aureus infections. Mater. Sci. Eng. C 2016, 61, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.T.; Ling, L.S.; Hussain, R. Injectable magnesium-doped brushite cement for controlled drug release application. J. Mater. Sci. 2016, 51, 7427–7439. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, D.; Robertson, S.; Vahabzadeh, S. Enhanced In Vivo Bone and Blood Vessel Formation by Iron Oxide and Silica Doped 3D Printed Tricalcium Phosphate Scaffolds. Ann. Biomed. Eng. 2018, 46, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Vahabzadeh, S.; Robertson, S.; Bose, S. Beta-phase Stabilization and Increased Osteogenic Differentiation of Stem Cells by Solid-State Synthesized Magnesium Tricalcium Phosphate. J. Mater. Res. 2021, 36, 3041–3049. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; DeVoe, K.; Bandyopadhyay, A.; Bose, S. Mechanical and In Vitro Biocompatibility of Brushite Cement Modified by Polyethylene Glycol. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 2145–2152. [Google Scholar] [CrossRef]
- Ke, D.; Tarafder, S.; Vahabzadeh, S.; Bose, S. Effects of MgO, ZnO, SrO, and SiO2 in tricalcium phosphate scaffolds on in vitro gene expression and in vivo osteogenesis. Mater. Sci. Eng. C 2019, 96, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Banerjee, D.; Ke, D. Enhanced osteogenic protein expression on human osteoblast-osteoclast co-culture system using doped hydroxyapatite plasma coatings for orthopedic and dental applications. Mater. Today Commun. 2019, 21, 100534. [Google Scholar] [CrossRef]
- Mommer, A.; Tabatabaei, F.; Tayebi, L.; Vahabzadeh, S. Role of magnesium on phase composition of tricalcium phosphate and its interaction with human dental pulp stem cells. J. Mater. Res. 2023, 38, 228–236. [Google Scholar] [CrossRef]
- Yang, N.; Wang, S.; Ding, P.; Sun, S.; Wei, Q.; Jafari, H.; Wang, L.; Han, Y.; Okoro, O.V.; Wang, T.; et al. Magnesium-doped biphasic calcium phosphate nanoparticles with incorporation of silver: Synthesis, cytotoxic and antibacterial properties. Mater. Lett. 2022, 322, 132478. [Google Scholar] [CrossRef]
- Krokhicheva, P.A.; Goldberg, M.A.; Fomin, A.S.; Khayrutdinova, D.R.; Antonova, O.S.; Baikin, A.S.; Leonov, A.V.; Merzlyak, E.M.; Mikheev, I.V.; Kirsanova, V.A.; et al. Zn-Doped Calcium Magnesium Phosphate Bone Cement Based on Struvite and Its Antibacterial Properties. Materials 2023, 16, 4824. [Google Scholar] [CrossRef]
- Famery, R.; Richard, N.; Boch, P. Preparation of α- and β-tricalcium phosphate ceramics, with and without magnesium addition. Ceram. Int. 1994, 20, 327–336. [Google Scholar] [CrossRef]
- Vahabzadeh, S.; Fleck, S.; Duvvuru, M.K.; Cummings, H. Effects of Cobalt on Physical and Mechanical Properties and In Vitro Degradation Behavior of Brushite Cement. JOM 2019, 71, 315–320. [Google Scholar] [CrossRef]
- Vahabzadeh, S.; Bandyopadhyay, A.; Bose, S.; Mandal, R.; Nandi, S.K. IGF-Loaded Silicon and Zinc Doped Brushite Cement: Physico-Mechanical Characterization and In Vivo Osteogenesis Evaluation. Integr. Biol. 2015, 7, 1561–1573. [Google Scholar] [CrossRef]
- Rau, J.V.; Fosca, M.; Graziani, V.; Egorov, A.A.; Zobkov, Y.V.; Fedotov, A.Y.; Ortenzi, M.; Caminiti, R.; Baranchikov, A.E.; Komlev, V.S. Silver-Doped Calcium Phosphate Bone Cements with Antibacterial Properties. J. Funct. Biomater. 2016, 7, 10. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Cabrejos-Azama, J.; Rodríguez, C.R.; Jerez, L.B.; Cabarcos, E.L. Magnesium substitution in brushite cements. Mater. Sci. Eng. C 2013, 33, 475–481. [Google Scholar] [CrossRef]
- AIto, A.; Sogo, Y.; Yamazaki, A.; Aizawa, M.; Osaka, A.; Hayakawa, S.; Kikuchi, M.; Yamashita, K.; Tanaka, Y.; Tadokoro, M.; et al. Interlaboratory studies on in vitro test methods for estimating in vivo resorption of calcium phosphate ceramics. Acta Biomater. 2015, 25, 347–355. [Google Scholar] [CrossRef]
- Giocondi, J.L.; El-Dasher, B.S.; Nancollas, G.H.; Orme, C.A. Molecular mechanisms of crystallization impacting calcium phosphate cements. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 1937–1961. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Grover, L.M.; Gbureck, U.; Young, A.M.; Wright, A.J.; Barralet, J.E. Temperature dependent setting kinetics and mechanical properties of β-TCP–pyrophosphoric acid bone cement. J. Mater. Chem. 2005, 15, 4955–4962. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, H.W.; Ginebra, M.P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012, 3, 2041731412439555. [Google Scholar] [CrossRef]
- Li, X.; Ito, A.; Sogo, Y.; Wang, X.; LeGeros, R.Z. Solubility of Mg-containing beta-tricalcium phosphate at 25 degrees C. Acta Biomater. 2009, 5, 508–517. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).