Writing Tiny Nanoclusters Using a Nanofountain Pen Operated by Spontaneous Evaporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoparticles-Dispersed Ink
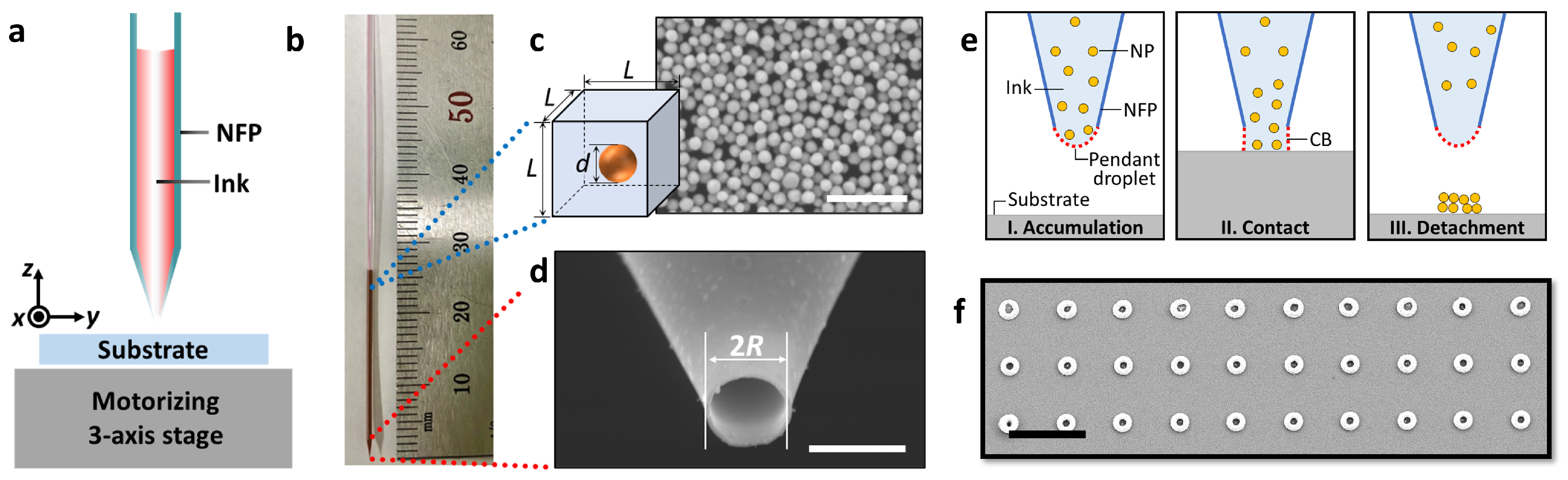
2.2. Preparing Micrometer-Sized Nozzle of NFPs
2.3. Fabrication Setup for Using NFPs
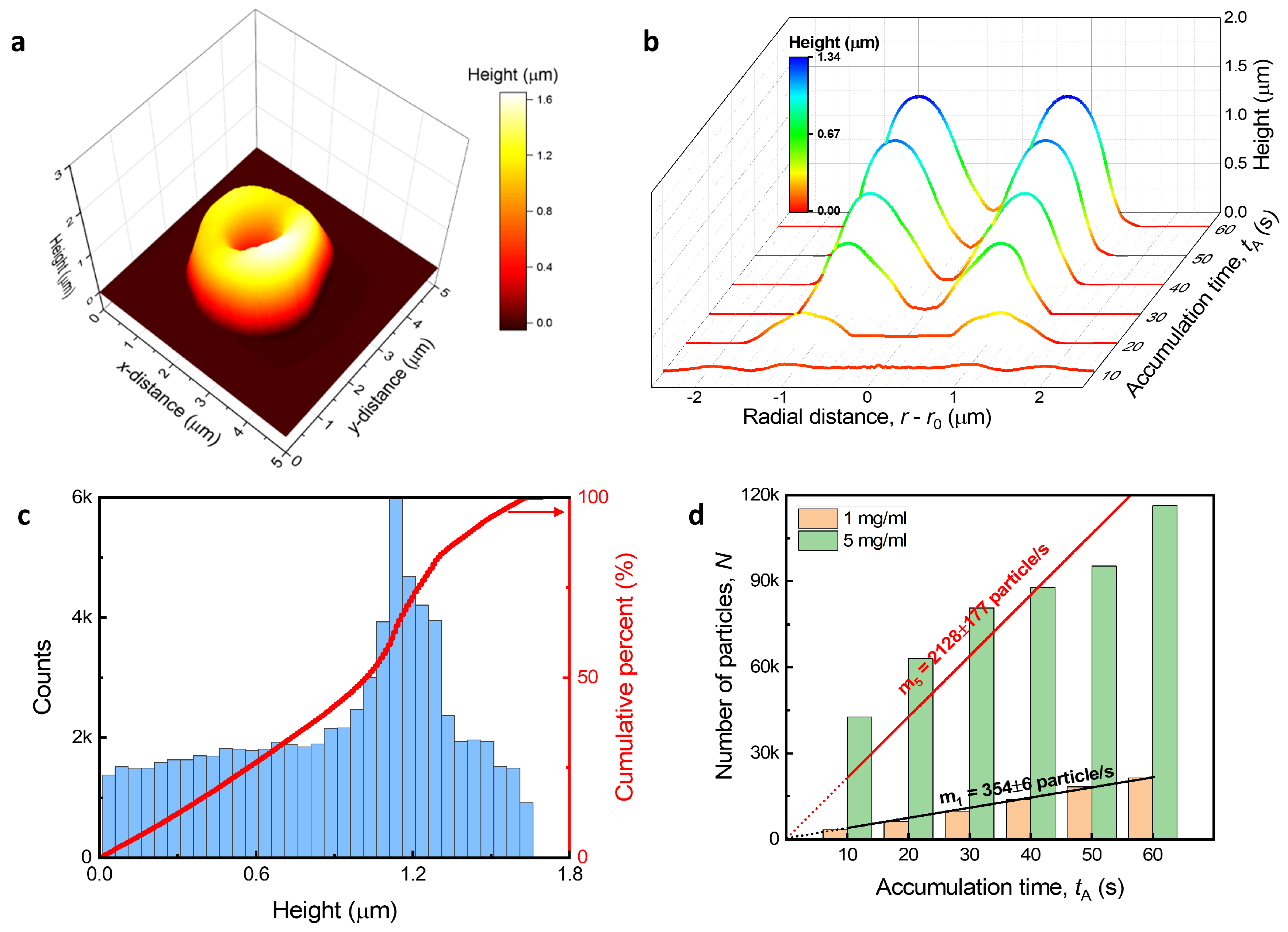
2.4. Measurement of Nanocluster Structures
3. Results and Discussion
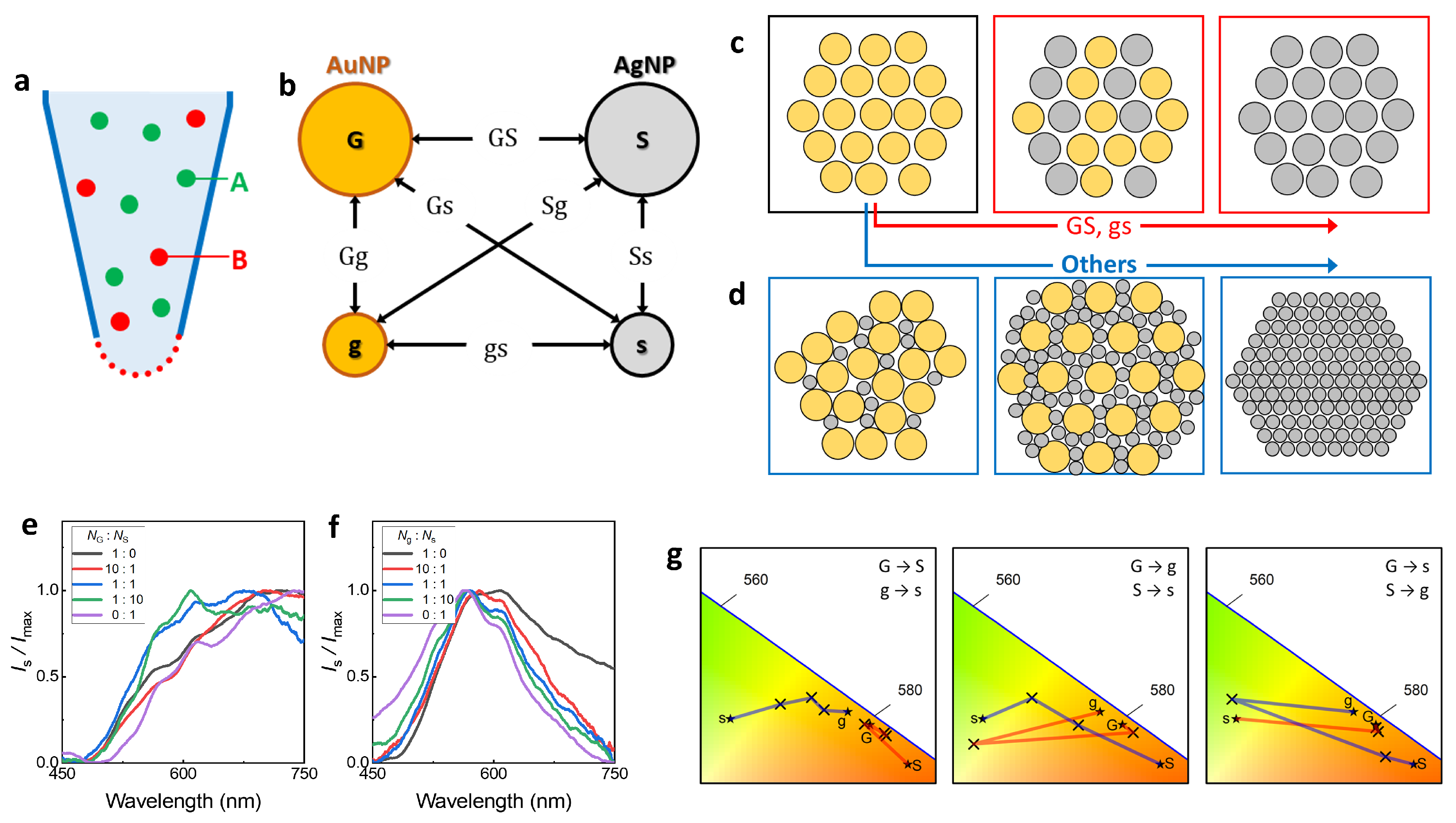
3.1. Nanoclusters Fabricated by a Nanofountain Pen
3.2. Structure Characteristics According to Fabrication Condition
3.3. Spontaneous Evaporation-Driven Fabrication Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NP | Nanoparticle |
| NFP | Nanofountain Pen |
| CB | Capillary Bridge |
| SEM | Scanning Electron Microscope |
| AFM | Atomic Force Microscope |
| LSPR | Localized Surface Plasmon Resonance |
| SERS | Surface-enhanced Raman scattering |
References
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Jensen, T.R.; Malinsky, M.D.; Haynes, C.L.; Van Duyne, R.P. Nanosphere lithography: Tunable localized surface plasmon resonance spectra of silver nanoparticles. J. Phys. Chem. 2000, 104, 10549–10556. [Google Scholar] [CrossRef]
- Ringe, E.; McMahon, J.M.; Sohn, K.; Cobley, C.; Xia, Y.; Huang, J.; Schatz, G.C.; Marks, L.D.; Van Duyne, R.P. Unraveling the effects of size, composition, and substrate on the localized surface plasmon resonance frequencies of gold and silver nanocubes: A systematic single-particle approach. J. Phys. Chem. C 2010, 114, 12511–12516. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localised surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, T.; Grattan, K.T. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Wu, J.L.; Chen, F.C.; Hsiao, Y.S.; Chien, F.C.; Chen, P.; Kuo, C.H.; Huang, M.H.; Hsu, C.S. Surface plasmonic effects of metallic nanoparticles on the performance of polymer bulk heterojunction solar cells. ACS Nano 2011, 5, 959–967. [Google Scholar] [CrossRef]
- Du, P.; Jing, P.; Li, D.; Cao, Y.; Liu, Z.; Sun, Z. Plasmonic Ag@ oxide nanoprisms for enhanced performance of organic solar cells. Small 2015, 11, 2454–2462. [Google Scholar] [CrossRef]
- Lee, H.B.; Kim, W.G.; Lee, M.; Lee, J.M.; He, S.; Kumar, N.; Devaraj, V.; Choi, E.J.; Jeon, I.; Song, M.; et al. Gap Plasmon of Virus-Templated Biohybrid Nanostructures uplifting the performance of organic optoelectronic devices. Adv. Opt. Mater. 2020, 8, 1902080. [Google Scholar] [CrossRef]
- Xiao, Q.; Jaatinen, E.; Zhu, H. Direct Photocatalysis for Organic Synthesis Using Plasmonic-Metal Nanoparticles Irradiated with Visible Light. Chem. Asian J. 2014, 9, 3046–3064. [Google Scholar] [CrossRef]
- Kolemen, S.; Ozdemir, T.; Lee, D.; Kim, G.M.; Karatas, T.; Yoon, J.; Akkaya, E.U. Remote-controlled release of singlet oxygen by the plasmonic heating of endoperoxide-modified gold nanorods towards a paradigm change in photodynamic therapy. Angew. Chem. 2016, 128, 3670–3674. [Google Scholar] [CrossRef]
- Hung, W.H.; Aykol, M.; Valley, D.; Hou, W.; Cronin, S.B. Plasmon resonant enhancement of carbon monoxide catalysis. Nano Lett. Mater. 2010, 10, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Liu, C.; Favraud, G.; Chen, Q.; Han, M.; Fratalocchi, A.; Zhang, S. Broadband Single-Molecule SERS Detection Design Using Warped Optical Spaces. Nat. Commun. 2018, 9, 5428. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, J.; Kneipp, H.; Kneipp, K. SERS: A single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 2008, 37, 1052–1060. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Liz-Marzán, L.M. SERS Detection of Small Inorganic Molecules and Ions. Angew. Chem. Int. Ed. 2012, 51, 11214–11223. [Google Scholar] [CrossRef]
- Sonnefraud, Y.; Verellen, N.; Sobhani, H.; Vandenbosch, G.A.; Moshchalkov, V.V.; Van Dorpe, P.; Nordlander, P.; Maier, S. Experimental realization of subradiant, superradiant, and Fano resonance in ring/disk plasmonic nanocavities. ACS Nano 2010, 4, 1664–1670. [Google Scholar] [CrossRef]
- Fan, J.A.; Bao, K.; Wu, C.; Bao, J.; Bardhan, R.; Halas, N.J.; Manoharan, V.N.; Shvets, G.; Nordlander, P.; Capasso, F. Fano-like interference in self-assembled plasmonic quadrumer clusters. Nano Lett. 2010, 10, 4680–4685. [Google Scholar] [CrossRef]
- Shao, T.; Wang, X.; Dong, H.; Liu, S.; Duan, D.; Li, Y.; Song, P.; Jiang, H.; Hou, Z.; Gao, C.; et al. A Stacked Plasmonic Metamaterial with a strong localized electric field enables highly efficient Broadband Light-Driven CO2 hydrogenation. Adv. Mater. 2022, 34, 2202367. [Google Scholar] [CrossRef]
- Ji, T.; Peng, L.; Zhu, Y.; Yang, F.; Cui, Y.; Wu, X.; Liu, L.; He, S.; Zhu, F.; Hao, Y. Plasmonic broadband absorber by stacking multiple metallic nanoparticle layers. Appl. Phys. Lett. 2015, 106, 161107. [Google Scholar] [CrossRef]
- Saeed, A.; Panaro, S.; Zaccaria, R.P.; Raja, W.; Liberale, C.; Dipalo, M.; Messina, G.C.; Wang, H.; De Angelis, F.; Toma, A. Stacked optical antennas for plasmon propagation in a 5 nm-confined cavity. Sci. Rep. 2015, 5, 11237. [Google Scholar] [CrossRef]
- Kim, W.G.; Lee, J.M.; Yang, Y.; Kim, H.; Devaraj, V.; Kim, M.; Jeong, H.; Choi, E.J.; Yang, J.; Jang, Y.; et al. Three-dimensional plasmonic nanocluster-driven light–matter interaction for photoluminescence enhancement and picomolar-level biosensing. Nano Lett. 2022, 22, 4702–4711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhen, Y.R.; Neumann, O.; Day, J.K.; Nordlander, P.; Halas, N.J. Coherent anti-Stokes Raman scattering with single-molecule sensitivity using plasmonic Fano resonance. Nat. Commun. 2014, 5, 4424. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.W.; Myroshnychenko, V.; Chen, C.H.; Deng, J.P.; Mou, C.Y.; García de Abajo, F.J. Probing bright and dark surface-plasmon modes in individual and coupled noble metal nanoparticles using an electron beam. Nano Lett. 2009, 9, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; You, E.A.; Hwang, D.W.; Kang, S.; Wi, J.S. Active Accumulation of Spherical analytes on plasmonic hot Spots of Double-Bent Au Strip Arrays by Multiple Dip Coating. Nanomaterials 2019, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Tahghighi, M.; Mannelli, I.; Janner, D.; Ignés-Mullol, J. Tailoring plasmonic Response by Langmuir-Blodgett gold nanoparticle templating for the Fabrication of SERS Substrates. Appl. Surf. Sci. 2018, 447, 416–422. [Google Scholar] [CrossRef]
- Hanske, C.; Tebbe, M.; Kuttner, C.; Bieber, V.; Tsukruk, V.V.; Chanana, M.; König, T.A.; Fery, A. Strongly coupled plasmonic modes in macroscopic areas via template-assisted colloidal self-assembly. Nano Lett. 2014, 14, 6863–6871. [Google Scholar] [CrossRef]
- Koster, H.J.; Guillen-Perez, A.; Gomez-Diaz, J.S.; Navas-Moreno, M.; Birkeland, A.C.; Carney, R.P. Fused Raman spectroscopic analysis of blood and saliva delivers high accuracy for head and neck cancer diagnostics. Sci. Rep. 2022, 12, 18464. [Google Scholar] [CrossRef]
- Schedin, F.; Lidorikis, E.; Lombardo, A.; Kravets, V.G.; Geim, A.K.; Grigorenko, A.N.; Novoselov, K.S.; Ferrari, A.C. Surface-enhanced Raman spectroscopy of graphene. ACS Nano 2010, 4, 5617–5626. [Google Scholar] [CrossRef]
- Shen, Y.; Yue, J.; Xu, W.; Xu, S. Recent progress of surface-enhanced Raman spectroscopy for subcellular compartment analysis. Theranostics 2021, 11, 4872. [Google Scholar] [CrossRef]
- Shi, R.; Liu, X.; Ying, Y. Facing challenges in real-life application of surface-enhanced Raman scattering: Design and nanofabrication of surface-enhanced Raman scattering substrates for rapid field test of food contaminants. J. Agric. Food Chem. 2017, 66, 6525–6543. [Google Scholar] [CrossRef]
- Li, X.; Keshavarz, M.; Kassanos, P.; Kidy, Z.; Roddan, A.; Yeatman, E.; Thompson, A.J. SERS Detection of Breast Cancer-Derived Exosomes Using a Nanostructured Pt-Black Template. Adv. Sens. Res. 2023, 2, 2200039. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef]
- Kudelski, A. Analytical applications of Raman spectroscopy. Talanta 2008, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lane, L.A.; Qian, X.; Nie, S. SERS nanoparticles in medicine: From label-free detection to spectroscopic tagging. Chem. Rev. 2015, 115, 10489–10529. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Yonzon, C.R.; Haynes, C.L.; Zhang, X.; Walsh, J.T.; Van Duyne, R.P. A glucose biosensor based on surface-enhanced Raman scattering: Improved partition layer, temporal stability, reversibility, and resistance to serum protein interference. Anal. Chem. 2004, 76, 78–85. [Google Scholar] [CrossRef]
- Camden, J.P.; Dieringer, J.A.; Wang, Y.; Masiello, D.J.; Marks, L.D.; Schatz, G.C.; Van Duyne, R.P. Probing the structure of single-molecule surface-enhanced Raman scattering hot spots. J. Am. Chem. Soc. 2008, 130, 12616–12617. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Yang, M.; Xu, J.; Goh, S.J.; Pan, M.; Chen, H. Measuring ensemble-averaged surface-enhanced Raman scattering in the hotspots of colloidal nanoparticle dimers and trimers. J. Am. Chem. Soc. 2010, 132, 3644–3645. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Etchegoin, P.G.; Meyer, M. Enhancement factor distribution around a single surface-enhanced Raman scattering hot spot and its relation to single molecule detection. J. Chem. Phys. 2006, 125, 204701. [Google Scholar] [CrossRef]
- Premasiri, W.R.; Lee, J.C.; Sauer-Budge, A.; Théberge, R.; Costello, C.E.; Ziegler, L.D. The biochemical origins of the surface-enhanced Raman spectra of bacteria: A metabolomics profiling by SERS. Anal. Bioanal. Chem. 2016, 408, 4631–4647. [Google Scholar] [CrossRef]
- Ren, Y.F.; Ye, Z.H.; Liu, X.Q.; Xia, W.J.; Yuan, Y.; Zhu, H.Y.; Chen, X.T.; Hou, R.Y.; Cai, H.M.; Li, D.X.; et al. Surface-enhanced Raman spectroscopy-based metabolomics for the discrimination of Keemun black teas coupled with chemometrics. LWT-Food Sci. Technol. 2023, 181, 114742. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, C.; Dai, C.; Littlepage, L.E.; Li, J.; Schultz, Z.D. Untargeted Tumor Metabolomics with Liquid Chromatography–Surface-Enhanced Raman Spectroscopy. Angew. Chem.-Int. Edit. 2020, 132, 3467–3471. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Tantra, R.; Brown, R.J.; Milton, M.J. Strategy to improve the reproducibility of colloidal SERS. J. Raman Spectrosc. 2007, 38, 1469–1479. [Google Scholar] [CrossRef]
- Tripp, R.A.; Dluhy, R.A.; Zhao, Y. Novel nanostructures for SERS biosensing. Nano Today 2008, 3, 31–37. [Google Scholar] [CrossRef]
- Jang, M.; Shin, J.; Kim, Y.H.; Jeong, T.Y.; Jo, S.; Kim, S.J.; Devaraj, V.; Kang, J.; Choi, E.J.; Lee, J.E.; et al. 3D superstructure based metabolite profiling for glaucoma diagnosis. Biosens. Bioelectron. 2023, 244, 115780. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, I.H.; Kim, W.G.; Hwang, Y.H.; Oh, J.W. Fountain-Pen-inspired 3D Colloidal Assembly, Consisting of Metallic Nanoparticles on a Femtoliter Scale. Nanomaterials 2023, 13, 2403. [Google Scholar] [CrossRef]
- Dullien, F.A. Porous Media: Fluid Transport and Pore Structure; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- CVR. Lange’s Handbook of Chemistry, 10th ed.; McGraw-Hill: New York, NY, USA, 1968; p. 449. [Google Scholar]
- Cui, J.; Ju, Y.; Liang, K.; Ejima, H.; Lörcher, S.; Gause, K.T.; Richardson, J.J.; Caruso, F. Nanoscale engineering of low-fouling surfaces through polydopamine immobilization of zwitterionic peptides. Soft Matter 2014, 10, 2656–2663. [Google Scholar] [CrossRef]
- Shuturminska, K.; Tarakina, N.V.; Azevedo, H.S.; Bushby, A.J.; Mata, A.; Anderson, P.; Al-Jawad, M. Elastin-like protein with statherin derived peptides controls fluorapatite formation and morphology. Front. Physiol. 2017, 8, 368. [Google Scholar] [CrossRef]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-J.; Yi, D.; Lee, I.H.; Kim, W.-G.; Kim, Y.-J.; Moon, J.-S.; Oh, J.-W. Writing Tiny Nanoclusters Using a Nanofountain Pen Operated by Spontaneous Evaporation. Crystals 2024, 14, 9. https://doi.org/10.3390/cryst14010009
Kim S-J, Yi D, Lee IH, Kim W-G, Kim Y-J, Moon J-S, Oh J-W. Writing Tiny Nanoclusters Using a Nanofountain Pen Operated by Spontaneous Evaporation. Crystals. 2024; 14(1):9. https://doi.org/10.3390/cryst14010009
Chicago/Turabian StyleKim, Sung-Jo, Dongwon Yi, Il Hyun Lee, Won-Geun Kim, Ye-Ji Kim, Jong-Sik Moon, and Jin-Woo Oh. 2024. "Writing Tiny Nanoclusters Using a Nanofountain Pen Operated by Spontaneous Evaporation" Crystals 14, no. 1: 9. https://doi.org/10.3390/cryst14010009
APA StyleKim, S.-J., Yi, D., Lee, I. H., Kim, W.-G., Kim, Y.-J., Moon, J.-S., & Oh, J.-W. (2024). Writing Tiny Nanoclusters Using a Nanofountain Pen Operated by Spontaneous Evaporation. Crystals, 14(1), 9. https://doi.org/10.3390/cryst14010009






