Thermal Conductivity and Orientation Structure of Liquid Crystalline Epoxy Thermosets Prepared by Latent Curing Catalyst
Abstract
:1. Introduction
2. Materials and Methods
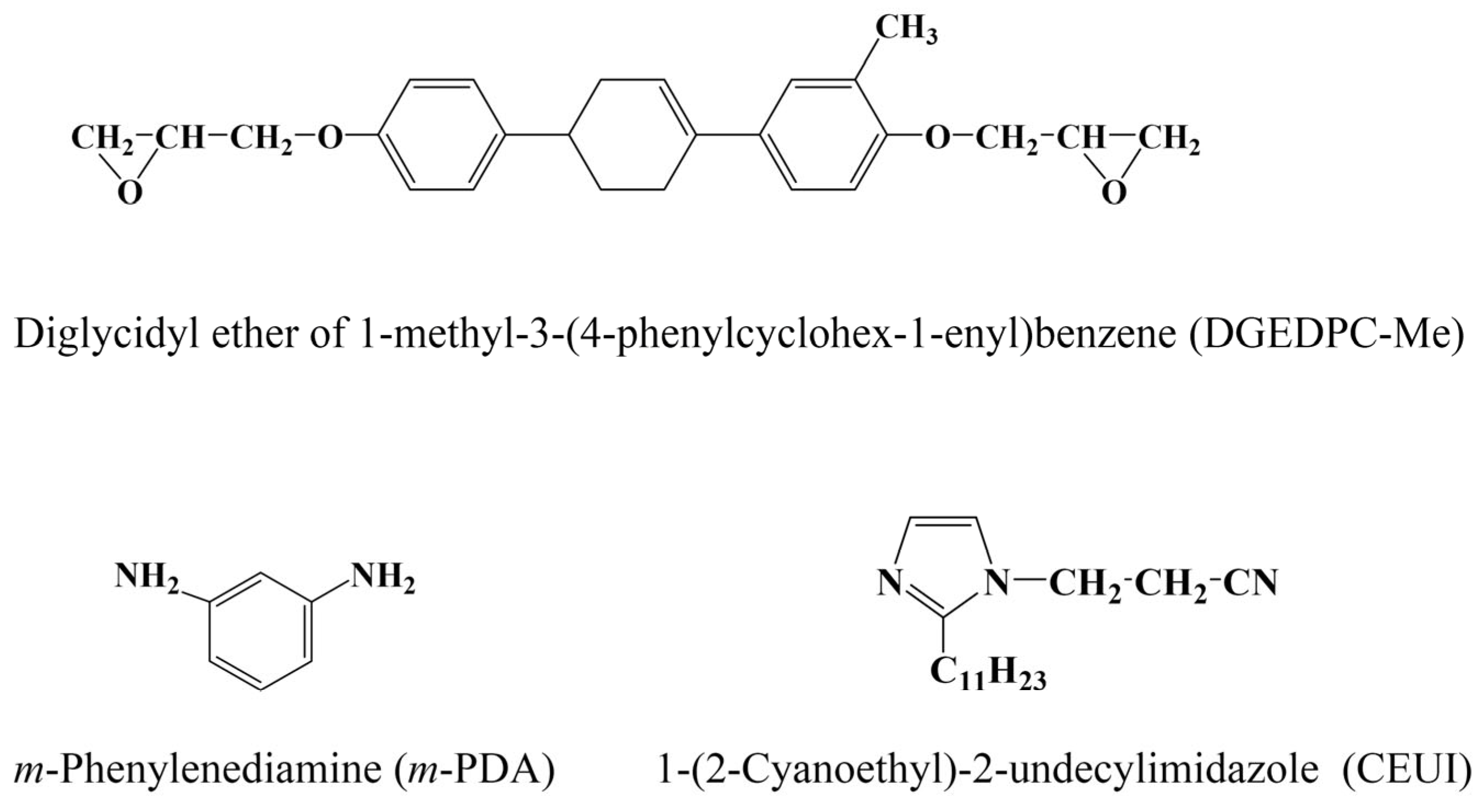
2.1. Materials
2.2. Synthesis of 1,4-Diphenyl-3-Methyl-Cyclohexene-Type Epoxy Resin (DGEDPC-Me)
2.3. Curing of Epoxy Resin and Al2O3 Composites
2.4. Surface Treatment of a Glass Substrate
2.5. Characterization Techniques
3. Results
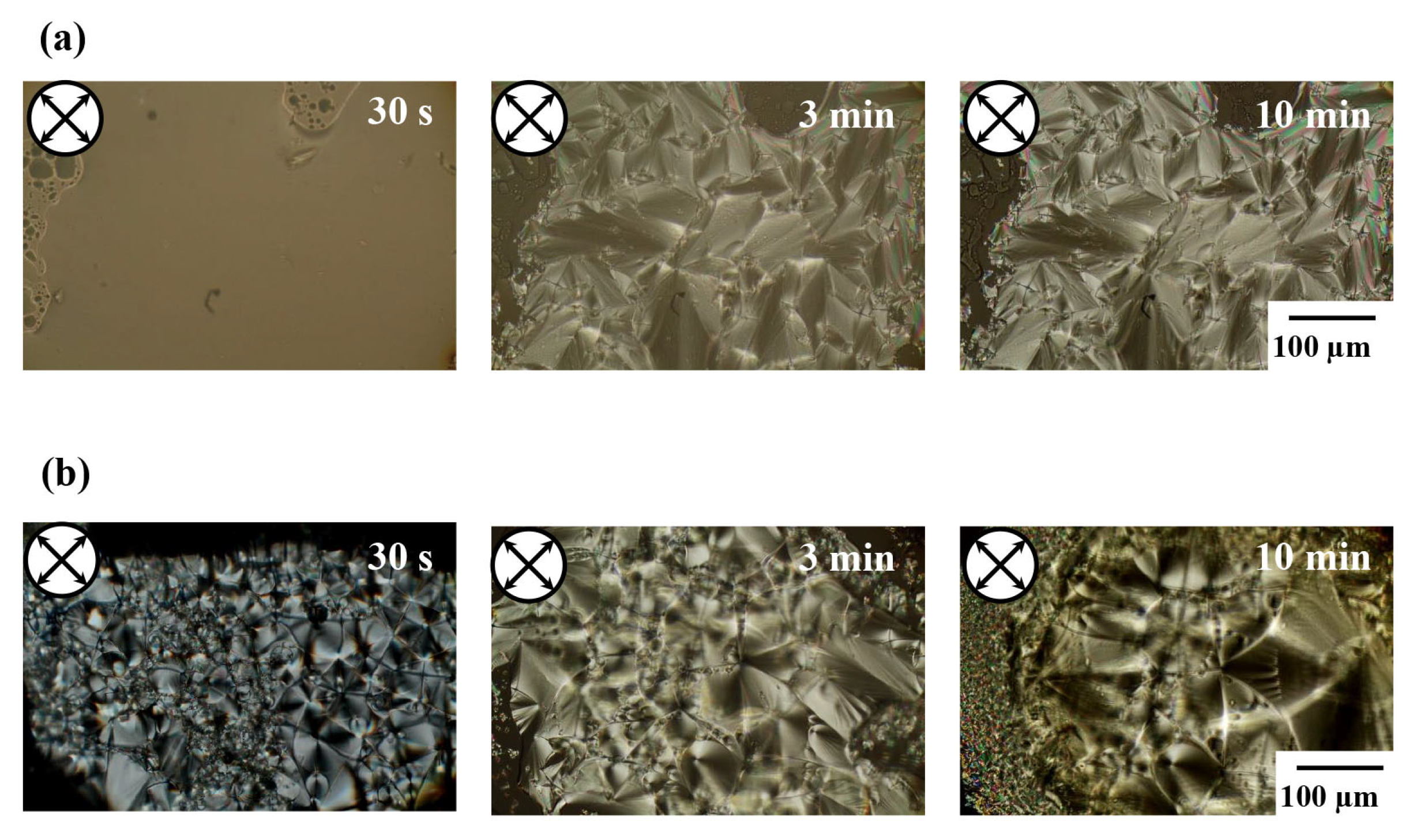
3.1. Curing Reaction of the DGEDPC-Me/m-PDA and CEUI Systems
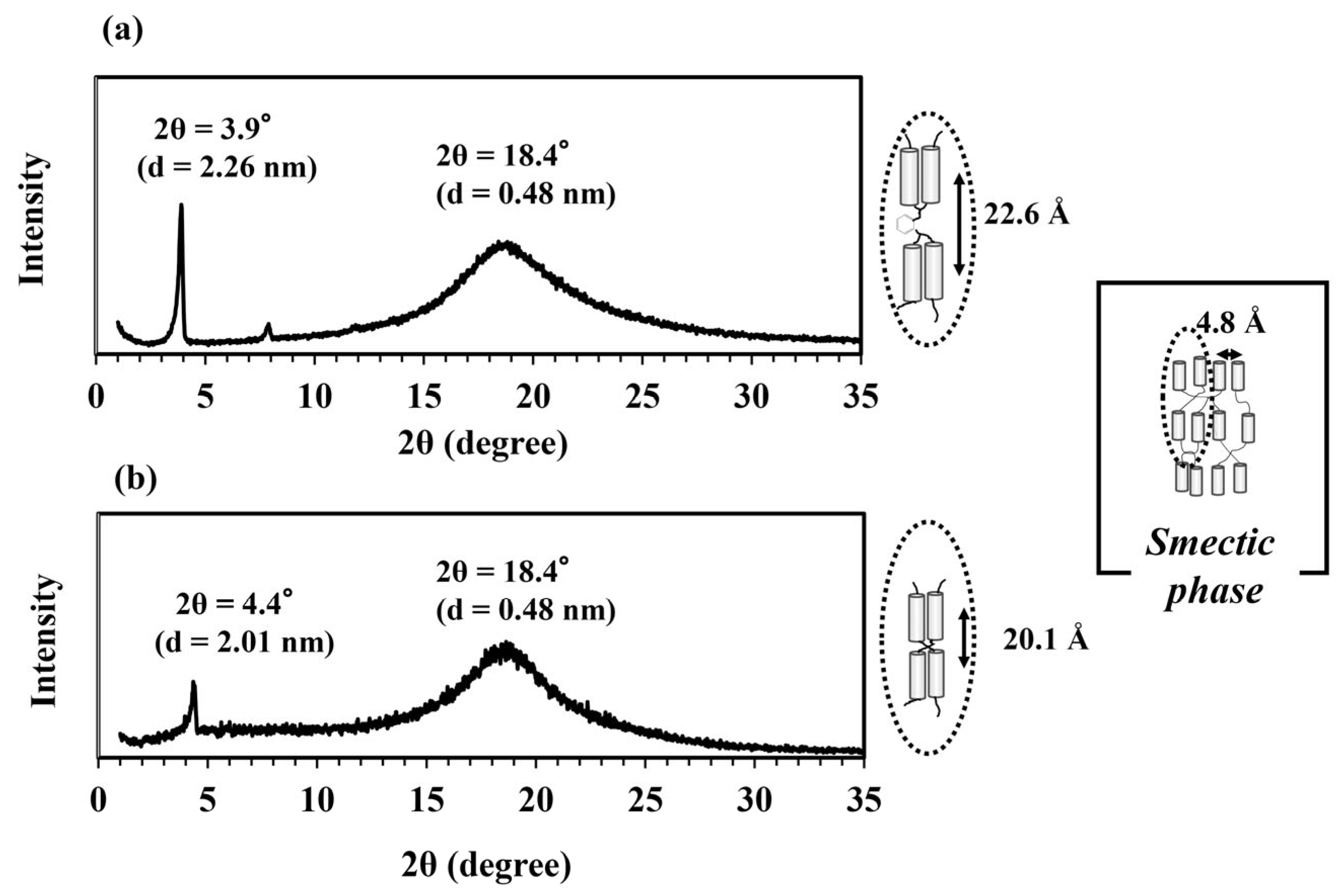
3.2. LC Phase Structure of the DGEDPC-Me/m-PDA and CEUI Systems
3.3. Thermal Conductivity of the DGEDPC-Me/m-PDA and CEUI Systems
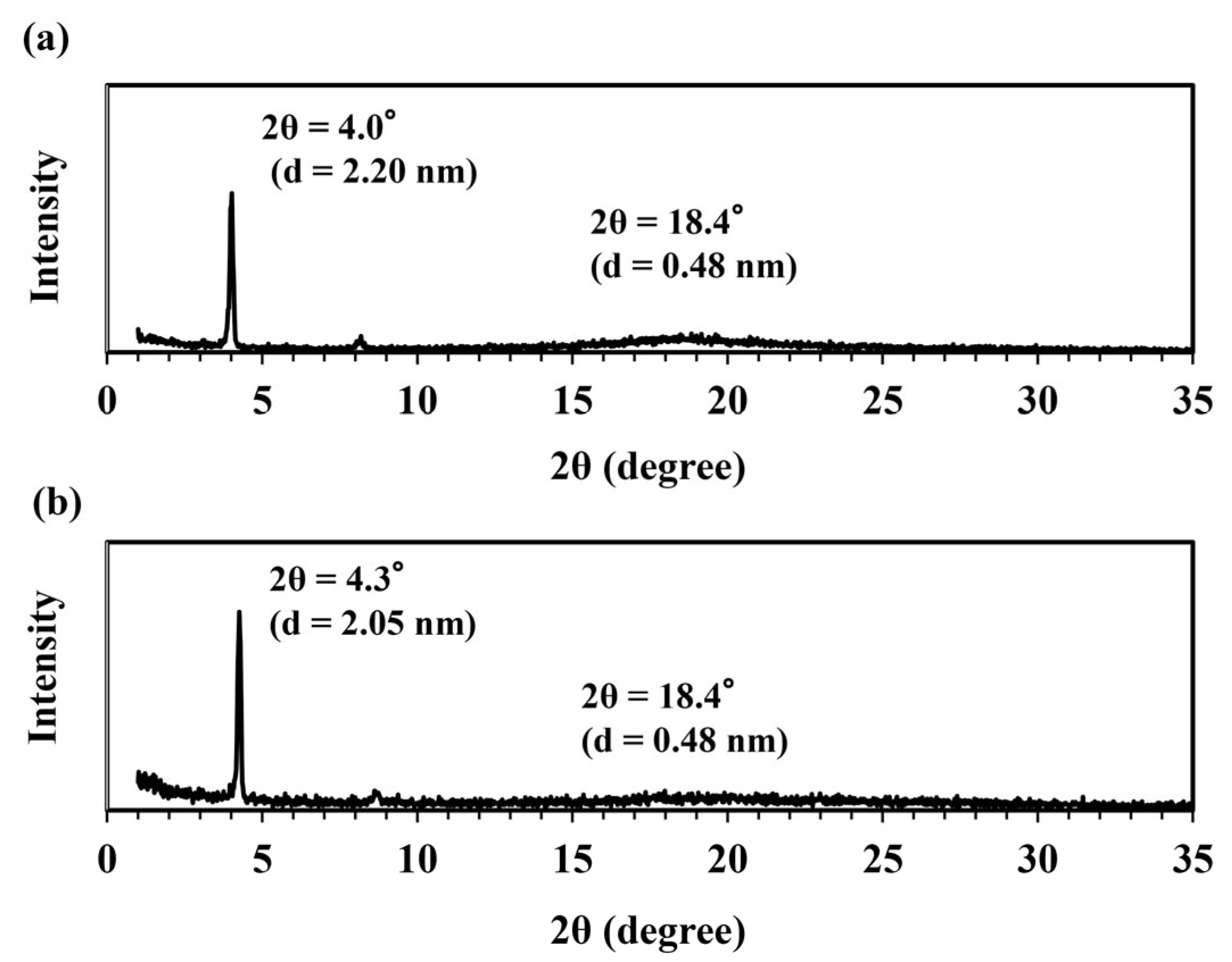
3.4. Alignment of the DGEDPC-Me/m-PDA and CEUI Systems on Glass Substrates
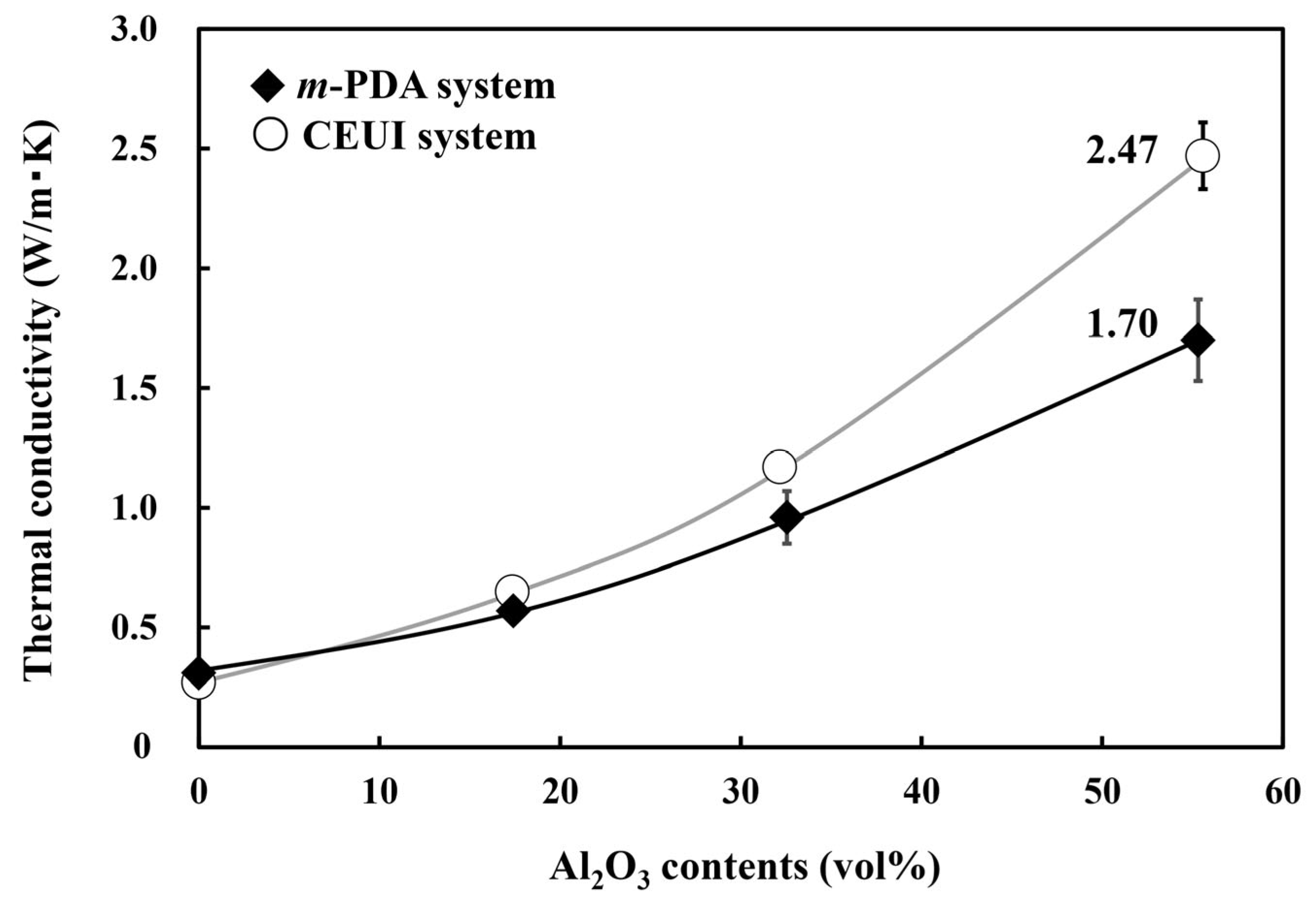
3.5. Thermal Conductivity of the DGEDPC-Me/m-PDA and CEUI/Al2O3 Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Smet, V.; Tummala, R. Review of packaging schemes for power module. IEEE J. Emerg. Sel. Top. Power Electron. 2020, 8, 1. [Google Scholar]
- Yetgin, H.; Veziroglu, S.; Aktas, O.; Yalçinkaya, T. Enhancing thermal conductivity of epoxy with a binary filler system of h-BN platelets and Al2O3 nanoparticles. Int. J. Adhes. Adhes. 2020, 98, 102540. [Google Scholar] [CrossRef]
- Li, H.; Zheng, W. Enhanced thermal conductivity of epoxy/alumina composite through multiscale-disperse packing. J. Compos. Mater. 2021, 55, 17–25. [Google Scholar] [CrossRef]
- Tian, F.; Cao, J.; Ma, W. Enhanced thermal conductivity and rheological performance of epoxy and liquid crystal epoxy composites with filled Al2O3 compound. Polym. Test. 2023, 120, 107940. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Liu, X. Novel functionalized BN nanosheets/epoxy composites with advanced thermal conductivity and mechanical properties. ACS Appl. Mater. Interfaces 2020, 12, 6503–6515. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, T.; Wang, J.; Yang, G.; Li, M.; Wu, W. The investigation of the effect of filler sizes in 3D-BN skeletons on thermal conductivity of epoxy-based composites. Nanomaterials 2022, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Aligning diamond particles inside BN honeycomb for significantly improving thermal conductivity of epoxy composite. Compos. Sci. Technol. 2022, 222, 109370. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, J.; Li, Z.; Tian, W.; Wang, L.; Luo, J.; Li, Q.; Fan, X.; Yao, Y. Enhanced through-plane thermal conductivity of boron nitride/epoxy composites. Compos. Part A 2017, 98, 25–31. [Google Scholar] [CrossRef]
- Bian, W.; Yao, T.; Chen, M.; Zhang, C.; Shao, T.; Yang, Y. The synergistic effects of the micro-BN and nano-Al2O3 in micro-nano composites on enhancing the thermal conductivity for insulating epoxy resin. Compos. Sci. Technol. 2018, 168, 420–428. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Yang, D.; Zhang, J.; Guo, Y.; Zhong, X.; Kong, J.; Gua, J. High-efficiency improvement of thermal conductivities for epoxy composites from synthesized liquid crystal epoxy followed by doping BN fillers. Compos. Part B Eng. 2020, 185, 107784. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Y.; Yeh, Y.; Li, C. Characteristic and Synthesis of High-Temperature Resistant Liquid Crystal Epoxy Resin Containing Boron Nitride Composite. Polymers 2022, 14, 1252. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; He, R.; Liu, Y.; Wang, Q. Multifunctional boron nitride nanosheets cured epoxy resins with highly thermal conductivity and enhanced flame retardancy for thermal management applications. Compos. Part A 2023, 164, 107309. [Google Scholar] [CrossRef]
- Akatsuka, M.; Takezawa, Y. Study of high thermal conductive epoxy resins containing controlled high-order structures. J. Appl. Polym. Sci. 2003, 89, 2464–2467. [Google Scholar] [CrossRef]
- Harada, M.; Ochi, M.; Tobita, M.; Kimura, T.; Ishigaki, T.; Shimoyama, N.; Aoki, H. Thermal-conductivity properties of liquid-crystalline epoxy resin cured under a magnetic field. J. Polym. Sci. Part A Polym. Phys. 2003, 41, 1739–1743. [Google Scholar] [CrossRef]
- Tokushige, N.; Mihara, T.; Koide, N. Thermal properties and photo-polymerization of diepoxy monomers with mesogenic group. Mol. Cryst. Liq. Cryst. 2005, 428, 33–47. [Google Scholar] [CrossRef]
- Song, S.; Katagi, H.; Takezawa, T. Study on high thermal conductivity of mesogenic epoxy resin with spherulite structure. Polymer 2012, 53, 4489–4492. [Google Scholar] [CrossRef]
- Harada, M.; Hamaura, N.; Ochi, M.; Agari, Y. Thermal conductivity of liquid crystalline epoxy/BN filler composites having ordered network structure. Compos. Part B 2013, 55, 306–313. [Google Scholar] [CrossRef]
- Li, Y.; Badrinarayanan, P.; Kessler, M. Liquid crystalline epoxy resin based on biphenyl mesogen: Thermal characterization. Polymer 2013, 54, 3017–3025. [Google Scholar] [CrossRef]
- Giang, T.; Kim, J. Effect of backbone moiety in diglycidylether-terminated liquid crystalline epoxy on thermal conductivity of epoxy/alumina composite. J. Ind. Eng. Chem. 2015, 30, 77–84. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, H.; Gan, J.; Liang, L.; Wu, K.; Lu, M. High thermal conductivity epoxies containing substituted biphenyl mesogenic. J. Mater. Sci. Mater. Electron. 2016, 27, 2754–2759. [Google Scholar] [CrossRef]
- Kawamoto, S.; Fujiwara, H.; Nishimura, S. Hydrogen characteristics and ordered structure of mono-mesogen type liquid-crystalline epoxy polymer. Int. J. Hydrog. Energy 2016, 41, 7500–7510. [Google Scholar] [CrossRef]
- Guo, H.; Lu, M.; Liang, L.; Wu, K.; Ma, D.; Xue, W. Liquid crystalline epoxies with lateral substituents showing a low dielectric constant and high thermal conductivity. J. Electron. Mater. 2017, 46, 982–991. [Google Scholar] [CrossRef]
- Kim, Y.; Yeo, H.; You, N.; Jang, S.G.; Ahn, S.; Jeong, J.; Lee, S.H.; Goh, M. Highly thermal conductive resins formed from wide-temperature-range eutectic mixtures of liquid crystalline epoxies bearing diglycidyl moieties at the side positions. Polym. Chem. 2017, 8, 2806–2814. [Google Scholar] [CrossRef]
- Tanaka, S.; Hojo, F.; Takezawa, Y.; Kanie, K.; Muramatsu, A. Layer structure formation of mesogenic liquid crystalline epoxy resin during curing reactions: A reactive coarse-grained molecular dynamics study. Polym.-Plast. Technol. Eng. 2018, 57, 269–275. [Google Scholar] [CrossRef]
- Tanaka, S.; Hojo, F.; Takezawa, Y.; Kanie, K.; Muramatsu, A. Homeotropically aligned monodomain-like smectic-A structure in liquid crystalline epoxy films: Analysis of the local ordering structure by microbeam small-angle X-ray scattering. ACS Omega 2018, 3, 3562–3570. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.; Kim, C.B.; Kang, D.; Jeong, K.; Jang, S.G.; You, N.; Ahn, S.; Lee, D.; Goh, M. Liquid crystalline epoxy resin with improved thermal conductivity by intermolecular dipole–dipole interactions. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 708–715. [Google Scholar] [CrossRef]
- Ota, S.; Yamaguchi, K.; Harada, M. Phase structure and thermal conductivity of liquid crystalline epoxy resins cured with the binary mixed curing agents. J. Netw. Polym. Jpn. 2019, 40, 278–286. [Google Scholar]
- Lin, Z.; Cong, Y.; Zhang, B.; Huang, H. Synthesis and characterization of a novel Y-shaped liquid crystalline epoxy and its effect on isotropic epoxy resin. Liq. Cryst. 2019, 46, 1467–1477. [Google Scholar] [CrossRef]
- Shen, W.; Cao, Y.; Zhang, C.; Yuan, X. Network morphology and electro-optical characterization of epoxy based polymer stabilized liquid crystals. Liq. Cryst. 2020, 47, 481–488. [Google Scholar] [CrossRef]
- Ota, S.; Harada, H. Filler surface adsorption of mesogenic epoxy for LC Epoxy/MgO composites with high thermal conductivity. Compos. Part C Open Access 2021, 4, 100087. [Google Scholar] [CrossRef]
- Ota, S.; Harada, M. Thermal conductivity enhancement of liquid crystalline epoxy/MgO composites by formation of highly ordered network structure. J. Appl. Polym. Sci. 2021, 138, 50367. [Google Scholar] [CrossRef]
- Harada, M.; Kawasaki, Y. High toughness and thermal conductivity of thermosets from liquid crystalline epoxy with low melting point. J. Appl. Polym. Sci. 2022, 139, 52391. [Google Scholar] [CrossRef]
- Zhong, X.; Yang, X.; Ruan, K.; Zhang, J.; Zhang, H.; Gu, J. Discotic Liquid Crystal Epoxy Resins Integrating Intrinsic High Thermal Conductivity and Intrinsic Flame Retardancy. Macromol. Rapid Commun. 2022, 43, 2100580. [Google Scholar] [CrossRef]
- Hossain, M.; Olamilekan, A.; Jeong, H. Diacetylene-containing dual-functional liquid crystal epoxy resin: Strategic phase control for topochemical polymerization of diacetylenes and thermal conductivity enhancement. Macromolecules 2022, 55, 11, 4402–4410. [Google Scholar] [CrossRef]
- Wang, M.; Yu, Y.; Wu, X.; Li, S. Synthesis and properties of cycloaliphatic epoxy resins containing imide and diphenyl sulfone. Polymer 2004, 45, 1253–1259. [Google Scholar] [CrossRef]
- Harada, M.; Yamaguchi, K. Fracture toughness of highly ordered liquid crystalline epoxy thermosets achieved by optimized composition of curing agents. J. Appl. Polym. Sci. 2021, 138, 50593. [Google Scholar] [CrossRef]
- Van Krevelen, D.; Te Nijenhuis, K. Properties of Polymers, 4th ed.; Elsevier: Oxford, UK, 2009. [Google Scholar]




| Curing System | Thermal Conductivity (W/(m·K)) | Domain Size (μm) | Relative Intensity * |
|---|---|---|---|
| m-PDA system | 0.31 ± 0.03 | 80 | 1.3 |
| CEUI system | 0.27 ± 0.02 | 120 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harada, M.; Matsumoto, T. Thermal Conductivity and Orientation Structure of Liquid Crystalline Epoxy Thermosets Prepared by Latent Curing Catalyst. Crystals 2024, 14, 47. https://doi.org/10.3390/cryst14010047
Harada M, Matsumoto T. Thermal Conductivity and Orientation Structure of Liquid Crystalline Epoxy Thermosets Prepared by Latent Curing Catalyst. Crystals. 2024; 14(1):47. https://doi.org/10.3390/cryst14010047
Chicago/Turabian StyleHarada, Miyuki, and Takuya Matsumoto. 2024. "Thermal Conductivity and Orientation Structure of Liquid Crystalline Epoxy Thermosets Prepared by Latent Curing Catalyst" Crystals 14, no. 1: 47. https://doi.org/10.3390/cryst14010047
APA StyleHarada, M., & Matsumoto, T. (2024). Thermal Conductivity and Orientation Structure of Liquid Crystalline Epoxy Thermosets Prepared by Latent Curing Catalyst. Crystals, 14(1), 47. https://doi.org/10.3390/cryst14010047






