A Foldable Metal–Organic Framework with cds Topology Assembled via Four-Connected Square-Planar Single Ni2+-Ion Nodes and Linear Bidentate Linkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
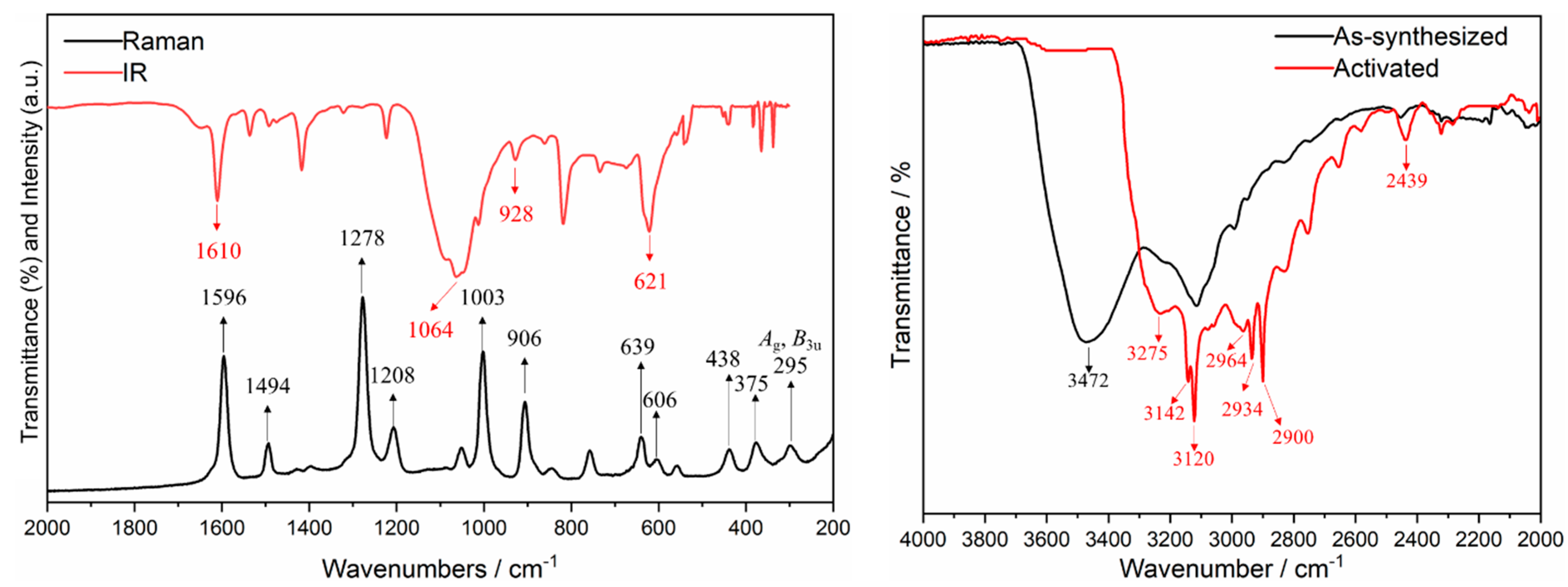
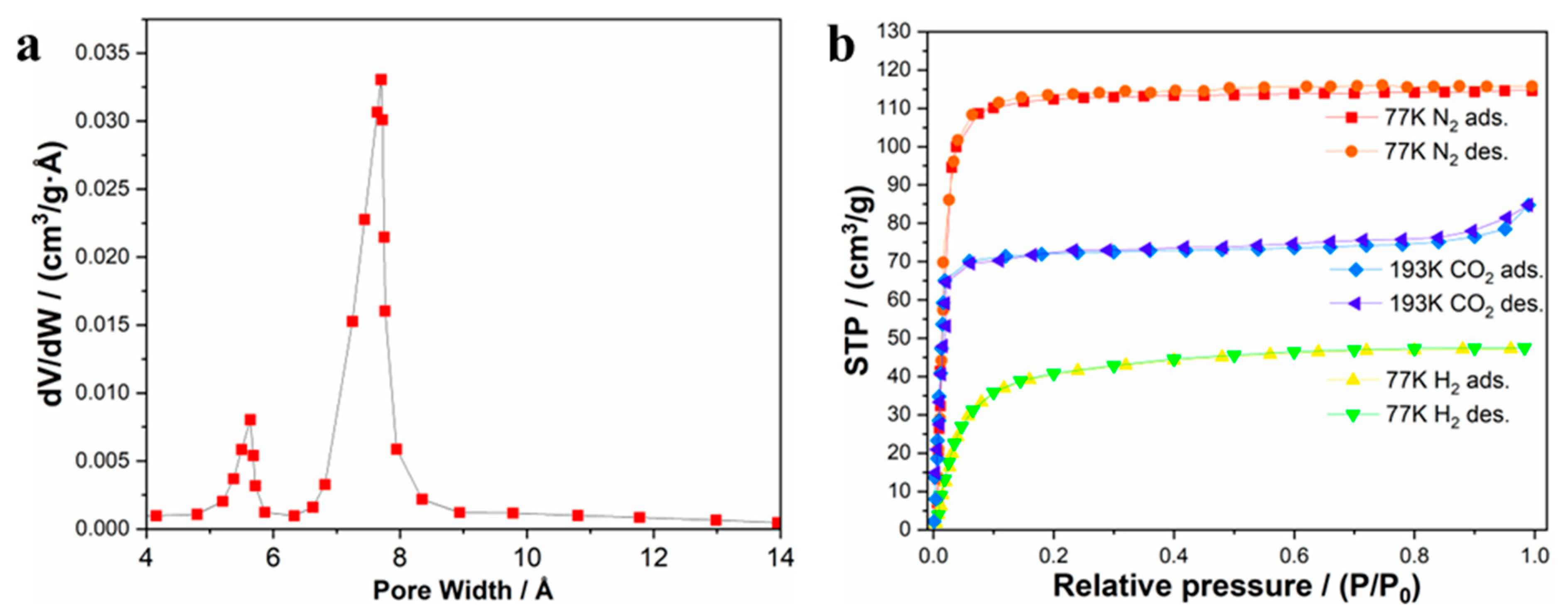
2.2. Physical Methods
2.3. Synthesis
3. Results and Discussion
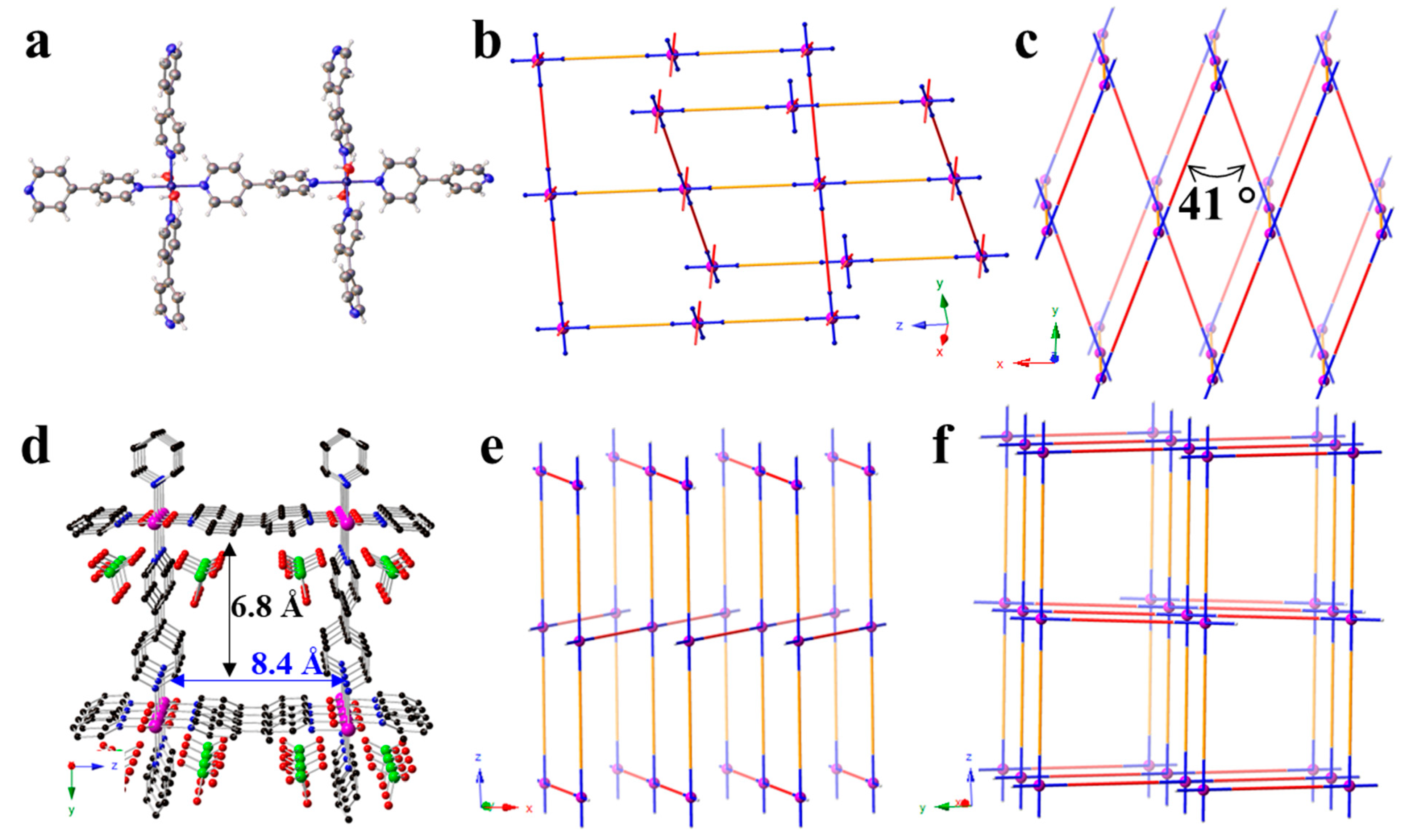
3.1. Structure Description
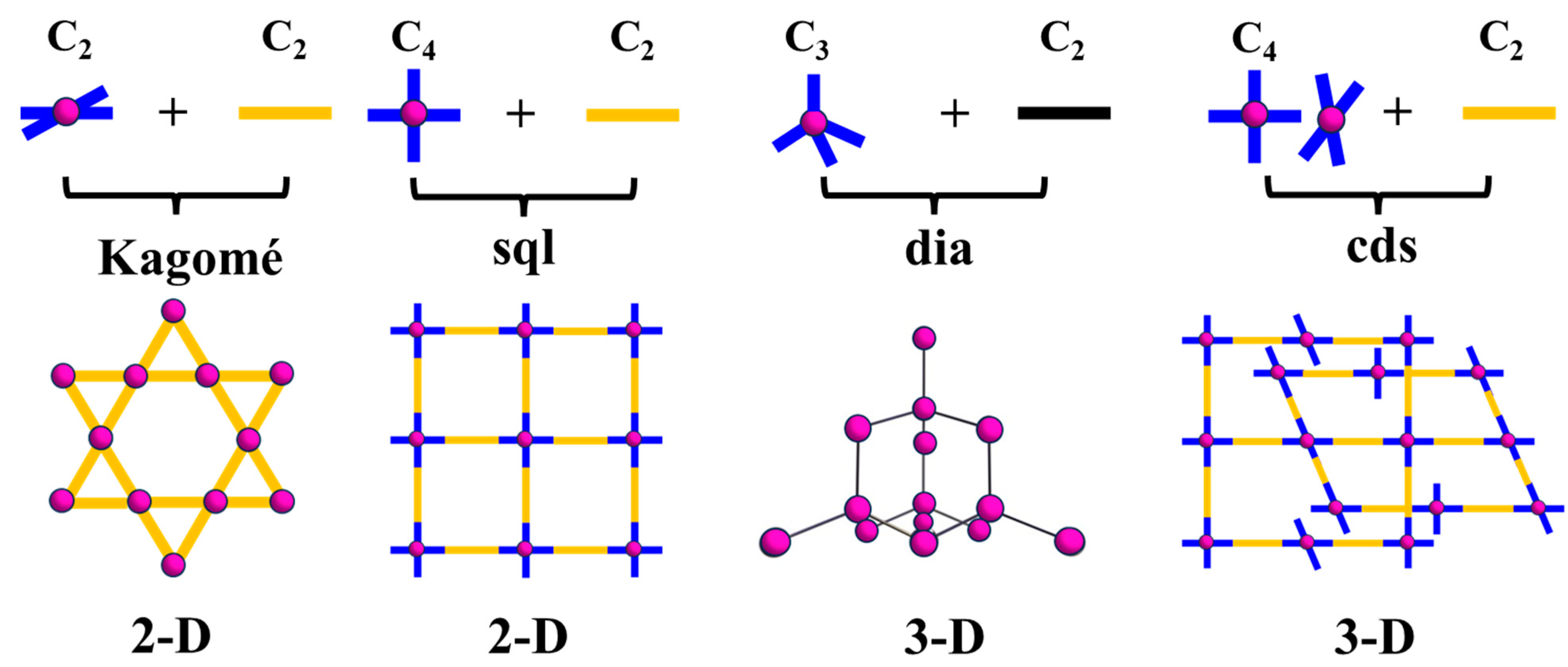
3.2. Topological Analysis
3.3. Packing Diagrams and Folding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dinca, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The current status of MOF and COF applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal-Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R. Metal-Organic Frameworks, Design and Application. In Topology and Interpenetration; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; Chapter 3. [Google Scholar]
- Severino, M.I.; Gkaniatsou, E.; Nouar, F.; Pinto, M.L.; Serre, C. MOFs industrialization: A complete assessment of production costs. Faraday Discuss. 2021, 231, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhou, Y.; Lv, Y.; Chen, W.; Su, X.; Zhang, T.; Xing, G.; Xu, G.; Terasaki, O.; Chen, L. Direct Construction of 2D Conductive Metal–Organic Frameworks from a Nonplanar Ligand: In Situ Scholl Reaction and Topological Modulation. J. Am. Chem. Soc. 2023, 145, 2739–2744. [Google Scholar] [CrossRef]
- Adams, C.J.; Munoz, M.C.; Waddington, R.E.; Real, J.A. Cooperative Spin Transition in the Two-Dimensional Coordination Polymer [Fe(4,4′-bipyridine)2(NCX)2]·4CHCl3 (X = S, Se). Inorg. Chem. 2011, 50, 10633–10642. [Google Scholar] [CrossRef]
- Zhang, Y.-S.; Enright, G.D.; Breeze, S.R.; Wang, S. Coordination polymers of cobalt(II): [Co(4,4′-bpy)2(O2CCF3)2]n and [Co(4,4′-bpy)(O2CCH3)2(H2O)2]n. New J. Chem. 1999, 23, 625–628. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H.; Groy, T.L. A Molecular Railroad with Large Pores: Synthesis and Structure of Ni(4,4′-bpy)2.5(H2O)2(ClO4)2·1.5(4,4′-bpy)·2H2O. Inorg. Chem. 1997, 36, 4292–4293. [Google Scholar] [CrossRef]
- Hu, F.; Yin, X.; Mi, Y.; Li, W.; Tang, X.; Ma, Z. Synthesis, structure and properties of a new copper (II) complex, [Cu2(4,4′-bpy)5(H2O)4](ClO4)4(4,4′-bpy)(DMF)2(H2O)2. Inorg. Chem. Commun. 2010, 13, 720–723. [Google Scholar] [CrossRef]
- Noro, S.-I.; Kitaura, R.; Kondo, M.; Kitagawa, S.; Ishii, T.; Matsuzaka, H.; Yamashita, M. Framework Engineering by Anions and Porous Functionalities of Cu(II)/4,4′-bpy Coordination Polymers. J. Am. Chem. Soc. 2002, 124, 2568–2583. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H. Hydrothermal synthesis of a metal-organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Subramanian, S.; Zaworotko, M.J. Porous solids by design: [Zn(4,4′-bpy)2(SiF6)]n ·xDMF, a single framework octahedral coordination polymer with large square channels. Angew. Chem. Int. Ed. Engl. 1995, 34, 2127–2129. [Google Scholar] [CrossRef]
- Friedrichs, O.D.; O’Keeffe, M.; Yaghi, O.M. The CdSO4, rutile, cooperite and quartz dual nets: Interpenetration and catenation. Solid State Sci. 2003, 5, 73–78. [Google Scholar] [CrossRef]
- Friedrichs, O.D.; O’Keeffe, M. Three-periodic tilings and nets: Face-transitive tilings and edge-transitive nets revisited. Acta Cryst. A 2007, 63, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Moulton, B.; Abourahma, H.; Bradner, M.W.; Lu, J.; McManus, G.J.; Zaworotko, M.J. A new 65.8 topology and a distorted 65.8 CdSO4 topology: Two new supramolecular isomers of [M2(bdc)2(L)2]n coordination polymers. Chem. Commun. 2003, 1342–1343. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, X.-Z.; Gu, H.-X.; Xu, Y.; Shen, X.; Zhu, D.-R. Two 3D metal–organic frameworks with different topologies, thermal stabilities and magnetic properties. Cryst. Eng. Comm. 2012, 14, 5905–5913. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Wu, J.; Jia, Z.-B.; Chen, H.-L.; Li, Q.-L.; Sakiyama, H.; Soares, T.; Fei, R.; Daiguebonne, C.; Guillou, O.; et al. Two isoreticular metal–organic frameworks with CdSO4-like topology: Selective gas sorption and drug delivery. Dalton Trans. 2014, 43, 17265–17273. [Google Scholar] [CrossRef]
- Tomar, K.; Rajak, R.; Sanda, S.; Konar, S. Synthesis and Characterization of Polyhedral-Based Metal–Organic Frameworks Using a Flexible Bipyrazole Ligand: Topological Analysis and Sorption Property Studies. Cryst. Growth Des. 2015, 15, 2732–2741. [Google Scholar] [CrossRef]
- Meng, D.-L.; Wang, X.; Tian, C.-B.; Wei, W.; Du, S.-W. Multistep Structural Transformation of a Magnetic Metal–Organic Framework: Possible Transformation Mechanism, Form Evolution, and Magnetic Properties. Cryst. Growth Des. 2020, 20, 1203–1210. [Google Scholar] [CrossRef]
- You, Z.-X.; Wang, C.; Xiao, Y.; Guan, Q.-L.; Li, J.-X.; Xing, Y.-H.; Gao, H.-W.; Sun, L.; Bai, F.-Y. Integrated Photoresponsive Alkaline Earth Metal Coordination Networks: Synthesis, Topology, Photochromism and Photoluminescence Investigation. Chem. Eur. J. 2021, 27, 9605–9619. [Google Scholar] [CrossRef] [PubMed]
- Amombo Noa, F.M.; Svensson Grape, E.; Brülls, S.M.; Cheung, O.; Malmberg, P.; Inge, A.K.; McKenzie, C.J.; Mårtensson, J.; Öhrström, L. Metal–Organic Frameworks with Hexakis(4-Carboxyphenyl)Benzene: Extensions to Reticular Chemistry and Introducing Foldable Nets. J. Am. Chem. Soc. 2020, 142, 9471–9481. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS. Program for Empirical Absorption Correction; University of Gottingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Long, G.J.; Clarke, P.J. Crystal and molecular structures of trans-tetrakis(pyridine)dichloroiron(II), -nickel(II), and -cobalt(II) and trans-tetrakis(pyridine)dichloroiron(II) monohydrate. Inorg. Chem. 1978, 17, 1394–1401. [Google Scholar] [CrossRef]
- Spec, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Lee, I.S.; Yang, D.C.; Moon, D.; Nam, J.; Cho, H.; Kang, E.; Lee, J.; Noh, H.-J.; Min, S.K.; et al. Origamic Metal-Organic Framework toward Mechanical Metamaterial. Nat. Commun. 2023, 14, 7938. [Google Scholar] [CrossRef]
- Holló, B.B.; Petruševski, V.M.; Kovács, G.B.; Franguelli, F.P.; Farkas, A.; Menyhárd, A.; Lendvay, G.; Sajó, I.E.; Nagy-Bereczki, L.; Pawar, R.P.; et al. Thermal and spectroscopic studies on a double-salt-type pyridine–silver perchlorate complex having κ1-O coordinated perchlorate ions. J. Therm. Anal. Calorim. 2019, 138, 1193–1205. [Google Scholar] [CrossRef]
- Yang, S.; Sun, J.; Ramirez-Cuesta, A.J.; Callear, S.K.; David, W.I.F.; Anderson, D.; Newby, R.; Blake, A.J.; Parker, J.E.; Tang, C.C.; et al. Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host. Nat. Chem. 2012, 4, 887–894. [Google Scholar] [CrossRef]
- Hönicke, I.M.; Senkovska, I.; Bon, V.; Baburin, I.A.; Bönisch, N.; Raschke, S.; Evans, J.D.; Kaskel, S. Balancing Mechanical Stability and Ultrahigh Porosity in Crystalline Framework Materials. Angew. Chem. Int. Ed. 2018, 57, 13780–13783. [Google Scholar] [CrossRef] [PubMed]
- Millward, A.R.; Yaghi, O.M. Metal−Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Rosen, A.S.; Notestein, J.M.; Snurr, R.Q. Structure–Activity Relationships That Identify Metal–Organic Framework Catalysts for Methane Activation. ACS Catal. 2019, 9, 3576–3587. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal–Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef]
- Cisterna, J.; Artigas, V.; Fuentealba, M.; Hamon, P.; Manzur, C.; Dorcet, V.; Hamon, J.-R.; Carrillo, D. Nickel(II) and copper(II) complexes of new unsymmetrically-substituted tetradentate Schiff base ligands: Spectral, structural, electrochemical and computational studies. Inorg. Chim. Acta 2017, 462, 266–280. [Google Scholar] [CrossRef]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef]


| {[trans-Ni(H2O)2(μ-4,4′-bpy)2](ClO4)2}n | |
|---|---|
| Empirical formula | C20H16Cl2N4NiO10 |
| FW | 606.01 |
| Temp (K) | 172 |
| Wavelength (Å) | 0.71073 |
| Crystal system | Orthorhombic |
| Space group | Ccc2 (No. 37) |
| a (Å) | 7.9447 (5) |
| b (Å) | 21.236 (1) |
| c (Å) | 22.519 (2) |
| V (Å3) | 3799.3 (4) |
| Z | 4 |
| D(calcd) (g/cm−3) | 1.059 |
| abs coeff (mm−1) | 0.692 |
| Total/indnt reflcns | 25,030/3255 |
| Refl I > 2σ(I)/param | 2240/103 |
| Rint | 0.0702 |
| R1 | 0.0841 |
| wR2 | 0.2198 |
| R1 (all data) | 0.1222 |
| wR2 (all data) | 0.2673 |
| F(000) | 1240.0 |
| GoF | 1.051 |
| CCDC No. | 2310766 |
| Compound | Ni-O Å | Ni-N Å | N-Ni-N | O-Ni-N | O-Ni-O |
|---|---|---|---|---|---|
| 1 | 2.097 (7) | 2.101 (17)–2.133 (4) | 177.592 (3), 180 | 89.165 (4)–90.8 (2) | 179.397 (4) |
| [Ni(H2O)2(py)4](ClO4)2 | 2.093 (6), 2.090 (6) | 2.096 (7)–2.115 (7) | 176.6 (3), 176.7 (3) | 87.7 (3)–93.3 (3) | 178.6 (3) |
| Element | Mass [%] | Atom [%] |
|---|---|---|
| Carbon | 23.5 (12) | 48.6 |
| Nitrogen | 6.9 (4) | 12.7 |
| Oxygen | 18.0 (9) | 28.7 |
| Chlorine | 10.0 (3) | 7.2 |
| Nickel | 6.3 (2) | 2.8 |
| Total | 64.7 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.-C.; Wang, X.; Drozd, V.; Raptis, R.G. A Foldable Metal–Organic Framework with cds Topology Assembled via Four-Connected Square-Planar Single Ni2+-Ion Nodes and Linear Bidentate Linkers. Crystals 2024, 14, 40. https://doi.org/10.3390/cryst14010040
Shi Z-C, Wang X, Drozd V, Raptis RG. A Foldable Metal–Organic Framework with cds Topology Assembled via Four-Connected Square-Planar Single Ni2+-Ion Nodes and Linear Bidentate Linkers. Crystals. 2024; 14(1):40. https://doi.org/10.3390/cryst14010040
Chicago/Turabian StyleShi, Zhi-Chun, Xiaoliang Wang, Vadym Drozd, and Raphael G. Raptis. 2024. "A Foldable Metal–Organic Framework with cds Topology Assembled via Four-Connected Square-Planar Single Ni2+-Ion Nodes and Linear Bidentate Linkers" Crystals 14, no. 1: 40. https://doi.org/10.3390/cryst14010040
APA StyleShi, Z.-C., Wang, X., Drozd, V., & Raptis, R. G. (2024). A Foldable Metal–Organic Framework with cds Topology Assembled via Four-Connected Square-Planar Single Ni2+-Ion Nodes and Linear Bidentate Linkers. Crystals, 14(1), 40. https://doi.org/10.3390/cryst14010040






