Optical Photon Propagation Characteristics and Thickness Optimization of LaCl3:Ce and LaBr3:Ce Crystal Scintillators for Nuclear Medicine Imaging
Abstract
:1. Introduction
2. Materials and Methods
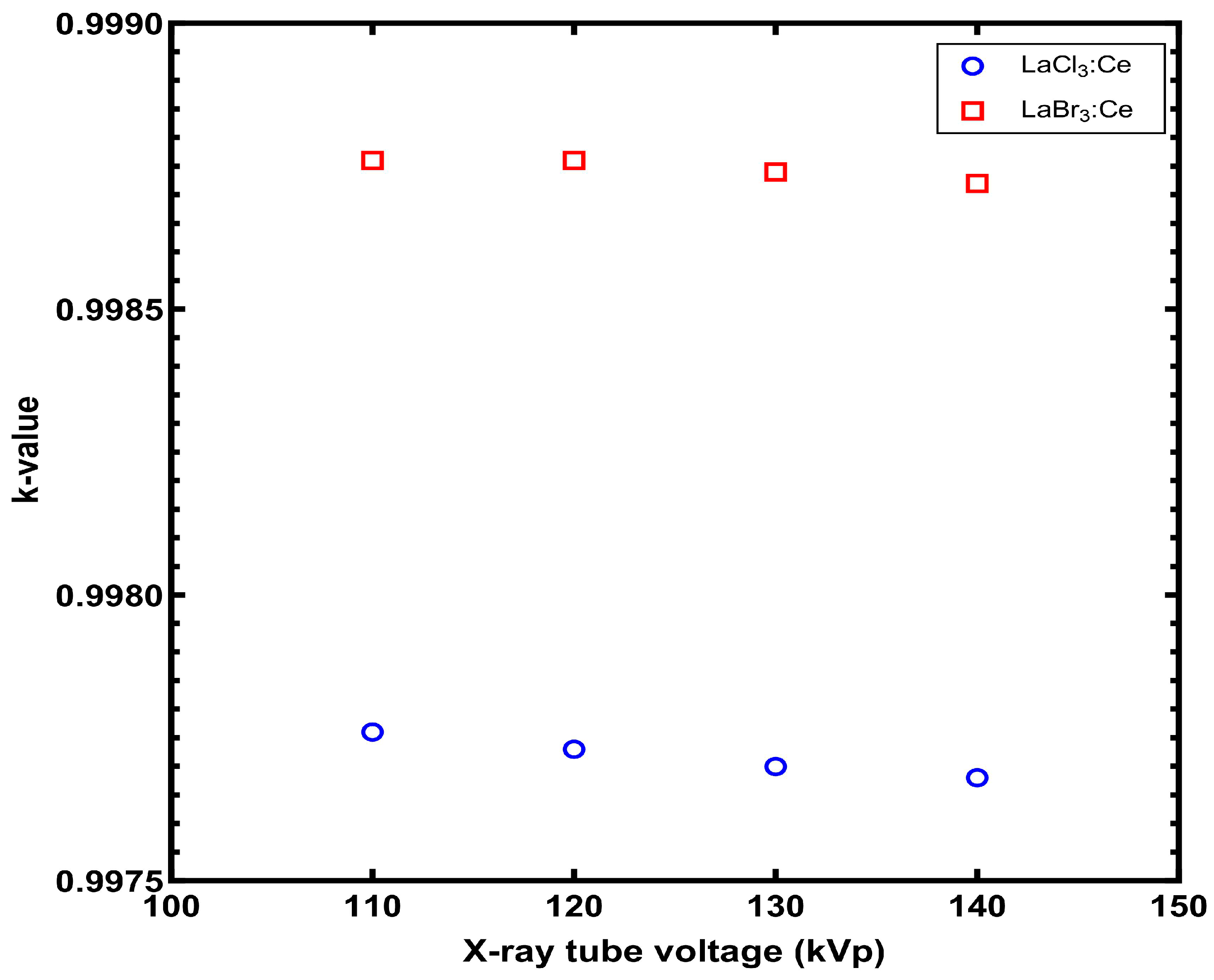
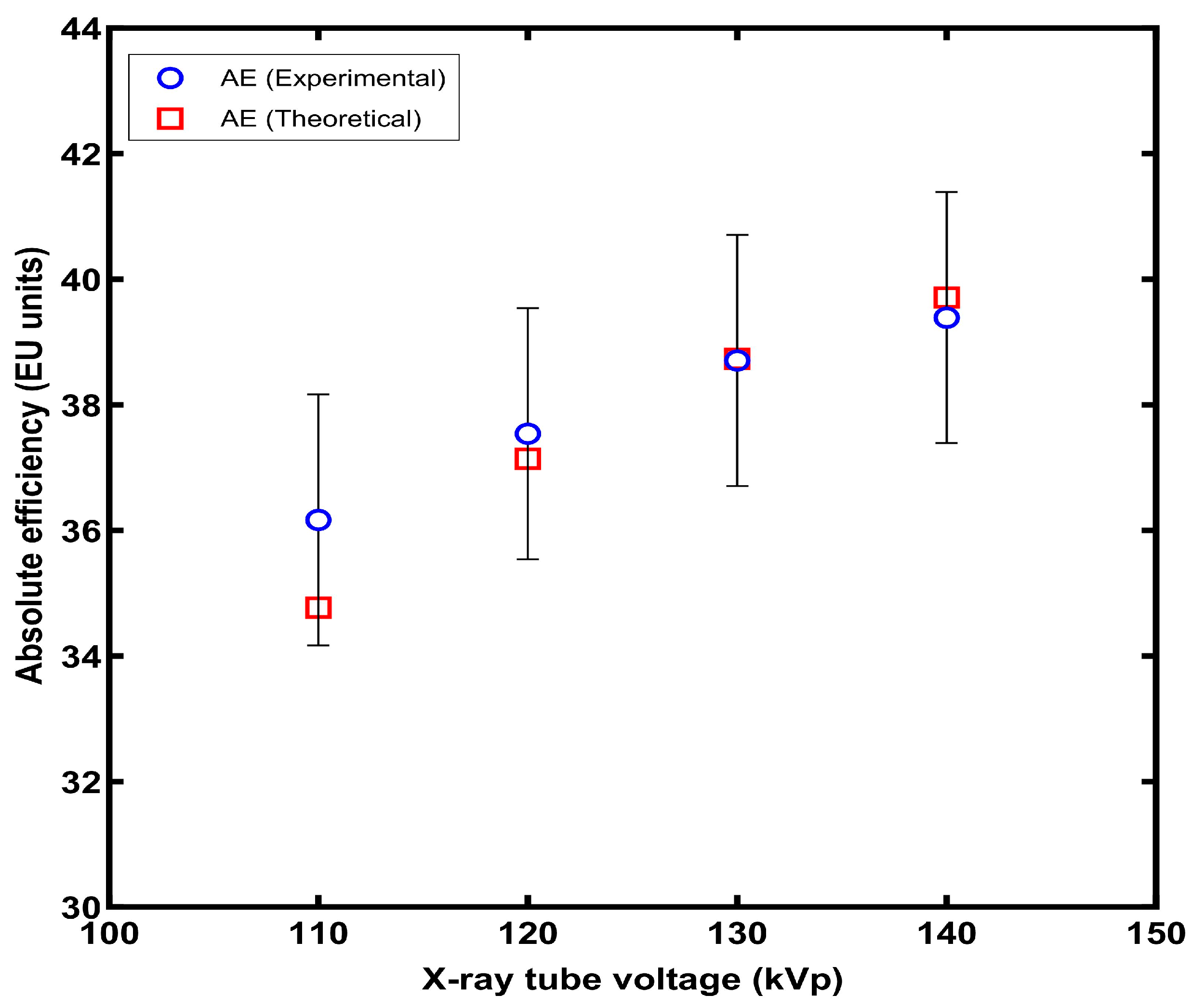
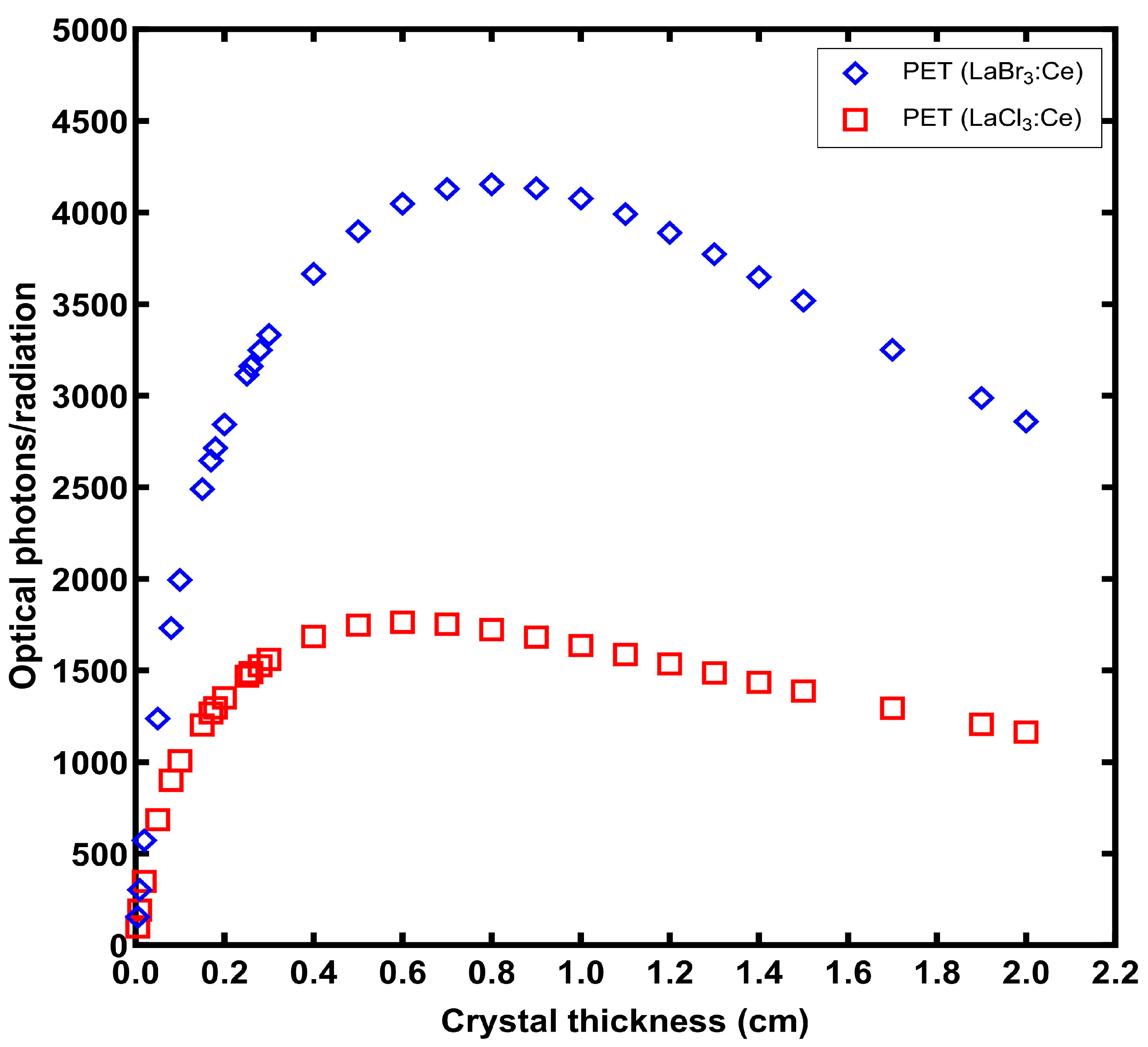
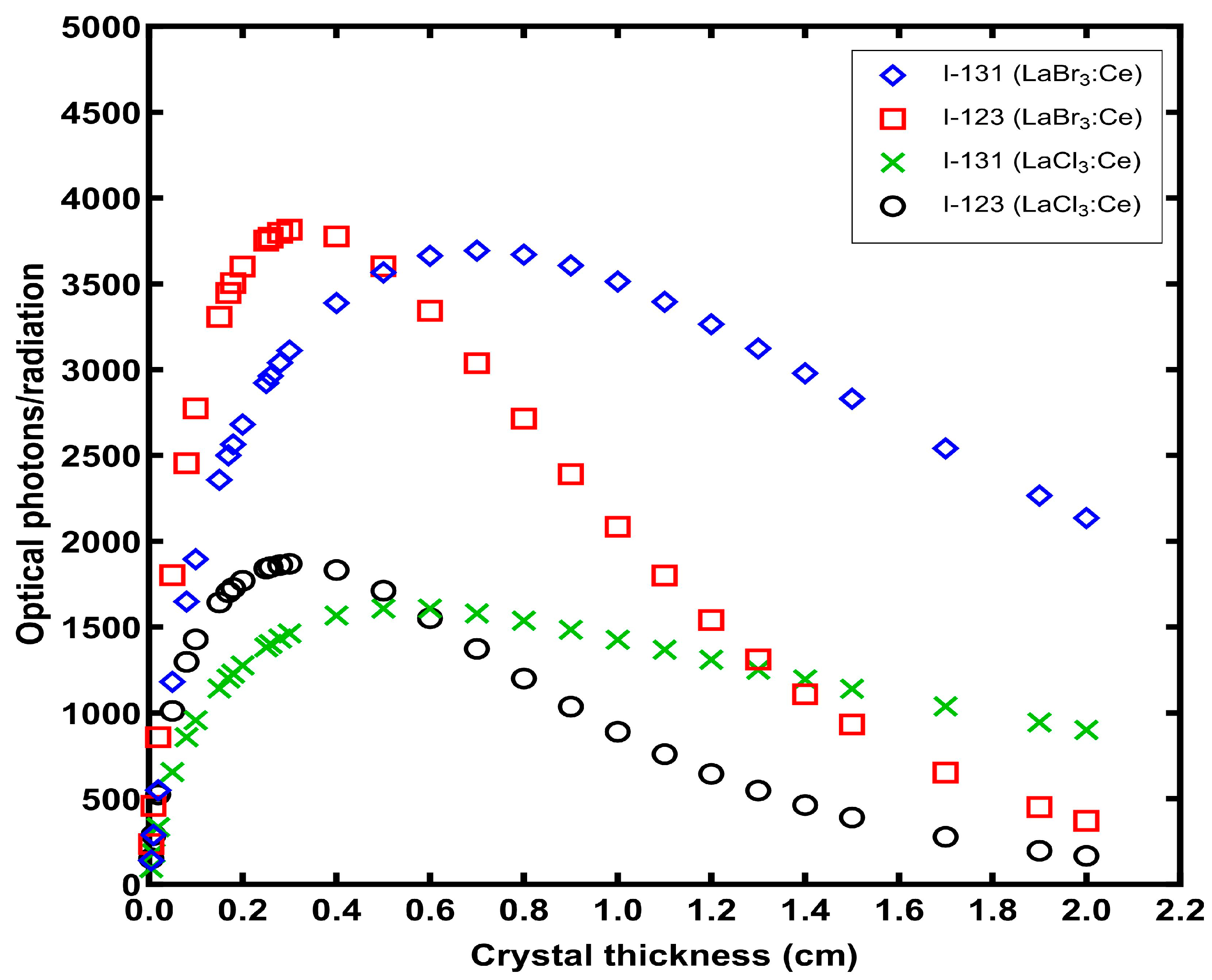
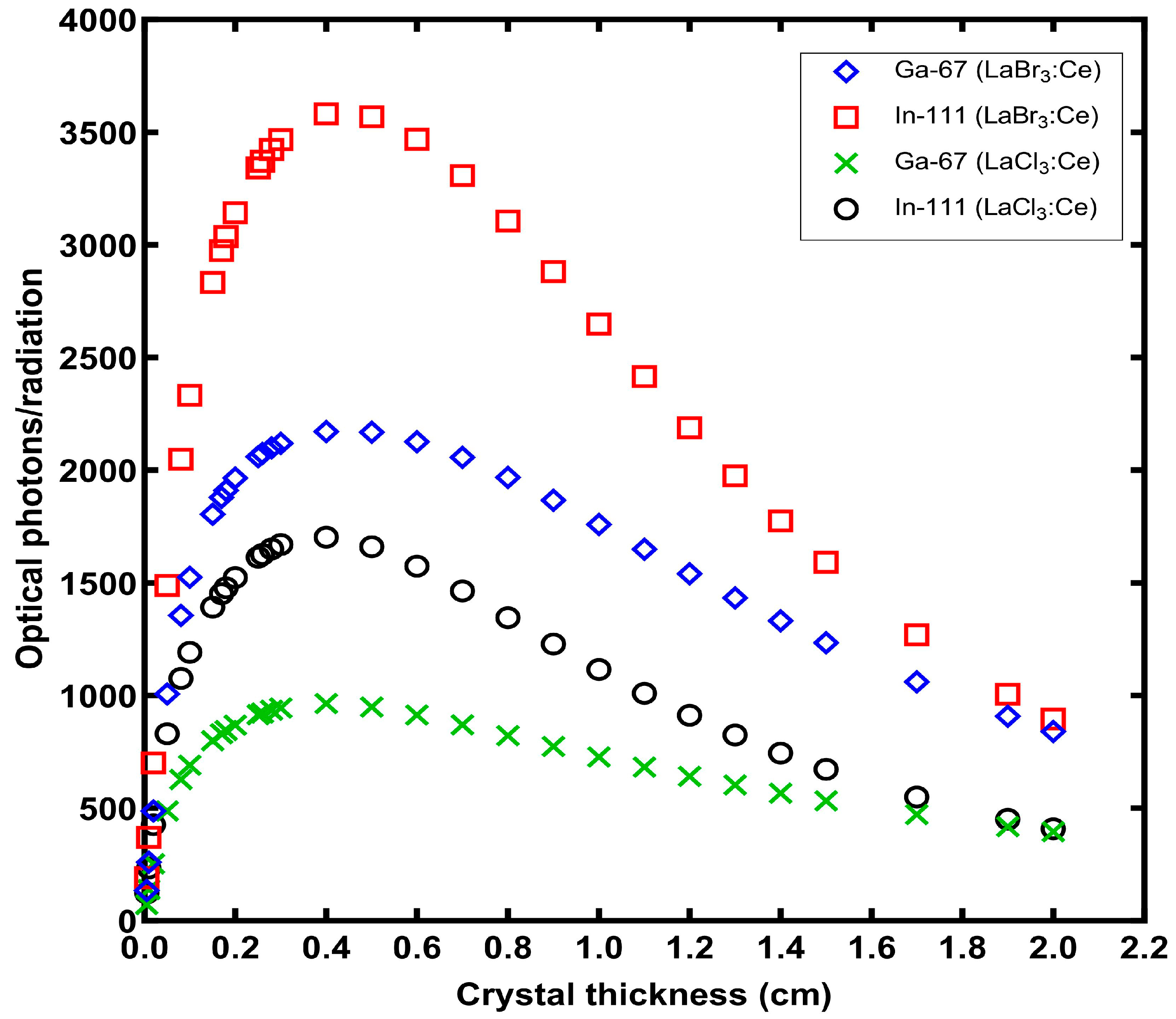
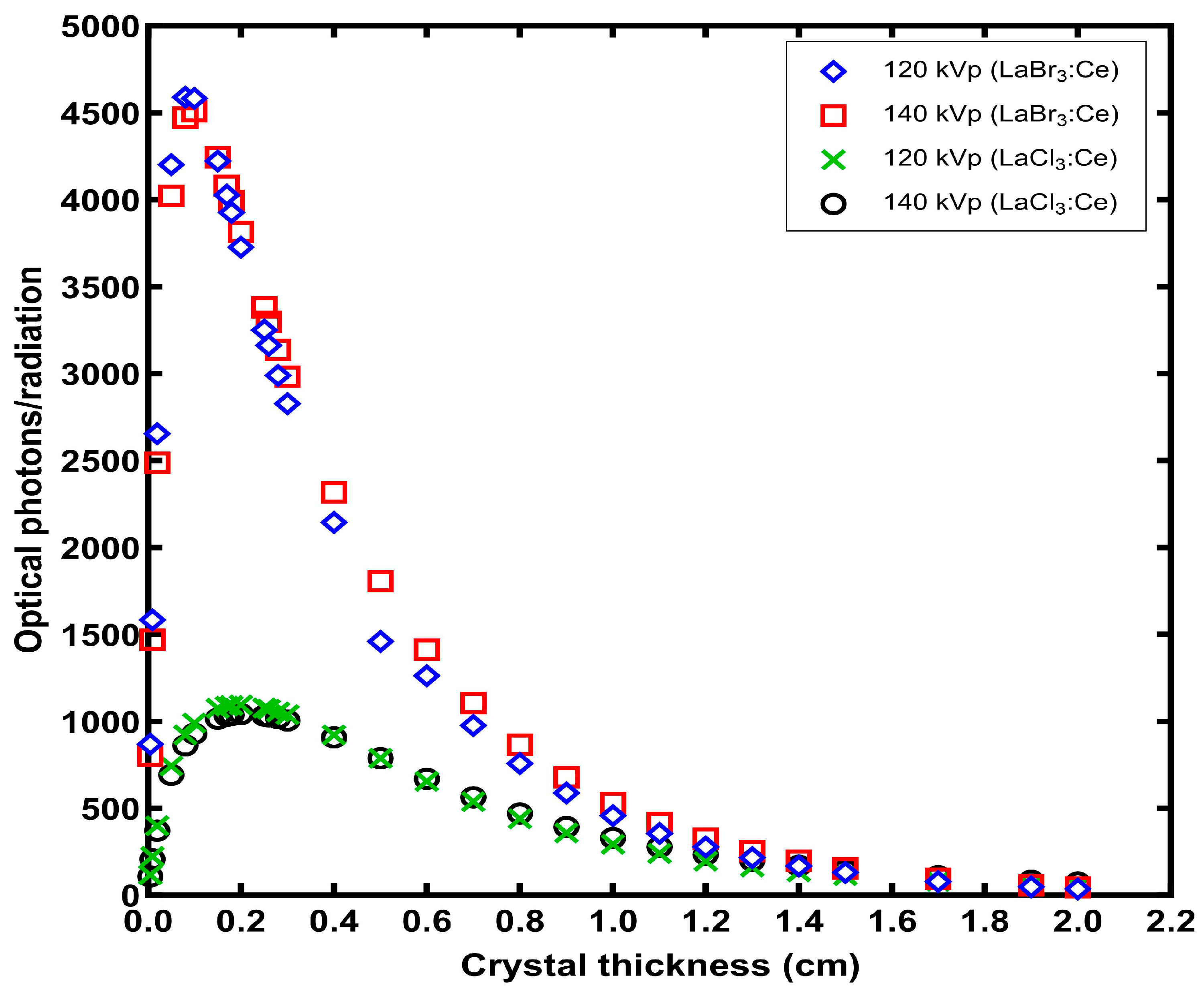
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. Physics in Nuclear Medicine, 4th ed.; Elsevier/Saunders: Philadelphia, PA, USA, 2012. [Google Scholar]
- Lecomte, R.; Granja, C.; Leroy, C.; Stekl, I. Biomedical Imaging: SPECT and PET. AIP Conf. Proc. 2007, 958, 115–122. [Google Scholar] [CrossRef]
- Eijk, C.W.E.V. Inorganic scintillators in medical imaging. Phys. Med. Biol. 2002, 47, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Morphis, M.; Van Staden, J.A.; du Raan, H.; Ljungberg, M. Evaluation of Iodine-123 and Iodine-131 SPECT activity quantification: A Monte Carlo study. EJNMMI Phys. 2021, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.T. Recent Advances in SPECT Imaging. J. Nucl. Med. 2007, 48, 661–673. [Google Scholar] [CrossRef]
- Zanzonico, P. Positron emission tomography: A review of basic principles, scanner design and performance, and current systems. Semin. Nucl. Med. 2004, 34, 87–111. [Google Scholar] [CrossRef]
- Chowdhury, F.U.; Scarsbrook, A.F. The role of hybrid SPECT-CT in oncology: Current and emerging clinical applications. Clin. Radiol. 2008, 63, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.L. Transmission scanning in emission tomography. Eur. J. Nucl. Med. Mol. Imaging 1998, 25, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Cal-Gonzalez, J.; Rausch, I.; Shiyam Sundar, L.K.; Lassen, M.L.; Muzik, O.; Moser, E.; Papp, L.; Beyer, T. Hybrid Imaging: Instrumentation and Data Processing. Front. Phys. 2018, 6, 47. [Google Scholar] [CrossRef]
- Beyer, T.; Freudenberg, L.S.; Townsend, D.W.; Czernin, J. The Future of Hybrid Imaging—Part 1: Hybrid Imaging Technologies and SPECT/CT. Insights Imaging 2011, 2, 161–169. [Google Scholar] [CrossRef]
- Beyer, T.; Townsend, D.W.; Czernin, J.; Freudenberg, L.S. The Future of Hybrid Imaging—Part 2: PET/CT. Insights Imaging 2011, 2, 225–234. [Google Scholar] [CrossRef]
- Guo, Z. The Principle and State-of-Art Facilities for PET. J. Phys. Conf. Ser. 2022, 2386, 012062. [Google Scholar] [CrossRef]
- Marsden, P.K. Detector technology challenges for nuclear medicine and PET. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2003, 513, 1–7. [Google Scholar] [CrossRef]
- Berard, P.; Pepin, C.M.; Rouleau, D.; Cadorette, J.; Lecomte, R. CT acquisition using PET detectors and electronics. IEEE Trans. Nucl. Sci. 2005, 52, 634–637. [Google Scholar] [CrossRef]
- Van Loef, E.V.D.; Dorenbos, P.; Van Eijk, C.W.E.V.; Kramer, K.; Gudel, H.U. Scintillation properties of LaCl3:Ce3+ crystals: Fast, efficient and high-energy-resolution scintillators. IEEE Nucl. Sci. Symp. Conf. Rec. 2000, 6, 31–34. [Google Scholar] [CrossRef]
- Iltis, A.; Mayhugh, M.R.; Menge, P.; Rozsa, C.M.; Selles, O.; Solovyev, V. Lanthanum halide scintillators: Properties and applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 563, 359–363. [Google Scholar] [CrossRef]
- Van Loef, E.V.D.; Dorenbos, P.; Van Eijk, C.W.E.V.; Krämer, K.; Güdel, H.U. High-energy-resolution scintillator: Ce3+ activated LaBr3. Appl. Phys. Lett. 2001, 79, 1573–1575. [Google Scholar] [CrossRef]
- Van Loef, E.V.D.; Dorenbos, P.; Van Eijk, C.W.E.V.; Krämer, K.; Güdel, H.U. Scintillation properties of LaBr3:Ce3+ crystals: Fast, efficient and high-energy-resolution scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2002, 486, 254–258. [Google Scholar] [CrossRef]
- Advatech, U.K. LaCl3: Ce-Lanthanum Chloride (Ce). Available online: https://www.advatech-uk.co.uk/lacl3_ce.html (accessed on 12 May 2023).
- Advatech, U.K. LaBr3: Ce-Lanthanum Bromide (Ce). Available online: https://www.advatech-uk.co.uk/labr3_ce.html (accessed on 12 May 2023).
- Tseremoglou, S.; Michail, C.; Valais, I.; Ninos, K.; Bakas, A.; Kandarakis, I.; Fountos, G.; Kalyvas, N. Evaluation of Cerium-Doped Lanthanum Bromide (LaBr3:Ce) Single-Crystal Scintillator’s Luminescence Properties under X-ray Radiographic Conditions. Appl. Sci. 2022, 13, 419. [Google Scholar] [CrossRef]
- Tseremoglou, S.; Michail, C.; Valais, I.; Ninos, K.; Bakas, A.; Kandarakis, I.; Fountos, G.; Kalyvas, N. Efficiency Properties of Cerium-Doped Lanthanum Chloride (LaCl3:Ce) Single Crystal Scintillator under Radiographic X-ray Excitation. Crystals 2022, 12, 655. [Google Scholar] [CrossRef]
- Bagheri, R.; Shirmardi, S.P.; Heidari, B.; Ahmadi, B.; Tuzemen, S. Comparison of LaBr3(CE) and NaI(TL) Scintillation Crystals Responses to Various Gamma Energies Using Monte Carlo Simulations. Int. J. Adv. Res. 2016, 4, 494–500. [Google Scholar] [CrossRef]
- Sharma, S.; Ranga, V.; Dhibar, M.; Rawat, S.; Kumar, G.A. Intrinsic Resolution of Compton Electrons in LaBr3: Ce and LaCl3: Ce Detectors Using Compton Coincidence Technique. Nucl. Phys. 2016, 61, 1020–1021. [Google Scholar]
- Sariyal, R.; Mazumdar, I.; Patel, S.M. Measurement of Absolute Light Yield and Quantum Efficiency of LaBr3: Ce Scintillator Detector Using SiPM. J. Inst. 2021, 16, P12024. [Google Scholar] [CrossRef]
- Dey Chaudhuri, S.; Banerjee, D.; Bhattacharjee, T.; Wasim Raja, S.; Acharya, R.; Pujari, P.K. Performance Study of LaBr3: Ce Detectors Coupled to R2083 PM Tube for Energy and Timing Characteristics. J. Radioanal. Nucl. Chem. 2020, 324, 829–835. [Google Scholar] [CrossRef]
- Cazzaniga, C.; Nocente, M.; Tardocchi, M.; Fazzi, A.; Hjalmarsson, A.; Rigamonti, D.; Ericsson, G.; Gorini, G. Thin YAP:Ce and LaBr3:Ce Scintillators as Proton Detectors of a Thin-Film Proton Recoil Neutron Spectrometer for Fusion and Spallation Sources Applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2014, 751, 19–22. [Google Scholar] [CrossRef]
- Nácher, E.; Mårtensson, M.; Tengblad, O.; Álvarez-Pol, H.; Bendel, M.; Cortina-Gil, D.; Gernhäuser, R.; Le Bleis, T.; Maj, A.; Nilsson, T.; et al. Proton Response of CEPA4: A Novel LaBr3(Ce)–LaCl3(Ce) Phoswich Array for High-Energy Gamma and Proton Spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2015, 769, 105–111. [Google Scholar] [CrossRef]
- Babiano, V.; Caballero, L.; Calvo, D.; Ladarescu, I.; Olleros, P.; Domingo-Pardo, C. γ -Ray Position Reconstruction in Large Monolithic LaCl3(Ce) Crystals with SiPM Readout. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2019, 931, 1–22. [Google Scholar] [CrossRef]
- Taggart, M.P.; Henderson, J. Fast-Neutron Response of LaBr3(Ce) and LaCl3(Ce) Scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 975, 164201. [Google Scholar] [CrossRef]
- Garnett, R.; Prestwich, W.V.; Atanackovic, J.; Wong, M.; Byun, S.H. Characterization of a LaBr3(Ce) Detector for Gamma-Ray Spectrometry for CANDU Power Reactors. Radiat. Meas. 2017, 106, 628–631. [Google Scholar] [CrossRef]
- Aldawood, S.; Castelhano, I.; Gernhäuser, R.; Van Der Kolff, H.; Lang, C.; Liprandi, S.; Lutter, R.; Maier, L.; Marinšek, T.; Schaart, D.R.; et al. Comparative Characterization Study of a LaBr3(Ce) Scintillation Crystal in Two Surface Wrapping Scenarios: Absorptive and Reflective. Front. Oncol. 2015, 5, 270. [Google Scholar] [CrossRef]
- Nikolopoulos, D.; Kalyvas, N.; Valais, I.; Argyriou, X.; Vlamakis, E.; Sevvos, T.; Kandarakis, I. A semi-empirical Monte Carlo based model of the Detector Optical Gain of Nuclear Imaging scintillators. J. Inst. 2012, 7, P11021. [Google Scholar] [CrossRef]
- David, S.; Michail, C.; Seferis, I.; Valais, I.; Fountos, G.; Liaparinos, P.; Kandarakis, I.; Kalyvas, N. Evaluation of Gd2O2S: Pr granular phosphor properties for X-ray mammography imaging. J. Lumin. 2016, 169, 706–710. [Google Scholar] [CrossRef]
- Kalyvas, N.; Valais, I.; Michail, C.; Fountos, G.; Kandarakis, I.; Cavouras, D. A theoretical study of CsI:Tl columnar scintillator image quality parameters by analytical modeling. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2015, 779, 18–24. [Google Scholar] [CrossRef]
- Kalyvas, N.; Valais, I.; David, S.; Michail, C.; Fountos, G.; Liaparinos, P.; Kandarakis, I. Studying the energy dependence of intrinsic conversion efficiency of single crystal scintillators under X-ray excitation. Opt. Spectrosc. 2014, 116, 743–747. [Google Scholar] [CrossRef]
- Roncali, E.; Stockhoff, M.; Cherry, S.R. An integrated model of scintillator-reflector properties for advanced simulations of optical transport. Phys. Med. Biol. 2017, 62, 4811–4830. [Google Scholar] [CrossRef] [PubMed]
- Bernabei, R.; Belli, P.; Montecchia, F.; Nozzoli, F.; d’Angelo, A.; Cappella, F.; Incicchitti, A.; Prosperi, D.; Castellano, S.; Cerulli, R.; et al. Performances and potentialities of a LaCl3:Ce scintillator. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2005, 555, 270–281. [Google Scholar] [CrossRef]
- Giaz, A.; Pellegri, L.; Riboldi, S.; Camera, F.; Blasi, N.; Boiano, C.; Bracco, A.; Brambilla, S.; Ceruti, S.; Coelli, S.; et al. Characterization of large volume 3.5″×8″ LaBr3:Ce detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2013, 729, 910–921. [Google Scholar] [CrossRef]
- RefractiveIndex. INFO. Available online: https://refractiveindex.info/ (accessed on 12 May 2023).
- TASMIP Spectra Calculator. Available online: https://www.solutioinsilico.com/medical-physics/applications/tasmip-app.php?ans=0 (accessed on 14 March 2023).
- Averyanov, D.; Blau, D. New Inogranic Scintillators’ Application in the Electromagnetic Calorimetry in High-Energy Physics. Appl. Sci. 2023, 13, 6189. [Google Scholar] [CrossRef]
- Nicolaidis, R.; Nozzoli, F.; Pepponi, G.; on behalf of the NUSES Collaboration. A Compact Particle Detector for Space-Based Applications: Development of a Low-Energy Module (LEM) for the NUSES Space Mission. Instruments 2023, 7, 40. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Elmahroug, Y.; Kumar, A.; Tekin, H.O.; Rekik, N.; Dong, M.; Lee, D.-E.; Yoon, J.; Park, T. Detailed Inspection of γ-Ray, Fast and Thermal Neutrons Shielding Competence of Calcium Oxide or Strontium Oxide Comprising Bismuth Borate Glasses. Materials 2021, 14, 2265. [Google Scholar] [CrossRef]
- Sarrut, D.; Bała, M.; Bardiès, M.; Bert, J.; Chauvin, M.; Chatzipapas, K.; Dupont, M.; Etxebeste, A.; M Fanchon, L.; Jan, S.; et al. Advanced Monte Carlo Simulations of Emission Tomography Imaging Systems with GATE. Phys. Med. Biol. 2021, 66, 10TR03. [Google Scholar] [CrossRef]
- Sarrut, D.; Arbor, N.; Baudier, T.; Borys, D.; Etxebeste, A.; Fuchs, H.; Gajewski, J.; Grevillot, L.; Jan, S.; Kagadis, G.C.; et al. The OpenGATE Ecosystem for Monte Carlo Simulation in Medical Physics. Phys. Med. Biol. 2022, 67, 184001. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, X.; Chen, Y.; Zhang, B.; Ma, X.; Ai, X.; Li, J. A Portable Three-Layer Compton Camera for Wide-Energy-Range Gamma-Ray Imaging: Design, Simulation and Preliminary Testing. Sensors 2023, 23, 8951. [Google Scholar] [CrossRef]
- Zarrini-Monfared, Z.; Karbasi, S.; Zamani, A.; Mosleh-Shirazi, M.A. Full modulation transfer functions of thick parallel- and focused-element scintillator arrays obtained by a Monte Carlo optical transport model. Med. Phys. 2023, 50, 3651–3660. [Google Scholar] [CrossRef]
- McIlwain, M.E.; Gao, D.; Thompson, N. First principle quantum description of the energetics associated with LaBr3, LaCl3, and Ce doped scintillators. In Proceedings of the 2007 IEEE Nuclear Science Symposium Conference Record, Honolulu, HI, USA, 26 October–3 November 2007; pp. 2460–2465. [Google Scholar] [CrossRef]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef]
- Pimlott, S.L.; Sutherland, A. Molecular tracers for the PET and SPECT imaging of disease. Chem. Soc. Rev. 2011, 40, 149–162. [Google Scholar] [CrossRef]
- Pagnanelli, R.A.; Basso, D.A. Myocardial Perfusion Imaging with 201Tl. J. Nucl. Med. Technol. 2010, 38, 1–3. [Google Scholar] [CrossRef]
- Bailey, D.; Sabanathan, D.; Aslani, A.; Campbell, D.; Walsh, B.; Lengkeek, N. RetroSPECT: Gallium-67 as a Long-Lived Imaging Agent for Theranostics. Asia Ocean. J. Nucl. Med. Biol. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Israel, O.; Pellet, O.; Biassoni, L.; De Palma, D.; Estrada-Lobato, E.; Gnanasegaran, G.; Kuwert, T.; la Fougere, C.; Mariani, G.; Massalha, S.; et al. Two decades of SPECT/CT—The coming of age of a technology: An updated review of literature evidence. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1990–2012. [Google Scholar] [CrossRef]
- Oohashi, M.; Toshima, H.; Hayama, K.; Ogura, I. Gallium-67 SPECT-CT for the evaluation of head and neck: Preliminary study on maximum standardised uptake value in lesions, and in the parotid and submandibular glands. Pol. J. Radiol. 2020, 85, 224–229. [Google Scholar] [CrossRef]
- Lewis, S.S.; Cox, G.M.; Stout, J.E. Clinical Utility of Indium 111–Labeled White Blood Cell Scintigraphy for Evaluation of Suspected Infection. Open Forum Infect. Dis. 2014, 1, ofu089. [Google Scholar] [CrossRef]
- Roca, M.; De Vries, E.F.J.; Jamar, F.; Israel, O.; Signore, A. Guidelines for the labelling of leucocytes with 111In-oxine. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 835–841. [Google Scholar] [CrossRef] [PubMed]


| Radionuclides | γ Energy (keV) and % Ratio |
|---|---|
| 123I | 159 (83%) |
| 201Tl | 71 (47%), 80 (20%), 167 (10%) |
| 67Ga | 93 (40%), 185 (20%), 300 (17%), 393 (20%) |
| 111In | 171 (90%), 245 (94%) |
| 131I | 365 (82%) |
| 99mTc | 140 (89%) |
| Properties | LaCl3:Ce | LaBr3:Ce |
|---|---|---|
| Wavelength, max emission (nm) | 350 | 380 |
| Decay Time (ns) | 28 | 25 |
| Light Yield (photons/MeV) | 49,000 | 63,000 |
| Radiation Length (cm) | 2.813 | 1.881 |
| Density (g/cm3) | 3.86 | 5.2 |
| Hardness (Mho) | 3 | 3 |
| Reflection Loss/Surface (%) | 6.8 | 6.8 |
| Lattice constant (nm) | 0.6196 | 0.6196 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseremoglou, S.; Michail, C.; Valais, I.; Ninos, K.; Bakas, A.; Kandarakis, I.; Fountos, G.; Kalyvas, N. Optical Photon Propagation Characteristics and Thickness Optimization of LaCl3:Ce and LaBr3:Ce Crystal Scintillators for Nuclear Medicine Imaging. Crystals 2024, 14, 24. https://doi.org/10.3390/cryst14010024
Tseremoglou S, Michail C, Valais I, Ninos K, Bakas A, Kandarakis I, Fountos G, Kalyvas N. Optical Photon Propagation Characteristics and Thickness Optimization of LaCl3:Ce and LaBr3:Ce Crystal Scintillators for Nuclear Medicine Imaging. Crystals. 2024; 14(1):24. https://doi.org/10.3390/cryst14010024
Chicago/Turabian StyleTseremoglou, Stavros, Christos Michail, Ioannis Valais, Konstantinos Ninos, Athanasios Bakas, Ioannis Kandarakis, George Fountos, and Nektarios Kalyvas. 2024. "Optical Photon Propagation Characteristics and Thickness Optimization of LaCl3:Ce and LaBr3:Ce Crystal Scintillators for Nuclear Medicine Imaging" Crystals 14, no. 1: 24. https://doi.org/10.3390/cryst14010024
APA StyleTseremoglou, S., Michail, C., Valais, I., Ninos, K., Bakas, A., Kandarakis, I., Fountos, G., & Kalyvas, N. (2024). Optical Photon Propagation Characteristics and Thickness Optimization of LaCl3:Ce and LaBr3:Ce Crystal Scintillators for Nuclear Medicine Imaging. Crystals, 14(1), 24. https://doi.org/10.3390/cryst14010024










