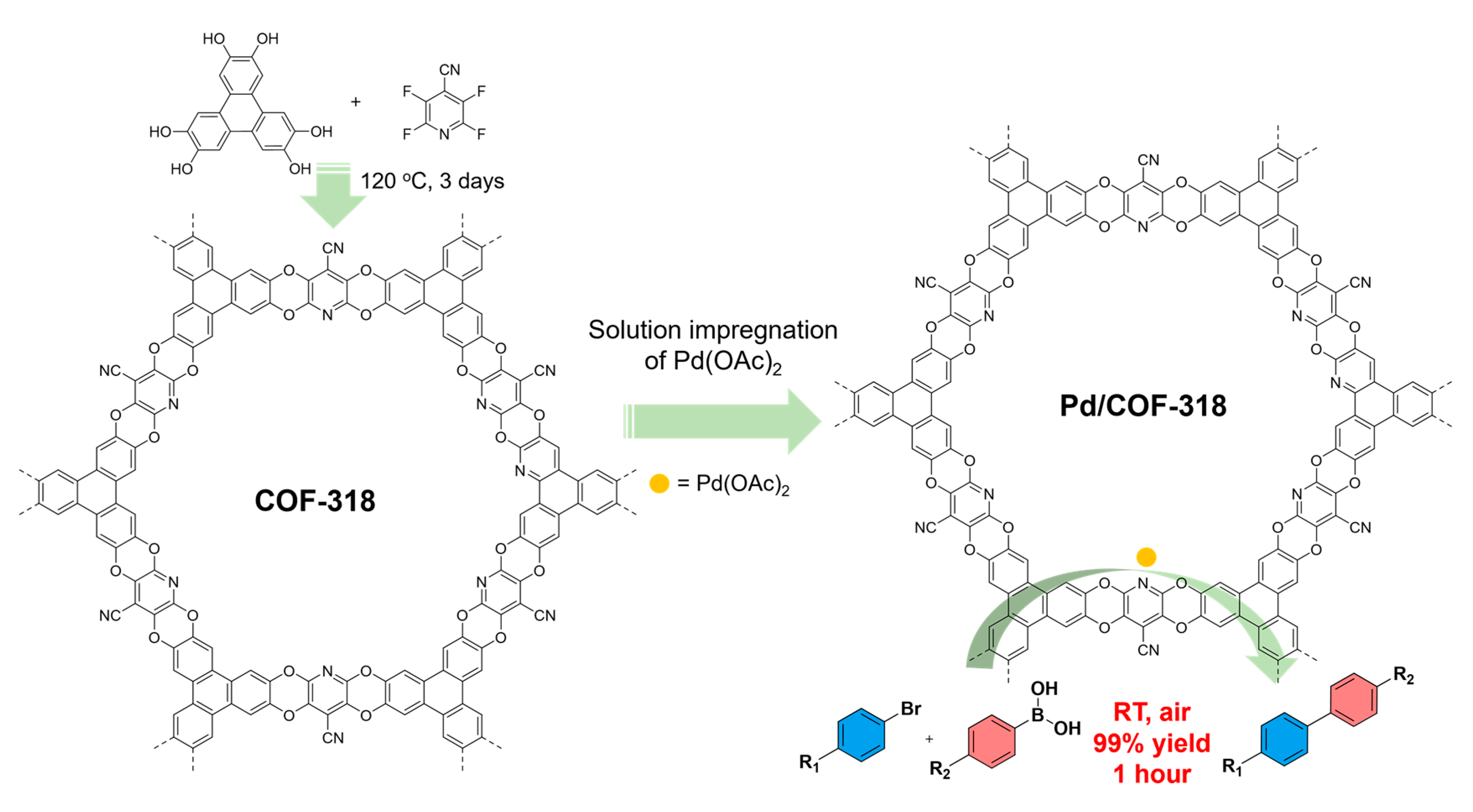
Dioxin-Linked Covalent Organic Framework-Supported Palladium Complex for Rapid Room-Temperature Suzuki–Miyaura Coupling Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
2.3. Synthesis
2.4. Catalysis
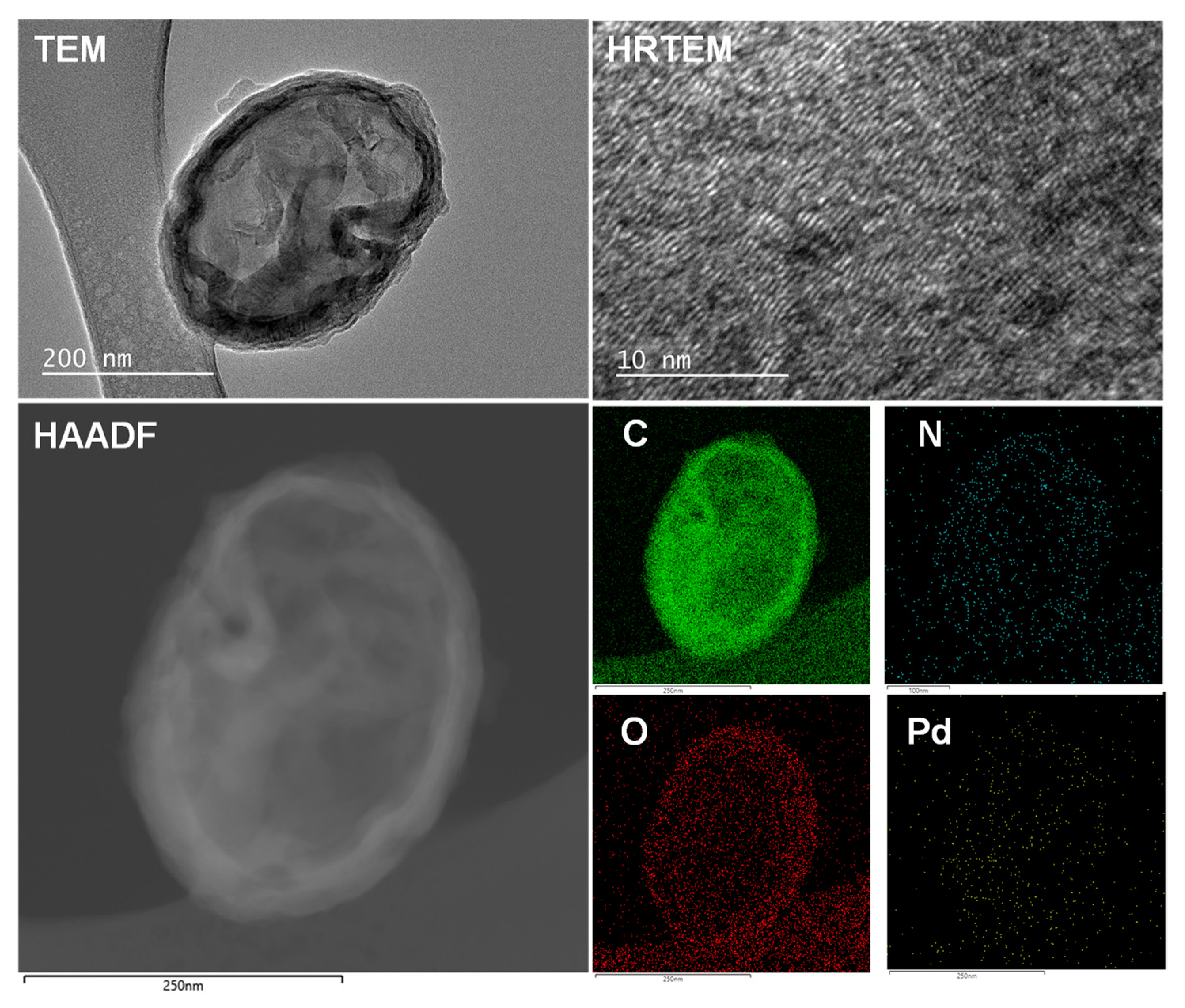
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ma, S.; Li, J.; Xiao, F.; Xiong, H. A water-compatible, highly active and reusable PEG-coated mesoporous silica-supported palladium complex and its application in Suzuki coupling reactions. Chem. Commun. 2006, 23, 2495–2497. [Google Scholar] [CrossRef] [PubMed]
- Kabalka, G.W.; Pagni, R.M.; Wang, L.; Namboodiri, V.; Hair, C.M. Microwave-assisted, solventless Suzuki coupling reactions on palladium-doped alumina. Green Chem. 2000, 2, 120–122. [Google Scholar] [CrossRef]
- Bulut, H.; Artok, L.; Yilmazu, S. Suzuki cross-coupling reaction of aryl halides with arylboronic acids catalysed by Pd (II)-NaY zeolite. Tetrahedron Lett. 2003, 44, 289–291. [Google Scholar] [CrossRef]
- Esteban, N.; Ferrer, M.L.; Ania, C.O.; de la Campa, J.G.; Lozano, Á.E.; Álvarez, C.; Miguel, J.A. Porous Organic Polymers Containing Active Metal Centers for Suzuki–Miyaura Heterocoupling Reactions. ACS Appl. Mater. Interfaces 2020, 12, 56974–56986. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Van Zeeland, R.; Maligal-Ganesh, R.V.; Pei, Y.; Power, G.; Stanley, L.; Huang, W. Impact of Linker Engineering on the Catalytic Activity of Metal–Organic Frameworks Containing Pd(II)–Bipyridine Complexes. ACS Catal. 2016, 6, 6324–6328. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Chen, H.; Zheng, Q.; Zhang, Q.; Mao, H.; Liu, Y.; Cai, S.; Sun, B.; Dun, C. Dynamic covalent synthesis of crystalline porous graphitic frameworks. Chem 2020, 6, 933–944. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Tang, X.-H.; Yan, Y.-L.; Li, S.-Q.; Zheng, S.; Fan, J.; Li, X.; Zhang, W.-G.; Cai, S. Facile and Site-Selective Synthesis of an Amine-Functionalized Covalent Organic Framework. ACS Macro Lett. 2021, 10, 1590–1596. [Google Scholar] [CrossRef]
- Li, R.; Tang, X.; Wu, J.; Zhang, K.; Zhang, Q.; Wang, J.; Zheng, J.; Zheng, S.; Fan, J.; Zhang, W.; et al. A sulfonate-functionalized covalent organic framework for record-high adsorption and effective separation of organic dyes. J. Chem. Eng. 2023, 464, 142706. [Google Scholar] [CrossRef]
- Alsudairy, Z.; Brown, N.; Yang, C.; Cai, S.; Akram, F.; Ambus, A.; Ingram, C.; Li, X. Facile Microwave-Assisted Synthesis of 2D Imine-Linked Covalent Organic Frameworks for Exceptional Iodine Capture. Precis. Chem. 2023, 1, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Alsudairy, Z.; Behera, R.; Akram, F.; Chen, K.; Smith-Petty, K.; Motley, B.; Williams, S.; Huang, W.; Ingram, C.; et al. Green mechanochemical synthesis of imine-linked covalent organic frameworks for high iodine capture. Green Chem. 2023, 25, 6287–6296. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, D. Covalent organic frameworks for heterogeneous catalysis: Principle, current status, and challenges. ACS Cent. Sci. 2020, 6, 869–879. [Google Scholar] [CrossRef]
- Yusran, Y.; Li, H.; Guan, X.; Fang, Q.; Qiu, S. Covalent organic frameworks for catalysis. EnergyChem 2020, 2, 100035. [Google Scholar] [CrossRef]
- Alsudairy, Z.; Brown, N.; Campbell, A.; Ambus, A.; Brown, B.; Smith-Petty, K.; Li, X. Covalent organic frameworks in heterogeneous catalysis: Recent advances and future perspective. Mater. Chem. Front. 2023, 7, 3298–3331. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.-G.; Su, C.-Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Lu, S.; Hu, Y.; Wan, S.; McCaffrey, R.; Jin, Y.; Gu, H.; Zhang, W. Synthesis of ultrafine and highly dispersed metal nanoparticles confined in a thioether-containing covalent organic framework and their catalytic applications. J. Am. Chem. Soc. 2017, 139, 17082–17088. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, X.; Sun, J.; Lin, S.; Qi, D.; Hong, R.; Li, D.; Xiao, X.; Jiang, J. Good Suzuki-coupling reaction performance of Pd immobilized at the metal-free porphyrin-based covalent organic framework. Microporous Mesoporous Mater. 2015, 214, 108–114. [Google Scholar] [CrossRef]
- Gonçalves, R.S.; de Oliveira, A.B.; Sindra, H.C.; Archanjo, B.S.; Mendoza, M.E.; Carneiro, L.S.; Buarque, C.D.; Esteves, P.M. Heterogeneous Catalysis by Covalent Organic Frameworks (COF): Pd(OAc)2@COF-300 in Cross-Coupling Reactions. ChemCatChem 2016, 8, 743–750. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jena, H.S.; Rawat, K.S.; Schmidt, J.; Leus, K.; Van Speybroeck, V.; Van Der Voort, P. Linker Engineering of 2D Imine Covalent Organic Frameworks for the Heterogeneous Palladium-Catalyzed Suzuki Coupling Reaction. ACS Appl. Mater. Interfaces 2022, 14, 50923–50931. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhan, H.; Wang, N.; Song, Y.; Wang, C.; Wang, X.; Ma, L.; Chen, L. Palladium nanoparticles on covalent organic framework supports as catalysts for Suzuki–Miyaura cross-coupling reactions. ACS Appl. Nano Mater. 2021, 4, 6239–6249. [Google Scholar] [CrossRef]
- Nailwal, Y.; Addicoat, M.A.; Gaurav, M.; Pal, S.K. Role of Intralayer Hydrogen Bonding in the Fast Crystallization of the Hydrazone-Linked Nanoporous Covalent Organic Framework for Catalytic Suzuki–Miyaura Cross-Coupling Reactions. ACS Appl. Nano Mater. 2023, 6, 1714–1723. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Chen, Q.; Chen, Z.; Hu, J. The synthesis of size-controlled hollow spherical covalent organic frameworks and its application in photocatalysis and Suzuki coupling reactions. J. Catal. 2022, 416, 29–38. [Google Scholar] [CrossRef]
- Salemi, H.; Debruyne, M.; Van Speybroeck, V.; Van Der Voort, P.; D’Hooghe, M.; Stevens, C.V. Covalent organic framework supported palladium catalysts. J. Mater. Chem. A 2022, 10, 20707–20729. [Google Scholar] [CrossRef]
- Wang, J.-C.; Liu, C.-X.; Kan, X.; Wu, X.-W.; Kan, J.-L.; Dong, Y.-B. Pd@COF-QA: A phase transfer composite catalyst for aqueous Suzuki–Miyaura coupling reaction. Green Chem. 2020, 22, 1150–1155. [Google Scholar] [CrossRef]
- López-Magano, A.; Mas-Ballesté, R.; Alemán, J. Predesigned Covalent Organic Frameworks as Effective Platforms for Pd(II) Coordination Enabling Cross-Coupling Reactions under Sustainable Conditions. Adv. Sustain. Syst. 2022, 6, 2100409. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Y.; Wu, X.; Liu, W.; Wang, Y.; Wang, J. An N-heterocyclic carbene-functionalised covalent organic framework with atomically dispersed palladium for coupling reactions under mild conditions. Green Chem. 2019, 21, 5267–5273. [Google Scholar] [CrossRef]
- Sadhasivam, V.; Balasaravanan, R.; Chithiraikumar, C.; Siva, A. Incorporating Pd (OAc) 2 on Imine Functionalized Microporous Covalent Organic Frameworks: A Stable and Efficient Heterogeneous Catalyst for Suzuki-Miyaura Coupling in Aqueous Medium. ChemistrySelect 2017, 2, 1063–1070. [Google Scholar] [CrossRef]
- Dong, Z.; Pan, H.; Gao, P.; Xiao, Y.; Fan, L.; Chen, J.; Wang, W. Palladium Immobilized on a Polyimide Covalent Organic Framework: An Efficient and Recyclable Heterogeneous Catalyst for the Suzuki–Miyaura Coupling Reaction and Nitroarene Reduction in Water. Catal. Lett. 2022, 152, 299–306. [Google Scholar] [CrossRef]
- Wu, S.; Ding, N.; Jiang, P.; Wu, L.; Feng, Q.; Zhao, L.; Wang, Y.; Su, Q.; Zhang, H.; Yang, Q. A two-dimensional amide-linked covalent organic framework anchored Pd catalyst for Suzuki-Miyaura coupling reaction in the aqueous phase at room temperature. Tetrahedron Lett. 2020, 61, 152656. [Google Scholar] [CrossRef]
- Li, X.; Cai, S.; Sun, B.; Yang, C.; Zhang, J.; Liu, Y. Chemically robust covalent organic frameworks: Progress and perspective. Matter 2020, 3, 1507–1540. [Google Scholar] [CrossRef]
- Li, X. sp 2 carbon-conjugated covalent organic frameworks: Synthesis, properties, and applications. Mater. Chem. Front. 2021, 5, 2931–2949. [Google Scholar] [CrossRef]
- Wei, P.-F.; Qi, M.-Z.; Wang, Z.-P.; Ding, S.-Y.; Yu, W.; Liu, Q.; Wang, L.-K.; Wang, H.-Z.; An, W.-K.; Wang, W. Benzoxazole-Linked Ultrastable Covalent Organic Frameworks for Photocatalysis. J. Am. Chem. Soc. 2018, 140, 4623–4631. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, L.; Chu, T.; Niu, H.; Wang, J.; Cai, Y. Constructing chemical sTable 4-carboxyl-quinoline linked covalent organic frameworks via Doebner reaction for nanofiltration. Nat. Commun. 2022, 13, 2615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pang, H.; Huang, D.; Liu, G.; Hu, J.; Xiang, Y. Construction of Ultrastable Nonsubstituted Quinoline-Bridged Covalent Organic Frameworks via Rhodium-Catalyzed Dehydrogenative Annulation. Angew. Chem. Int. Ed. 2022, 61, e202208833. [Google Scholar] [CrossRef]
- Yang, S.; Yang, C.; Dun, C.; Mao, H.; Khoo, R.S.H.; Klivansky, L.M.; Reimer, J.A.; Urban, J.J.; Zhang, J.; Liu, Y. Covalent Organic Frameworks with Irreversible Linkages via Reductive Cyclization of Imines. J. Am. Chem. Soc. 2022, 144, 9827–9835. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Cai, S.; Lei, X.; Altoe, V.; Hong, F.; Urban, J.J.; Ciston, J.; Chan, E.M.; Liu, Y. Facile transformation of imine covalent organic frameworks into ultrastable crystalline porous aromatic frameworks. Nat. Commun. 2018, 9, 2998. [Google Scholar] [CrossRef]
- Qian, C.; Feng, L.; Teo, W.L.; Liu, J.; Zhou, W.; Wang, D.; Zhao, Y. Imine and imine-derived linkages in two-dimensional covalent organic frameworks. Nat. Rev. Chem. 2022, 6, 881–898. [Google Scholar] [CrossRef]
- Volkov, A.; Mi, J.; Lalit, K.; Chatterjee, P.; Jing, D.; Carnahan, S.L.; Chen, Y.; Sun, S.; Rossini, A.J.; Huang, W.; et al. General Strategy for Incorporation of Functional Group Handles into Covalent Organic Frameworks via the Ugi Reaction. J. Am. Chem. Soc. 2023, 145, 6230–6239. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, M.; Mao, H.; Pei, X.; Alshmimri, S.A.; Reimer, J.A.; Yaghi, O.M. Crystalline dioxin-linked covalent organic frameworks from irreversible reactions. J. Am. Chem. Soc. 2018, 140, 12715–12719. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Li, H.; Ma, Y.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; Qiu, S. Chemically stable polyarylether-based covalent organic frameworks. Nat. Chem. 2019, 11, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, M.; Liu, C.-G.; Liu, J.; Shang, L.-J.; Wang, M.; Chang, J.-N.; Li, S.-L.; Lan, Y.-Q. Stable Dioxin-Linked Metallophthalocyanine Covalent Organic Frameworks (COFs) as Photo-Coupled Electrocatalysts for CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 4864–4871. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, K.; Zheng, Q.; Li, X.; Mao, H.; Zhong, W.; Chen, C.; Sun, B.; Zheng, H.; Zhuang, X. Chemically Stable Polyarylether-Based Metallophthalocyanine Frameworks with High Carrier Mobilities for Capacitive Energy Storage. J. Am. Chem. Soc. 2021, 143, 17701–17707. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, R.; Zhang, P.; Yang, M.; Zhao, R.; Wang, Y.; Dai, X.; Liu, W. Functionalized polyarylether-based COFs for rapid and selective extraction of uranium from aqueous solution. J. Chem. Eng. 2022, 434, 134623. [Google Scholar] [CrossRef]
- Ji, W.; Guo, Y.-S.; Xie, H.-M.; Wang, X.; Jiang, X.; Guo, D.-S. Rapid microwave synthesis of dioxin-linked covalent organic framework for efficient micro-extraction of perfluorinated alkyl substances from water. J. Hazard. Mater. 2020, 397, 122793. [Google Scholar] [CrossRef]
- Lei, Z.; Lucas, F.W.; Moya, E.C.; Huang, S.; Rong, Y.; Wesche, A.; Li, P.; Bodkin, L.; Jin, Y.; Holewinski, A. Highly stable dioxin-linked metallophthalocyanine covalent organic frameworks. Chin. Chem. Lett. 2021, 32, 3799–3802. [Google Scholar] [CrossRef]
- Huang, N.; Lee, K.H.; Yue, Y.; Xu, X.; Irle, S.; Jiang, Q.; Jiang, D. A stable and conductive metallophthalocyanine framework for electrocatalytic carbon dioxide reduction in water. Angew. Chem. Int. Ed. 2020, 132, 16730–16736. [Google Scholar]
- Liu, Y.; Wu, C.; Sun, Q.; Hu, F.; Pan, Q.; Sun, J.; Jin, Y.; Li, Z.; Zhang, W.; Zhao, Y. Spirobifluorene-Based Three-Dimensional Covalent Organic Frameworks with Rigid Topological Channels as Efficient Heterogeneous Catalyst. CCS Chem. 2021, 3, 2418–2427. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, C.; Pan, Q.; Zhang, B.; Liu, Y.; Lu, X.; Sun, J.; Sun, L.; Zhao, Y. Three-Dimensional Covalent-Organic Frameworks Loaded with Highly Dispersed Ultrafine Palladium Nanoparticles as Efficient Heterogeneous Catalyst. ChemNanoMat 2021, 7, 95–99. [Google Scholar] [CrossRef]
- Kaleeswaran, D.; Antony, R.; Sharma, A.; Malani, A.; Murugavel, R. Catalysis and CO2 Capture by Palladium-Incorporated Covalent Organic Frameworks. ChemPlusChem 2017, 82, 1253–1265. [Google Scholar] [CrossRef] [PubMed]


 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Base | Solvent | Yield b |
| 1 | Pd/COF-318 | Na2CO3 | EtOH | 87% |
| 2 | Pd/COF-318 | K2CO3 | EtOH | >99% |
| 3 | Pd/COF-318 | Cs2CO3 | EtOH | 82% |
| 4 | Pd/COF-318 | K2CO3 | MeOH | 94% |
| 5 | Pd/COF-318 | K2CO3 | Acetonitrile | 90% |
| 6 | Pd/COF-318 | K2CO3 | CH2Cl2 | 4% |
| 7 | Pd/COF-318 | K2CO3 | THF | 53% |
| 8 | COF-318 | K2CO3 | EtOH | / |
| 9 | 5% Pd/carbon | K2CO3 | EtOH | 90% |
| Entry | COF Catalyst | Linkage | Pd Valence | T | Time | Yield | Stability (Runs) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Pd/COF-318 | Dioxin | Pd2+ | RT | 1 h | 99% | 5 | This work |
| 2 | Pd@COF-NHC | Imine | Pd0 | RT | 1 h | 99% | 8 | [28] |
| 3 | Pd/TATAE | Imine | Pd2+ | RT | 2 h | 98% | 4 | [29] |
| 4 | Pd@OC-MA | Imine | Pd2+ | RT | 5 h | 99% | 4 | [30] |
| 5 | Pd@COF-TM | Amide | Pd2+ | RT | 6 h | 99% | 9 | [31] |
| 6 | Pd/Phen-COF | Imine | Pd2+ | RT | 16 h | 99% | 5 | [27] |
| 7 | PdNPs@Thio-COF | Imine | Pd0 | 50 °C | 3 h | 85% | 5 | [18] |
| 8 | Pd@COF-QA | Imine | Pd0 | 50 °C | 6 h | 99% | 10 | [26] |
| 9 | Pd(OAc)2@COF-300 | Imine | Pd2+ | 70 °C | 0.3 h | 100% | 5 | [20] |
| 10 | Pd(II)@SP-3D-COF-Bpy | Imine | Pd2+ | 70 °C | 2 h | 98% | 5 | [49] |
| 11 | Pd@TPM-3D-COF-Bpy | Imine | Pd0 | 70 °C | 5 h | 98% | 5 | [50] |
| 12 | Pd/COF-SMC2 | Imine | Pd0, Pd2+ | 80 °C | 1 h | 96% | 4 | [22] |
| 13 | Pd/H2P-Bph-COF | Imine | Pd2+ | 110 °C | 1.5 h | 98% | 5 | [19] |
| 14 | PdII/TAT–DHBD | Imine | Pd2+ | 120 °C | 24 h | 56% | / | [51] |
 | |||
|---|---|---|---|
| Entry | R1-Ar-Br | R2-Ar-B(OH)2 | Yield b |
| 1 |  |  | >99% |
| 2 |  |  | >99% |
| 3 |  |  | >99% |
| 4 | 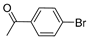 |  | >99% |
| 5 |  |  | >99% |
| 6 |  | 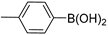 | >99% |
| 7 |  | 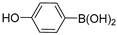 | >99% |
| 8 |  |  | >99% |
| 9 |  | 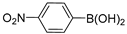 | >99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, A.; Alsudairy, Z.; Dun, C.; Akram, F.; Smith-Petty, K.; Ambus, A.; Bingham, D.; Dinadayalane, T.; Ingram, C.; Li, X. Dioxin-Linked Covalent Organic Framework-Supported Palladium Complex for Rapid Room-Temperature Suzuki–Miyaura Coupling Reaction. Crystals 2023, 13, 1268. https://doi.org/10.3390/cryst13081268
Campbell A, Alsudairy Z, Dun C, Akram F, Smith-Petty K, Ambus A, Bingham D, Dinadayalane T, Ingram C, Li X. Dioxin-Linked Covalent Organic Framework-Supported Palladium Complex for Rapid Room-Temperature Suzuki–Miyaura Coupling Reaction. Crystals. 2023; 13(8):1268. https://doi.org/10.3390/cryst13081268
Chicago/Turabian StyleCampbell, Allea, Ziad Alsudairy, Chaochao Dun, Fazli Akram, Kayla Smith-Petty, Abrianna Ambus, Danielle Bingham, Tandabany Dinadayalane, Conrad Ingram, and Xinle Li. 2023. "Dioxin-Linked Covalent Organic Framework-Supported Palladium Complex for Rapid Room-Temperature Suzuki–Miyaura Coupling Reaction" Crystals 13, no. 8: 1268. https://doi.org/10.3390/cryst13081268
APA StyleCampbell, A., Alsudairy, Z., Dun, C., Akram, F., Smith-Petty, K., Ambus, A., Bingham, D., Dinadayalane, T., Ingram, C., & Li, X. (2023). Dioxin-Linked Covalent Organic Framework-Supported Palladium Complex for Rapid Room-Temperature Suzuki–Miyaura Coupling Reaction. Crystals, 13(8), 1268. https://doi.org/10.3390/cryst13081268







