Zn(II) Metal–Organic Frameworks with a Long Spacer Ligand and a Tricarboxylate Coligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Preparation of [Zn2(bpeb)(btb)(OH)]·DMF·H2O (1)
2.3. Preparation of [Zn3(btb)2(bpeb)]·xSolvent (2)
2.4. [Zn2(Hbtb)2(bpeb)][Zn2(Hbtb)2(bpeb)][Zn4(Hbtb)4(bpeb)2]·xSolvent (3)
2.5. X-ray Crystallographic Analysis
3. Results and Discussion
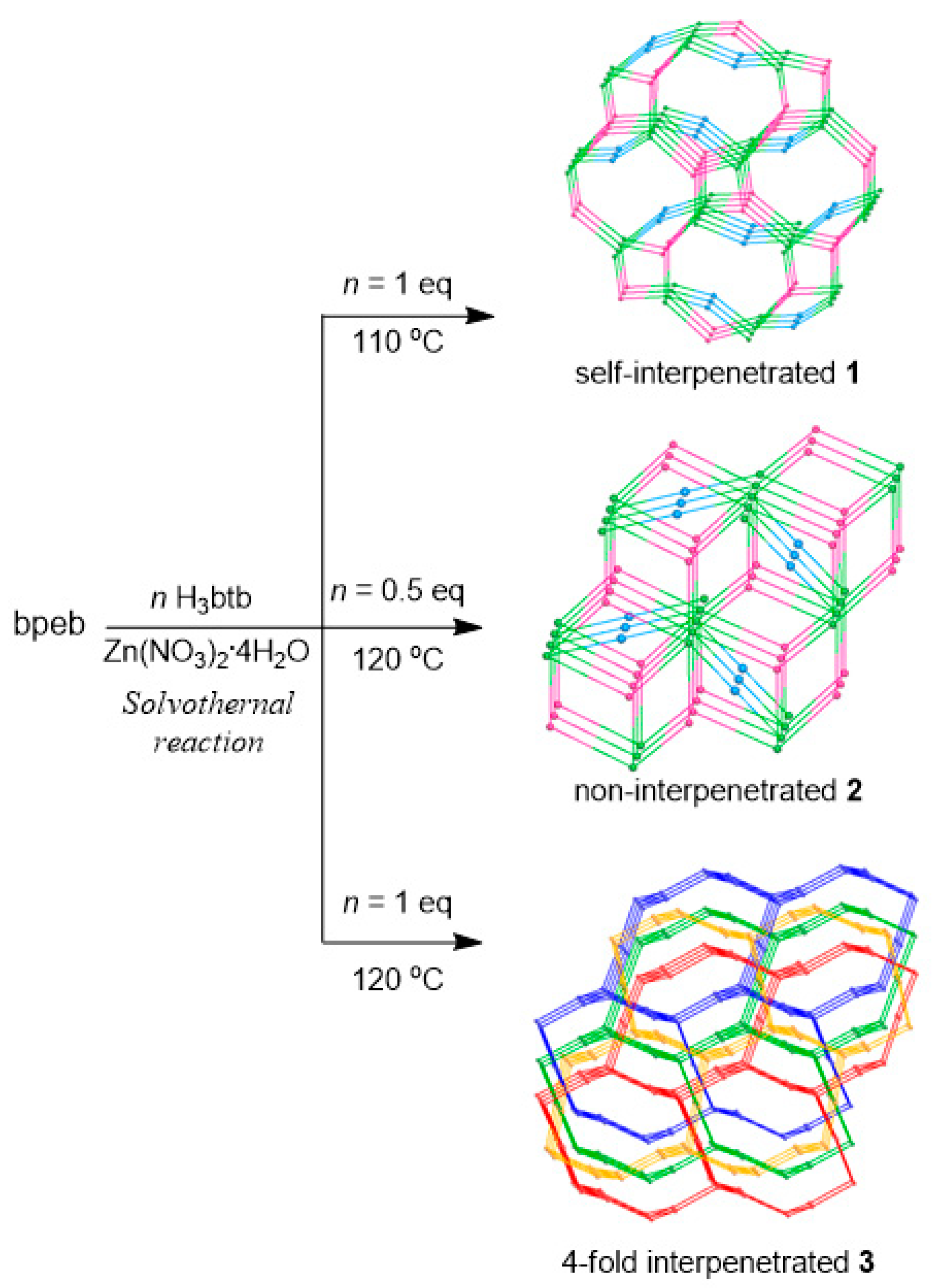
3.1. Syntheses of CPs 1–3
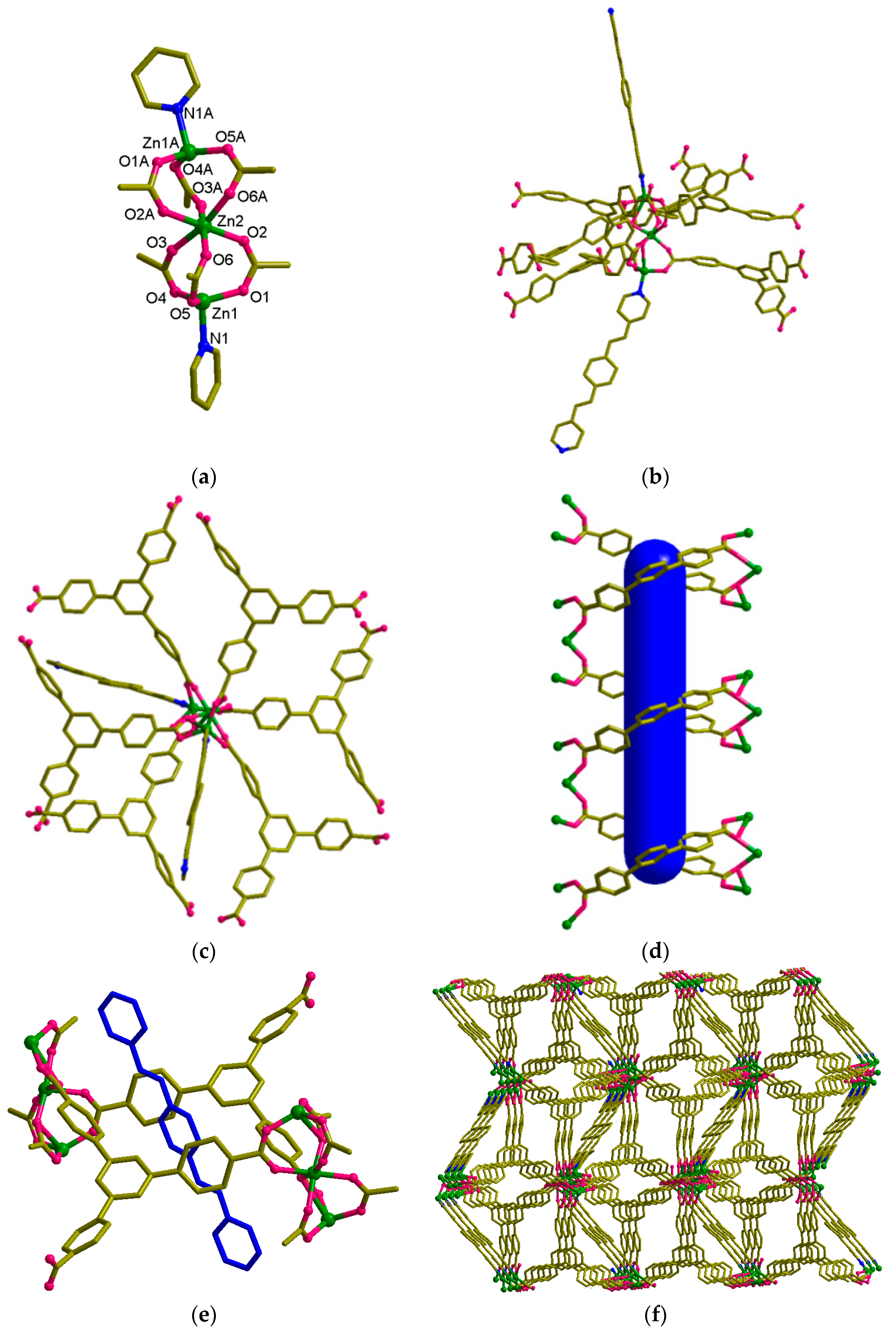
3.2. Structural Description of [Zn2(bpeb)(btb)(OH)]·DMF·H2O (1)
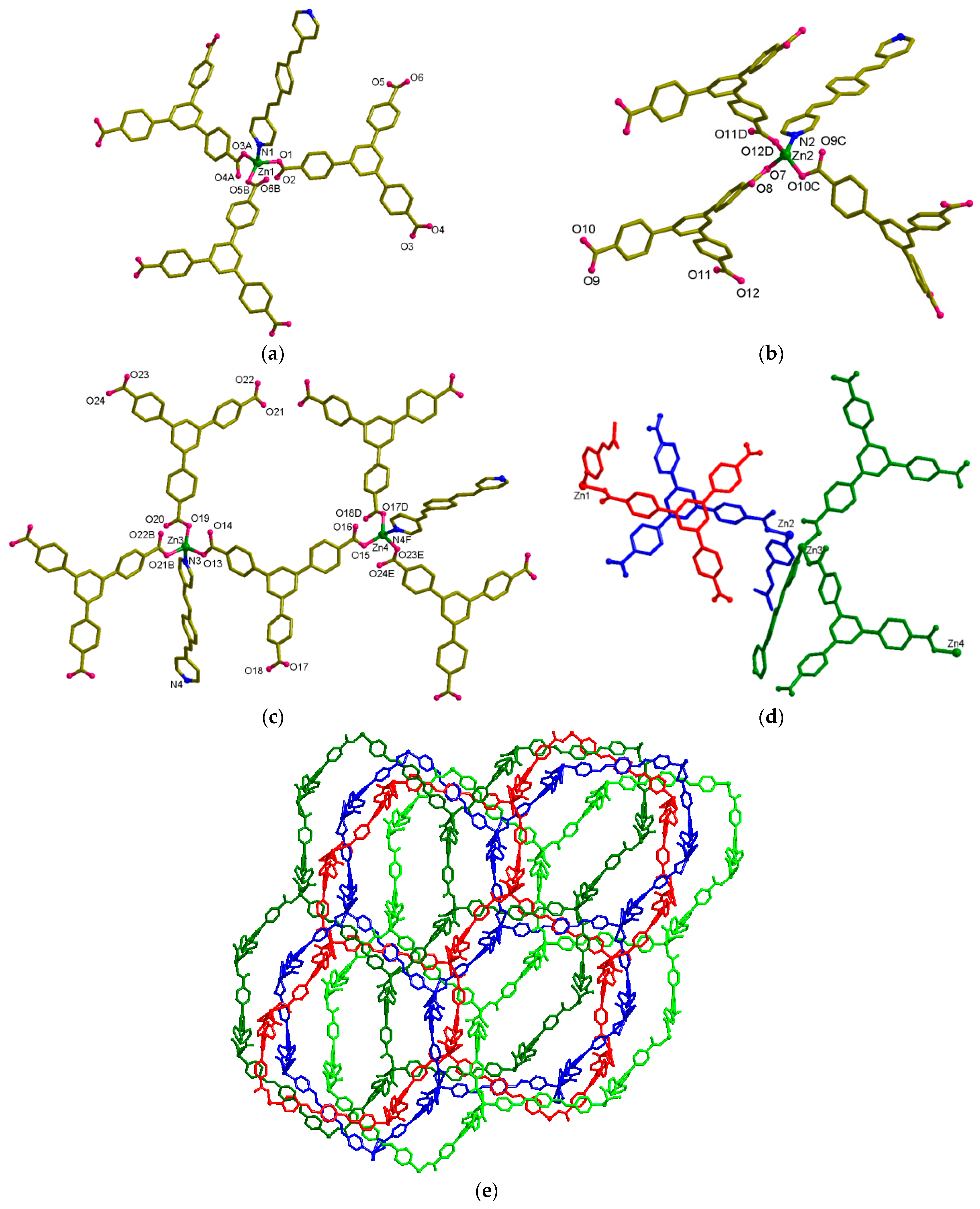
3.3. Structural Description of [Zn3(btb)2(bpeb)]·xSolvent (2)
3.4. Structural Description of [Zn2(Hbtb)2(bpeb)][Zn2(Hbtb)2(bpeb)][Zn4(Hbtb)4(bpeb)2]·xSolvent (3)
3.5. Thermal Stability
3.6. Topological Analysis
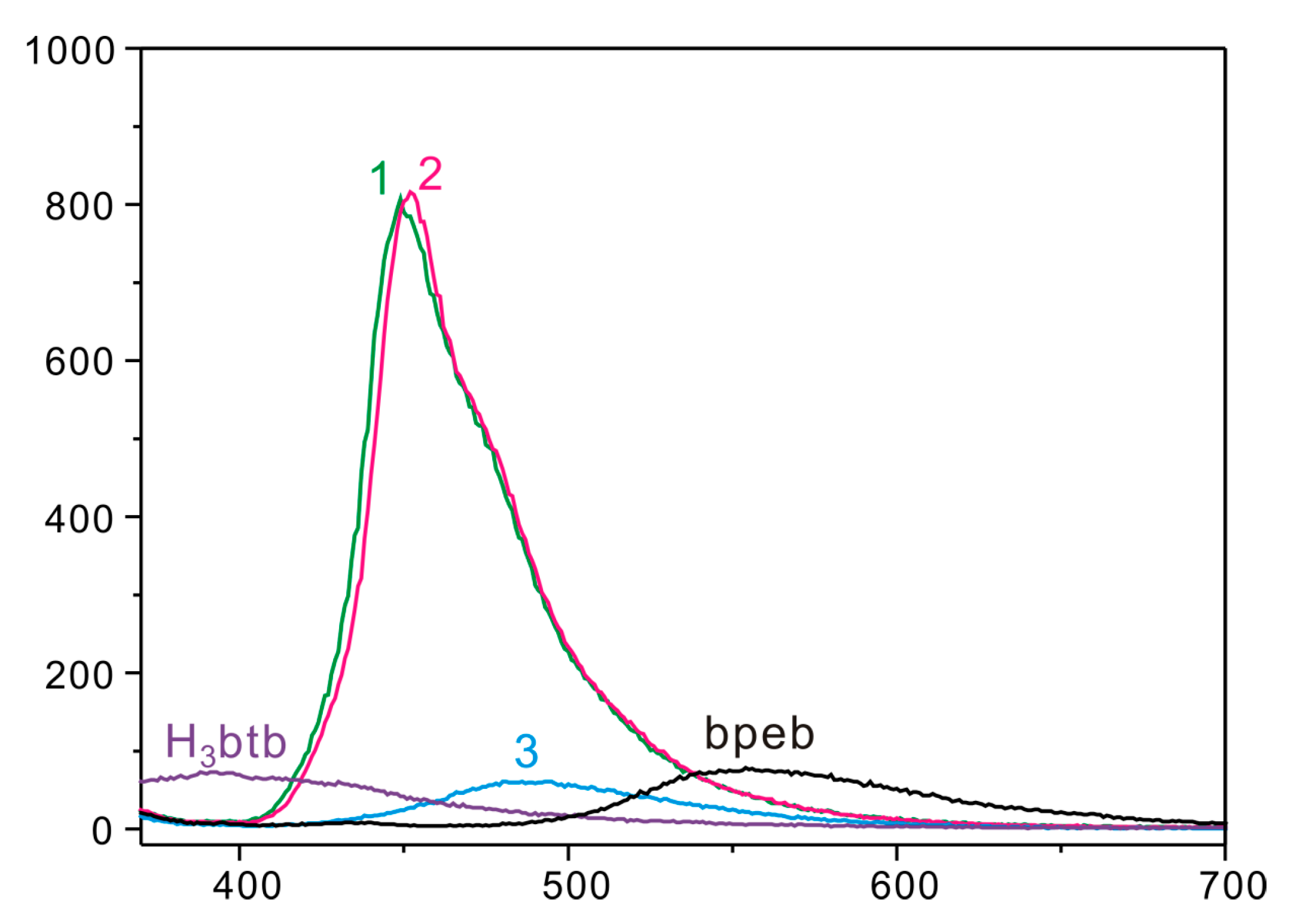
3.7. Solid-State Emission
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Morris, R.E.; Brammer, L. Coordination change, lability and hemilability in metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 5444–5462. [Google Scholar] [CrossRef] [PubMed]
- Kole, G.K.; Vittal, J.J. Solid-state reactivity and structural transformations involving coordination polymers. Chem. Soc. Rev. 2013, 42, 1755–1775. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Moulton, B.; Zaworotko, M.J. From Molecules to Crystal Engineering: Supramolecular Isomerism and Polymorphism in Network Solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Modifying MOFs: New chemistry, new materials. Chem. Sci. 2010, 1, 32–36. [Google Scholar] [CrossRef]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef]
- Chakraborty, G.; Park, I.-H.; Medishetty, R.; Vittal, J.J. Two-Dimensional Metal-Organic Framework Materials: Synthesis, Structures, Properties and Applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Houck, H.A.; Du Prez, F.E.; Barner-Kowollik, C. Controlling thermal reactivity with different colors of light. Nat. Commun. 2017, 8, 1869. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.I. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Park, I.-H.; Mulijanto, C.E.; Lee, H.-H.; Kang, Y.; Lee, E.; Chanthapally, A.; Lee, S.S.; Vittal, J.J. Influence of Interpenetration in Diamondoid Metal–Organic Frameworks on the Photoreactivity and Sensing Properties. Cryst. Growth Des. 2016, 16, 2504–2508. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469. [Google Scholar] [CrossRef]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational Design, Synthesis, Purification, and Activation of Metal–Organic Framework Materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wojtas, L.; Larsen, R.W.; Eddaoudi, M.; Zaworotko, M.J. Temperature and Concentration Control over Interpenetration in a Metal–Organic Material. J. Am. Chem. Soc. 2009, 131, 17040–17041. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.; Senkovska, I.; Gedrich, K.; Stoeck, U.; Henschel, A.; Mueller, U.; Kaskel, S. A Mesoporous Metal–Organic Framework. Angew. Chem. Int. Ed. 2009, 48, 9954–9957. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Batten, S.R.; Robson, R. Interpenetrating Nets: Ordered, Periodic Entanglement. Angew. Chem. Int. Ed. 1998, 37, 1460–1494. [Google Scholar] [CrossRef]
- Gupta, M.; Vittal, J.J. Control of interpenetration and structural transformations in the interpenetrated MOFs. Coord. Chem. Rev. 2021, 435, 213789. [Google Scholar] [CrossRef]
- Chen, Z.; Gallo, G.; Sawant, V.A.; Zhang, T.; Zhu, M.; Liang, L.; Chanthapally, A.; Bolla, G.; Quah, H.S.; Liu, X.; et al. Giant Enhancement of Second Harmonic Generation Accompanied by the Structural Transformation of 7-Fold to 8-Fold Interpenetrated Metal–Organic Frameworks (MOFs). Angew. Chem. Int. Ed. 2020, 59, 833–838. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Mohamed, M.H.; Wojtas, L.; Chanthapally, A.; Pham, T.; Space, B.; Vittal, J.J.; Zaworotko, M.J. Putting the Squeeze on CH4 and CO2 through Control over Interpenetration in Diamondoid Nets. J. Am. Chem. Soc. 2014, 136, 5072–5077. [Google Scholar] [CrossRef]
- Yang, G.-P.; Hou, L.; Luan, X.-J.; Wu, B.; Wang, Y.-Y. Molecular braids in metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 6992–7000. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Makal, T.A.; Zhou, H.-C. Interpenetration control in metal–organic frameworks for functional applications. Coord. Chem. Rev. 2013, 257, 2232–2249. [Google Scholar] [CrossRef]
- Dezotti, Y.; Ribeiro, M.A.; Pirota, K.R.; Barros, W.P. Influence of the Metal Ion on the Topology and Interpenetration of Pyridylvinyl(benzoate) Based Metal–Organic Frameworks. Cryst. Growth Des. 2019, 19, 5592–5603. [Google Scholar] [CrossRef]
- Bae, C.; Gu, M.; Jeon, Y.; Kim, D.; Kim, J. Metal–organic frameworks for NH3 adsorption by different NH3 operating pressures. Bull. Korean Chem. Soc. 2023, 44, 112–124. [Google Scholar] [CrossRef]
- Choi, I.-H.; Gu, J.-M.; Kim, H.-C.; Kim, Y.; Huh, S. Gas sorption properties of a new Zn-BTB metal–organic framework with permanent porosity. Bull. Korean Chem. Soc. 2023. Early view. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Joung, H.; Jang, G.J.; Han, S.Y. Probing emergence of biomolecular coronas around drug-loaded liposomal nanoparticles in the solution by using nanoparticle tracking analysis. Bull. Korean Chem. Soc. 2023, 44, 551–557. [Google Scholar] [CrossRef]
- Kim, J.; Na, C.; Son, Y.; Prabu, M.; Yoon, M. Stilbene ligand-based metal–organic frameworks for efficient dye adsorption and nitrobenzene detection. Bull. Korean Chem. Soc. 2023, 44, 507–515. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.; Son, Y.; Kim, J.Y.; Cha, S.; Kwak, D.; Lee, J.; Kwak, J.; Yoon, M.; Kim, M. Uncoordinated tetrazole ligands in metal–organic frameworks for proton-conductivity studies. Bull. Korean Chem. Soc. 2022, 43, 912–917. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, W.R.; Kim, Y.; Lee, H.J.; Ryu, D.W.; Phang, W.J.; Hong, C.S. Interpenetration Control, Sorption Behavior, and Framework Flexibility in Zn(II) Metal–Organic Frameworks. Cryst. Growth Des. 2014, 14, 699–704. [Google Scholar] [CrossRef]
- Shekhah, O.; Wang, H.; Paradinas, M.; Ocal, C.; Schüpbach, B.; Terfort, A.; Zacher, D.; Fischer, R.A.; Wöll, C. Controlling interpenetration in metal–organic frameworks by liquid-phase epitaxy. Nat. Mater. 2009, 8, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, S.-T.; Choi, S.B.; Sim, J.; Kim, J.; Ahn, W.-S. Control of catenation in CuTATB-n metal–organic frameworks by sonochemical synthesis and its effect on CO2 adsorption. J. Mater. Chem. 2011, 21, 3070–3076. [Google Scholar] [CrossRef]
- Evans, O.R.; Lin, W. Rational Design of Nonlinear Optical Materials Based on 2D Coordination Networks. Chem. Mater. 2001, 13, 3009–3017. [Google Scholar] [CrossRef]
- Ma, L.; Lin, W. Chirality-Controlled and Solvent-Templated Catenation Isomerism in Metal–Organic Frameworks. J. Am. Chem. Soc. 2008, 130, 13834–13835. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Sun, Z.-M. Solvents control over the degree of interpenetration in metal-organic frameworks and their high sensitivities for detecting nitrobenzene at ppm level. J. Mater. Chem. 2012, 22, 15939–15946. [Google Scholar] [CrossRef]
- Evans, O.R.; Lin, W. Crystal Engineering of Nonlinear Optical Materials Based on Interpenetrated Diamondoid Coordination Networks. Chem. Mater. 2001, 13, 2705–2712. [Google Scholar] [CrossRef]
- Song, F.; Wang, C.; Falkowski, J.M.; Ma, L.; Lin, W. Isoreticular Chiral Metal–Organic Frameworks for Asymmetric Alkene Epoxidation: Tuning Catalytic Activity by Controlling Framework Catenation and Varying Open Channel Sizes. J. Am. Chem. Soc. 2010, 132, 15390–15398. [Google Scholar] [CrossRef]
- Evans, O.R.; Lin, W. Crystal Engineering of NLO Materials Based on Metal–Organic Coordination Networks. Acc. Chem. Res. 2002, 35, 511–522. [Google Scholar] [CrossRef]
- Katz, M.J.; Ramnial, T.; Yu, H.-Z.; Leznoff, D.B. Polymorphism of Zn[Au(CN)2]2 and Its Luminescent Sensory Response to NH3 Vapor. J. Am. Chem. Soc. 2008, 130, 10662–10673. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.-X.; Liu, L.-L.; Wang, H.-M.; Li, N.-Y.; Ren, Z.-G.; Lang, J.-P. How do substituent groups in the 5-position of 1,3-benzenedicarboxylate affect the construction of supramolecular frameworks? CrystEngComm 2010, 12, 3708–3716. [Google Scholar] [CrossRef]
- Li, F.F.; Zhang, L.; Gong, L.L.; Yan, C.S.; Gao, H.Y.; Luo, F. Reversible photo/thermoswitchable dual-color fluorescence through single-crystal-to-single-crystal transformation. Dalton Trans. 2017, 46, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Quah, H.S.; Nalla, V.; Zheng, K.; Lee, C.A.; Liu, X.; Vittal, J.J. Tuning Two-Photon Absorption Cross Section in Metal Organic Frameworks. Chem. Mater. 2017, 29, 7424–7430. [Google Scholar] [CrossRef]
- Park, I.-H.; Ju, H.; Kim, K.; Lee, S.S.; Vittal, J.J. Isomerism in double-pillared-layer coordination polymers—Structures and photoreactivity. IUCrJ 2018, 5, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Gutov, A.V.; Rusanov, E.B.; Chepeleva, L.V.; Garasevich, S.G.; Ryabitskii, A.B.; Chernega, A.N. New viologen analogs: 1,4-bis[2-(pyridin-4-yl)ethenyl]benzene quaternary salts. Russ. J. Gen. Chem. 2009, 79, 1513–1518. [Google Scholar] [CrossRef]
- Bruker, A.J.B.A.I. Saint and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. SADABS, Version 2.03; University of Göttingen: Göttingen, Germany, 2002.
- Bruker, A.I.; Madison, W. SHELXTL, Version 6.10; Bruker AXS Inc.: Madison, WI, USA, 2000.
- Spek, A. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Park, I.-H.; Kim, K.; Lee, S.S.; Vittal, J.J. An Unusual Interweaving in a 3-Fold Interpenetrated Pillared-Layer Zn(II) Coordination Polymer with a Long Spacer Ligand. Cryst. Growth Des. 2012, 12, 3397–3401. [Google Scholar] [CrossRef]
- Park, I.-H.; Medishetty, R.; Kim, J.-Y.; Lee, S.S.; Vittal, J.J. Distortional Supramolecular Isomers of Polyrotaxane Coordination Polymers: Photoreactivity and Sensing of Nitro Compounds. Angew. Chem. Int. Ed. 2014, 53, 5591–5595. [Google Scholar] [CrossRef]
- Park, I.-H.; Medishetty, R.; Lee, S.S.; Vittal, J.J. Solid-state polymerization in a polyrotaxane coordination polymer via a [2 + 2] cycloaddition reaction. Chem. Commun. 2014, 50, 6585–6588. [Google Scholar] [CrossRef]
- Park, I.-H.; Herng, T.S.; Ju, H.; Ding, J.; Lee, S.S.; Vittal, J.J. Synthesis, structures and magnetic properties of isoreticular polyrotaxane-type two-dimensional coordination polymers. RSC Adv. 2017, 7, 45582–45586. [Google Scholar] [CrossRef]
- Park, I.-H.; Ju, H.; Herng, T.S.; Kang, Y.; Lee, S.S.; Ding, J.; Vittal, J.J. Supramolecular Isomerism and Polyrotaxane-Based Two-Dimensional Coordination Polymers. Cryst. Growth Des. 2016, 16, 7278–7285. [Google Scholar] [CrossRef]
- Ju, H.; Shin, M.; Park, I.-H.; Jung, J.H.; Vittal, J.J.; Lee, S.S. Construction of 2D Interdigitated Polyrotaxane Layers and their Transformation to a 3D Polyrotaxane by a Photocycloaddition Reaction between Wheels. Inorg. Chem. 2021, 60, 8285–8292. [Google Scholar] [CrossRef]
- Blatov, V.A. Nanocluster analysis of intermetallic structures with the program package TOPOS. Struct. Chem. 2012, 23, 955–963. [Google Scholar] [CrossRef]
- Alexandrov, E.V.; Blatov, V.A.; Kochetkov, A.V.; Proserpio, D.M. Underlying nets in three-periodic coordination polymers: Topology, taxonomy and prediction from a computer-aided analysis of the Cambridge Structural Database. CrystEngComm 2011, 13, 3947–3958. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Li, S.-L.; Lan, Y.-Q.; Ma, J.-F.; Fu, Y.-M.; Yang, J.; Ping, G.-J.; Liu, J.; Su, Z.-M. Structures and Luminescent Properties of a Series of Zinc(II) and Cadmium(II) 4,4′-Oxydiphthalate Coordination Polymers with Various Ligands Based on Bis(pyridyl imidazole) under Hydrothermal Conditions. Cryst. Growth Des. 2008, 8, 1610–1616. [Google Scholar] [CrossRef]
- Guo, H.-D.; Guo, X.-M.; Batten, S.R.; Song, J.-F.; Song, S.-Y.; Dang, S.; Zheng, G.-L.; Tang, J.-K.; Zhang, H.-J. Hydrothermal Synthesis, Structures, and Luminescent Properties of Seven d10 Metal–Organic Frameworks Based on 9,9-Dipropylfluorene-2,7-Dicarboxylic Acid (H2DFDA). Cryst. Growth Des. 2009, 9, 1394–1401. [Google Scholar] [CrossRef]
- Bauer, C.A.; Timofeeva, T.V.; Settersten, T.B.; Patterson, B.D.; Liu, V.H.; Simmons, B.A.; Allendorf, M.D. Influence of Connectivity and Porosity on Ligand-Based Luminescence in Zinc Metal–Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 7136–7144. [Google Scholar] [CrossRef]
- Duan, J.; Higuchi, M.; Foo, M.L.; Horike, S.; Rao, K.P.; Kitagawa, S. A Family of Rare Earth Porous Coordination Polymers with Different Flexibility for CO2/C2H4 and CO2/C2H6 Separation. Inorg. Chem. 2013, 52, 8244–8249. [Google Scholar] [CrossRef]
- Henke, S.; Schmid, R.; Grunwaldt, J.-D.; Fischer, R.A. Flexibility and Sorption Selectivity in Rigid Metal–Organic Frameworks: The Impact of Ether-Functionalised Linkers. Chem. Eur. J. 2010, 16, 14296–14306. [Google Scholar] [CrossRef]
- Kaneko, W.; Ohba, M.; Kitagawa, S. A Flexible Coordination Polymer Crystal Providing Reversible Structural and Magnetic Conversions. J. Am. Chem. Soc. 2007, 129, 13706–13712. [Google Scholar] [CrossRef]


| 1 | 2 | 3 | |
|---|---|---|---|
| Formula | C47H31N2O7Zn2 | C74H46N2O12Zn3 | C296H184N8O48Zn8 |
| Formula weight | 866.48 | 1351.24 | 5143.46 |
| Temperature (K) | 173 | 173 | 173 |
| Crystal system | Orthorhombic | Monoclinic | Monoclinic |
| Space group | Fdd2 | C2/c | P21/c |
| Z | 16 | 4 | 2 |
| a (Å) | 60.5386(9) | 11.4402(4) | 16.3093(12) |
| b (Å) | 62.2276(10) | 30.8405(10) | 27.4589(19) |
| c (Å) | 5.90860(10) | 27.0931(9) | 48.052(3) |
| α (°) | 90 | 90 | 90 |
| β (°) | 90 | 96.884(2) | 97.288(4) |
| γ (°) | 90 | 90 | 90 |
| V (Å3) | 22,258.7(6) | 9490.1(6) | 21,346(3) |
| Dcalc (g/cm3) | 1.034 | 0.946 | 0.800 |
| 2θmax (°) | 52.00 | 52.00 | 52.00 |
| R1, wR2 [I > 2σ(I)] | 0.0866, 0.2656 | 0.0620, 0.1673 | 0.0836, 0.1800 |
| R1, wR2 [all data] | 0.0980, 0.2826 | 0.1161, 0.1845 | 0.1939, 0.2066 |
| Goodness-of-fit on F2 | 1.010 | 0.994 | 0.819 |
| No. of reflection used [>2σ(I)] | 10834 [Rint = 0.0513] | 9302 [Rint = 0.1134] | 41682 [Rint = 0.1771] |
| Refinement | 55166 | 77799 | 62360 |
| Largest diff. peak and hole | 1.956 and −1.655 e·Å−3 | 0.406 and −0.431 e·Å−3 | 1.153 and −1.232 e·Å−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.H.; Park, I.-H. Zn(II) Metal–Organic Frameworks with a Long Spacer Ligand and a Tricarboxylate Coligand. Crystals 2023, 13, 1266. https://doi.org/10.3390/cryst13081266
Lee DH, Park I-H. Zn(II) Metal–Organic Frameworks with a Long Spacer Ligand and a Tricarboxylate Coligand. Crystals. 2023; 13(8):1266. https://doi.org/10.3390/cryst13081266
Chicago/Turabian StyleLee, Dong Hee, and In-Hyeok Park. 2023. "Zn(II) Metal–Organic Frameworks with a Long Spacer Ligand and a Tricarboxylate Coligand" Crystals 13, no. 8: 1266. https://doi.org/10.3390/cryst13081266
APA StyleLee, D. H., & Park, I.-H. (2023). Zn(II) Metal–Organic Frameworks with a Long Spacer Ligand and a Tricarboxylate Coligand. Crystals, 13(8), 1266. https://doi.org/10.3390/cryst13081266









