Development of Composite Microencapsulated Phase Change Materials for Multi-Temperature Thermal Energy Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication Method for MPCM
2.3. Characterization Methods
3. Results and Discussions
3.1. Structural Analysis of MEPCMs
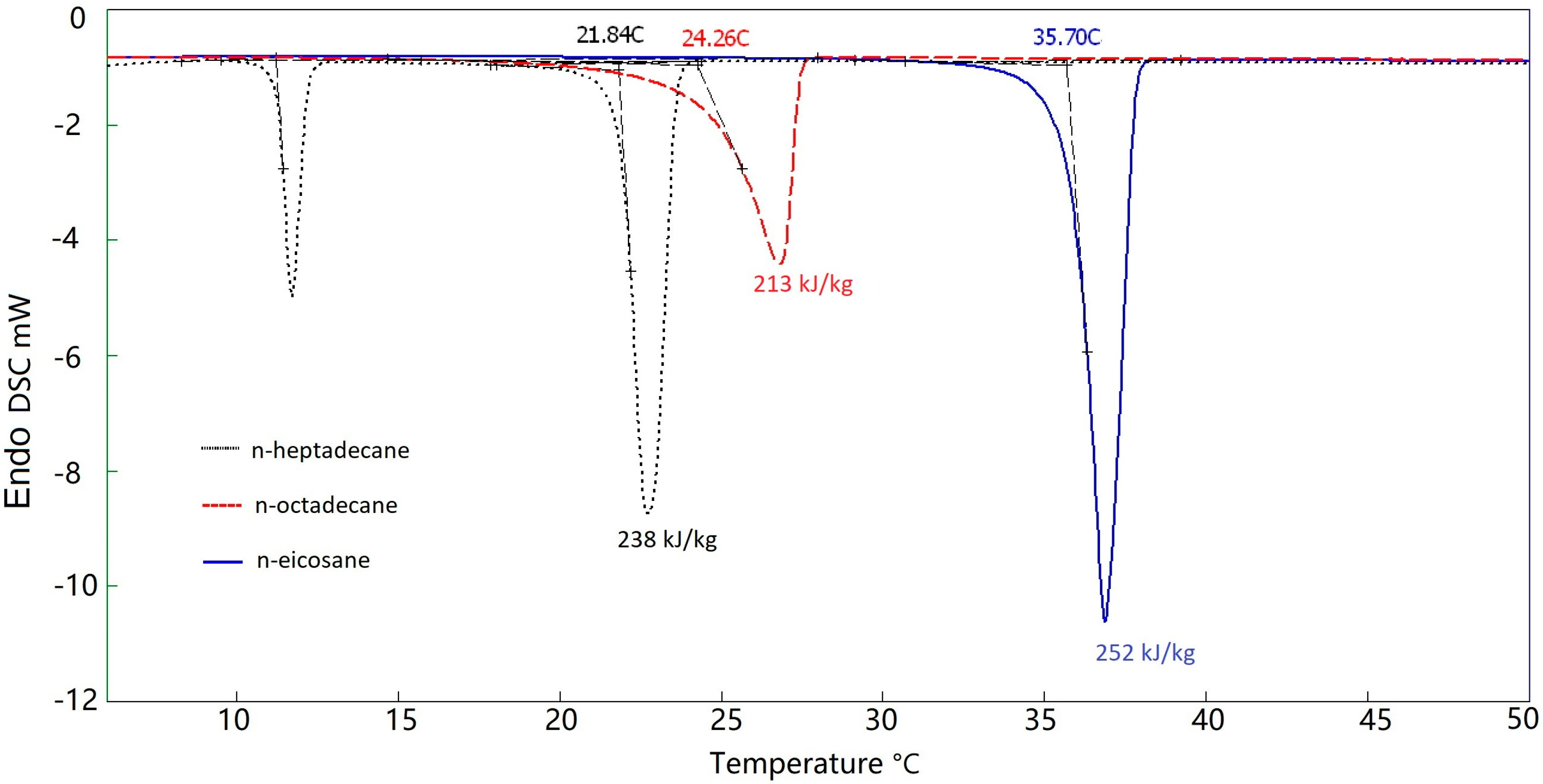
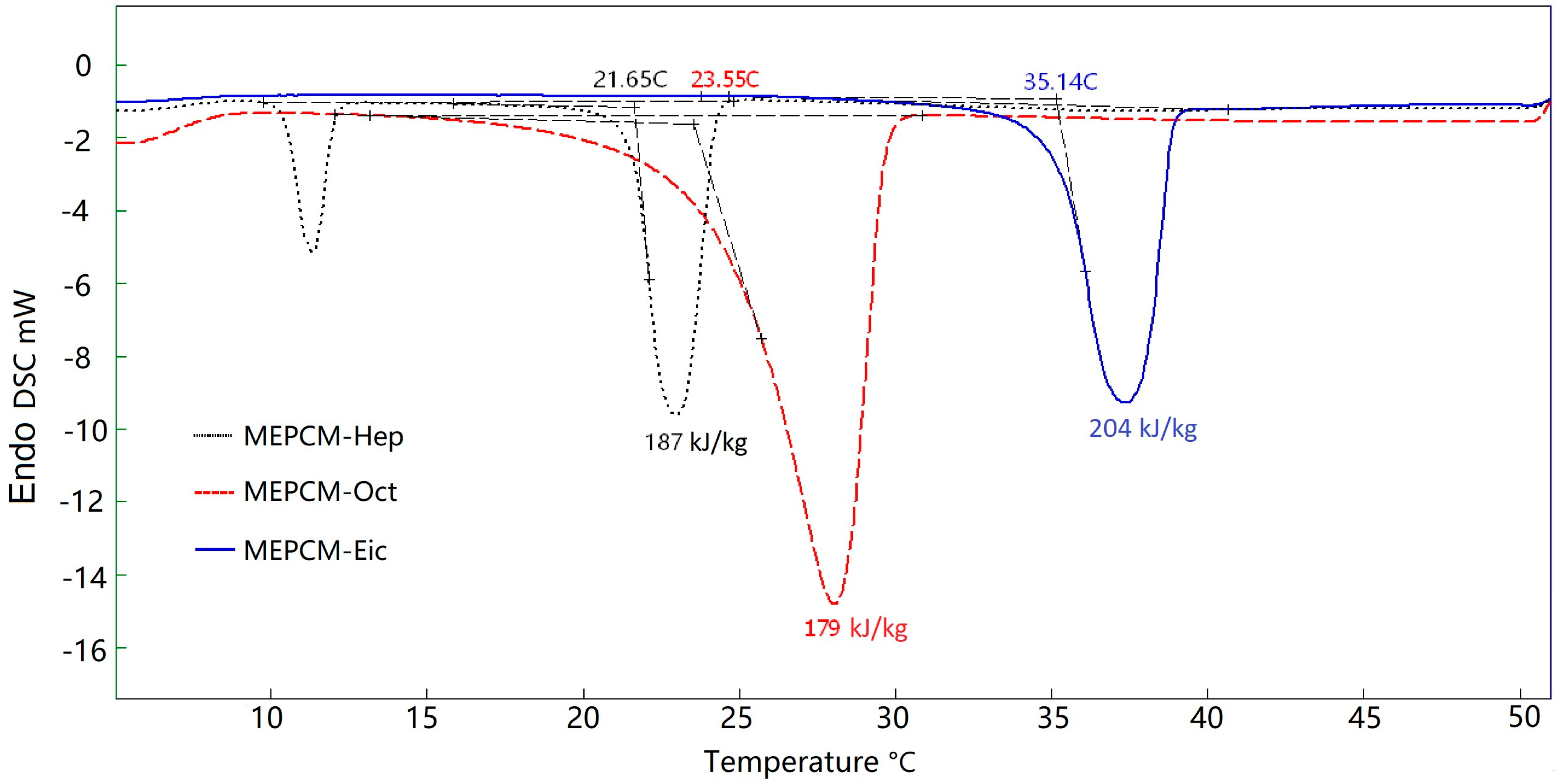
3.2. Thermal Energy Storage Analysis of Pure PCM and MEPCMs
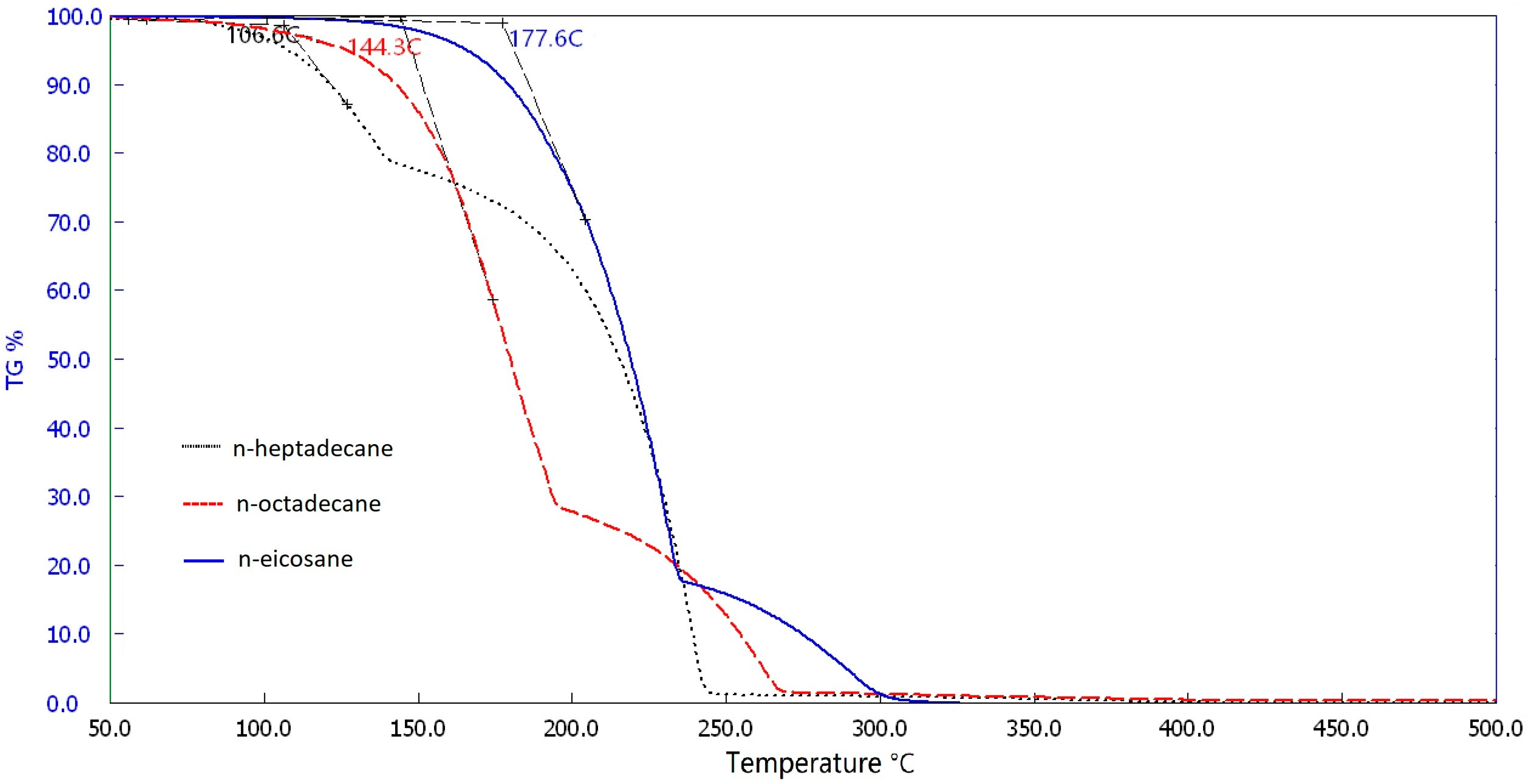
3.3. Thermogravimetric Analysis of Pure PCM and MEPCM Samples
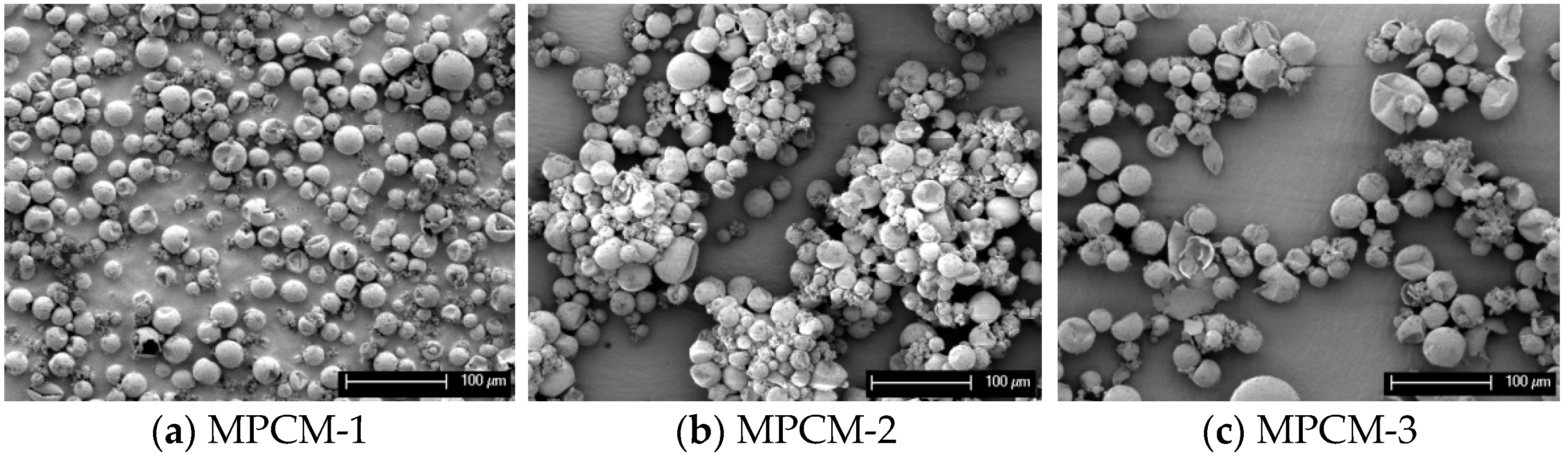
3.4. SEM Analysis of MPCMs
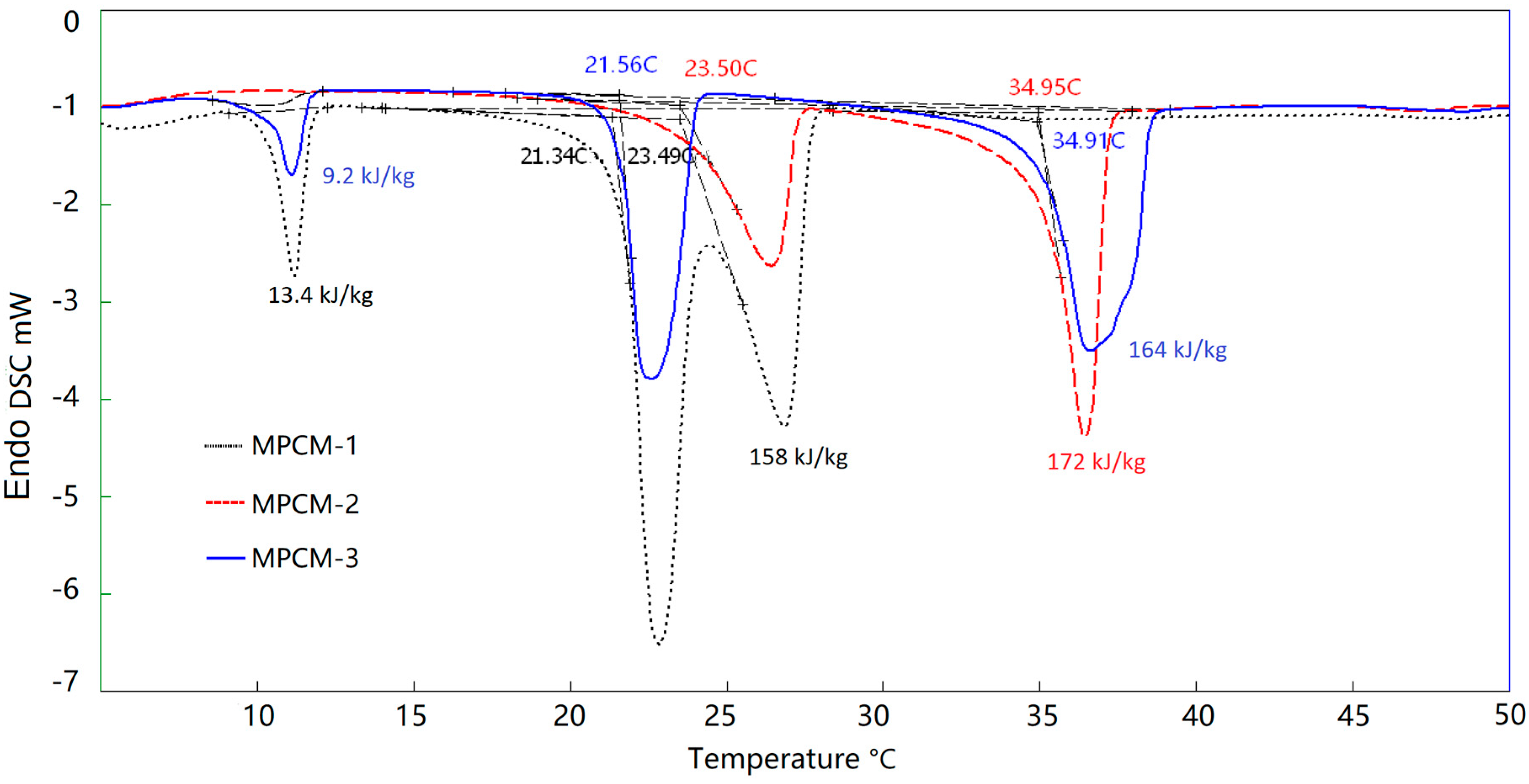
3.5. Thermal Energy Storage Analysis of MPCMs
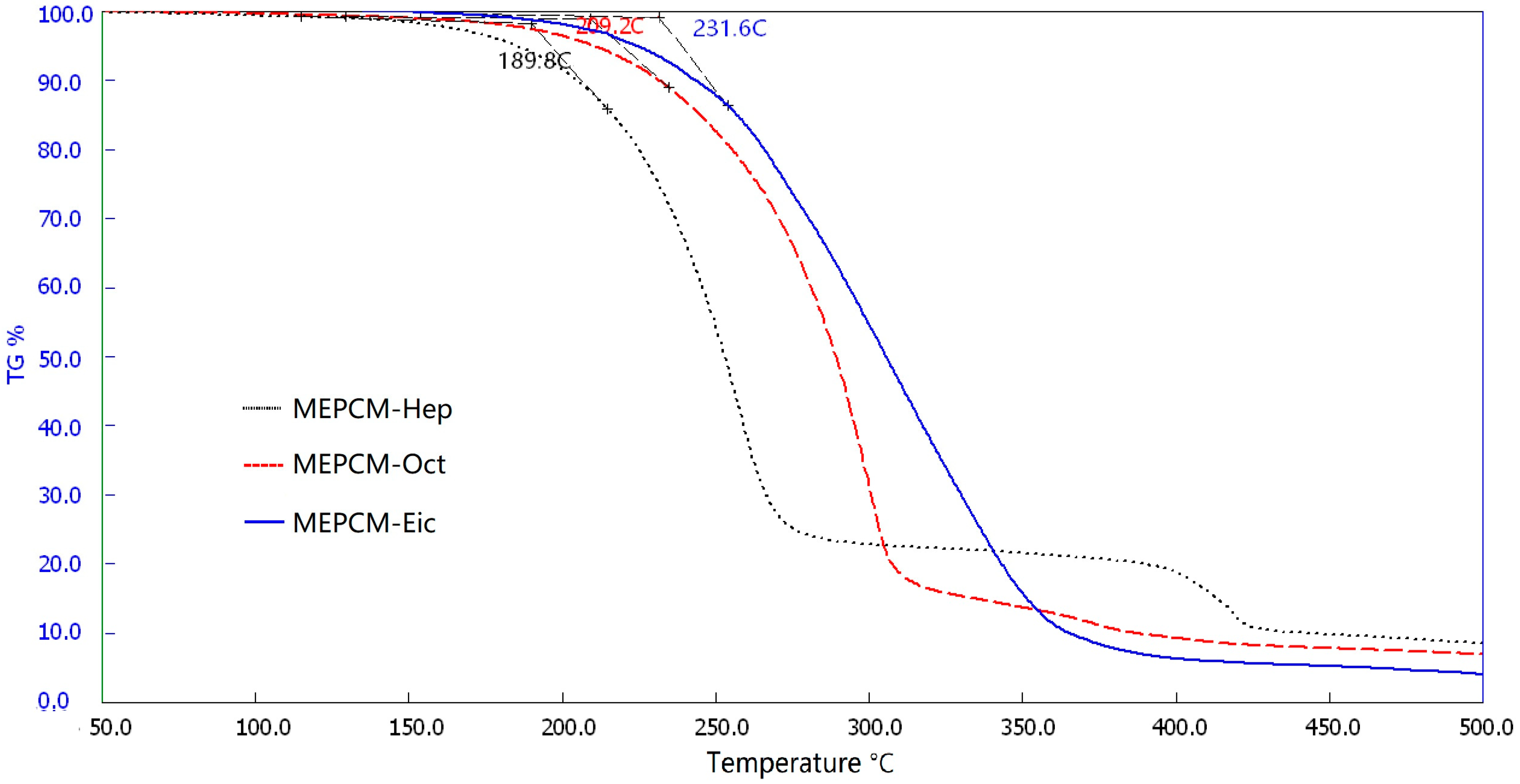
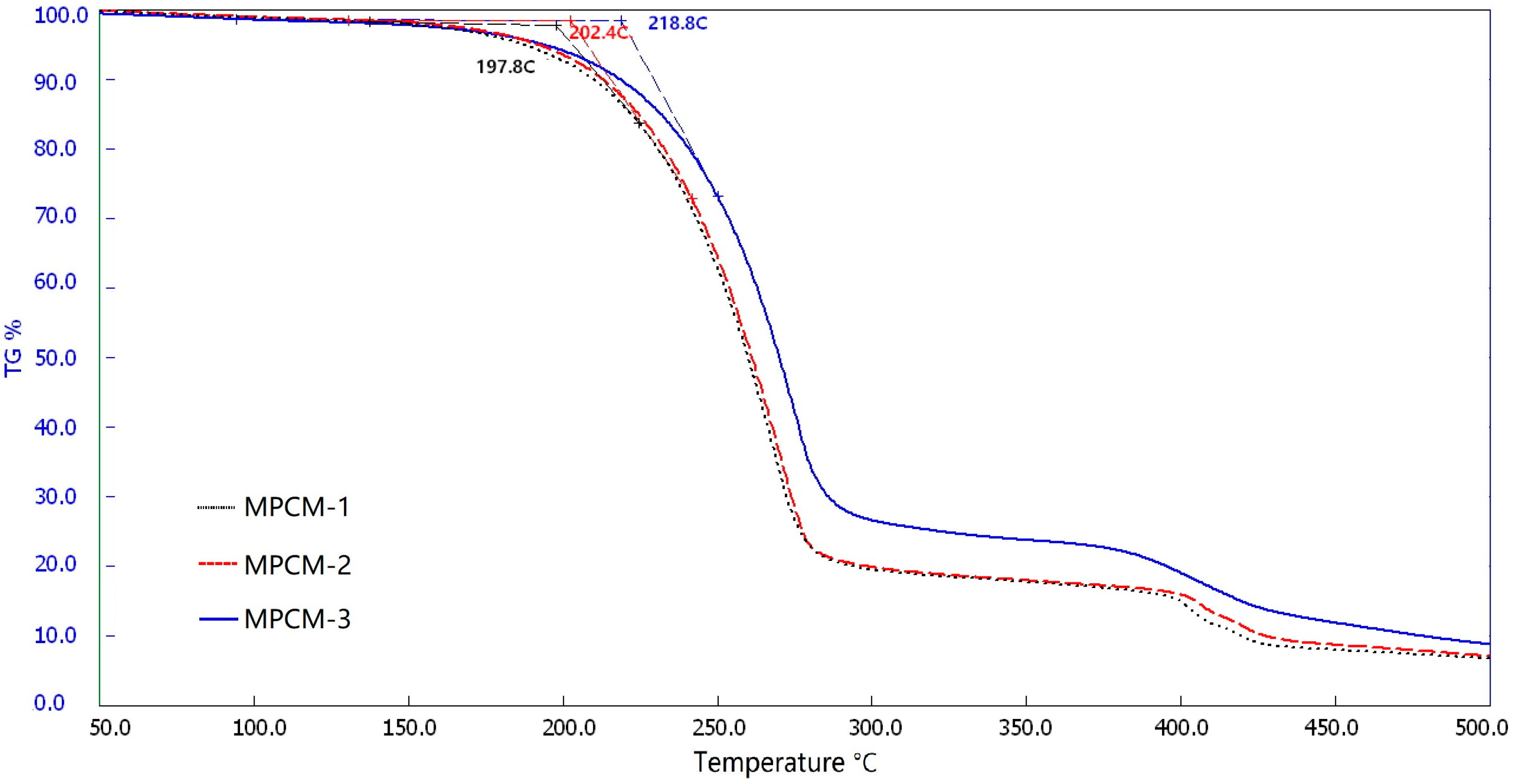
3.6. Thermal Stability Analysis of MPCMs
4. Conclusions
- In comparison with pure PCMs, the melting points of the MEPCMs were reduced by 0.19–0.75 °C after encapsulation.
- The differential core material content between the theoretical and experimental values for the MEPCMs was between 1.43–4.03%
- The phase change temperatures of the MPCMs were slightly reduced by 0.09–0.31 °C as compared with the MEPCMs samples.
- The measured energy storage capacities for the MPCM samples were reduced by about 6.3–11.4% as compared with the theoretical values. They however, displayed relatively good thermal stability behavior of up to 197.8–218.8 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| C | carbon |
| DSC | differential scanning calorimetric |
| EDS | energy-dispersive X-ray spectroscopy |
| EGDS | ethylene glycole distearate |
| MEPCM | microencapsulated phase change material |
| MEPCM-Hep | MEPCM with n-heptadecane as a core |
| MEPCM-Oct | MEPCM with n-octadecane as a core |
| MEPCM-Eic | MEPCM with n-eicosane as a core |
| MPCM | multiphase change materials |
| N | nitrogen |
| O | oxygen |
| O/W | oil-in-water |
| PCM | phase change material |
| SEM | Scanning electron microscope |
| Si | silicon |
| TG | Thermogravimetry |
| Latent heat, kJ/kg | |
| latent heat of MEPCM-x, kJ/kg | |
| latent heat of MEPCM-y, kJ/kg | |
| tm1 | the first melting point of MPCM, °C |
| tm2 | the second melting points of MPCM, °C |
| tm | melting temperature, °C |
| the weight percentages of MEPCM-x, % | |
| the weight percentages of MEPCM-y, % |
References
- Da Cunha, S.R.L.; de Aguiar, J.L.B. Phase change materials and energy efficiency of buildings: A review of knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Saafi, K.; Daouas, N. Energy and cost efficiency of phase change materials integrated in building envelopes under Tunisia Mediterranean climate. Energy 2019, 187, 115987. [Google Scholar] [CrossRef]
- Tunçbilek, E.; Arıcı, M.; Bouadila, S.; Wonorahardjo, S. Seasonal and annual performance analysis of PCM-integrated building brick under the climatic conditions of Marmara region. J. Therm. Anal. Calorim. 2020, 141, 613–624. [Google Scholar] [CrossRef]
- Zhang, H.; Xing, F.; Cui, H.-Z.; Chen, D.-Z.; Ouyang, X.; Xu, S.-Z.; Wang, J.-X.; Huang, Y.-T.; Zuo, J.-D.; Tang, J.-N. A novel phase-change cement composite for thermal energy storage: Fabrication, thermal and mechanical properties. Appl. Energy 2016, 170, 130–139. [Google Scholar] [CrossRef]
- Pilehvar, S.; Szczotok, A.M.; Carmona, M.; Pamies, R.; Kjøniksen, A. The effect of microencapsulated phase change materials on the rheology of geopolymer and Portland cement mortars. J. Am. Ceram. Soc. 2020, 103, 5852–5869. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M. Nanostructures encapsulated phase change materials for sustained thermal energy storage in concrete: An overall assessment. Mater. Today Proc. 2021, 42, 2457–2463. [Google Scholar] [CrossRef]
- Li, G.; Hwang, Y.; Radermacher, R.; Chun, H.-H. Review of cold storage materials for subzero applications. Energy 2013, 51, 1–17. [Google Scholar] [CrossRef]
- Podara, C.V.; Kartsonakis, I.A.; Charitidis, C.A. Towards Phase Change Materials for Thermal Energy Storage: Classification, Improvements and Applications in the Building Sector. Appl. Sci. 2021, 11, 1490. [Google Scholar] [CrossRef]
- Su, W.; Hu, M.; Wang, L.; Kokogiannakis, G.; Chen, J.; Gao, L.; Li, A.; Xu, C. Microencapsulated phase change materials with graphene-based materials: Fabrication, characterisation and prospects. Renew. Sustain. Energy Rev. 2022, 168, 112806. [Google Scholar] [CrossRef]
- Konuklu, Y.; Ostry, M.; Paksoy, H.O.; Charvat, P. Review on using microencapsulated phase change materials (PCM) in building applications. Energy Build. 2015, 106, 134–155. [Google Scholar] [CrossRef]
- Zhou, T.; Darkwa, J.; Kokogiannakis, G. Thermal evaluation of laminated composite phase change material gypsum board under dynamic conditions. Renew. Energy 2015, 78, 448–456. [Google Scholar] [CrossRef]
- Young, B.A.; Falzone, G.; Wei, Z.; Sant, G.; Pilon, L. Reduced-scale experiments to evaluate performance of composite building envelopes containing phase change materials. Constr. Build. Mater. 2018, 162, 584–595. [Google Scholar] [CrossRef]
- Berthou, Y.; Biwole, P.H.; Achard, P.; Sallée, H.; Tantot-Neirac, M.; Jay, F. Full scale experimentation on a new translucent passive solar wall combining silica aerogels and phase change materials. Sol. Energy 2015, 115, 733–742. [Google Scholar] [CrossRef]
- Tokuç, A.; Başaran, T.; Yesügey, S.C. An experimental and numerical investigation on the use of phase change materials in building elements: The case of a flat roof in Istanbul. Energy Build. 2015, 102, 91–104. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, P.; Hu, P.; Liu, F.; Jiang, Z. Modeling and simulation on the performance of a novel double shape-stabilized phase change materials wallboard. Energy Build. 2015, 107, 181–190. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, F.; Liu, P.; Hu, P.; Wu, M. Energy saving potential of a novel phase change material wallboard in typical climate regions of China. Energy Build. 2016, 128, 360–369. [Google Scholar] [CrossRef]
- Li, G.; Hwang, Y.; Radermacher, R. Cold Thermal Energy Storage Materials and Applications Toward Sustainability. In Energy Solutions to Combat Global Warming; Dincer, I., Zhang, X., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Numerical thermal evaluation of laminated binary microencapsulated phase change material drywall systems. Build. Simul. 2020, 13, 89–98. [Google Scholar] [CrossRef]
- Huang, Z.; Ye, Q.; Shi, Z.; Tang, J.-N.; Ouyang, X.; Chen, D.-Z. Multiple phase change-stimulated shape memory and self-healing epoxy composites with thermal regulation function. Chem. Eng. J. 2020, 409, 127382. [Google Scholar] [CrossRef]
- Meng, X.; Qin, S.; Fan, H.; Huang, Z.; Hong, J.; Xu, X.; Ouyang, X.; Chen, D.-Z. Long alkyl chain-grafted carbon nanotube-decorated binary-core phase-change microcapsules for heat energy storage: Synthesis and thermal properties. Sol. Energy Mater. Sol. Cells 2020, 212, 110589. [Google Scholar] [CrossRef]
- Ma, Y.; Chu, X.; Li, W.; Tang, G. Preparation and characterization of poly(methyl methacrylate-co-divinylbenzene) microcapsules containing phase change temperature adjustable binary core materials. Sol. Energy 2012, 86, 2056–2066. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, S.; Li, J.; Tang, G. Preparation and thermal reliabilities of microencapsulated phase change materials with binary cores and acrylate-based polymer shells. Thermochim. Acta 2014, 588, 38–46. [Google Scholar] [CrossRef]
- Ma, Y.; Chu, X.; Tang, G.; Yao, Y. The effect of different soft segments on the formation and properties of binary core microencapsulated phase change materials with polyurea/polyurethane double shell. J. Colloid Interface Sci. 2013, 392, 407–414. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Luo, R.; Zhu, C.; Akiyama, T.; Zhang, Z. Microencapsulation of phase change materials with binary cores and calcium carbonate shell for thermal energy storage. Appl. Energy 2016, 171, 113–119. [Google Scholar] [CrossRef]
- Yataganbaba, A.; Ozkahraman, B.; Kurtbas, I. Worldwide trends on encapsulation of phase change materials: A bibliometric analysis (1990–2015). Appl. Energy 2017, 185, 720–731. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Development of microencapsulated phase change material for solar thermal energy storage. Appl. Therm. Eng. 2017, 112, 1205–1212. [Google Scholar] [CrossRef]
- Su, W.; Li, Y.; Zhou, T.; Darkwa, J.; Kokogiannakis, G.; Li, Z. Microencapsulation of Paraffin with Poly (Urea Methacrylate) Shell for Solar Water Heater. Energies 2019, 12, 3406. [Google Scholar] [CrossRef]
- Climate.OneBuilding.Org. China. 2021. Available online: http://climate.onebuilding.org/WMO_Region_2_Asia/CHN_China/index.html (accessed on 12 May 2023).
- Zhang, H.; Wang, X. Fabrication and performances of microencapsulated phase change materials based on n-octadecane core and resorcinol-modified melamine–formaldehyde shell. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 129–138. [Google Scholar] [CrossRef]
- Su, W.; Gao, L.; Wang, L.; Zhi, H. Calibration of differential scanning calorimeter (DSC) for thermal properties analysis of phase change material. J. Therm. Anal. Calorim. 2021, 143, 2995–3002. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Nanosilicon dioxide hydrosol as surfactant for preparation of microencapsulated phase change materials for thermal energy storage in buildings. Int. J. Low-Carbon Technol. 2018, 13, 301–310. [Google Scholar] [CrossRef]
- Vélez, C.; de Zárate, J.M.O.; Khayet, M. Thermal properties of n-pentadecane, n-heptadecane and n-nonadecane in the solid/liquid phase change region. Int. J. Therm. Sci. 2015, 94, 139–146. [Google Scholar] [CrossRef]
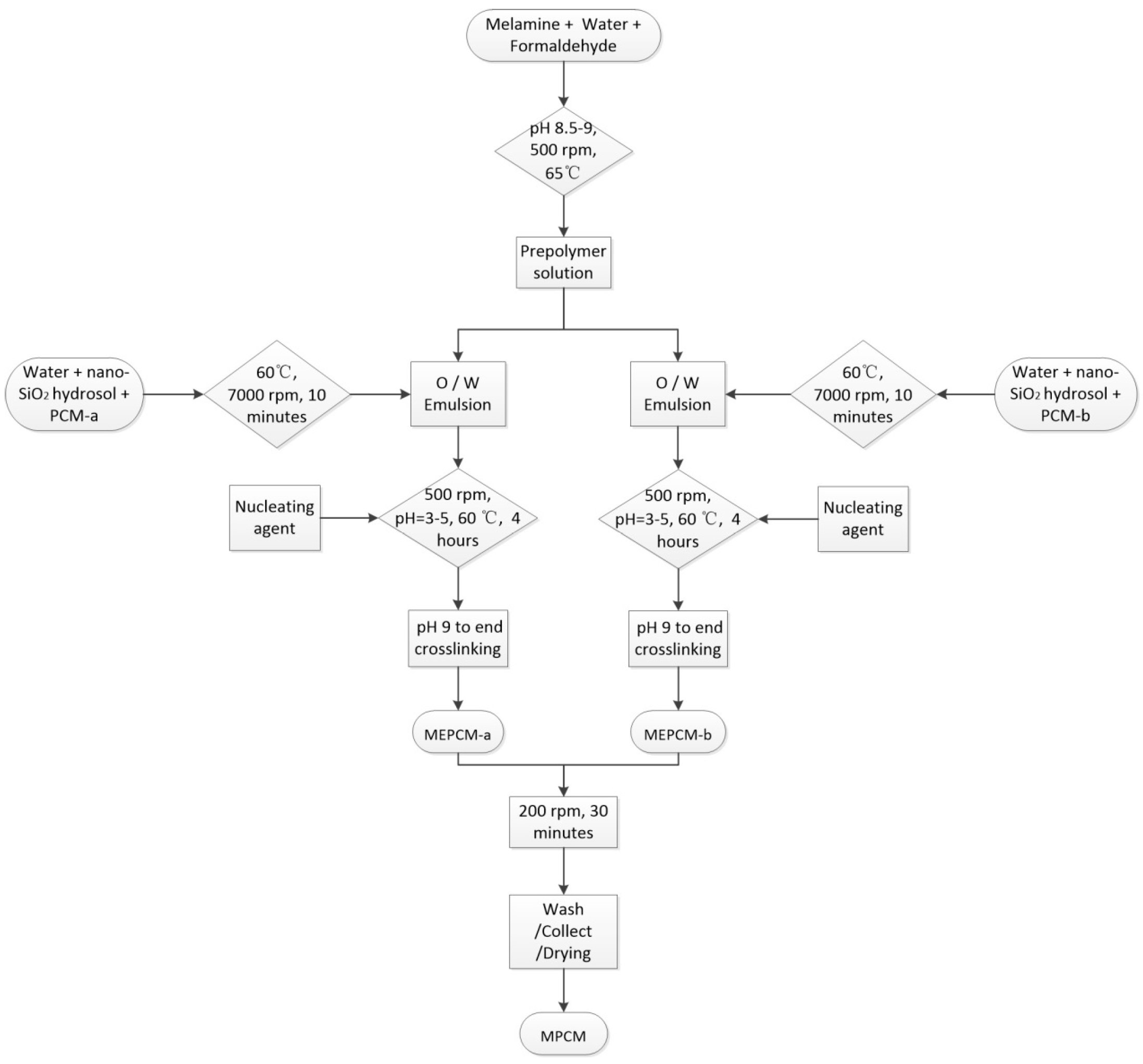
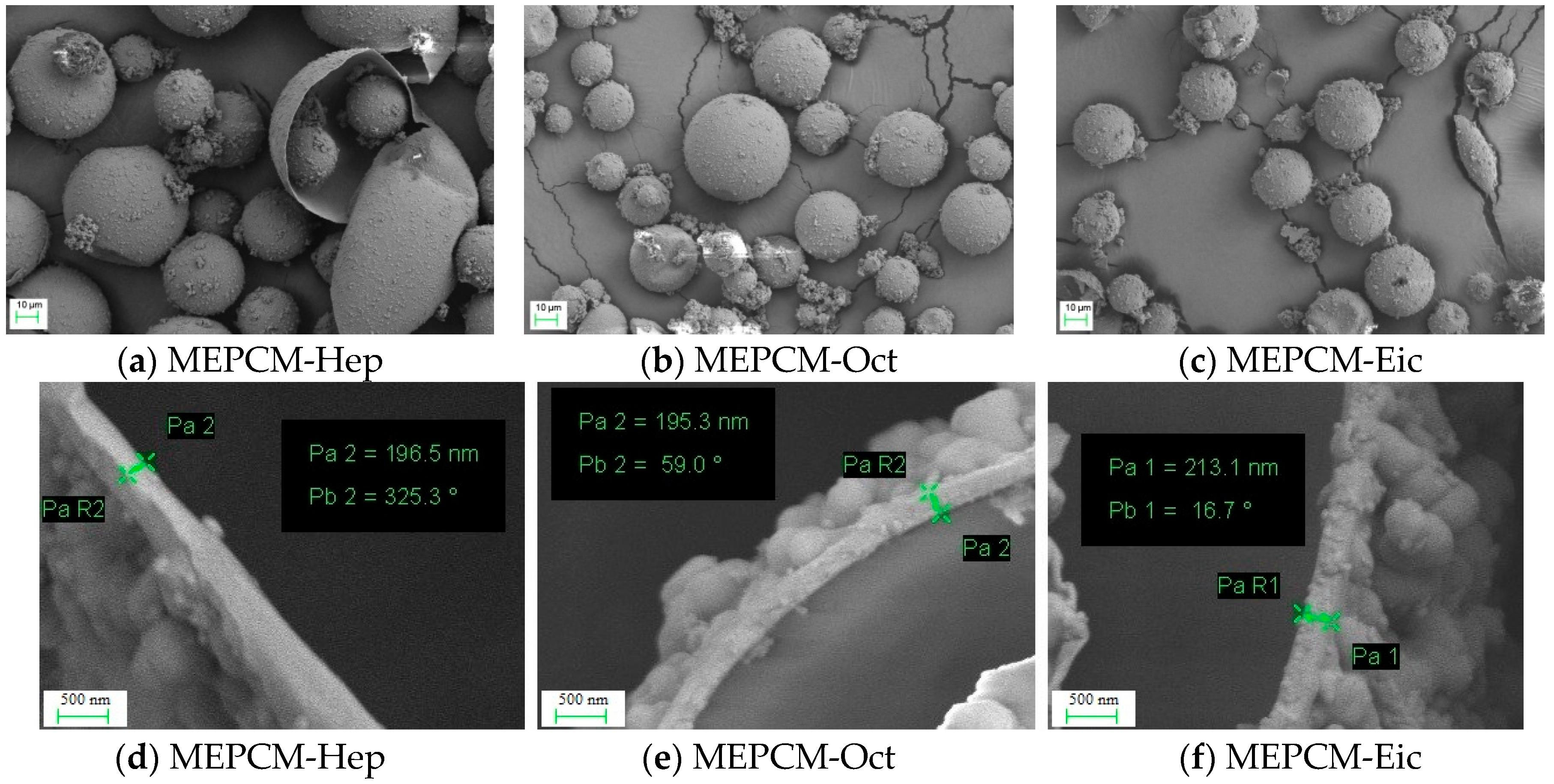
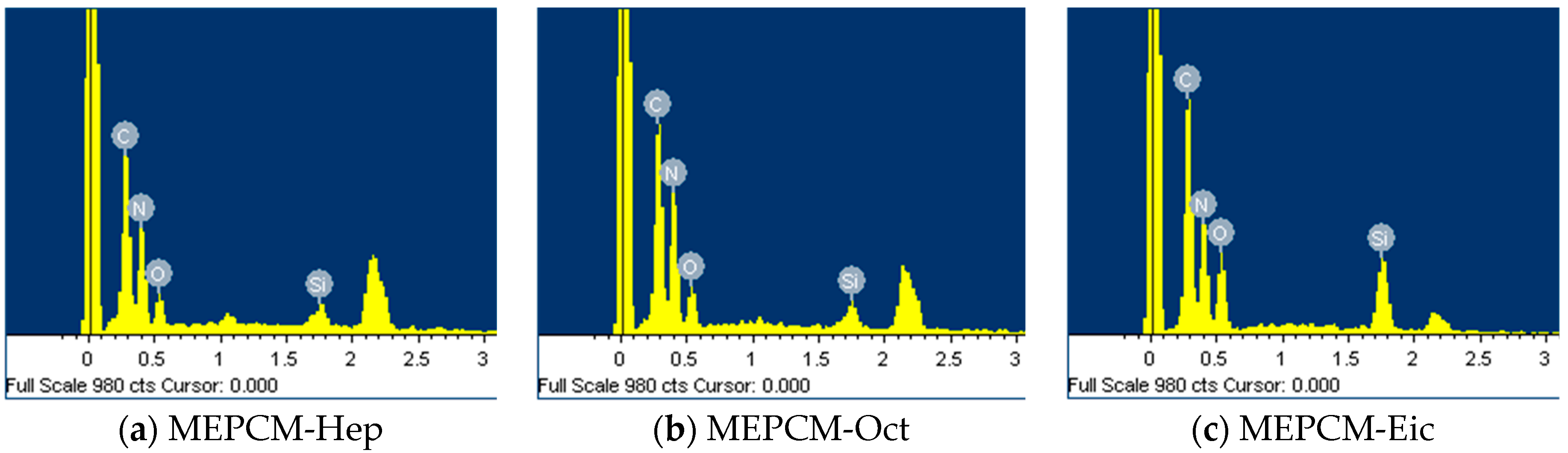
| Name | Melamine (g) | Formaldehyde (g) | Nano SiO2 Hydrosol (g) | Core Material | Core Material Weight (g) | Ammonium Chloride (g) |
|---|---|---|---|---|---|---|
| MEPCM-Hep | 2.0 | 3.2 | 1.2 | n-heptadecane | 10 | 0.125 |
| MEPCM-Oct | 2.0 | 3.2 | 1.2 | n-octadecane | 10 | 0.125 |
| MEPCM-Eic | 2.0 | 3.2 | 1.2 | n-eicosan | 10 | 0.125 |
| Name | MEPCM-Hep (wt%) | MEPCM-Oct (wt%) | MEPCM-Eic (wt%) |
|---|---|---|---|
| MPCM-1 | 50 | 50 | 0 |
| MPCM-2 | 0 | 50 | 50 |
| MPCM-3 | 50 | 0 | 50 |
| Name | tm (°C) | (kJ/kg) | Core Material (%) |
|---|---|---|---|
| n-heptadecane | 21.84 | 238 | -- |
| n-octadecane | 24.26 | 213 | -- |
| n-eicosane | 35.70 | 252 | -- |
| MEPCM-Hep | 21.65 | 187 | 78.57 |
| MEPCM-Oct | 23.55 | 179 | 84.03 |
| MEPCM-Eic | 35.14 | 204 | 80.95 |
| Items | Item | tm1 (°C) | tm2 (°C) | (kJ/kg) |
|---|---|---|---|---|
| Theoretical | MPCM-1 | 21.65 | 23.55 | 183 |
| MPCM-2 | 23.55 | 35.14 | 191.5 | |
| MPCM-3 | 21.65 | 35.14 | 195.5 | |
| Experimental | MPCM-1 | 21.34 | 23.49 | 171.4 |
| MPCM-2 | 23.50 | 34.95 | 172 | |
| MPCM-3 | 21.56 | 34.91 | 173.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.; Darkwa, J.; Zhou, T.; Du, D.; Kokogiannakis, G.; Li, Y.; Wang, L.; Gao, L. Development of Composite Microencapsulated Phase Change Materials for Multi-Temperature Thermal Energy Storage. Crystals 2023, 13, 1167. https://doi.org/10.3390/cryst13081167
Su W, Darkwa J, Zhou T, Du D, Kokogiannakis G, Li Y, Wang L, Gao L. Development of Composite Microencapsulated Phase Change Materials for Multi-Temperature Thermal Energy Storage. Crystals. 2023; 13(8):1167. https://doi.org/10.3390/cryst13081167
Chicago/Turabian StyleSu, Weiguang, Jo Darkwa, Tongyu Zhou, Dengfeng Du, Georgios Kokogiannakis, Yilin Li, Li Wang, and Liying Gao. 2023. "Development of Composite Microencapsulated Phase Change Materials for Multi-Temperature Thermal Energy Storage" Crystals 13, no. 8: 1167. https://doi.org/10.3390/cryst13081167
APA StyleSu, W., Darkwa, J., Zhou, T., Du, D., Kokogiannakis, G., Li, Y., Wang, L., & Gao, L. (2023). Development of Composite Microencapsulated Phase Change Materials for Multi-Temperature Thermal Energy Storage. Crystals, 13(8), 1167. https://doi.org/10.3390/cryst13081167











