Characterization of Ca-Dicarboxylate Salt Hydrates as Thermochemical Energy Storage Materials
Abstract
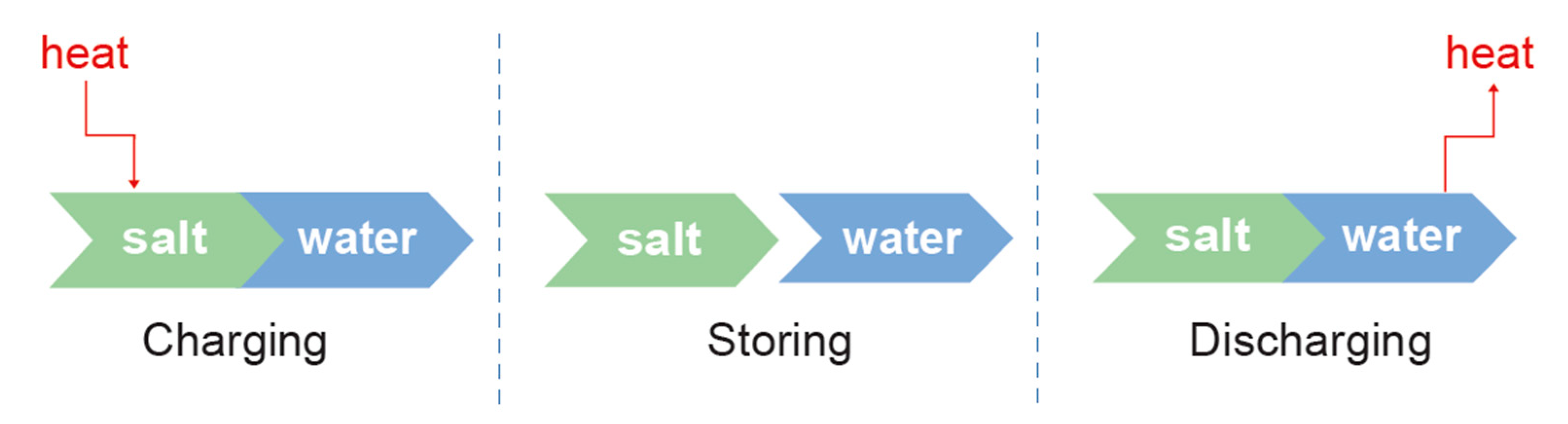
:1. Introduction
2. Materials and Methods
2.1. Materials
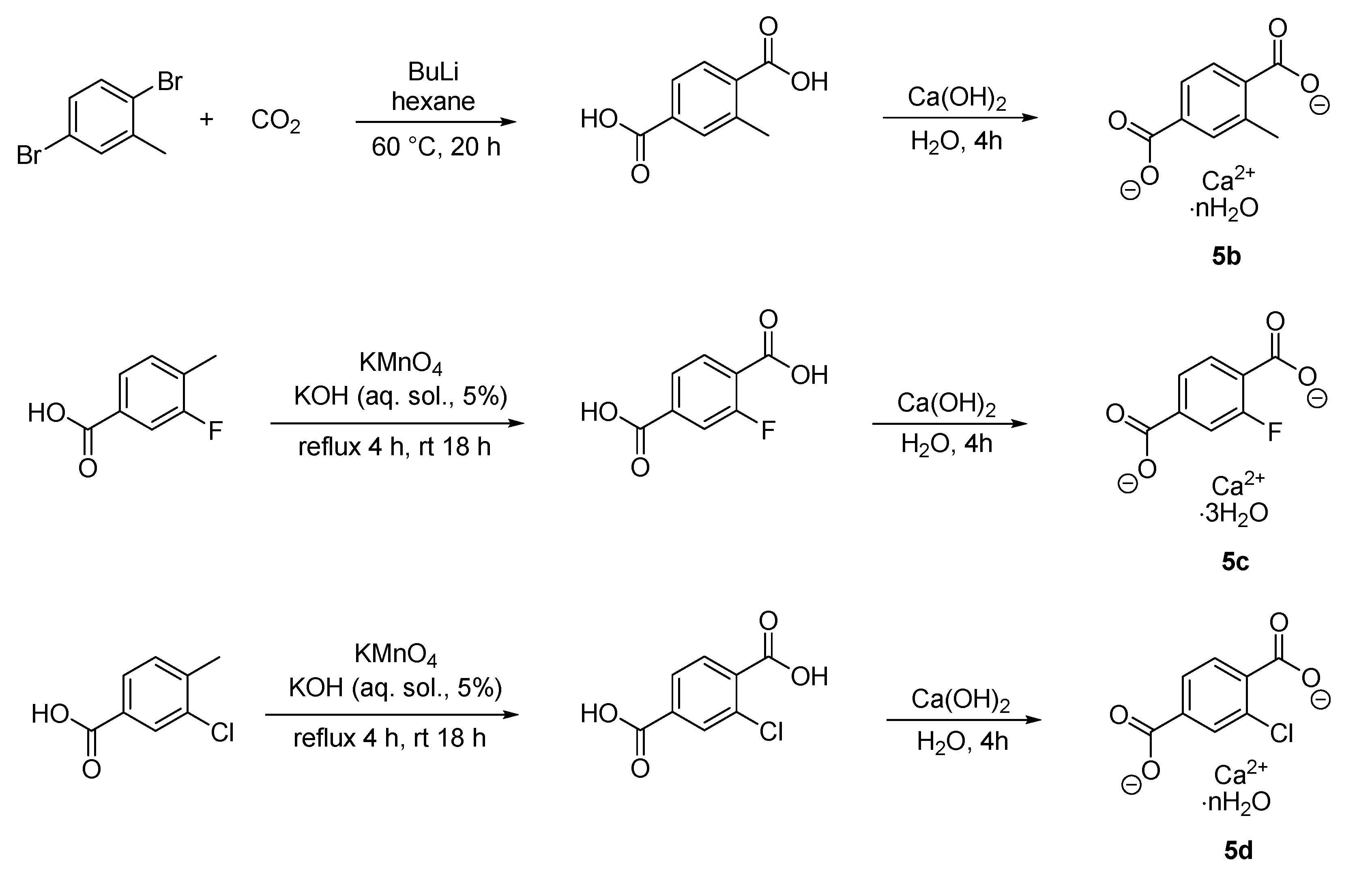
2.2. Synthesis
2.3. Single Crystal Growth
2.4. Thermal Analysis
2.5. Powder X-ray Diffraction
2.6. Single-Crystal X-ray Diffraction
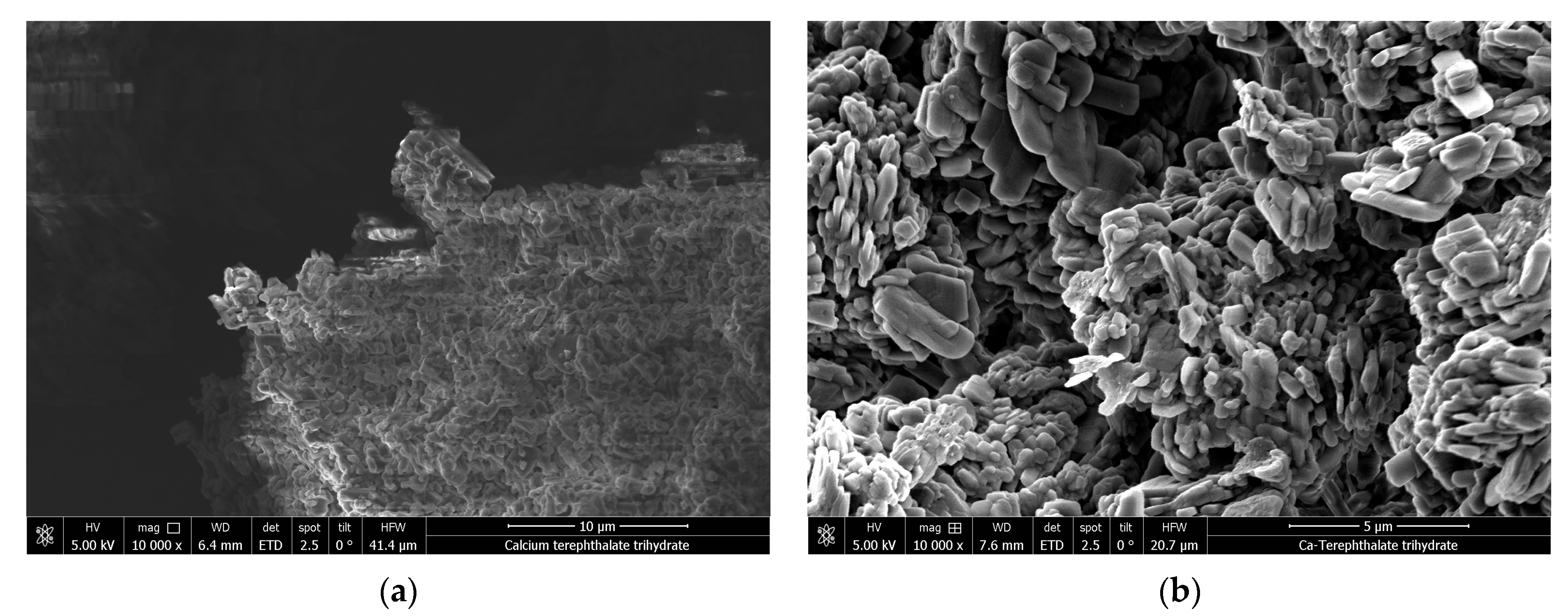
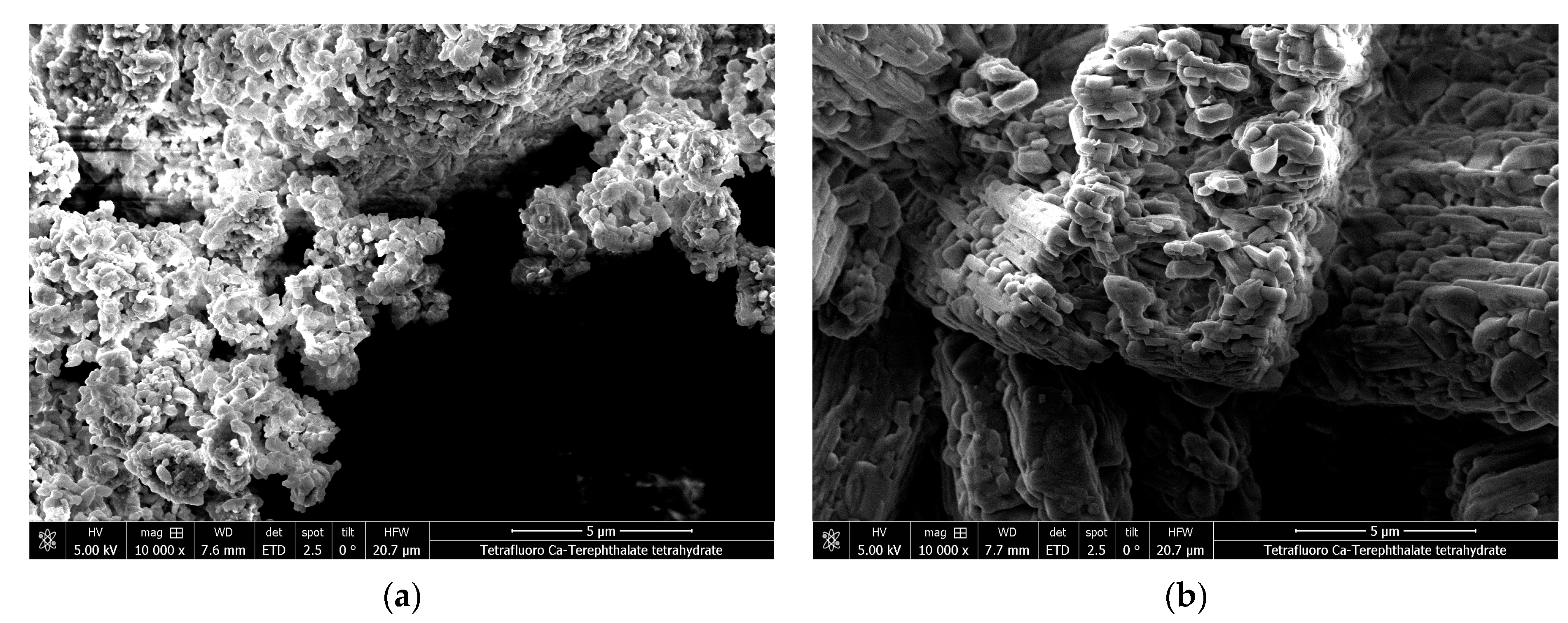
2.7. Scanning Electron Microscopy
3. Results and Discussion
3.1. Synthesis
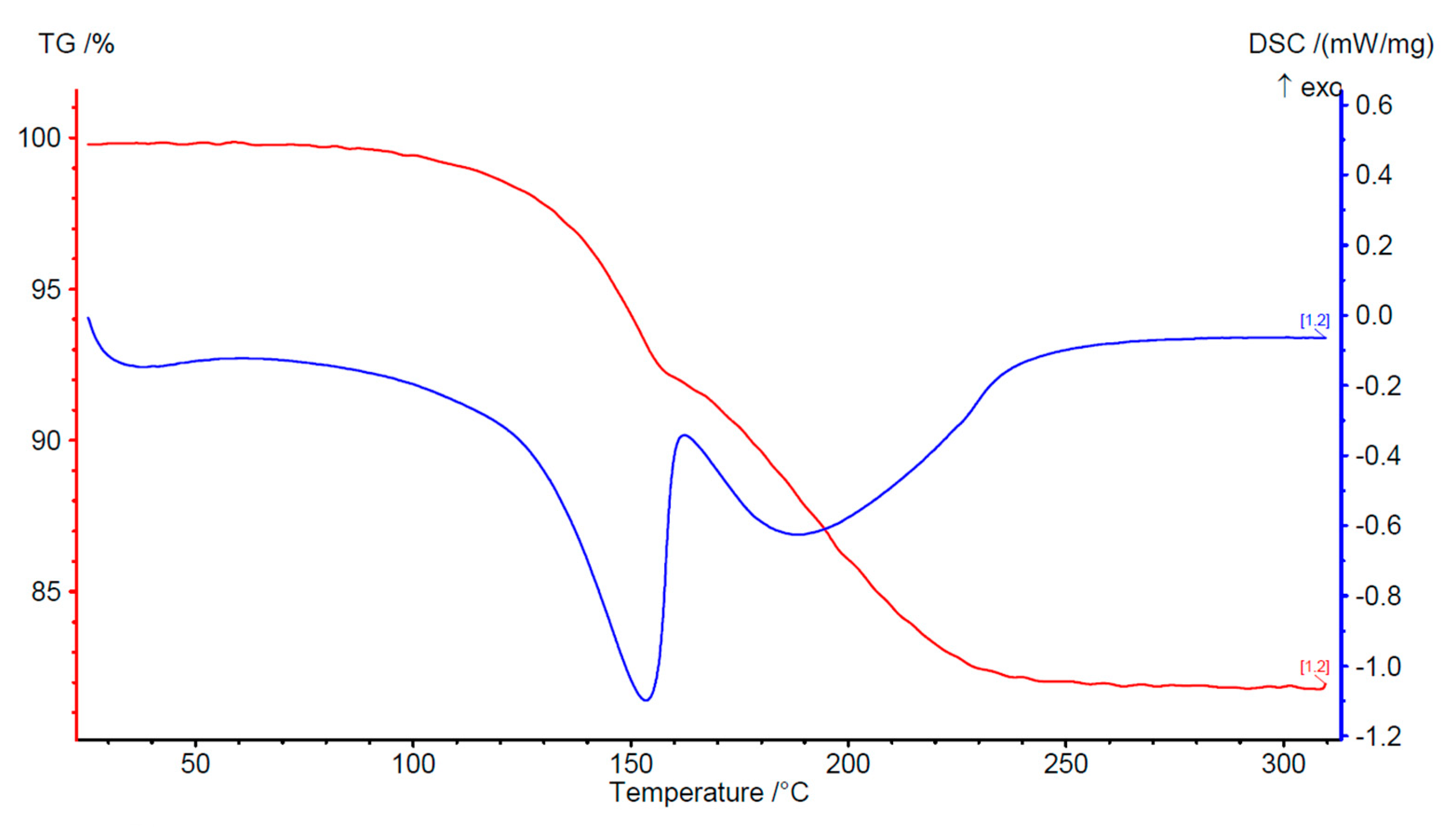
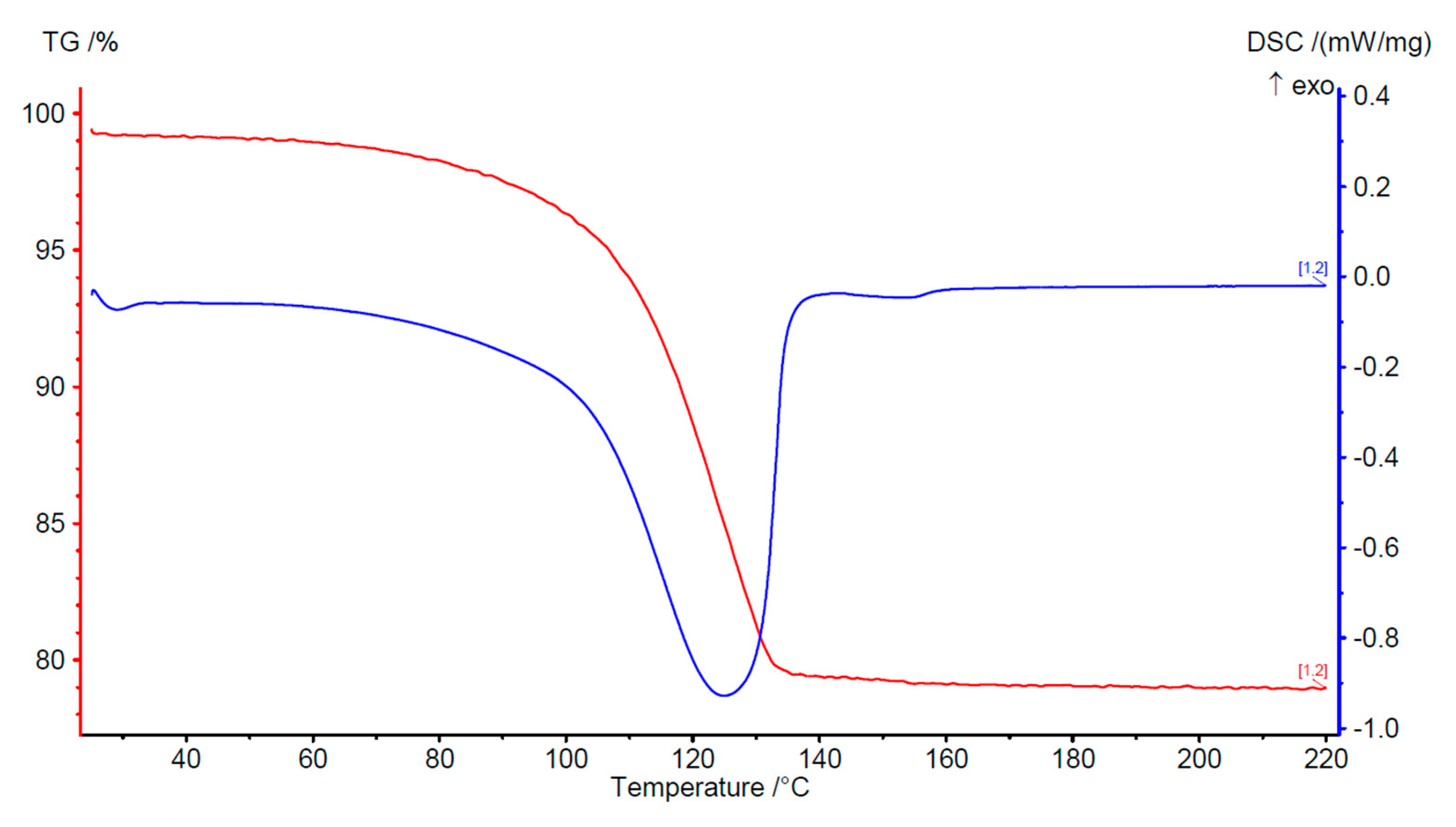
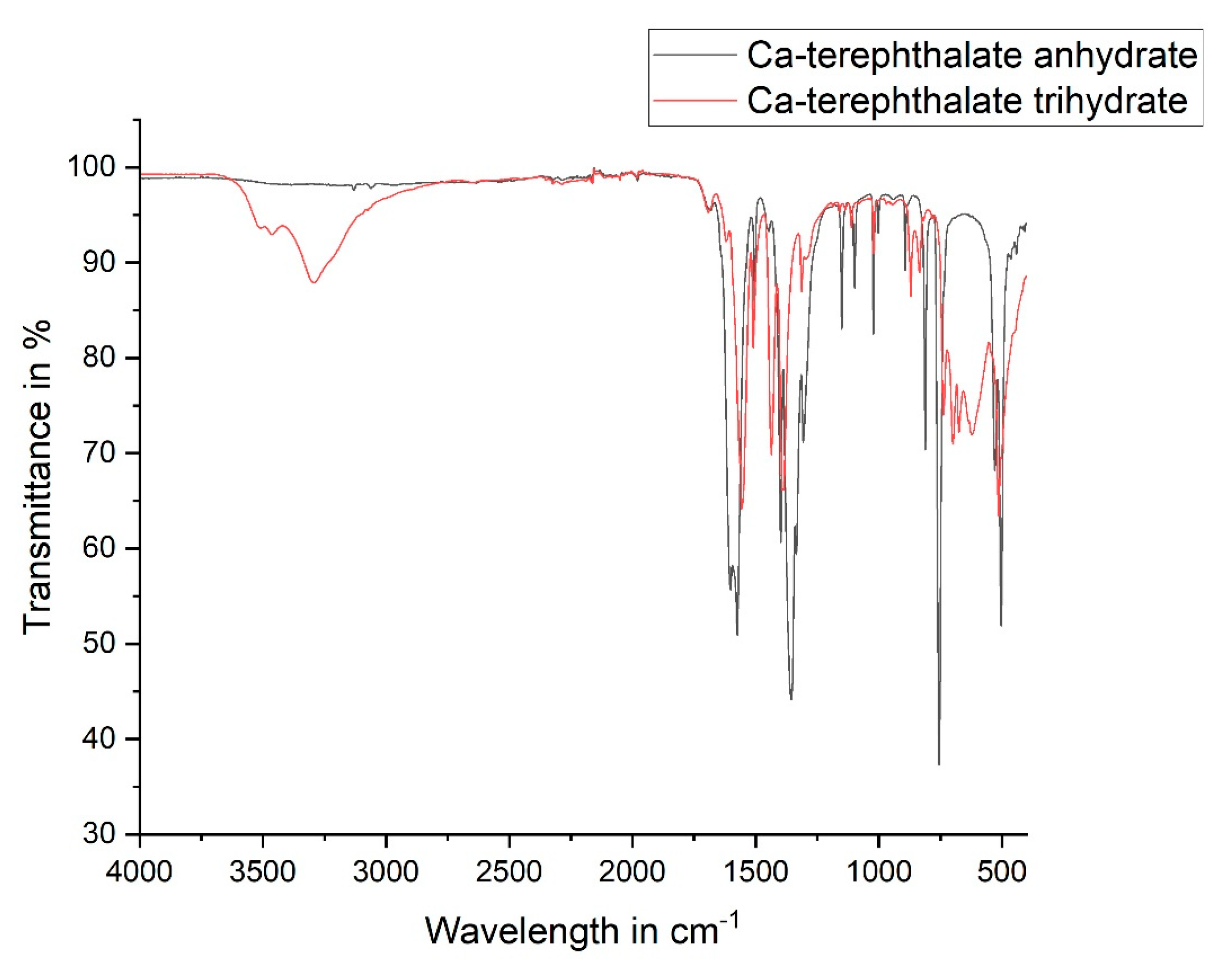
3.2. Characterization and Thermal Dehydration Experiments
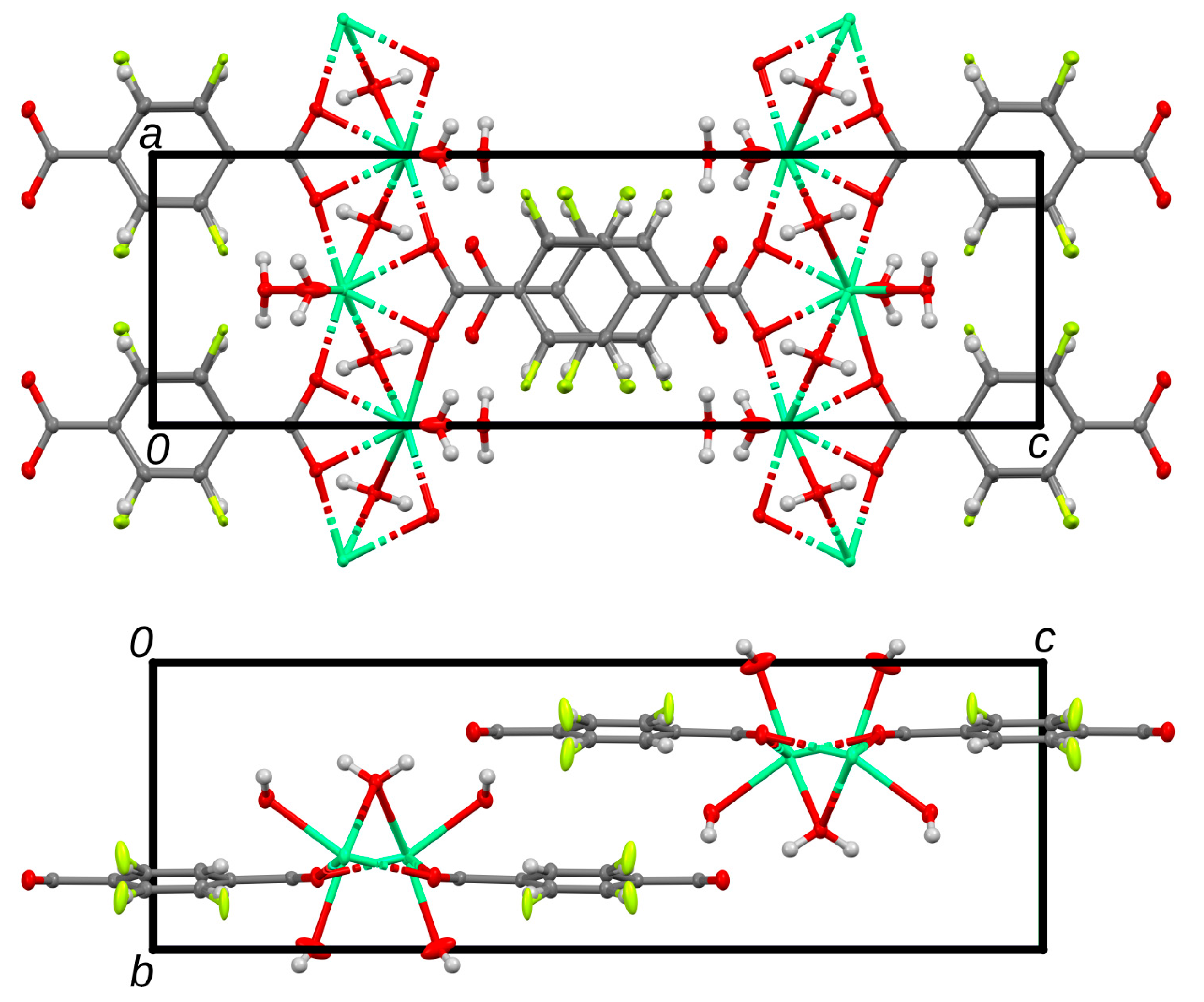
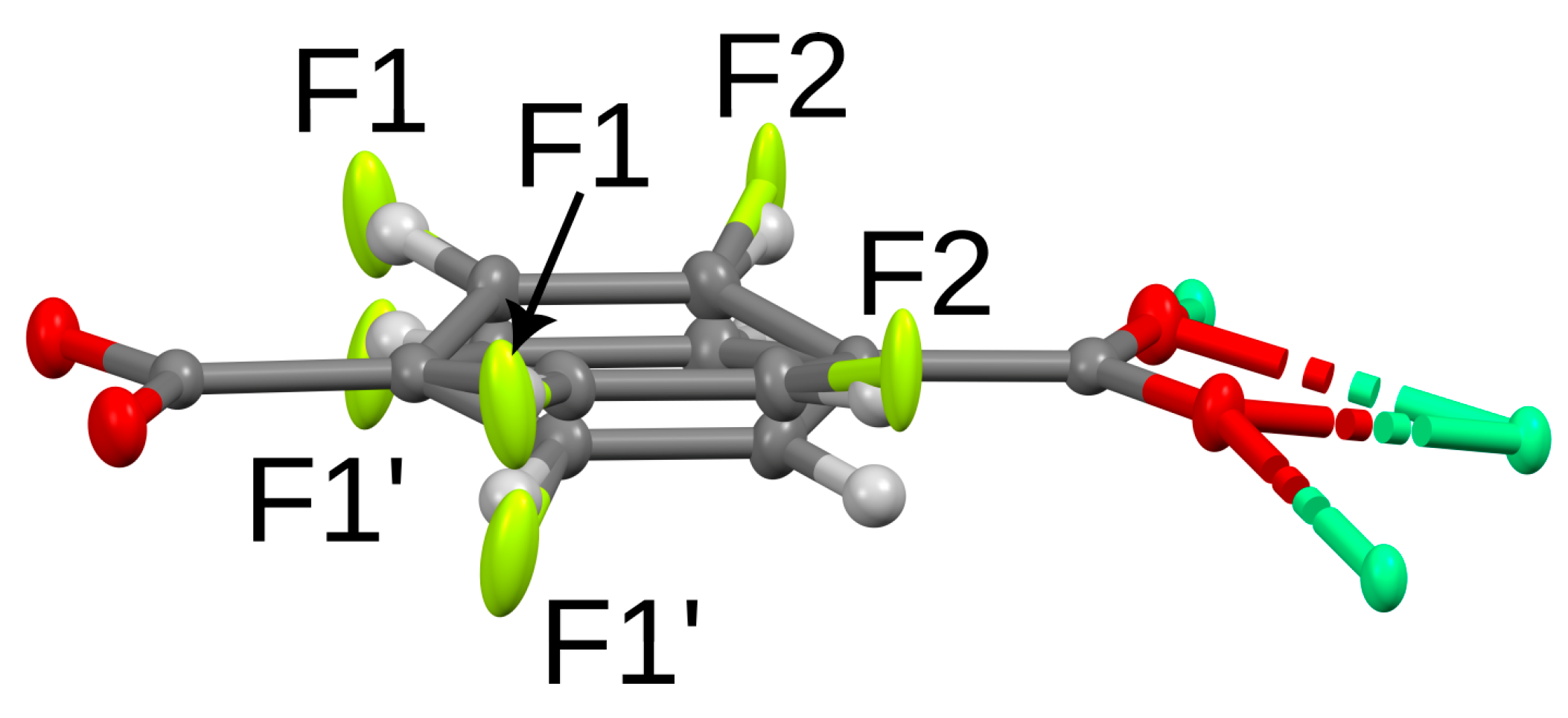
3.3. Structure Description of 2-Fluoro Ca-Terephthalate Trihydrate
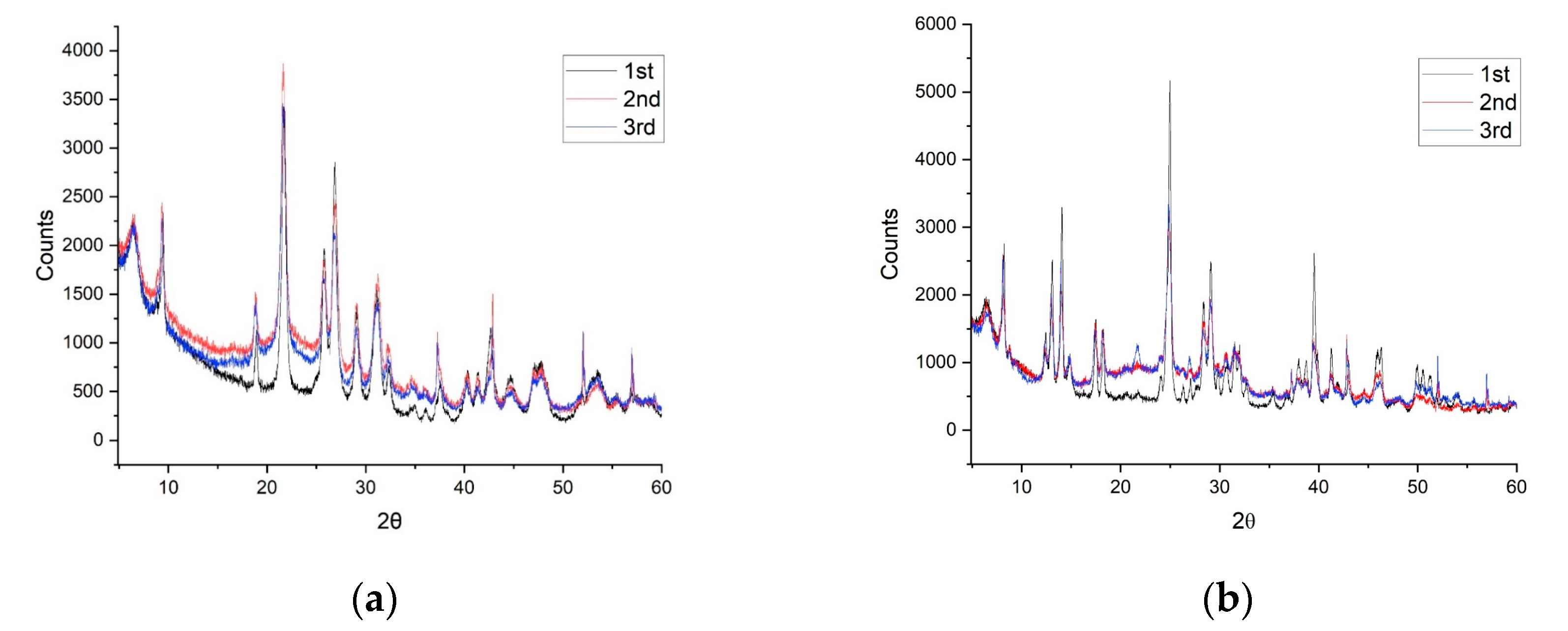
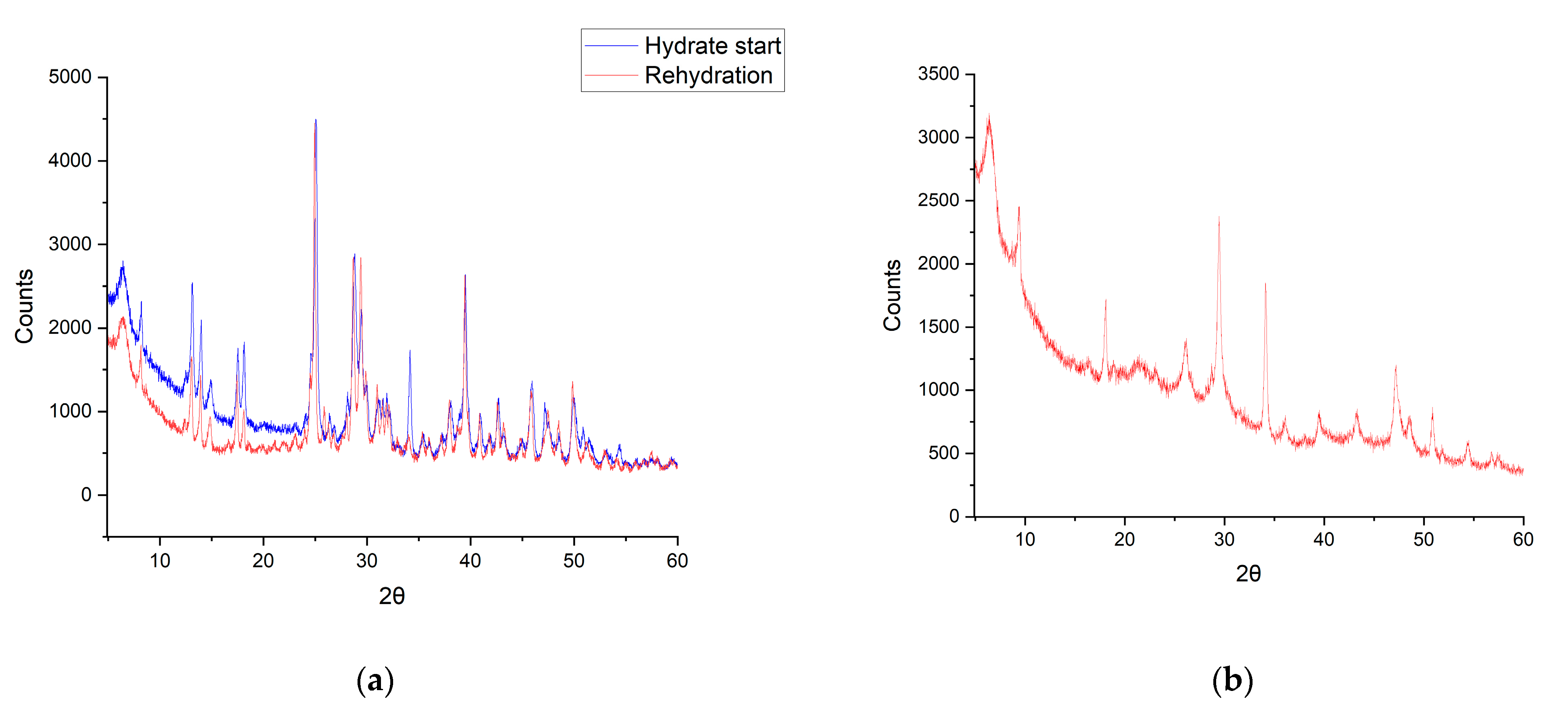
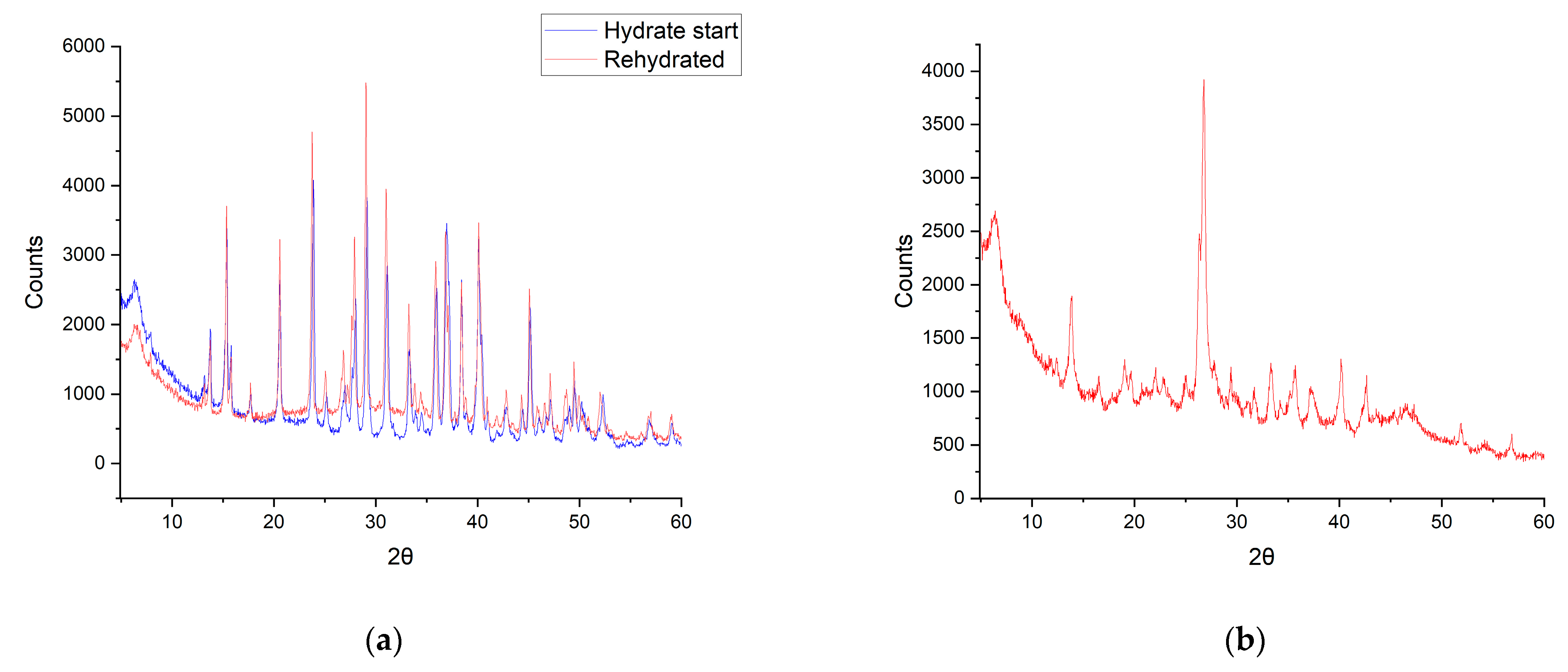
3.4. In Situ De- and Rehydration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. Co-Generation and Renewables; IEA: Paris, Fance, 2011. [Google Scholar]
- IEA. Heating Without Global Warming; IEA: Paris, France, 2014. [Google Scholar]
- Cot-Gores, J.; Castell, A.; Cabeza, L.F. Thermochemical energy storage and conversion: A-state-of-the-art review of the experimental research under practical conditions. Renew. Sustain. Energy Rev. 2012, 16, 5224. [Google Scholar] [CrossRef]
- Dale, S. BP Statistical Review of World Energy; BP: London, UK, 2021; p. 60. [Google Scholar]
- Knoll, C. Investigations of the Reaction Kinetics of Thermochemical Energy Storage Materials. Ph.D. Thesis, University of Vienna, Vienna, Austria, 2017; p. 200. [Google Scholar]
- Hasnain, S.M. Review on sustainable thermal energy storage technologies, Part I: Heat storage materials and techniques. Energy Convers. Manag. 1998, 39, 1138. [Google Scholar] [CrossRef]
- Shine, K.P.; Fuglestvedt, J.S.; Hailemariam, K.; Stuber, N. Alternatives to the Global Warming Potential for Comparing Climate Impacts of Emissions of Greenhouse Gases. Clim. Chang. 2005, 68, 281–302. [Google Scholar] [CrossRef]
- Clark, R.-J.; Mehrabadi, A.; Farid, M. State of the art on salt hydrate thermochemical energy storage systems for use in building applications. J. Energy Storage 2020, 27, 101145. [Google Scholar] [CrossRef]
- Miró, L.; Gasia, J.; Cabeza, L.F. Thermal energy storage (TES) for industrial waste heat (IWH) recovery: A review. Appl. Energy 2016, 179, 284–301. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A review of promising candidate reactions for chemical heat storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Ding, Y.; Riffat, S.B. Thermochemical energy storage technologies for building applications: A state-of-the-art review. Int. J. Low Carbon Technol. 2013, 8, 116. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Sögütoglu, L.C.; Huinink, H.P.; Fischer, H.R.; Adan, O.C.G. A review of salt hydrates for seasonal heat storage in domestic applications. Appl. Energy 2017, 199, 68. [Google Scholar] [CrossRef]
- Hua, W.; Yan, H.; Zhang, X.; Xu, X.; Zhang, L.; Shi, Y. Review of salt hydrates-based thermochemical adsorption thermal storage technologies. J. Energy Storage 2022, 56, 106158. [Google Scholar] [CrossRef]
- Dixit, P.; Reddy, V.J.; Parvate, S.; Balwani, A.; Singh, J.; Maiti, T.K.; Dasari, A.; Chattopadhyay, S. Salt hydrate phase change materials: Current state of art and the road ahead. J. Energy Storage 2022, 51, 104360. [Google Scholar] [CrossRef]
- Du, R.; Wu, M.; Wang, S.; Wu, S.; Wang, R.; Li, T. Experimental investigation on high energy-density and power-density hydrated salt-based thermal energy storage. Appl. Energy 2022, 325, 119870. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Martorell, I.; Miró, L.; Fernández, A.I.; Barreneche, C. Introduction to Thermal Energy Storage (TES) Systems; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; p. 28. [Google Scholar] [CrossRef]
- Linder, M. Using thermochemical reactions in thermal energy storage systems. In Advances in Thermal Energy Storage Systems; Woodhead Publishing: Sawston, UK, 2020; p. 495. [Google Scholar] [CrossRef]
- van Essen, V.M.; Cot Gores, J.; Bleijendaal, L.P.J.; Zondag, H.A.; Schuitema, R.; Bakker, M.; van Helden, W.G.J. Characterization of Salt Hydrates for Compact Seasonal Thermochemical Storage. In Proceedings of the ASME 2009 3rd International Conference on Energy Sustainability collocated with the Heat Transfer and InterPACK09 Conferences, San Francisco, CA, USA, 19–23 July 2009; pp. 825–830. [Google Scholar]
- Solé, A.; Martorell, I.; Cabeza, L.F. State of the art on gas–solid thermochemical energy storage systems and reactors for building applications. Renew. Sustain. Energy Rev. 2015, 47, 386–398. [Google Scholar] [CrossRef]
- Deutsch, M.; Müller, D.; Aumeyr, C.; Jordan, C.; Gierl-Mayer, C.; Weinberger, P.; Winter, F.; Werner, A. Systematic search algorithm for potential thermochemical energy storage systems. Appl. Energy 2016, 183, 120. [Google Scholar] [CrossRef]
- Knoll, C.; Müller, D.; Artner, W.; Welch, J.M.; Werner, A.; Harasek, M.; Weinberger, P. Probing cycle stability and reversibility in thermochemical energy storage—CaC2O4·H2O as perfect match? Appl. Energy 2017, 187, 1–9. [Google Scholar] [CrossRef]
- Garofalo, L.; Vitiello, F.V.; Montagnaro, F.; Bürgmayr, H.; Winter, F. Salt Hydrates for Thermochemical Storage of Solar Energy: Modeling the Case Study of Calcium Oxalate Monohydrate Dehydration/Rehydration under Suspension Reactor Conditions. Ind. Eng. Chem. Res. 2021, 60, 11357–11372. [Google Scholar] [CrossRef]
- Kazuo, M. Thermal behavior of alkaline earth metal malonate hydrates and their anhydrides. Thermochim. Acta 1996, 286, 198. [Google Scholar] [CrossRef]
- Galwey, A.K.; Abdel Aziz Mohamed, M. Thermal decomposition of calcium malonate dihydrate. Solid State Ion. 1990, 42, 135–145. [Google Scholar] [CrossRef]
- Brusau, E.V.; Narda, G.E.; Pedregosa, J.C.; Varetti, E.L. A low temperature infrared study of the coordinated water in calcium malonate dihydrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2002, 58, 1769–1774. [Google Scholar] [CrossRef]
- Christy, D.S.; Mahadevan, C.K.; Shajan, X.S. Growth by free evaporation method and physico—Chemical properties of calcium succinate single crystals. Optik 2017, 145, 418–427. [Google Scholar] [CrossRef]
- Binitha, M.P.; Pradyumnan, P.P. Studies on growth, thermal and dielectric behavior of calcium succinate trihydrate single crystals. J. Cryst. Growth 2014, 396, 44. [Google Scholar] [CrossRef]
- Li, Y.; Yi, H.; Ge, M.; Yao, D. Scale-Up Synthesis of High Purity Calcium Terephthalate from Polyethylene Terephthalate Waste: Purification, Characterization, and Quantification. Macromol. Mater. Eng. 2021, 306, 2100591. [Google Scholar] [CrossRef]
- Mazaj, M.; Mali, G.; Rangus, M.; Žunkovič, E.; Kaučič, V.; Zabukovec Logar, N. Spectroscopic Studies of Structural Dynamics Induced by Heating and Hydration: A Case of Calcium-Terephthalate Metal–Organic Framework. J. Phys. Chem. C 2013, 117, 7552–7564. [Google Scholar] [CrossRef]
- Groeneman, R.H.; Atwood, J.L. Terephthlate bridged coordination polymers based upon group two metals. Cryst. Eng. 1999, 2, 249. [Google Scholar] [CrossRef]
- Dominici, F.; Sarasini, F.; Luzi, F.; Torre, L.; Puglia, D. Thermomechanical and morphological properties of poly(ethylene terephthalate)/anhydrous calcium terephthalate nanocomposites. Polymers 2020, 12, 276. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Dermanaki Farahani, S. Experimental design optimization and isotherm modeling for removal of copper(II) by calcium-terephthalate MOF synthesized from recycled PET waste. J. Chemom. 2023, 37, e3396. [Google Scholar] [CrossRef]
- Mazaj, M.; Zabukovec Logar, N. Phase Formation Study of Ca-Terephthalate MOF-Type Materials. Cryst. Growth Des. 2015, 15, 617–624. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Iitaka, Y. The crystal structure of calcium terephthalate trihydrate. Acta Crystallogr. Sect. B 1972, 28, 1977–1981. [Google Scholar] [CrossRef]
- Barsk, A.; Yazdani, M.R.; Kankkunen, A.; Seppälä, A. Exceptionally high energy storage density for seasonal thermochemical energy storage by encapsulation of calcium chloride into hydrophobic nanosilica capsules. Sol. Energy Mater. Sol. Cells 2023, 251, 112154. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Adan, O.C.G.; Smeulders, D.M.J. Hydration/Dehydration Processes in Stabilized CaCl2. In Poromechanics VI; American Society of Civil Engineers: Reston, VA, USA, 2017; pp. 656–663. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Beckert, S.; Pel, L.; Stallmach, F.; Steiger, M.; Adan, O.C.G. Water Transport in MgSO4·7H2O During Dehydration in View of Thermal Storage. J. Phys. Chem. C 2015, 119, 28711–28720. [Google Scholar] [CrossRef]
- Van Essen, V.; Zondag, H.; Gores, J.C.; Bleijendaal, L.; Bakker, M.; Schuitema, R.; Van Helden, W.; He, Z.; Rindt, C. Characterization of MgSO4 hydrate for thermochemical seasonal heat storage. J. Sol. Energy Eng. 2009, 131, 041014. [Google Scholar] [CrossRef]
- Whiting, G.; Grondin, D.; Bennici, S.; Auroux, A. Heats of water sorption studies on zeolite–MgSO4 composites as potential thermochemical heat storage materials. Sol. Energy Mater. Sol. Cells 2013, 112, 112–119. [Google Scholar] [CrossRef]
- Ferchaud, C.; Zondag, H.; Veldhuis, J.; De Boer, R. Study of the reversible water vapour sorption process of MgSO4.7H2O and MgCl2.6H2O under the conditions of seasonal solar heat storage. J. Phys. Conf. Ser. 2012, 395, 012069. [Google Scholar] [CrossRef]
- Clark, R.-J.; Farid, M. Hydration reaction kinetics of SrCl2 and SrCl2-cement composite material for thermochemical energy storage. Sol. Energy Mater. Sol. Cells 2021, 231, 111311. [Google Scholar] [CrossRef]
- Blijlevens, M.A.R.; Mazur, N.; Kooijman, W.; Fischer, H.R.; Huinink, H.P.; Meekes, H.; Vlieg, E. A study of the hydration and dehydration transitions of SrCl2 hydrates for use in heat storage. Sol. Energy Mater. Sol. Cells 2022, 242, 111770. [Google Scholar] [CrossRef]
- Al-Terkawi, A.-A.; Scholz, G.; Prinz, C.; Zimathies, A.; Emmerling, F.; Kemnitz, E. Hydrated and dehydrated Ca-coordination polymers based on benzene-dicarboxylates: Mechanochemical synthesis, structure refinement, and spectroscopic characterization. Electronic supplementary information (ESI) available: Powder X-ray diffraction data, ATR-infrared spectra, EXAFS spectra, EXAFS data, SEM images, adsorption isotherms, and crystal data. CCDC Four files containing the crystal parameters of compounds 1 (CCDC 1582672), (1-H2O) (CCDC 1582673), 2 (CCDC 1582675), and 3 (CCDC 1582674). CrystEngComm. 2018, 20, 946–961. [Google Scholar] [CrossRef]
- X-Area 1.31.175.0, LANA 2.6.2.0; STOE & Cie GmbH: Darmstadt, Germany, 2021.
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Für Krist.-Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Ben Chanaa, M.b.; Lallemant, M.; Bertrand, G. Exploration systematique de la cinetique de rehydratation d’un sel renversable. Exemple de la reaction CaC2O4(s) + H2O(g) → CaC2O4·H2O(s). Thermochim. Acta 1986, 97, 369–385. [Google Scholar] [CrossRef]
- Gaisford, S.; Kett, V.; Haines, P. Principles of Thermal Analysis and Calorimetry, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Watts, B.A.; Ruck, W.K.L. A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage. Appl. Energy 2014, 124, 1–16. [Google Scholar] [CrossRef]
- Dornberger-Schiff, K.; Grell-Niemann, H. On the theory of order-disorder (OD) structures. Acta Crystallogr. 1961, 14, 167–177. [Google Scholar] [CrossRef]
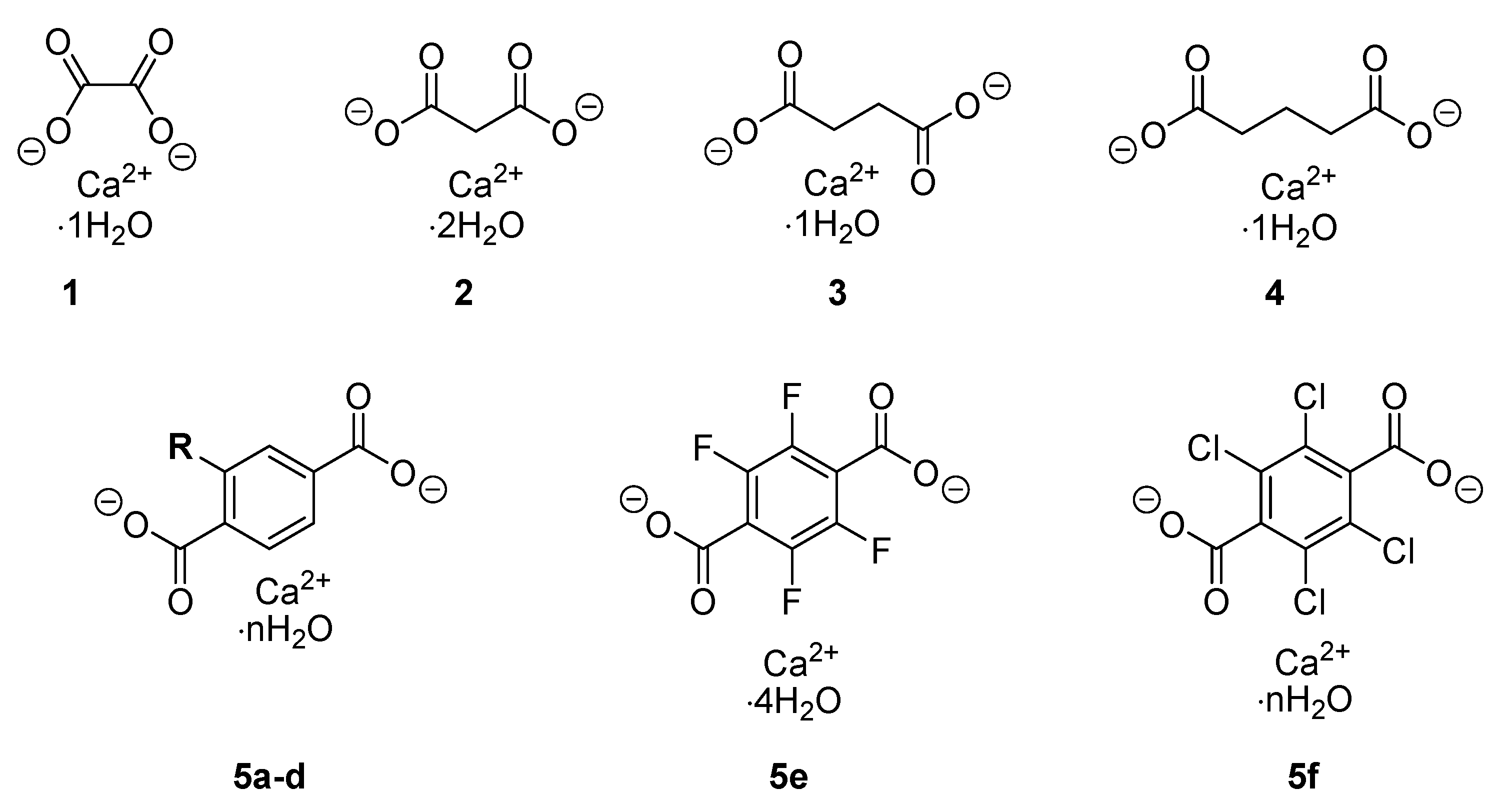

| Compound Number | Compound Name |
|---|---|
| 1 | Calcium oxalate monohydrate |
| 2 | Calcium malonate dihydrate |
| 3 | Calcium succinate monohydrate |
| 4 | Calcium glutarate monohydrate |
| 5a (R=H) | Calcium terephthalate trihydrate |
| 5b (R=CH3) | 2-methyl calcium terephthalate n-hydrate |
| 5c (R=F) | 2-fluoro calcium terephthalate trihydrate |
| 5d (R=Cl) | 2-chloro calcium terephthalate trihydrate |
| 5e | Tetrafluoro calcium terephthalate tetrahydrate |
| 5f | Tetrachloro calcium terephthalate n-hydrate |
| Substance | Theoretical Mass Loss in % | Observed Mass Loss in % | Onset Temperature of Dehydration in °C | ΔHdeh in J/g |
|---|---|---|---|---|
| Ca-oxalate monohydrate | 12.3 | 12.2 | 156 | 489 |
| Ca-malonate dihydrate | 20.2 | 17.9 | 127 | 637 |
| Ca-succinate monohydrate | 10.3 | 10.2 | 166 | 314 |
| Ca-glutarate monohydrate | 9.6 | 12.5 | 145 | 418 |
| Ca-terephthalate trihydrate | 20.9 | 20.1 | 104 | 695 |
| 2-methyl Ca-terephthalate n-hydrate | 15.7 | 15.8 | 93 | 220 |
| 2-fluoro Ca-terephthalate trihydrate | 19.5 | 15.6 | 126 | 483 |
| Tetrafluoro Ca-terephthalate tetrahydrate | 19.2 | 19.2 | 99 | 657 |
| TCM | Moles of Crystal Water | Moles of Water Lost in TGA | Δdehh | Δdehh/n H2O | Δhnet_25 |
|---|---|---|---|---|---|
| kJ/mol | kJ/mol H2O | kJ/mol | |||
| 1 | 1 | 0.99 | 71.33 | 72.20 | 27.87 |
| 2 | 2 | 1.72 | 110.26 | 64.10 | 34.59 |
| 3 | 1 | 0.98 | 54.60 | 55.46 | 11.29 |
| 4 | 1 | 1.35 | 81.36 | 60.29 | 21.99 |
| 5a | 3 | 2.85 | 177.52 | 62.26 | 52.09 |
| 5c | 3 * | 2.29 | 127.73 | 34.65 | 27.00 |
| 5e | 4 | 3.65 | 224.66 | 61.55 | 64.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, J.; Smith, J.; Stöger, B.; Artner, W.; Werner, A.; Weinberger, P. Characterization of Ca-Dicarboxylate Salt Hydrates as Thermochemical Energy Storage Materials. Crystals 2023, 13, 1518. https://doi.org/10.3390/cryst13101518
Werner J, Smith J, Stöger B, Artner W, Werner A, Weinberger P. Characterization of Ca-Dicarboxylate Salt Hydrates as Thermochemical Energy Storage Materials. Crystals. 2023; 13(10):1518. https://doi.org/10.3390/cryst13101518
Chicago/Turabian StyleWerner, Jakob, Jakob Smith, Berthold Stöger, Werner Artner, Andreas Werner, and Peter Weinberger. 2023. "Characterization of Ca-Dicarboxylate Salt Hydrates as Thermochemical Energy Storage Materials" Crystals 13, no. 10: 1518. https://doi.org/10.3390/cryst13101518
APA StyleWerner, J., Smith, J., Stöger, B., Artner, W., Werner, A., & Weinberger, P. (2023). Characterization of Ca-Dicarboxylate Salt Hydrates as Thermochemical Energy Storage Materials. Crystals, 13(10), 1518. https://doi.org/10.3390/cryst13101518







