Abstract
The tensile creep of Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) was tested at 150–250 °C and 125–350 MPa, and the effect of Ag on the high-temperature creep of Al-Cu-Mg alloys was discussed. After the addition of Ag, the high-temperature creep performances of the alloy were significantly improved at 150 °C/300 MPa and 200 °C/(150 MPa, 175 MPa). Then, constitutive relational models of the alloy during high-temperature creep were built, and the activation energy was calculated to be 136.65 and 104.06 KJ/mol. Based on the thermal deformation mechanism maps, the high-temperature creep mechanism of the alloy was predicted. After the addition of Ag, the creep mechanism of the alloy at 150 °C transitioned from lattice diffusion control to grain boundary diffusion control. At 250 °C, the mechanism was still controlled by grain boundary slip, but as the stress index increased and after Ag was added, the alloy fractures lead to the formation of dimples, thus improving the high-temperature creep performance.
1. Introduction
Heat-resistant 2xxx Al alloys (e.g., 2124, 2219 and 2618 alloys) are extensively applied in aerospace, due to their upper lightness and high heat resistance [1,2,3,4]. As the design requirements for aerospace and aircraft are progressively increased, the required operation temperature of Al alloys is increased accordingly. For this reason, researchers have devoted their energies to improving the heat resistance of Al-Cu-Mg Al alloys.
As for the design of material composition, the second phase in the balanced Al-Cu-Mg alloys mainly consists of θ, S and T phases [5]. The precipitate-phase precipitation series can be altered by regulating the Cu/Mg ratio of Al-Cu-Mg Al alloys and through ageing treatment. In other words, at a Cu/Mg ratio of >8, the main intensified phase is the θ’ phase. At a Cu/Mg ratio of 4–8, the main intensified phases are the θ’ and S’ phases. The main intensified phase is the S’ phase at a Cu/Mg ratio of 1.5–4. The coherent or semicoherent precipitate phases formed above can more effectively improve the alloy strength, and the S’ phase can strengthen the heat resistance of alloys. However, when the operation temperature is above 150 °C, the mechanical properties of the alloys are significantly weakened, due to the coarsening of intensified phases, which are unable to meet the requirements for key components in aerospace aircraft. For this reason, Ag is added to high-Cu/Mg-ratio Al-Cu-Mg alloys, in order to alter the ageing precipitate series. As a result, the Ω phase—which is consistently below 200 °C and does not aggregate or grow upwards—in minimally sized and dispersed distribution is dispersed, thus improving the room-temperature or high-temperature strength of the alloys and increasing their thermal stability [6,7]. As for research on the creep behaviors of aged alloys, the existing findings on the Ag and Mg distributions of aged Al-Cu-Mg-Ag alloys are listed in Table 1 [8,9,10,11,12,13]. As has previously been reported, Al-Cu-Mg-Ag alloys exhibit outstanding creep performance at high temperature, and the steady-state creep rate at 125 °C/265 MPa is 1.6 × 10−5 h−1, which is lower than the 2.8 × 10−5 h−1 of Al-Cu-Mg alloys at the same conditions [14,15,16]. The steady-state creep rate of Al-Cu-Mg alloys at 150 °C/265 MPa is 3.9 × 10−4 h−1, which is higher than the 1.3 × 10−4 h−1 of Al-Cu-Mg-Ag alloys. The creep performances of Al-Cu-Mg-Ag alloys after different ageing treatments have been studied. The steady-state creep rate of under-aged alloys (185 °C, 2 h) was 3.5 × 10−10 s−1, and that of peak-aged alloys (185 °C, 10 h) was 1.12 × 10−9 s−1. The above results indicate that composition and ageing treatment critically affect the creep properties of alloys.

Table 1.
Distribution of Mg and Ag atoms in Al-Cu-Mg-Ag alloys after ageing [8,9,10,11,12,13].
Generally, the creep behaviors of metal materials under service conditions decide the service life of alloys [17], but the existing literature on the structural performance control of Al-Cu-Mg alloys has focused primarily on three aspects [18,19,20,21,22,23,24,25,26]: (1) effect of Ag on microstructures of alloys; (2) effect of ageing on room-temperature or high-temperature mechanical properties of alloys; (3) creep behaviors under deformation and thermal treatment cooperative control. However, thus far, no researcher has added rare earth elements, Sc or Ag, to Al-Cu-Mg ternary alloys to prepare peak-aged structures after ageing treatment, and studied the creep behaviors of this peak-aged alloy system. According to the analysis of existing research results, it is not sufficient to improve the high-temperature creep performance of an Al-Cu-Mg alloy by adjusting its composition alone and combining it with heat treatment methods. If a small amount of Zr, Sc and Ag are added on the basis of the alloy system, combined with aging treatment to give full reign to the peak-aging effect, the high-temperature mechanical properties and high-temperature creep properties of the alloy can be significantly improved. In this study, Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) alloy has been prepared, and the microstructures and mechanical properties of peak-aged alloys after ageing treatment have been obtained. In particular, the effects of Ag on the creep properties of peak-aged alloys at different temperatures and stresses were explored. Constitutive relational models of creep were also built, and the deformation mechanism map involving dislocation quantity was plotted. Together with microstructure characterization, the high-temperature creep fracture mechanism of Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) was uncovered. Thereby, the findings provide a reliable theoretical basis for the practical aerospace applications of this alloy system.
2. Materials and Methods
High-purity Al, Mg, Sc and Ag of industrial purity, and Al-Cu, Al-Mn and Al-Zr intermediate alloys were used to prepare Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) alloy cast ingots (wt%) via cast-ingot metallurgy. The composition of the alloy was listed in Table 2. Then, the cast ingots were homogenized at 500 °C for 24 h, and then extruded at 430 °C into bars in diameter of 20 mm. The bars were subjected to solution treatment and ageing in an SX-4-10-box-type resistance furnace. The solution treatment was conducted at 510 °C, with heat preservation for 2 h followed by water-cooling. The ageing treatment was conducted at 180 °C for 2–10 h, followed by air cooling. The peak-aged Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) alloy under extruded deformation was subjected to static tensile creep experiments under constant load in an SRD-100-microcomputer-controlled electronic creep tester. The experiment conditions were 150 °C/300 MPa, 150 °C/325 MPa, 150 °C/350 MPa; 200 °C/150 MPa, 200 °C/175 MPa, 200 °C/200 MPa; 250 °C/100 MPa, 250 °C/125 MPa, 250 °C/150 MPa. All static tensile creep experiments were conducted until the specimens fractured. The corresponding time of fracture upon creep was considered as the creep fracture life of the alloy. The microstructures of the Al alloys after different thermal treatments were observed and analyzed under an Axio Observer A1m optical microscope, and the corrosion reagent was 2.5%HNO3 + 1.5%HCl + 0.5%HF. The microstructures of the alloys in the creep deformation zone after different thermal treatments were observed under a JEM-2100 transmission electron microscope (TEM). The electrolytic solution was 30%HNO3 + 70%CH3OH and controlled around −30 °C, and the voltage was 21 V. The fracture morphology of the creep-fractured samples was observed and analyzed with an S-3400N scanning electron microscope (SEM).

Table 2.
Chemical composition of alloys (wt.%).
3. Results
3.1. Effects of Ageing on Mechanical Properties of Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) Alloy
Figure 1 shows the mechanical performance curves of Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) after certain duration of ageing. Clearly, after certain duration of ageing treatment, the room-temperature tensile strength of Al-5Cu-0.8Mg-0.15Zr-0.2Sc was enhanced after the addition of Ag (Figure 1a), indicating that Ag can effectively improve the ageing strengthening effect on alloys. After certain ageing time, the above alloys showed an evident ageing strengthening effect, which was mainly divided into three stages: under-ageing, peak ageing, and over-ageing. At the under-ageing stage, the tensile strength was enhanced with the prolonged ageing time, and peak ageing occurred at 8th h when the tensile strength was maximized to 468.2 MPa (Al-5Cu-0.8Mg-0.15Zr-0.2Sc-0.5Ag) and 455.2 MPa (Al-5Cu-0.8Mg-0.15Zr-0.2Sc). At the over-ageing stage, the tensile strength was slightly weakened with the prolonged ageing time. The elongation at the break of the under-aged Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) was substantial, but the elongation at the break of the over-aged alloy was significantly weakened (Figure 1b).
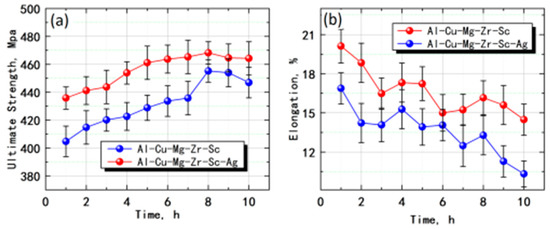
Figure 1.
Effects of ageing time on room temperature mechanical properties of Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) (a) tensile strength; (b) elongation at break.
Figure 2 shows the microstructure of an Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) alloy in under-aged (4 h), peak-aged (8 h) and over-aged (10 h) states. It can be seen from the figure that the precipitate phase exists both inside the grain and at the grain boundaries of the Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) alloy in the solution and aging state. At the same aging time, the grains of the two alloys are equiaxed, and the grain size of Al-5Cu-0.8Mg-0.15Zr-0.2Sc-0.5Ag alloys is relatively small. The intercept method can be used to calculate the average grain size under different holding times in Figure 2, which is about 15 μm–22 μm. The grain size first increases then decreases with the increase in temperature, and the amount of precipitate phase in the alloy is significantly higher than that of Al-5Cu-0.8Mg-0.15Zr-0.2Sc alloy. At peak aging (8 h), the precipitate phase quantity of both alloys showed an increasing trend, and at over-aging (10 h), the precipitate phase quantity of both alloys showed a decreasing trend. The TEM electron microscopic analysis was performed on the precipitate phase in Figure 2, as shown in Figure 3.
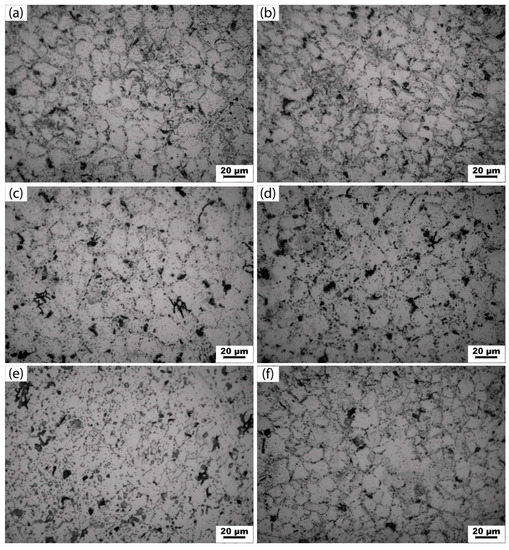
Figure 2.
Microstructures of Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) alloys subjected to aging treatment for various durations (a,c,e): Al-5Cu-0.8Mg-0.15Zr-0.2Sc at 4, 8, 10 h; (b,d,f) Al-5Cu-0.8Mg-0.15Zr-0.2Sc-0.5Ag at 4, 8, 10 h.
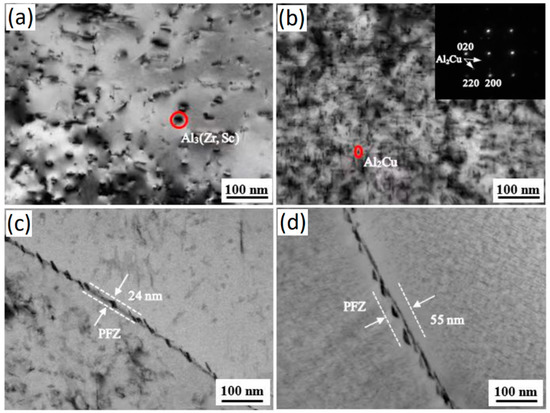
Figure 3.
TEM microstructure images of Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) after 8 h of ageing. (a,b) Intracrystalline precipitate phase of Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag); (c,d) no precipitate band on crystal boundary of Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag).
It can be seen from the figure that a large amount of precipitate phase was produced in the grain of the peak-aged Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) alloy. According to the characterization results of Figure 3a,b, the precipitate phase with a rod-like shape is θ phase (CuAl2). The precipitate phase is an Al3Sc phase, and no S phase (CuMgAl2) was found. The Al2Cu phase preferentially aggregates at the grain boundaries, which is consistent with the results obtained in the studies of Al-Cu-Mg-Ag series alloys [24,25,26,27,28]. During the aging process, in addition to the formation of Al2Cu precipitates inside the grains, there are also intermittent distributions of precipitates at the grain boundaries, and precipitation-free zones (PFZ) appear near the grain boundaries, as shown in Figure 3c,d. The addition of the Ag element slightly increased the size of the precipitate phase at the grain boundaries of Al-5Cu-0.8Mg-0.15Zr-0.2Sc alloy, and the width of the precipitated band at the grain boundary decrease. Therefore, the peak aging state Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) alloy with good comprehensive mechanical properties was selected to study the creep behavior at high temperatures.
3.2. Effects of Ag on Tensile Creep Performance of Peak-Aged Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag)
Figure 4 demonstrates the tensile creep curves of peak-aged Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) at 150, 200, 250 °C and external stresses of 300, 325, 350 MPa. After the addition of Ag, the creep life of the alloys was prolonged (Figure 4a) and in particular, the creep life was significantly extended at the external stress of 300 or 325 MPa. This was because after Ag was added into Al-5Cu-0.8Mg-0.2Sc-0.15Zr, the content of the Ω phase (Al2Cu) rose in the peak ageing stage achieved after long-time ageing, and the alloy showed high thermal stability. The second-phase strengthening effect was significant during the creep, but hindered dislocation motion, forming a dislocation pile-up cluster that prevented creep. As a result, the creep speed was decelerated, and the stable creep stage was extended, thus prolonging the creep life of the alloys. When the external stress was increased to 350 MPa, which was large, the dislocation motion was aggravated, and the dislocations were easily removed from the constraint by the second phase. As a result, the creep rate gradually increased and the steady-state creep stage was significantly shortened, so the creep life was not significantly prolonged. Figure 4b demonstrates the tensile creep curves of peak-aged Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) at 200 °C and different external stresses. Clearly, the creep life was significantly prolonged after the addition of Ag, and the creep life of Al-5Cu-0.8Mg-0.2Sc-0.15Zr-0.5Ag at 200 °C/200MPa was longer than that of Al-5Cu-0.8Mg-0.2Sc-0.15Zr at 200 °C/150 MPa. This was because with the temperature rise, the atom and cavity diffusion speeds were accelerated, which facilitated dislocation motion and creep deformation. With the presence of Ag, the peak-aged alloys separated out abundant Ω phase, which pinned and inhibited the dislocations. At the early creep stage, the dislocation motion was intense, and the Ω phase hindered dislocation motion, thus decelerating creep speed. At the steady-state creep stage, the Ω phase grew upwards, but this process depended on the diffusion and redistribution of Cu, Mg and Ag. At 200 °C, however, the above atom diffusion and redistribution were limited, so the Ω phase growing speed was slow, which thereby pinned the dislocation and crystal boundaries and inhibited the increase in steady-state creep speed. Figure 4c shows the tensile creep curves of peak-aged Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) at 250 °C and external stresses of 100, 125 and 150 MPa. Clearly, the presence of Ag prolonged the steady-state creep stage and extended the creep life of the alloys to an extent. In comparison with 150 or 200 °C, however, the creep life of alloys was significantly shortened after the addition of Ag. The reason for this was that although the newly added Ag formed abundant thermally stable Ω phase, the Ω phase grew upwards after the temperature rise, so the second phase intensifying was weakened. Moreover, dislocation motion was intensified. These changes contributed to creep. Hence, at this temperature, the effect of Ag on prolonging the creep life of alloys was limited.
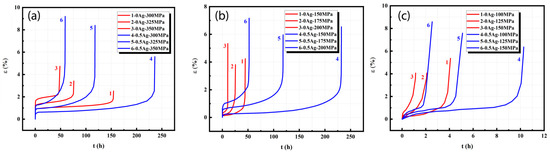
Figure 4.
Tensile creep curves of peak-aged Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) at different temperatures (a) 150 °C, (b) 200 °C, (c) 250 °C.
The high-temperature tensile creep curves of peak-aged Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) under different conditions at the steady-state creep stage were linearly fitted, and the steady-state creep speeds can be calculated thereby (Table 3).

Table 3.
Steady-state creep rates of peak-aged Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) under the same conditions.
At the same temperature, the steady-state creep speed of Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) rose in tandem with external stress, but that of the alloys increased alongside the creep temperature and the drop of external stress, and the alloys were very sensitive to temperature (Table 2). The creep properties of alloys before and after the addition of 0.5 Ag were compared. Clearly, the steady-state creep speed at 150 °C increased after the addition of Ag, but the increasing rate was significantly correlated with the increase in external stress. It was apparent that the creep properties of the alloys with added Ag were weakened by the decrease in external stress at 150 °C. The steady-state creep rate decreased at both 200 and 250 °C, but did not change significantly with the increment of applied stress. It may be that the addition of 0.5 Ag enhances the high-temperature creep properties of Al-5Cu-0.8Mg-0.2Sc-0.15Zr to an extent.
In all, Ag substantially prolonged the tensile creep life of peak-aged Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag), and this effect was significant under low-temperature and high-stress conditions (150 °C/300 MPa), as well as medium-temperature and low-stress conditions (200 °C/(150MPa, 175 MPa)).
3.3. Constitutive Relational Model of Alloy Creep
The peak-aged Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) had a substantial high-temperature creep resistance (Figure 4). Reportedly, steady-state creep speed is associated with creep temperature and stress [29]:
where is steady-state creep rate, T is creep temperature, σ is stress, A is constant, n is stress index, Q is activation energy, and R is mol gas constant. Equation (1) is mathematically transformed:
Based on the above equation, when the stress is invariable, the slope of the and 1/T curve is −Q/R. When the temperature is invariable, the slope on the curve of against lnσ is n and the intercept is lnA − (Q/(RT)). On this basis, the stress index n and constant A can be determined. Thus, the data on the high-temperature creep curve of peak-aged Al-5Cu-0.8Mg-0.2Sc-0.15Zr under different conditions in Figure 4 were chosen and fitted to obtain − 1/T and −lnσ curves (Figure 5). Furthermore, we determined that n = 5.97, A = 1.89 × 10−5, and Q = 136.65 kJ/mol.
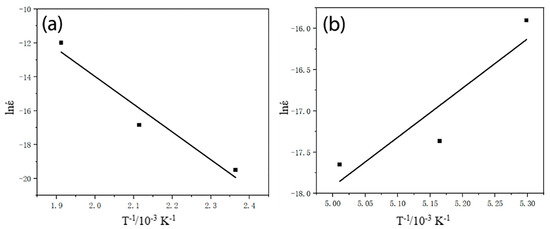
Figure 5.
Relation curve of high-temperature creep of Al-5Cu-0.8Mg-0.2Sc-0.15Zr (a) and 1/T curve; (b) −lnσ curve.
With the above method, we determined that n = 4.81, A = 4.27 × 10−5 and Q = 104.06 kJ/mol during the creep of Al-5Cu-0.8Mg-0.2Sc-0.15Zr-0.5Ag. Thus, at the steady-state creep stage of Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) at 150–250 °C, and 100–350 MPa, the relationships of creep speed with temperature and external stress can be obtained:
Constitutive relational model of Al-5Cu-0.8Mg-0.2Sc-0.15Zr:
έ = 1.89 × 10−5 σ5.97 exp(−136,650/RT),
Constitutive relational model of Al-5Cu-0.8Mg-0.2Sc-0.15Zr-0.5Ag:
έ = 4.27 × 10−5 σ4.81 exp(−104,060/RT),
3.4. Alloy Creep Life Equation and Prediction
Figure 6 shows the double-logarithm curves of steady-state creep rate and creep fracture life of Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) at 150, 200 and 250 °C. Clearly, the double-logarithm curves of creep fracture time and steady-state creep speed are linear, so the creep life meets the Monkman-Grant relation [30]:
where is steady-state creep speed, tf is time of fracture, CMG is constant (related to the material and testing temperature), β is the slope of the double-logarithm curve between creep life and steady-state creep speed. Then, the β and CMG were calculated under different creep conditions (Table 4). Based on the Monkman–Grant relational models, the creep life of Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) was predicted and compared with the testing results (Figure 7).
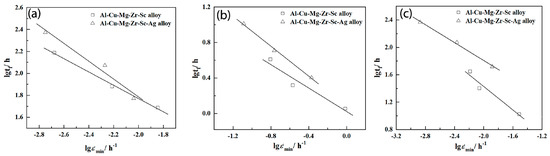
Figure 6.
Double-logarithm curve of creep life and steady-state creep rate of Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) (a) 150 °C; (b) 200 °C; (c) 250 °C.

Table 4.
The β, and CMG of Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) under different conditions.
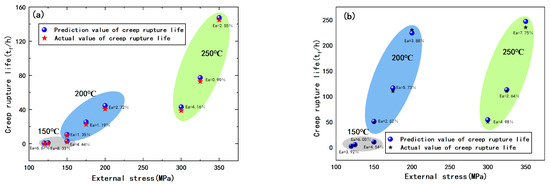
Figure 7.
Comparison between predicted value and actual value of creep life of (a) Al-5Cu-0.8Mg-0.2Sc-0.15Zr; (b) Al-5Cu-0.8Mg-0.2Sc-0.15Zr-0.5Ag.
The Monkman–Grant models can accurately predict the creep life of Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) at 150, 200 or 250 °C and under various external stresses (Figure 7). The maximum and minimum relative errors of the predicted results are 8.33% and 0.90%, respectively, indicating that the creep life models are applicable.
4. Discussion
Deformation mechanism maps, also called the phase maps of metal hot deformation, are theoretically based on the constitutive relational models based on diffusion control, the constitutive relational models based on grain boundary slide, and the constitutive relational models based on dislocation slide control. The high-temperature deformation mechanisms of metal materials all obey a velocity control equation:
where i is the steady-state strain rate; σi is stress; E is Young’s modulus; di is grain size, b is the Burgers vector; k is Boltzmann’s constant; D is the diffusion coefficient (including the grain boundary diffusion coefficient Dgb and the lattice diffusion coefficient DL); and Ai, n and P are material constants. The main parameters used in the deformation mechanism map plotting are listed in Table 5. Constitutive relational models ①–⑤, ②–⑤, ⑤–⑥, ⑥–⑦, ⑥–②, ②–③, ②–①, ②–④, ⑥–③, ⑦–③, ①–④ and ③–④ in Table 4 were all solved, and the coordinates (σ/E,d/b)i of the key points in the deformation mechanism maps were determined. The internal dislocation root count of single crystal grains can be computed as follows [31]:
where is the number of internal dislocation roots in the crystal grains, is Poisson’s ratio and τi is the shear stress (MPa), τi = 0.5σi. The ni was solved and marked at the intersection point on each deformation mechanism map. The above constitutive equations were solved. Then, RWS deformation mechanism maps were plotted on a plotting software with modulus compensation stress as X-axis, and grain size with Burgers vector compensation as Y-axis (Figure 8).

Table 5.
The main parameters of the deformation mechanism map for Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag).
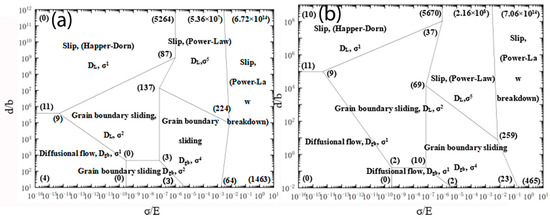
Figure 8.
Steady-state controlling deformation mechanism maps for aluminum alloy at different temperatures (a) 150 °C; (b) 250 °C.
The experimental data of creep of Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) under various conditions were analyzed to determine the grain size with Burgers vector compensation and to determine the stress with modulus compensation under different temperatures and different strain rates (Table 6).

Table 6.
Experimentally calculated data on creep of Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag).
The deformation mechanism of the creep of 5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) was deduced from Table 5 and Figure 8. At the deformation temperature of 150 °C, the high-temperature creep of Al-5Cu-0.8Mg-0.2Sc-0.15Zr fell within the dislocation polygon (137)(224)(64)(13)(3)(3), indicating that the creep mechanism of this alloy was a dislocation glide mechanism with a stress index of 4 and was controlled by grain boundary slip. At the deformation temperature of 250 °C, during deformation, this alloy fell within the dislocation polygon (2)(10)(2)(0), indicating that the creep mechanism at this temperature was a grain boundary slip system with a stress index of 1 and controlled by diffusion. At the deformation temperature of 150 °C, the high-temperature creep of Al-5Cu-0.8Mg-0.2Sc-0.15Zr-0.5Ag fell within the dislocation polygon (9)(87)(137)(3)(0), indicating that the creep mechanism of this alloy was a dislocation glide mechanism with a stress index of 2 and controlled by grain boundary sliding. At the deformation temperature of 250 °C, during deformation, this alloy fell within the dislocation polygon (10)(12)(23)(259)(69), indicating that the creep mechanism at this temperature was a grain boundary slip mechanism with a stress index of 4 and controlled by diffusion. It was observed that after the addition of Ag, the creep mechanism at 150 °C transitioned from lattice diffusion control to grain boundary diffusion control, but at 250 °C, the mechanism was controlled by grain boundary slip, and the stress index was observed to increase. It is apparent that the addition of Ag enhances the high-temperature creep properties of Al-5Cu-0.8Mg-0.2Sc-0.15Zr.
Figure 9 shows the creep fracture morphology of Al-5Cu-0.8Mg-0.2Sc-0.15Zr at 150 °C and at stresses of 300 or 350 MPa. The creep fractures of the alloy at 150 °C/300 MPa contained certain amounts of dimples and torn edges as well as a few shallow pores on the crystal boundary (Figure 9a,b). As the stress increased further to 350 MPa, the number of cavities at the crystal boundary of creep fractures also increased significantly, and the pores were aggregated. This was because, during creeping of aged alloys, as the stress was increased, the dislocations further reduced the constraint of the second-phase particles, so that the crystal boundaries were weakened and slip and torsion occurred more easily, leading to the formation of hollows. As a result, the number of hollows was increased. At the same time, as the creep proceeded, the small hollows gradually grew upwards, aggregated and expanded to form long strips and even fractures. Nevertheless, the fractures of this alloy under the above creep conditions were clearly ductile.
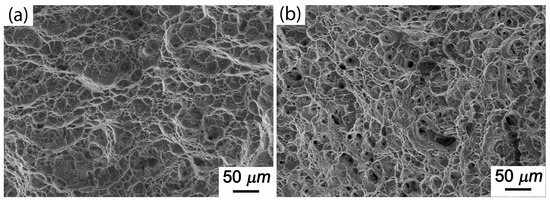
Figure 9.
Creep fracture morphology of Al-5Cu-0.8Mg-0.2Sc-0.15Zr under different stresses (150 °C): (a) 300 MPa; (b) 350 MPa.
Figure 10 shows the creep fracture morphology of Al-5Cu-0.8Mg-0.2Sc-0.15Zr-0.5Ag at 150 °C and under stress conditions of either 300 or 350 MPa. Clearly, the creep fractures of the alloy with added 0.5Ag were still ductile, and the number of dimples increased. When the external stress was at 350 MPa, the speed of pore aggregation, growth and expansion was reduced, which inhibited the expansion of holes near crystal boundaries. It was observed that the addition of 0.5Ag enhanced the creep property of alloys.
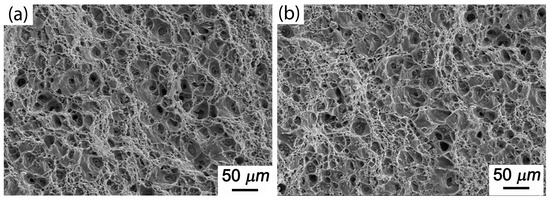
Figure 10.
Creep fracture morphology of Al-5Cu-0.8Mg-0.2Sc-0.15Zr-0.5Ag under different stresses (150 °C): (a) 300 MPa; (b) 350 MPa.
Figure 11 shows the creep fracture morphology of Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) at 200 °C and under stress conditions of 150 MPa. Clearly, after the addition of 0.5Ag, dimples increased notably in both number and depth, and were found to coexist with torn edges. This was because more precipitate phase was formed after the addition of Ag, which caused the dimples to form and be distributed evenly. Hence, the creep life of Al-5Cu-0.8Mg-0.2Sc-0.15Zr-0.5Ag was significantly prolonged under the above creep conditions.
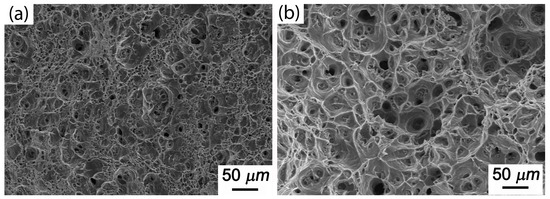
Figure 11.
Creep fracture morphology of Al-5Cu-0.8Mg-0.2Sc-0.15Zr-0.5Ag under different stresses (200 °C): (a) Al-5Cu-0.8Mg-0.2Sc-0.15Zr; (b) Al-5Cu-0.8Mg-0.2Sc-0.15Zr-0.5Ag.
5. Conclusions
The peak-aged Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) alloy, which had outstanding comprehensive mechanical properties, was tested under different conditions in terms of its high-temperature creep performance.
(1) Ag prolonged the high-temperature creep life of peak-aged Al-5Cu-0.8Mg-0.2Sc-0.15Zr, and this effect was significant under conditions of 150 °C/300 MPa or 200 °C/(150 MPa, 175 MPa).
(2) The stress indices of peak-aged Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) during high-temperature tensile creep were 5.97 and 4.81, and the activation energy was 136.65 and 104.06 KJ/mol. Accordingly, constitutive relational models between creep speed and creep temperature or stress were built:
Constitutive relational model of Al-5Cu-0.8Mg-0.2Sc-0.15Zr:
έ = 1.89 × 10−5 σ5.97 exp(−136,650/RT),
Constitutive relational model of Al-5Cu-0.8Mg-0.2Sc-0.15Zr-0.5Ag:
έ = 4.27 × 10−5 σ4.81 exp(−104,060/RT),
(3) Monkman–Grant creep life models of Al-5Cu-0.8Mg-0.2Sc-0.15Zr(-0.5Ag) were built and utilized in order to predict the creep life of the alloys at temperatures of 150, 200 and 250 °C, and under different stresses. The maximum and minimum relative errors of the predicted results are 8.33% and 0.90%, respectively, indicating that the models are applicable.
(4) RWS deformation mechanism maps involving dislocation quantity were plotted, and the high-temperature creep mechanism of Al-5Cu-0.8Mg-0.15Zr-0.2Sc(-0.5Ag) was predicted. At 150 °C, the creep mechanism, after the addition of Ag, transitioned from lattice diffusion control to grain boundary diffusion control, but at 250 °C, the mechanism was still controlled by grain boundary slip, and the stress index was observed to increase. After Ag was added, dimples were formed upon fracturing and tended to be evenly distributed, which effectively enhanced the high-temperature creep properties of the alloys.
Author Contributions
Investigation, Y.W. and G.Z.; conceptualization, F.L., X.C. and L.C.; formal analysis, Y.W., L.C. and G.Z.; writing—original draft preparation, Y.W. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Liaoning Provincial Science and Technology Department Applied Basic Research Program Project and the grant number [No. 2022JH2/101300078].
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sukumaran, K.; Ravikumar, K.K.; Pillai, S.G.K.; Rajan, T.P.D.; Ravi, M.; Pillai, R.M.; Pai, B.C. Studies on squeeze casting of Al 2124 alloy and 2124-10% SiCp metal matrix composite. Mater. Sci. Eng. A 2008, 490, 235–241. [Google Scholar] [CrossRef]
- Raju, P.N.; Rao, K.S.; Reddy, G.M.; Kamaraj, M.; Rao, K.P. Microstructure and high temperature stability of age hardenable AA2219 aluminium alloy modified by Sc, Mg and Zr additions. Mater. Sci. Eng. A 2007, 464, 192–201. [Google Scholar] [CrossRef]
- Wang, J.; Yi, D.; Su, X.; Yin, F. Influence of deformation ageing treatment on microstructure and properties of aluminum alloy 2618. Mater. Charact. 2008, 59, 965–968. [Google Scholar] [CrossRef]
- Yu, K.; Li, W.; Li, S.; Zhao, J. Mechanical properties and microstructure of aluminum alloy 2618 with Al3 (Sc, Zr) phases. Mater. Sci. Eng. A 2004, 368, 88–93. [Google Scholar] [CrossRef]
- Villars, P. Handbook of Ternary Alloy Phase Diagrams; ASM International: Detroit, MI, USA, 1995; Volume 7, pp. 8754–8755. [Google Scholar]
- Chang, C.H.; Lee, S.L.; Lin, J.C.; Yeh, M.S.; Jeng, R.R. Effect of Ag content and heat treatment on the stress corrosion cracking of Al–4.6 Cu–0.3 Mg alloy. Mater. Chem. Phys. 2005, 91, 454–462. [Google Scholar] [CrossRef]
- Xiao, D.H.; Wang, J.N.; Ding, D.Y.; Chen, S.P. Effect of Cu content on the mechanical properties of an Al–Cu–Mg–Ag alloy. J. Alloy. Compd. 2002, 343, 77–81. [Google Scholar] [CrossRef]
- Kerry, S.; Scott, V.D. Structure and orientation relationship of precipitates formed in Al-Cu-Mg-Ag alloys. Met. Sci. 1984, 18, 289–294. [Google Scholar] [CrossRef]
- Scott, V.D.; Kerry, S.; Trumper, R.L. Nucleation and growth of precipitates in Al–Cu–Mg–Ag alloys. Mater. Sci. Technol. 1987, 3, 827–835. [Google Scholar] [CrossRef]
- Rainforth, W.M.; Rylands, L.M.; Jones, H. Nano-beam analysis of {Omega} precipitates in a Al-Cu-Mg-Ag alloy. Scr. Mater. 1996, 35, 261–265. [Google Scholar] [CrossRef]
- Auld, J.H.; Cousland, S.M. On the structure of the M’phase in Al–Zn–Mg alloys. J. Appl. Crystallogr. 1985, 18, 47–48. [Google Scholar] [CrossRef]
- Sano, N.; Hono, K.; Sakurai, T.; Hirano, K. Atom-probe analysis of Ω and θ′ phases in an Al-Cu-Mg-Ag alloy. Scr. Metall. Et Mater. 1991, 25, 491–496. [Google Scholar] [CrossRef]
- Hutchinson, C.R.; Fan, X.; Pennycook, S.J.; Shiflet, G.J. On the origin of the high coarsening resistance of Ω plates in Al–Cu–Mg–Ag alloys. Acta Mater. 2001, 49, 2827–2841. [Google Scholar] [CrossRef]
- Bakavos, D.; Prangnell, P.B.; Bes, B.; Eberl, F. The effect of silver on microstructural evolution in two 2xxx series Al-alloys with a high Cu: Mg ratio during ageing to a T8 temper. Mater. Sci. Eng. A 2008, 491, 214–223. [Google Scholar] [CrossRef]
- Reddy, A.S. Fatigue and creep deformed microstructures of aged alloys based on Al–4% Cu–0.3% Mg. Mater. Des. 2008, 29, 763–768. [Google Scholar] [CrossRef]
- Lumley, R.N.; Morton, A.J.; Polmear, I.J. Enhanced creep performance in an Al–Cu–Mg–Ag alloy through underageing. Acta Mater. 2002, 50, 3597–3608. [Google Scholar] [CrossRef]
- Liu, M.T.; Tian, Y.; Wang, Y.; Wang, K.L.; Zhang, K.M.; Lu, S.Q. Critical Conditions for Dynamic Recrystallization of S280 Ultra-High-Strength Stainless Steel Based on Work Hardening Rate. Metals 2022, 12, 1122. [Google Scholar] [CrossRef]
- Lin, Y.C.; Jiang, Y.Q.; Xia, Y.C.; Zhang, X.C.; Zhou, H.M.; Deng, J. Effects of creep-aging processing on the corrosion resistance and mechanical properties of an Al–Cu–Mg alloy. Mater. Sci. Eng. A 2014, 605, 192–202. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C. Creep resistance of cast and aged Al–0.1 Zr and Al–0.1 Zr–0.1 Ti (at.%) alloys at 300–400 °C. Scr. Mater. 2008, 59, 387–390. [Google Scholar] [CrossRef]
- Xu, Y.; Zhan, L.; Xu, L.; Huang, M. Experimental research on creep aging behavior of Al-Cu-Mg alloy with tensile and compressive stresses. Mater. Sci. Eng. A 2017, 682, 54–62. [Google Scholar] [CrossRef]
- Xu, F.S.; Zhang, J.; Deng, Y.L.; Zhang, X.M. Precipitation orientation effect of 2124 aluminum alloy in creep aging. Trans. Nonferrous Met. Soc. China 2014, 24, 2067–2071. [Google Scholar] [CrossRef]
- Zhan, L.H.; Li, Y.G.; Huang, M.H. Effects of process parameters on mechanical properties and microstructures of creep aged 2124 aluminum alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 2232–2238. [Google Scholar] [CrossRef]
- Farkoosh, A.R.; Pekguleryuz, M. The effects of manganese on the Τ-phase and creep resistance in Al–Si–Cu–Mg–Ni alloys. Mater. Sci. Eng. A 2013, 582, 248–256. [Google Scholar] [CrossRef]
- Erdeniz, D.; Nasim, W.; Malik, J.; Yost, A.R.; Park, S.; De Luca, A.; Dunand, D.C. Effect of vanadium micro-alloying on the microstructural evolution and creep behavior of Al-Er-Sc-Zr-Si alloys. Acta Mater. 2017, 124, 501–512. [Google Scholar] [CrossRef]
- Song, Y.; Pan, Q.; Cao, S.; Wang, Y.; Li, C. Effects of Ag Content on Microstructures and Properties of Al-Cu-Mg Alloy. J. Aeronaut. Mater. 2013, 33, 7–13. [Google Scholar]
- Ringer, S.P.; Hono, K.; Polmear, I.J.; Sakurai, T. Nucleation of precipitates in aged AlCuMg (Ag) alloys with high Cu: Mg ratios. Acta Mater. 1996, 44, 1883–1898. [Google Scholar] [CrossRef]
- Gazizov, M.; Kaibyshev, R. High cyclic fatigue performance of Al–Cu–Mg–Ag alloy under T6 and T840 conditions. Trans. Nonferrous Met. Soc. China 2017, 27, 1215–1223. [Google Scholar] [CrossRef]
- Li, J.; An, Z.; Hage, F.S.; Wang, H.; Xie, P.; Jin, S.; Sha, G. Solute clustering and precipitation in an Al–Cu–Mg–Ag–Si model alloy. Mater. Sci. Eng. A 2019, 760, 366–376. [Google Scholar] [CrossRef]
- Yin, X.; Zhan, L.; Zhan, J. Establishment of steady creep constitutive equation of 2219 aluminum alloy. Chin. J. Nonferrous Met. 2014, 24, 7. [Google Scholar]
- Monkman, F.C. An empirical relationship between rupture life and minimum creep rate in creep-rupture tests. Proc. ASTM 1956, 56, 593–620. [Google Scholar]
- Liu, C.; Wang, X.; Zhou, G.; Li, F.; Zhang, S.; Zhang, H.; Liu, H. Dislocation-controlled low-temperature superplastic deformation of Ti-6Al-4V alloy. Front. Mater. 2021, 7, 606092. [Google Scholar] [CrossRef]
- Coble, R.L. A model for boundary diffusion controlled creep in polycrystalline materials. J. Appl. Phys. 1963, 34, 1679–1682. [Google Scholar] [CrossRef]
- Lüthy, H.; White, R.A.; Sherby, O.D. Grain boundary sliding and deformation mechanism maps. Mater. Sci. Eng. 1979, 39, 211–216. [Google Scholar] [CrossRef]
- Ruano, O.A.; Miller, A.K.; Sherby, O.D. The influence of pipe diffusion on the creep of fine-grained materials. Mater. Sci. Eng. 1981, 51, 9–16. [Google Scholar] [CrossRef]
- Harper, J.; Dorn, J.E. Viscous creep of aluminum near its melting temperature. Acta Metall. 1957, 5, 654–665. [Google Scholar] [CrossRef]
- Robinson, S.L.; Sherby, O.D. Mechanical behavior of polycrystalline tungsten at elevated temperature. Acta Metall. 1969, 17, 109–125. [Google Scholar] [CrossRef]
- Vaidya, M.; Pradeep, K.G.; Murty, B.S.; Wilde, G.; Divinski, S.V. Bulk tracer diffusion in CoCrFeNi and CoCrFeMnNi high entropy alloys. Acta Mater. 2018, 146, 211–224. [Google Scholar] [CrossRef]
- Fuentes-Samaniego, R.; Nix, W.D. Appropriate diffusion coefficients for describing creep processes in solid solution alloys. Scr. Metall. 1981, 15, 15–20. [Google Scholar] [CrossRef]
- Hasaka, M.; Morimura, T.; Uchiyama, Y.; Kondo, S.; Furuse, T.; Watanabe, T.; Hisatsune, K. Diffusion of copper, aluminum and boron in nickel. Scr. Metall. Mater. USA 1993, 29, 959–962. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).