Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
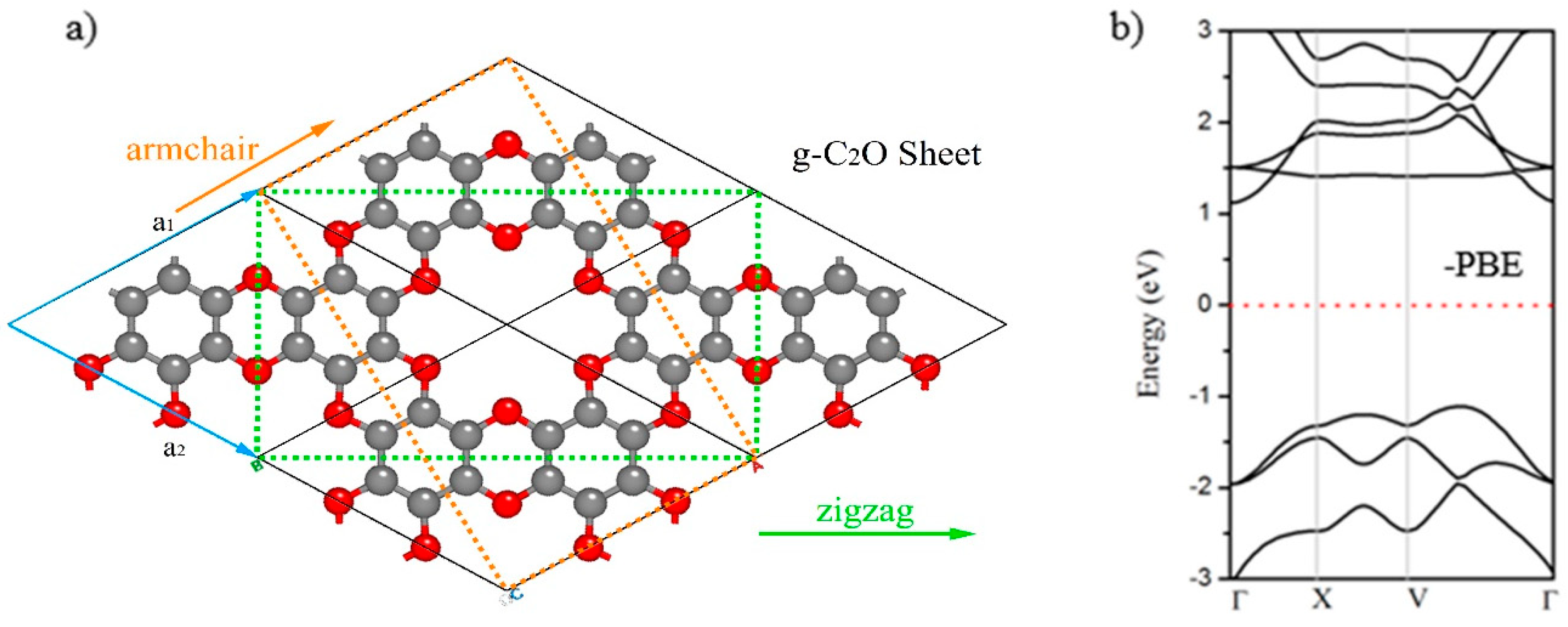
3.1. Electronic Properties
3.2. Thermoelectric Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Zhao, C.H.; Wu, G.F.; Chen, J.H.; Li, Y.Q. DFT study on the electronic structure and optical properties of N, Al, and N-Al doped graphene. Appl. Surf. Sci. 2018, 459, 354–362. [Google Scholar] [CrossRef]
- Feng, J.G.; Dong, H.Z.; Pang, B.L.; Shao, F.F.; Zhang, C.K.; Yu, L.Y.; Dong, L.F. Theoretical study on the optical and electronic properties of graphene quantum dots doped with heteroatoms. Phys. Chem. Chem. Phys. 2018, 20, 15244–15252. [Google Scholar] [CrossRef] [PubMed]
- Avouris, P. Graphene: Electronic and Photonic Properties and Devices. Nano Lett. 2010, 10, 4285–4294. [Google Scholar] [CrossRef]
- Alzebdeh, K.I. Asme, Evaluation of Effective Elastic Mechanical Properties of Graphene Sheets; Amer Soc Mechanical Engineers: New York, NY, USA, 2013; pp. 1205–1210. [Google Scholar]
- Bafekry, A.; Stampfl, C.; Ghergherehchi, M.; Shayesteh, S.F. A first-principles study of the effects of atom impurities, defects, strain, electric field and layer thickness on the electronic and magnetic properties of the C2N nanosheet. Carbon 2020, 157, 371–384. [Google Scholar] [CrossRef]
- Xu, B.; Xiang, H.; Wei, Q.; Liu, J.Q.; Xia, Y.D.; Yin, J.; Liu, Z.G. Two-dimensional graphene-like C2N: An experimentally available porous membrane for hydrogen purification. Phys. Chem. Chem. Phys. 2015, 17, 15115–15118. [Google Scholar] [CrossRef]
- Bafekry, A.; Stampfl, C.; Shayesteh, S.F. A First-Principles Study of C3N Nanostructures: Control and Engineering of the Electronic and Magnetic Properties of Nanosheets, Tubes and Ribbons. ChemPhysChem 2020, 12, 164–174. [Google Scholar] [CrossRef]
- Bafekry, A.; Shayesteh, S.F.; Peeters, F.M. C3N Monolayer: Exploring the Emerging of Novel Electronic and Magnetic Properties with Adatom Adsorption, Functionalizations, Electric Field, Charging, and Strain. J. Phys. Chem. C 2019, 123, 12485–12499. [Google Scholar] [CrossRef]
- Yu, T.; Hu, Z.M.; Wang, H.M.; Tan, X. Enhanced visible-light-driven hydrogen evolution of ultrathin narrow-band-gap g-C3N4 nanosheets. J. Mater. Sci. 2020, 55, 2118–2128. [Google Scholar] [CrossRef]
- Liu, X.Q.; Kang, W.; Zeng, W.; Zhang, Y.X.; Qi, L.; Ling, F.L.; Fang, L.; Chen, Q.; Zhou, M. Structural, electronic and photocatalytic properties of g-C3N4 with intrinsic defects: A first-principles hybrid functional investigation. Appl. Surf. Sci. 2020, 499, 6. [Google Scholar] [CrossRef]
- Yang, S.W.; Li, W.; Ye, C.C.; Wang, G.; Tian, H.; Zhu, C.; He, P.; Ding, G.Q.; Xie, X.M.; Liu, Y.; et al. C3N-A 2D Crystalline, Hole-Free, Tunable-Narrow-Bandgap Semiconductor with Ferromagnetic Properties. Adv. Mater. 2017, 29, 7. [Google Scholar]
- Luo, Z.Q.; Lim, S.H.; Tian, Z.Q.; Shang, J.Z.; Lai, L.F.; MacDonald, B.; Fu, C.; Shen, Z.X.; Yu, T.; Lin, J.Y. Pyridinic N doped graphene: Synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 2011, 21, 8038–8044. [Google Scholar] [CrossRef]
- Matar, S.F.; Weihrich, R. Strong p-magnetism in carbon suboxide C2O devised from first principles. Chem. Phys. Lett. 2017, 674, 115–119. [Google Scholar] [CrossRef]
- Prias-Barragan, J.J.; Gross, K.; Ariza-Calderon, H.; Prieto, P. Graphene oxide multilayers: Synthesis, properties and possible applications in electronics. In Proceedings of the 2019 Latin American Electron Devices Conference (LAEDC), Armenia, Colombia, 24–27 February 2019; IEEE: New York, NY, USA, 2019; pp. 61–64. [Google Scholar]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224, Erratum in Nat. Nanotechnol. 2010, 5, 309–309. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, G.H.; Li, Y.P.; Kong, L.J.; Liu, P.F. Structural Stability, Electronic and Magnetic Properties of O-doped Monolayer C2N. Chin. J. Struct. Chem. 2019, 38, 76–82. [Google Scholar]
- Liu, J.J.; Cheng, B. New understanding of photocatalytic properties of zigzag and armchair g-C3N4 nanotubes from electronic structures and carrier effective mass. Appl. Surf. Sci. 2018, 430, 348–354. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.Y.; Miao, M.S. Macrocycles inserted in graphene: From coordination chemistry on graphene to graphitic carbon oxide. Nanoscale 2016, 8, 17976–17983. [Google Scholar] [CrossRef]
- Qin, G.; Cui, Q.; Yun, B.; Sun, L.; Du, A.; Sun, Q. High capacity and reversible hydrogen storage on two dimensional C2N monolayer membrane. Int. J. Hydrog. Energy 2018, 43, 9895–9901. [Google Scholar] [CrossRef]
- Liu, X.F.; Chang, X.; Zhu, L.; Li, X.F. High-efficiency helium separation through g-C2O membrane: A theoretical study. Comput. Mater. Sci. 2019, 157, 1–5. [Google Scholar] [CrossRef]
- Nikkho, S.; Mirzaei, M.; Sabet, J.K.; Moosavian, M.A.; Hedayat, S.M. Enhanced quality of transfer-free graphene membrane for He/CH4 separation. Sep. Purif. Technol. 2020, 232, 9. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, C.M.; Wang, X.B.; Wang, C.; Fu, L. Density functional calculations of efficient H-2 separation from impurity gases (H2, N2, H2O, CO, Cl2, and CH4) via bilayer g-C3N4 membrane. Chin. Phys. B 2019, 28, 8. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, Q.Z.; Li, X.F.; Wu, T.T.; Jin, Y.K.; Xing, W. C2N: An excellent two-dimensional monolayer membrane for He separation. J. Mater. Chem. A 2015, 3, 21351–21356. [Google Scholar] [CrossRef]
- Wu, J.F.; Ding, S.Y.; Ye, S.H.; Lai, C. Grafting polymeric sulfur onto carbon nanotubes as highly-active cathode for lithium-sulfur batteries. J. Energy Chem. 2020, 42, 27–33. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.J.; Cho, J.Y.; Jeong, S.; Kim, H.Y.; Kim, J.H.; Seo, S.H.; Jeong, H.J.; Jeong, S.Y.; Lee, G.W.; et al. Highly Exfoliated and Functionalized Single-Walled Carbon Nanotubes as Fast-Charging, High-Capacity Cathodes for Rechargeable Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Fellah, M.F. A DFT study on Pt doped (4,0) SWCNT: CO adsorption and sensing. Appl. Surf. Sci. 2020, 504, 9. [Google Scholar] [CrossRef]
- Zeb, S.; Peng, X.J.; Yuan, G.Z.; Zhao, X.X.; Qin, C.Y.; Sun, G.X.; Nie, Y.; Cui, Y.; Jiang, X.C. Controllable synthesis of ultrathin WO3 nanotubes and nanowires with excellent gas sensing performance. Sens. Actuator B-Chem. 2020, 305, 12. [Google Scholar] [CrossRef]
- Park, K.R.; Cho, H.B.; Lee, J.; Song, Y.; Kim, W.B.; Choa, Y.H. Design of highly porous SnO2-CuO nanotubes for enhancing H2S gas sensor performance. Sens. Actuator B-Chem. 2020, 302, 7. [Google Scholar] [CrossRef]
- Hsu, J.H.; Yu, C. Sorting-free utilization of semiconducting carbon nanotubes for large thermoelectric responses. Nano Energy 2020, 67, 9. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, P.; Xue, J.M. Ti2CO2 Nanotubes with Negative Strain Energies and Tunable Band Gaps Predicted from First-Principles Calculations. J. Phys. Chem. Lett. 2016, 7, 5280–5284. [Google Scholar] [CrossRef]
- Jalil, A.; Agathopoulos, S.; Khan, N.Z.; Khan, S.A.; Kiani, M.; Khan, K.; Zhu, L. New physical insight in structural and electronic properties of InSb nano-sheet being rolled up into single-wall nanotubes. Appl. Surf. Sci. 2019, 487, 550–557. [Google Scholar] [CrossRef]
- Abbasi, A.; Sardroodi, J.J.; Ebrahimzadeh, A.R.; Yaghoobi, M. Theoretical study of the structural and electronic properties of novel stanene-based buckled nanotubes and their adsorption behaviors. Appl. Surf. Sci. 2018, 435, 733–742. [Google Scholar] [CrossRef]
- Abbasi, A.; Sardroodi, J.J. Interaction of sulfur trioxide molecules with armchair and zigzag stanene-based nanotubes: Electronic properties exploration by DFT calculations. Adsorpt. J. Int. Adsorpt. Soc. 2018, 24, 443–458. [Google Scholar] [CrossRef]
- Eslami, M.; Moradi, M.; Moradi, R. DFT investigation of hydrogen adsorption on the C3N nanotube. Vacuum 2016, 133, 7–12. [Google Scholar] [CrossRef]
- Tan, G.J.; Ohta, M.; Kanatzidis, M.G. Thermoelectric power generation: From new materials to devices. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2019, 377, 20180450. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Zhu, T.J.; Liu, Y.T.; Fu, C.G.; Heremans, J.P.; Snyder, J.G.; Zhao, X.B. Compromise and Synergy in High-Efficiency Thermoelectric Materials. Adv. Mater. 2017, 29, 1605884. [Google Scholar] [CrossRef]
- Sun, Y.; Di, C.A.; Xu, W.; Zhu, D.B. Advances in n-Type Organic Thermoelectric Materials and Devices. Adv. Electron. Mater. 2019, 5, 1800825. [Google Scholar] [CrossRef]
- Perumal, S.; Roychowdhury, S.; Biswas, K. Reduction of thermal conductivity through nanostructuring enhances the thermoelectric figure of merit in Ge1-xBixTe. Inorg. Chem. Front. 2016, 3, 125–132. [Google Scholar] [CrossRef]
- Jood, P.; Ohta, M.; Kunii, M.; Hu, X.K.; Nishiate, H.; Yamamoto, A.; Kanatzidis, M.G. Enhanced average thermoelectric figure of merit of n-type PbTe1-xIx-MgTe. J. Mater. Chem. C 2015, 3, 10401–10408. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.Y.; Duan, W.H. Thermal and Thermoelectric Properties of Graphene. Small 2014, 10, 2182–2199. [Google Scholar] [CrossRef] [PubMed]
- Avery, A.D.; Zhou, B.H.; Lee, J.; Lee, E.S.; Miller, E.M.; Ihly, R.; Wesenberg, D.; Mistry, K.S.; Guillot, S.L.; Zink, B.L.; et al. Tailored semiconducting carbon nanotube networks with enhanced thermoelectric properties. Nat. Energy 2016, 1, 16033. [Google Scholar] [CrossRef]
- Brownlie, L.; Shapter, J. Advances in carbon nanotube n-type doping: Methods, analysis and applications. Carbon 2018, 126, 257–270. [Google Scholar] [CrossRef]
- Elapolu, M.S.R.; Tabarraei, A.; Reihani, A.; Ramazani, A. Phononic thermal transport properties of C3N nanotubes. Nanotechnology 2020, 31, 035705. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, L. Giant reduction of thermal conductivity in a two-dimensional nitrogenated holey C2N nanosheet. Phys. Chem. Chem. Phys. 2017, 19, 1757–1761. [Google Scholar] [CrossRef] [PubMed]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef]
- Gopejenko, V.; Gopejenko, A. Using Applications and Tools to Visualize ab initio Calculations Performed in VASP. In Augmented Reality, Virtual Reality, and Computer Graphics, Avr 2018, Pt I; DePaolis, L.T., Bourdot, P., Eds.; Springer: Cham, Switzerland, 2018; Volume 10850, pp. 489–496. [Google Scholar]
- Hacene, M.; Anciaux-Sedrakian, A.; Rozanska, X.; Klahr, D.; Guignon, T.; Fleurat-Lessard, P. Accelerating VASP electronic structure calculations using graphic processing units. J. Comput. Chem. 2012, 33, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Hongzhiwei, Technology, Device Studio, Version 2021A, China. 2021. Available online: http://iresearch.net.cn/cloudSoftware (accessed on 1 March 2023.).
- Korth, M.; Thiel, W. Benchmarking Semiempirical Methods for Thermochemistry, Kinetics, and Noncovalent Interactions: OMx Methods Are Almost As Accurate and Robust As DFT-GGA Methods for Organic Molecules. J. Chem. Theory Comput. 2011, 7, 2929–2936. [Google Scholar] [CrossRef]
- Togo, A.; Oba, F.; Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 2008, 78, 134106. [Google Scholar] [CrossRef]
- Shuichi, N. Constant Temperature Molecular Dynamics Methods. Prog. Theor. Phys. 1991, 103, 1–46. [Google Scholar]
- Jiang, J.W.; Wang, J.S.; Li, B.W. A nonequilibrium Green’s function study of thermoelectric properties in single-walled carbon nanotubes. J. Appl. Phys. 2011, 109, 014326. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baseggio, O.; Bonfa, P.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; de Gironcoli, S.; Delugas, P.; Ruffino, F.F.; et al. Quantum ESPRESSO toward the exascale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef] [PubMed]
- Majidi, R.; Odelius, M.; AlTaha, S. Structural and electronic properties of nitrogenated holey nanotubes: A density functional theory study. Diam. Relat. Mat. 2018, 82, 96–101. [Google Scholar] [CrossRef]
- Alam, H.; Ramakrishna, S. A review on the enhancement of figure of merit from bulk to nano-thermoelectric materials. Nano Energy 2013, 2, 190–212. [Google Scholar] [CrossRef]

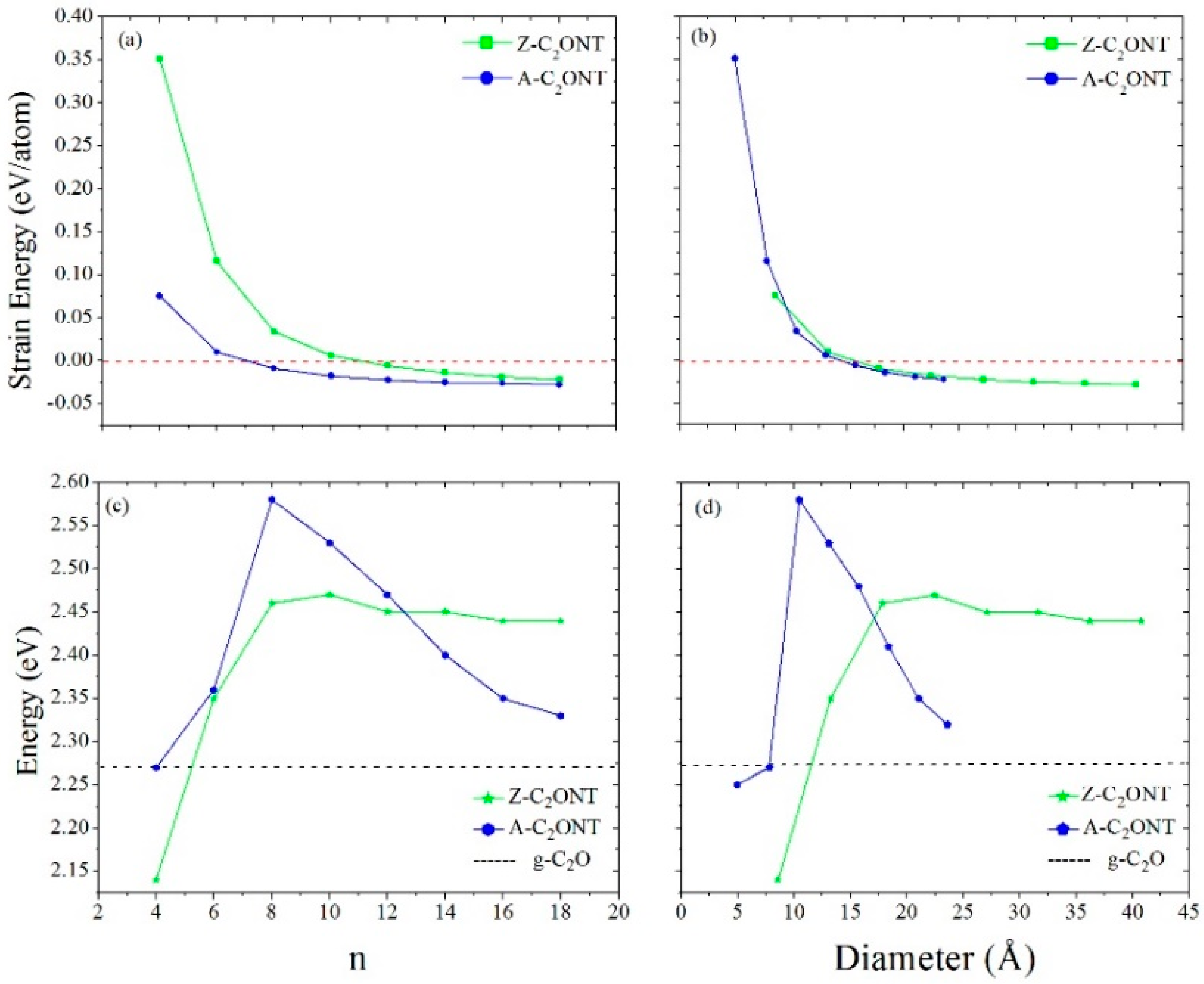



(meV/Unit Cell) | Diameter (Å) | |||
|---|---|---|---|---|
| Nanosheet | (0, 0) | - | - | 2.27 i |
| Zigzag (n, 0) | (2, 0) | 533.0 | 3.78 | 1.07 i |
| (4, 0) | 75.3 | 8.55 | 2.14 i | |
| (6, 0) | 9.9 | 13.24 | 2.35 i | |
| (8, 0) | −8.9 | 17.80 | 2.46 i | |
| (10, 0) | −17.5 | 22.45 | 2.47 i | |
| (12, 0) | −22.2 | 27.08 | 2.45 i | |
| (14, 0) | −25.0 | 31.62 | 2.45 i | |
| (16, 0) | −26.2 | 36.21 | 2.44 i | |
| (18, 0) | −27.9 | 40.75 | 2.44 i | |
| Armchair (n, n) | (2, 2) | 1911.8 | 2.69 | 0.41 d |
| (4, 4) | 351.1 | 4.96 | 2.25 d | |
| (6, 6) | 115.6 | 7.80 | 2.27 d | |
| (8, 8) | 34.0 | 10.45 | 2.58 d | |
| (10, 10) | 6.1 | 13.06 | 2.53 d | |
| (12, 12) | −5.6 | 15.71 | 2.48 d | |
| (14, 14) | −14.0 | 18.36 | 2.41 d | |
| (16, 16) | −18.9 | 21.04 | 2.35 d | |
| (18, 18) | −22.1 | 23.59 | 2.34 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Jiang, L.; Li, X.; Yin, Z.
Wu J, Jiang L, Li X, Yin Z.
Wu, Jianbao, Liyuan Jiang, Xiaoyi Li, and Zhixiang Yin.
2023. "
Wu, J., Jiang, L., Li, X., & Yin, Z.
(2023).







