A DFT Study and Hirshfeld Surface Analysis of the Molecular Structures, Radical Scavenging Abilities and ADMET Properties of 2-Methylthio(methylsulfonyl)-[1,2,4]triazolo [1,5-a]quinazolines: Guidance for Antioxidant Drug Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Single-Crystal X-ray Diffraction
2.2. Computational Methods
2.2.1. Hirshsfeld Surface Analysis
2.2.2. Density Functional Theory (DFT)
3. Results and Discussion
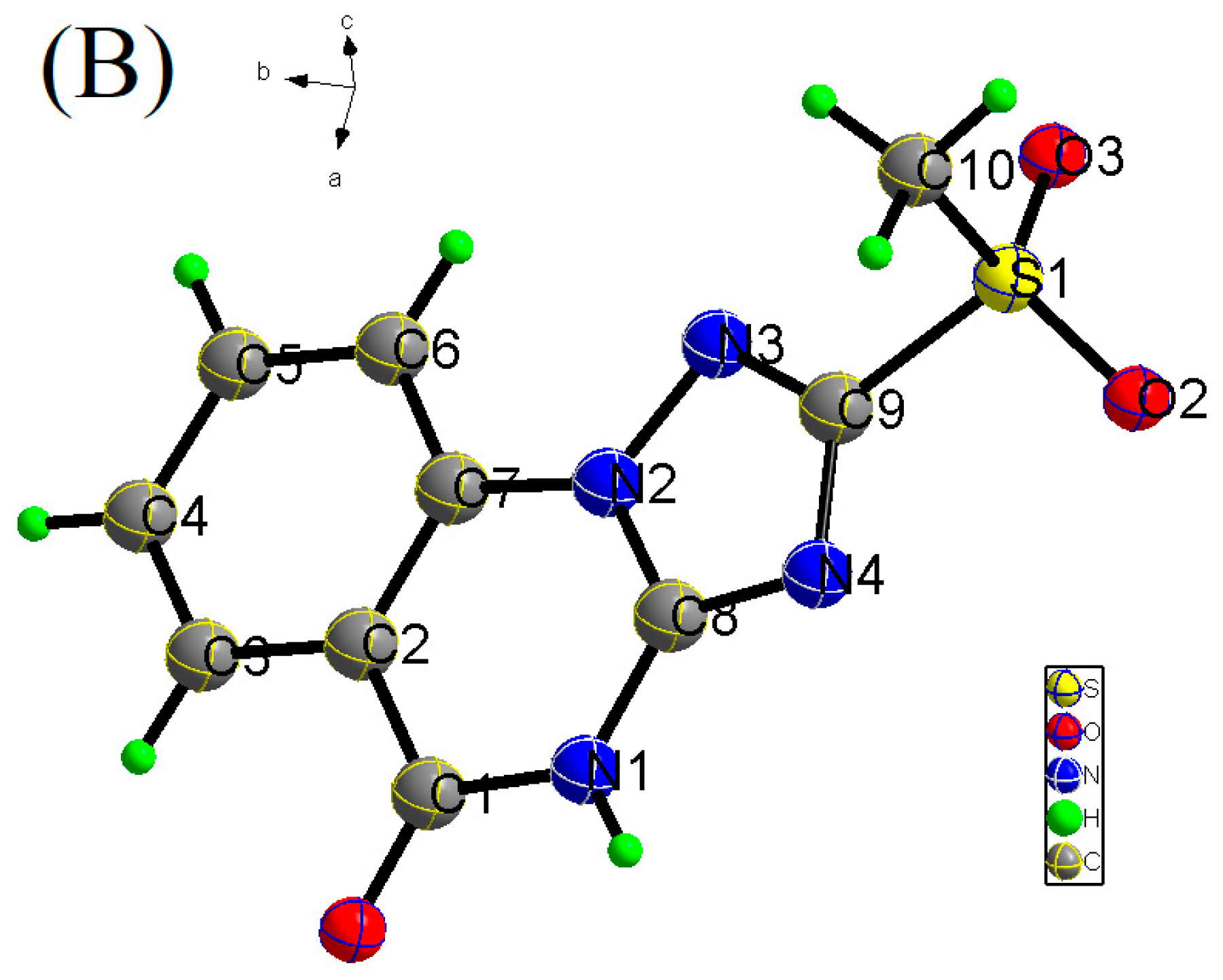
3.1. Exploration of the Crystal Structure of Investigated Targets
3.2. Molecular Geometries of Compounds 1 and 2
3.3. Intermolecular Interactions of the Compounds 1 and 2
3.4. Dimers’ Energetic and Structural Characterization Scenarios
3.5. Hirshfeld Surface Analysis
3.6. Topological Study of Non-Covalent Interactions: NCI-RDG and QTAIM Analyses
3.6.1. Hydrogen Bonds
3.6.2. Intermolecular Van Der Waals Interactions
3.7. Evaluation of Non-Covalent Interactions Using a Method Called Reduced Density Gradient Reduction
3.8. Chemical Reactivity Properties: A CDFT Reactivity Indices Analysis
3.8.1. Global Reactivity Indices
3.8.2. Local Reactivity Indices
3.9. Electrostatic Characteristics of Interactions Examined through Molecular Electrostatic Potential (MESP)
3.10. Antioxidant Reactivity Study
3.10.1. Radicalophilic Study
3.10.2. Electron Scavenging Activity
3.11. Mechanism Actions of the Antioxidants
3.12. Drug-Likeness Properties
3.13. The ADMET Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Salahi, R.; Al-Omar, M.A.; Alswaidan, I.; Marzouk, M.; El-Senousy, W.M.; Amr, A.E.-G.E. Antiviral activities of some synthesized methylsulfanyl triazoloquinazoline derivatives. Res. Chem. Intermed. 2015, 41, 151–161. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Al-Salahi, R. An overview of triazoloquinazolines: Pharmacological significance and recent developments. Bioorg. Chem. 2021, 115, 105263. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; El-Tahir, K.-E.; Alswaidan, I.; Lolak, N.; Hamidaddin, M.; Marzouk, M. Biological effects of a new set 1,2,4-triazolo [1,5-a]quinazolines on heart rate and blood pressure. Chem. Cent. J. 2014, 8, 3. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Gamal-Eldeen, A.M.; Alanazi, A.M.; Al-Omar, M.A.; Marzouk, M.A.; Fouda, M.M.G. Cytotoxic and anti-inflammatory active methylsulfanyltriazoloquinazolines. J. Pure Appl. Microbiol. 2013, 7, 189–198. [Google Scholar]
- Al-Salahi, R.; Alswaidan, I.A.; Marzouk, M.S.; El-Eraky, W.; Saleh, D. Antihistamine activity of 1,2,4-triazolo [1,5-a]quinazolines. Asian J. Chem. 2014, 26, 8617–8619. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Marzouk, S.M.; Ghabbour, A.H.; Kun, H.F. Synthesis of Novel 2-(Methylthio)benzo[g][1,2,4]triazolo[1, 5-a] quinazolin-5-(4H)-one and its Derivatives. Lett. Org. Chem. 2014, 11, 759–767. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Geffken, D. Synthesis and reactivity of [1,2,4]triazolo-annelated quinazolines. Molecules 2010, 15, 7016–7034, Erratum in Molecules 2010, 15, 8143. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Al-Omar, M.; Abbas, M.; Ng, S.W. 2-Methylsulfanyl-1,2,4-triazolo [1,5-a]quinazolin-5(4H)-one. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 6, o1805. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Marzouk, M. Synthesis of novel 2-amino-benzo[de]isoquinolin-1,3-dione derivatives. Asian J. Chem. 2014, 26, 2166–2172. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Marzouk, M.; Abbas, M.; Ng, S.W. 2-Methylsulfonyl-1, 2, 4-triazolo [1, 5-a] quinazolin-5 (4H)-one. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o1806. [Google Scholar] [CrossRef]
- Al-Salahi, R.A.; Marzouk, M.S. Synthesis of novel 2-phenoxybenzo[g][1,2,4]triazolo [1,5-a]quinazoline and its derivatives starting with diphenyl-N-cyanoimidocarbonate. Russ. J. Gen. Chem. 2016, 86, 1741–1746. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Anouar, E.H.; Marzouk, M.; Taie, H.A.A.; Abuelizz, H.A. Screening and evaluation of antioxidant activity of some 1,2,4-triazolo [1,5-a]quinazoline derivatives. Future Med. Chem. 2018, 10, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09W, Revision A. 02; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Jeng-Da, C.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar]
- Baur, W.H. Prediction of hydrogen bonds and hydrogen atom positions in crystalline solids. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 1456–1465. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F.J.M.P. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Bakheit, A.H.; Al-Salahi, R.; Al-Majed, A.A. Thermodynamic and Computational (DFT) Study of Non-Covalent Interaction Mechanisms of Charge Transfer Complex of Linagliptin with 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and Chloranilic acid (CHA). Molecules 2022, 27, 6320. [Google Scholar] [CrossRef] [PubMed]
- Bakheit, A.H.; Ghabbour, H.A.; Hussain, H.; Al-Salahi, R.; Ali, E.A.; Mostafa, G.A.E. Synthesis and Computational and X-ray Structure of 2, 3, 5-Triphenyl Tetrazolium, 5-Ethyl-5-phenylbarbituric Acid Salt. Crystals 2022, 12, 1706. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Jayatilaka, D.; Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Spackman, M.A. CrystalExplorer: A tool for displaying Hirshfeld surfaces and visualising intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. A Found. Crystallogr. 2006, 62, s90. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll, Version 19.10. 12; TK Gristmill Software: Overland Park, KS, USA, 2019. [Google Scholar]
- Abuelizz, H.A.; Taie, H.A.A.; Bakheit, A.H.; Marzouk, M.; Abdellatif, M.M.; Al-Salahi, R. Biological Evaluation of 4-(1H-triazol-1-yl) benzoic Acid Hybrids as Antioxidant Agents: In Vitro Screening and DFT Study. Appl. Sci. 2021, 11, 11642. [Google Scholar] [CrossRef]
- Ashfaq, M.; Tahir, M.N.; Muhammad, S.; Munawar, K.S.; Ali, A.; Bogdanov, G.; Alarfaji, S.S. Single-crystal investigation, Hirshfeld surface analysis, and DFT study of third-order NLO properties of unsymmetrical acyl thiourea derivatives. ACS Omega 2021, 6, 31211–31225. [Google Scholar] [CrossRef]
- Li, S.; Bu, R.; Gou, R.; Zhang, C. Hirshfeld surface method and its application in energetic crystals. Cryst. Growth Des. 2021, 21, 6619–6634. [Google Scholar] [CrossRef]
- Hajji, M.; Mtiraoui, H.; Amiri, N.; Msaddek, M.; Guerfel, T. Crystallographic and first-principles density functional theory study on the structure, noncovalent interactions, and chemical reactivity of 1, 5-benzodiazepin-2-ones derivatives. Int. J. Quantum Chem. 2019, 119, e26000. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Bakheit, A.H.; Marzouk, M.; Abdellatif, M.M.; Al-Salahi, R. Reactivity of 4, 5-Dichlorophthalic Anhydride towards Thiosemicarbazide and Amines: Synthesis, Spectroscopic Analysis, and DFT Study. Molecules 2022, 27, 3550. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.A.E.; Bakheit, A.H.; Al-Agamy, M.H.; Al-Salahi, R.; Ali, E.A.; Alrabiah, H. Synthesis of 4-Amino-N-[2 (diethylamino) Ethyl] Benzamide Tetraphenylborate Ion-Associate Complex: Characterization, Antibacterial and Computational Study. Molecules 2023, 28, 2256. [Google Scholar] [CrossRef] [PubMed]
- Lardy, S.W.; Luong, K.C.; Schmidt, V.A. Formal Aniline Synthesis from Phenols through Deoxygenative N-Centered Radical Substitution. Chem. Eur. J. 2019, 25, 15267–15271. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cifuentes, M.; Weiss-López, B.; Araya-Maturana, R. A computational study of structure and reactivity of N-substitued-4-piperidones curcumin analogues and their radical anions. Molecules 2016, 21, 1658. [Google Scholar] [CrossRef]
- Sarkar, A.; Middya, T.R.; Jana, A.D. A QSAR study of radical scavenging antioxidant activity of a series of flavonoids using DFT based quantum chemical descriptors–the importance of group frontier electron density. J. Mol. Model. 2012, 18, 2621–2631. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Eskandrani, R.; Al-Rasheed, L.S.; Ansari, S.A.; Bakheit, A.H.; Almehizia, A.A.; Almutairi, M.; Alkahtani, H.M. Targeting Transcriptional CDKs 7, 8, and 9 with Anilinopyrimidine Derivatives as Anticancer Agents: Design, Synthesis, Biological Evaluation and In Silico Studies. Molecules 2023, 28, 4271. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]














| Identification Code | Compound 1 | Compound 2 |
|---|---|---|
| CCDC | 887013 | 887014 |
| Empirical formula | C10H8N4OS | C10H8N4O3S |
| Formula weight | 232.26 | 264.26 |
| Temperature/K | 294(2) | 294(2) |
| Crystal system | Monoclinic | monoclinic |
| Space group | P21/c | P21 |
| a/Å | 10.41500(10) | 9.6216(2) |
| b/Å | 5.06310(10) | 4.92060(10) |
| c/Å | 18.6564(3) | 12.1623(3) |
| α/° | 90 | 90 |
| β/° | 96.8570(10) | 106.628(2) |
| γ/° | 90 | 90 |
| Volume/Å3 | 976.76(3) | 551.73(2) |
| Z | 4 | 2 |
| ρcalcg/cm3 | 1.579 | 1.591 |
| μ/mm−1 | 2.813 | 2.711 |
| F(000) | 480 | 272 |
| Crystal size/mm3 | 0.35 × 0.15 × 0.1 | 0.2 × 0.1 × 0.02 |
| Radiation | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) |
| 2Θ range for data collection/° | 8.56 to 153.78 | 7.58 to 153.36 |
| Index ranges | −13 ≤ h ≤ 13, −6 ≤ k ≤ 6, −23 ≤ l ≤ 23 | −12 ≤ h ≤ 12, −6 ≤ k ≤ 6, −14 ≤ l ≤ 15 |
| Reflections collected | 15,980 | 9640 |
| Independent reflections | 2052 [Rint = 0.0328, Rsigma = 0.0130] | 2304 [Rint = 0.0247, Rsigma = 0.0176] |
| Data/restraints/parameters | 2052/0/151 | 2304/1/168 |
| Goodness-of-fit on F2 | 1.054 | 1.039 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0303, wR2 = 0.0883 | R1 = 0.0299, wR2 = 0.0807 |
| Final R indexes [all data] | R1 = 0.0310, wR2 = 0.0894 | R1 = 0.0311, wR2 = 0.0820 |
| Largest diff. peak/hole/e Å−3 | 0.24/−0.21 | 0.15/−0.26 |
| Compound 1 | Compound 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Length/Å | Length/Å | ||||||||
| Atom | SC-XRD | DFT | MAE | MSE | Atom | SC-XRD | DFT | MAE | MSE |
| S1—C9 | 1.7402(14) | 1.7522 | 0.012 | 0.000144 | S1—O2 | 1.4421(16) | 1.391 | 0.0511 | 0.002611 |
| S1—C10 | 1.7980(15) | 1.814 | 0.016 | 0.000256 | S1—O3 | 1.4296(16) | 1.3927 | 0.0369 | 0.001362 |
| O1—C7 | 1.2145(18) | 1.2131 | 0.0014 | 1.96 × 10−6 | S1—C9 | 1.7726(17) | 1.723 | 0.0496 | 0.00246 |
| N1—N2 | 1.3848(15) | 1.3678 | 0.017 | 0.000289 | S1—C10 | 1.744(2) | 1.6803 | 0.0637 | 0.004058 |
| N1—C1 | 1.3897(17) | 1.3904 | 0.0007 | 4.90 × 10−7 | O1—C1 | 1.211(2) | 1.2417 | 0.0307 | 0.000942 |
| N1—C8 | 1.3443(15) | 1.3528 | 0.0085 | 7.22 × 10−5 | N1—C1 | 1.370(3) | 1.404 | 0.034 | 0.001156 |
| N2—C9 | 1.3237(17) | 1.3188 | 0.0049 | 2.40 × 10−5 | N1—C8 | 1.364(2) | 1.3869 | 0.0229 | 0.000524 |
| N3—C8 | 1.3192(18) | 1.3109 | 0.0083 | 6.89 × 10−5 | N2—N3 | 1.3718(19) | 1.3354 | 0.0364 | 0.001325 |
| N3—C9 | 1.3759(16) | 1.3711 | 0.0048 | 2.30 × 10−5 | N2—C7 | 1.397(2) | 1.4122 | 0.0152 | 0.000231 |
| N4—C7 | 1.3800(19) | 1.3918 | 0.0118 | 0.000139 | N2—C8 | 1.348(2) | 1.4471 | 0.0991 | 0.009821 |
| N4—C8 | 1.3614(17) | 1.3668 | 0.0054 | 2.92 × 10−5 | N3—C9 | 1.312(2) | 1.3664 | 0.0544 | 0.002959 |
| C1—C2 | 1.3942(17) | 1.3946 | 0.0004 | 1.60 × 10−7 | N4—C8 | 1.321(2) | 1.3662 | 0.0452 | 0.002043 |
| C1—C6 | 1.4008(18) | 1.4032 | 0.0024 | 5.76 × 10−6 | N4—C9 | 1.355(2) | 1.4124 | 0.0574 | 0.003295 |
| C2—C3 | 1.378(2) | 1.3868 | 0.0088 | 7.74 × 10−5 | C1—C2 | 1.479(3) | 1.4802 | 0.0012 | 1.44 × 10−6 |
| C3—C4 | 1.390(2) | 1.3996 | 0.0096 | 9.22 × 10−5 | C2—C3 | 1.397(3) | 1.3999 | 0.0029 | 8.41 × 10−6 |
| C4—C5 | 1.3800(19) | 1.3853 | 0.0053 | 2.81 × 10−5 | C2—C7 | 1.395(2) | 1.4193 | 0.0243 | 0.00059 |
| C5—C6 | 1.391(2) | 1.3973 | 0.0063 | 3.97 × 10−5 | C3—C4 | 1.377(3) | 1.3912 | 0.0142 | 0.000202 |
| C6—C7 | 1.4828(18) | 1.4828 | 0 | 0 | C4—C5 | 1.387(3) | 1.3965 | 0.0095 | 9.03 × 10−5 |
| 0.006867 | 7.17 × 10−5 | C5—C6 | 7.17 × 10−5 | 1.3917 | 0.0127 | 0.000161 | |||
| C6—C7 | 1.385(2) | 1.4081 | 0.0231 | 0.000534 | |||||
| 0.034225 | 0.001719 | ||||||||
| Compound 1 | Angle/° | Compound 2 | Angle/° | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Atoms | SC-XRD | DFT | MAE | MSE | Atoms | SC-XRD | DFT | MAE | MSE |
| C9—S1—C10 | 100.67(7) | 99.4244 | 1.2456 | 1.551519 | O2—S1—C9 | 105.18(9) | 108.6153 | 3.4353 | 11.80129 |
| N2—N1—C1 | 126.72(10) | 127.0981 | 0.3781 | 0.14296 | O2—S1—C10 | 109.57(10) | 110.4953 | 0.9253 | 0.85618 |
| C8—N1—N2 | 109.76(10) | 109.1637 | 0.5963 | 0.355574 | O3—S1—O2 | 119.93(11) | 117.6775 | 2.2525 | 5.073756 |
| C8—N1—C1 | 123.49(11) | 123.7383 | 0.2483 | 0.061653 | O3—S1—C9 | 108.24(9) | 109.1524 | 0.9124 | 0.832474 |
| C9—N2—N1 | 101.13(10) | 101.6318 | 0.5018 | 0.251803 | O3—S1—C10 | 109.44(11) | 109.7427 | 0.3027 | 0.091627 |
| C8—N3—C9 | 102.03(11) | 101.5335 | 0.4965 | 0.246512 | C10—S1—C9 | 103.09(9) | 99.6218 | 3.4682 | 12.02841 |
| C8—N4—C7 | 123.00(11) | 123.6761 | 0.6761 | 0.457111 | C8—N1—C1 | 122.54(16) | 120.7368 | 1.8032 | 3.25153 |
| N1—C1—C2 | 122.47(12) | 122.0837 | 0.3863 | 0.149228 | N3—N2—C7 | 126.61(13) | 129.5362 | 2.9262 | 8.562646 |
| N1—C1—C6 | 116.55(11) | 116.7211 | 0.1711 | 0.029275 | C8—N2—N3 | 109.28(14) | 109.4571 | 0.1771 | 0.031364 |
| C2—C1—C6 | 120.97(12) | 121.1952 | 0.2252 | 0.050715 | C8—N2—C7 | 124.09(14) | 121.0067 | 3.0833 | 9.506739 |
| C3—C2—C1 | 118.83(13) | 118.6206 | 0.2094 | 0.043848 | C9—N3—N2 | 100.69(13) | 105.4517 | 4.7617 | 22.67379 |
| C2—C3—C4 | 121.01(13) | 121.0563 | 0.0463 | 0.002144 | C8—N4—C9 | 100.67(15) | 103.4791 | 2.8091 | 7.891043 |
| C5—C4—C3 | 119.85(13) | 119.8135 | 0.0365 | 0.001332 | O1—C1—N1 | 120.47(19) | 117.9045 | 2.5655 | 6.58179 |
| C4—C5—C6 | 120.60(13) | 120.2804 | 0.3196 | 0.102144 | O1—C1—C2 | 123.5(2) | 124.0836 | 0.5836 | 0.340589 |
| C1—C6—C7 | 121.28(13) | 121.6514 | 0.3714 | 0.137938 | N1—C1—C2 | 116.01(16) | 118.0117 | 2.0017 | 4.006803 |
| C5—C6—C1 | 118.70(12) | 119.0339 | 0.3339 | 0.111489 | C3—C2—C1 | 120.22(17) | 118.8431 | 1.3769 | 1.895854 |
| C5—C6—C7 | 120.00(12) | 119.3147 | 0.6853 | 0.469636 | C7—C2—C1 | 121.66(16) | 121.8593 | 0.1993 | 0.03972 |
| O1—C7—N4 | 121.17(13) | 120.9174 | 0.2526 | 0.063807 | C7—C2—C3 | 118.12(18) | 119.2976 | 1.1776 | 1.386742 |
| O1—C7—C6 | 123.63(14) | 124.4552 | 0.8252 | 0.680955 | C4—C3—C2 | 120.10(18) | 120.3188 | 0.2188 | 0.047873 |
| N4—C7—C6 | 115.18(12) | 114.6274 | 0.5526 | 0.305367 | C3—C4—C5 | 120.47(18) | 120.1428 | 0.3272 | 0.10706 |
| N1—C8—N4 | 120.29(12) | 119.5857 | 0.7043 | 0.496038 | C6—C5—C4 | 120.88(19) | 120.929 | 0.049 | 0.002401 |
| N3—C8—N1 | 111.11(11) | 111.5024 | 0.3924 | 0.153978 | C5—C6—C7 | 118.24(18) | 119.2141 | 0.9741 | 0.948871 |
| N3—C8—N4 | 128.60(11) | 128.9119 | 0.3119 | 0.097282 | C2—C7—N2 | 115.60(15) | 117.3809 | 1.7809 | 3.171605 |
| N2—C9—S1 | 125.39(9) | 123.963 | 1.427 | 2.036329 | C6—C7—N2 | 122.22(16) | 122.5214 | 0.3014 | 0.090842 |
| N2—C9—N3 | 115.95(12) | 116.1686 | 0.2186 | 0.047786 | C6—C7—C2 | 122.18(17) | 120.0977 | 2.0823 | 4.335973 |
| N3—C9—S1 | 118.65(10) | 119.8684 | 1.2184 | 1.484499 | N2—C8—N1 | 119.95(15) | 121.0028 | 1.0528 | 1.108388 |
| 0.493488 | 0.366574 | N4—C8—N1 | 128.56(17) | 130.6879 | 2.1279 | 4.527958 | |||
| N4—C8—N2 | 111.47(16) | 108.3091 | 3.1609 | 9.991289 | |||||
| N3—C9—S1 | 121.05(13) | 124.8163 | 3.7663 | 14.18502 | |||||
| N3—C9—N4 | 117.88(15) | 113.3029 | 4.5771 | 20.94984 | |||||
| N4—C9—S1 | 120.94(13) | 121.8281 | 0.8881 | 0.788722 | |||||
| 1.808658 | 5.068006 | ||||||||
| Atoms | D–H…A | d(D-H)/Å | d(H-A)/Å | d(D-A)/Å | D-H-A/° | Symmetry Codes |
|---|---|---|---|---|---|---|
| Comp 1 | N4–H4…N3 | 0.85(2) | 2.05(2) | 2.896(2) | 174(2) | X,1-Y,-Z |
| Comp 2 | N1–H1…O2 | 0.880(10) | 2.230(10) | 3.072(2) | 160(3) | 1-X,1/2+Y,-Z |
| Conformation | ∆E (kcal/mole) | BSSE (kcal/mole) | ∆E(BSSE) (kcal/mole) |
|---|---|---|---|
| A | 1.19 | 4.969 | 6.16 |
| B | −5.08 | 1.010 | −4.08 |
| C | −12.05 | 7.097 | −4.96 |
| D | −22.56 | 5.095 | −17.47 |
| E | −4.52 | 1.286 | −3.24 |
| F | −24.63 | 5.993 | −18.64 |
| BCP | Atoms | ρ(r) | ∇2ρ(r) | Ellipticity | K | BPL | V | G | |V(r)|/G(r) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cp. 1 | 2 | N4—H35 | 0.006289 | 0.022212 | 0.113048 | 0.000705 | 5.140017 | −0.00414 | 0.004849 | 0.854609 |
| 26 | C32—O74 | 0.002331 | 0.007681 | 0.373475 | 0.000422 | 8.496853 | −0.00108 | 0.001499 | 0.718479 | |
| 28 | H22—O74 | 0.008058 | 0.033277 | 0.112983 | 0.000988 | 4.8339 | −0.00634 | 0.007332 | 0.865248 | |
| 35 | N27—C86 | 0.002116 | 0.007772 | 1.994151 | 0.000359 | 7.571653 | −0.00123 | 0.001585 | 0.773502 | |
| 37 | C40—C88 | 0.002703 | 0.007564 | 1.070424 | 0.000347 | 7.568092 | −0.0012 | 0.001545 | 0.775405 | |
| 58 | H48—S49 | 0.009736 | 0.044927 | 0.042777 | 0.001263 | 5.028214 | −0.00871 | 0.009968 | 0.873295 | |
| 61 | N52—H83 | 0.006285 | 0.0222 | 0.11302 | 0.000705 | 5.140599 | −0.00414 | 0.004846 | 0.854519 | |
| 63 | N30—C84 | 0.001735 | 0.007364 | 0.982007 | 0.000343 | 7.38067 | −0.00116 | 0.001499 | 0.771181 | |
| 84 | O26—C80 | 0.00233 | 0.007679 | 0.373457 | 0.000422 | 8.497276 | −0.00108 | 0.001498 | 0.718291 | |
| 85 | O26—H70 | 0.008056 | 0.033269 | 0.113215 | 0.000988 | 4.834342 | −0.00634 | 0.007329 | 0.865193 | |
| 91 | C38—N75 | 0.002115 | 0.00777 | 2.007461 | 0.000358 | 7.572067 | −0.00123 | 0.001584 | 0.77399 | |
| 95 | S1—H96 | 0.009735 | 0.044921 | 0.042777 | 0.001264 | 5.02833 | −0.0087 | 0.009967 | 0.873181 | |
| 96 | C36—N78 | 0.001736 | 0.007364 | 0.980273 | 0.000342 | 7.380474 | −0.00116 | 0.001499 | 0.771848 | |
| Cp. 2 | 28 | H26—H43 | 0.00125 | 0.004449 | 1.398681 | 0.000312 | 5.768847 | −0.00049 | 0.0008 | 0.61 |
| 47 | O4—C44 | 0.003221 | 0.012971 | 0.939037 | 0.000607 | 6.92691 | −0.00203 | 0.002637 | 0.769814 | |
| 48 | N8—H43 | 0.006246 | 0.024646 | 0.232877 | 0.0012 | 5.249185 | −0.00376 | 0.004962 | 0.758162 | |
| 49 | H19—C42 | 0.00253 | 0.007617 | 0.381515 | 0.000456 | 6.695947 | −0.00099 | 0.001448 | 0.685083 | |
| 54 | C18—O56 | 0.003229 | 0.012955 | 0.922371 | 0.000601 | 6.906885 | −0.00204 | 0.002637 | 0.772089 | |
| 61 | H51—O55 | 0.006083 | 0.028012 | 0.799943 | 0.001387 | 5.645358 | −0.00423 | 0.005616 | 0.753027 | |
| 63 | N34—O55 | 0.003969 | 0.016565 | 0.327863 | 0.00065 | 7.549581 | −0.00284 | 0.003492 | 0.81386 | |
| 64 | N34—O56 | 0.003614 | 0.016076 | 0.130798 | 0.000491 | 6.45756 | −0.00304 | 0.003528 | 0.860828 | |
| 66 | C44—O56 | 0.001118 | 0.006521 | 0.708403 | 0.000421 | 7.459908 | −0.00079 | 0.001209 | 0.651778 | |
| 67 | H52—O56 | 0.00249 | 0.013368 | 0.190436 | 0.000822 | 5.731315 | −0.0017 | 0.002521 | 0.673939 | |
| 70 | C40—N59 | 0.004426 | 0.012864 | 0.469134 | 0.00035 | 7.335367 | −0.00252 | 0.002867 | 0.877921 | |
| 71 | C44—N60 | 0.003265 | 0.010471 | 0.854723 | 0.000425 | 7.250512 | −0.00177 | 0.002192 | 0.806113 | |
| 74 | H17—N60 | 0.006257 | 0.024645 | 0.235346 | 0.001199 | 5.250095 | −0.00376 | 0.004962 | 0.758364 | |
| 78 | C37—N61 | 0.003655 | 0.010319 | 0.466641 | 0.000393 | 7.100146 | −0.00179 | 0.002187 | 0.820302 | |
| 79 | C46—C74 | 0.00326 | 0.009824 | 1.03613 | 0.000481 | 7.154223 | −0.00149 | 0.001974 | 0.756332 | |
| 92 | C16—H71 | 0.002573 | 0.007717 | 0.433497 | 0.000461 | 6.787062 | −0.00101 | 0.001469 | 0.686181 | |
| 100 | H17—H78 | 0.001246 | 0.004458 | 8.173816 | 0.000309 | 5.807382 | −0.0005 | 0.000805 | 0.616149 |
| Global Descriptors | Compound 1 | Compound 2 |
|---|---|---|
| E_HOMO(N) (eV) | −6.0688 | −7.2636 |
| E_HOMO(N+1) (eV) | 1.7367 | 1.2328 |
| E_HOMO(N−1) (eV) | −10.9226 | −11.8528 |
| Vertical (IP) (eV) | 7.7485 | 8.894 |
| Vertical (EA) (eV) | 0.0255 | 0.4702 |
| Mulliken electronegativity (eV) | 3.887 | 4.6821 |
| Chemical potential (μ) (eV) | −3.887 | −4.6821 |
| Hardness (η) (eV) | 7.723 | 8.4238 |
| Softness (S) (eV−1) | 0.1295 | 0.1187 |
| Electrophilicity index (ω) (eV) | 0.9782 | 1.3012 |
| Nucleophilicity index (N) (eV) | 3.0524 | 1.8576 |
| Compound 2 | Compound 1 | ||||||
|---|---|---|---|---|---|---|---|
| Atoms | P− | P+ | f0 | Atoms | (P+) | (P−) | f0 |
| C1 | 447.28 | 166.77 | 121.56 | C1 | 195.43 | −7.35 | 124.45 |
| C2 | 476.92 | 181.24 | 122.09 | C2 | −116.57 | 938.72 | 185.1 |
| C3 | 340.39 | −30.09 | 133.11 | C3 | 304.5 | −291.51 | 134.16 |
| C4 | −97.8 | 556.11 | 174.86 | C4 | −57.1 | 684.78 | 142.83 |
| C5 | 849.93 | −176.33 | 185.89 | C5 | 151.19 | 318.02 | 103.44 |
| C6 | −254.2 | 280.53 | 122.35 | C6 | −7.61 | 223.25 | 74.56 |
| H7 | −21.58 | 0.21 | 77.45 | H7 | −10.66 | −5.99 | 68 |
| H8 | 0.62 | −28.07 | 95.83 | H8 | 4.59 | −61.33 | 97.93 |
| H9 | −55.9 | 6.59 | 102.92 | H9 | −14.83 | 12.15 | 82.97 |
| H10 | 9.14 | −15.88 | 72.2 | H10 | 1.98 | −41.27 | 76.93 |
| N11 | −65.61 | 438.06 | 68 | C11 | −69.6 | 447.77 | 142.04 |
| N12 | 137.13 | 275.75 | 112.63 | N12 | 348.88 | 22.13 | 68.26 |
| H13 | −9.45 | −12.42 | 78.77 | N13 | 114.53 | 72.15 | 85.85 |
| C14 | 342.34 | −136.03 | 136.79 | H14 | −5.93 | −9.01 | 68.53 |
| O15 | 289.44 | 388.28 | 249.95 | C15 | 90.49 | −7.12 | 64.59 |
| N16 | 39.92 | 114.61 | 99.51 | N16 | −52.4 | −1.76 | 85.85 |
| C17 | 145.22 | 348.13 | 123.14 | C17 | 23.91 | −0.26 | 78.5 |
| C18 | 35.67 | 175.46 | 71.68 | N18 | 430.67 | −3.02 | 116.83 |
| N19 | −26.23 | −117.04 | 103.44 | O19 | 211.64 | 335.37 | 227.63 |
| S20 | 25.01 | −56.16 | 50.41 | S20 | 1049.34 | −0.19 | 389.1 |
| C21 | 1.84 | −3.44 | 34.13 | C21 | −43.53 | 0.01 | 56.71 |
| H22 | 0.03 | −0.29 | 22.05 | H22 | 38.47 | −0.01 | 47 |
| H23 | −0.2 | 0.96 | 18.9 | H23 | −0.37 | −0.02 | 57.24 |
| H24 | 6.16 | 2.31 | 49.62 | H24 | 38.47 | −0.01 | 47 |
| O25 | 3.48 | 102.09 | 89.53 | ||||
| O26 | 5.93 | 164.14 | 108.96 | ||||
| H Atoms | HAT | SET-PT | SPLET | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BDE | IP | PDE | PA | ETE | ||||||
| Cp. 1 | Cp. 2 | Cp. 1 | Cp. 2 | Cp. 1 | Cp. 2 | Cp. 1 | Cp. 2 | Cp. 1 | Cp. 2 | |
| H-7 | 482.57 | 481.37 | 734.82 | 853.66 | 1063.64 | 943.61 | 1609.3 | 1597.83 | 189.16 | 199.44 |
| H-8 | 470.52 | 474.35 | 734.82 | 853.66 | 1051.6 | 936.59 | 1618.94 | 1603.12 | 167.48 | 187.12 |
| H-9 | 473.75 | 472.06 | 734.82 | 853.66 | 1054.82 | 934.3 | 1630.51 | 1594.98 | 159.14 | 192.98 |
| H-10 | 480.28 | 483.89 | 734.82 | 853.66 | 1061.36 | 946.13 | 1627.06 | 1577.74 | 169.12 | 222.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakheit, A.H.; Abuelizz, H.A.; Al-Salahi, R. A DFT Study and Hirshfeld Surface Analysis of the Molecular Structures, Radical Scavenging Abilities and ADMET Properties of 2-Methylthio(methylsulfonyl)-[1,2,4]triazolo [1,5-a]quinazolines: Guidance for Antioxidant Drug Design. Crystals 2023, 13, 1086. https://doi.org/10.3390/cryst13071086
Bakheit AH, Abuelizz HA, Al-Salahi R. A DFT Study and Hirshfeld Surface Analysis of the Molecular Structures, Radical Scavenging Abilities and ADMET Properties of 2-Methylthio(methylsulfonyl)-[1,2,4]triazolo [1,5-a]quinazolines: Guidance for Antioxidant Drug Design. Crystals. 2023; 13(7):1086. https://doi.org/10.3390/cryst13071086
Chicago/Turabian StyleBakheit, Ahmed H., Hatem A. Abuelizz, and Rashad Al-Salahi. 2023. "A DFT Study and Hirshfeld Surface Analysis of the Molecular Structures, Radical Scavenging Abilities and ADMET Properties of 2-Methylthio(methylsulfonyl)-[1,2,4]triazolo [1,5-a]quinazolines: Guidance for Antioxidant Drug Design" Crystals 13, no. 7: 1086. https://doi.org/10.3390/cryst13071086
APA StyleBakheit, A. H., Abuelizz, H. A., & Al-Salahi, R. (2023). A DFT Study and Hirshfeld Surface Analysis of the Molecular Structures, Radical Scavenging Abilities and ADMET Properties of 2-Methylthio(methylsulfonyl)-[1,2,4]triazolo [1,5-a]quinazolines: Guidance for Antioxidant Drug Design. Crystals, 13(7), 1086. https://doi.org/10.3390/cryst13071086








